Microbial Profile and Antibiotic Resistance Patterns in Bile Aspirates from Patients with Acute Cholangitis: A Multicenter International Study
Abstract
1. Introduction
2. Results
2.1. Patients and General Characteristics
2.2. Etiologies of Acute Cholangitis
2.3. Microbial Agents Associated with AC
2.4. Resistance Patterns
2.5. Multidrug-Resistant Bacteria Involvement
2.6. Antimicrobial Treatment
2.7. Risk Factors Associated with MDR Bacteria Infections
3. Discussion
3.1. Risk Factors Associate with MDR Bacteria Infections
3.2. Antimicrobial Resistance Rates
4. Materials and Methods
4.1. Study Design and Ethical Considerations
4.2. Patient Selection and Bile Samples Collection
4.3. Data Acquisition and Study Variables
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | antibiotic resistance |
| AC | acute cholangitis |
| CBD | common bile duct |
| ERCP | endoscopic retrograde cholangiopancreatography |
| AMR | antimicrobial resistance |
| RF | risk factors |
| TG18 | 2018 Tokyo Guidelines |
| PTC | ultrasonographic-guided percutaneous transhepatic cholangiography |
| ESGE | European Society of Gastrointestinal Endoscopy |
| ESGENA | European Society of Gastroenterology Nurses and Associates |
| AGREE | Adverse Events for Gastrointestinal Endoscopy Classification |
| CCH | Colentina Clinical Hospital |
| HLH | Haut-Lévêque Hospital |
| CRP | C-reactive protein |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| ICU | intensive care unit |
References
- Miuțescu, B.; Vuletici, D.; Burciu, C.; Turcu-Stiolica, A.; Bende, F.; Rațiu, I.; Moga, T.; Sabuni, O.; Anjary, A.; Dalati, S.; et al. Identification of Microbial Species and Analysis of Antimicrobial Resistance Patterns in Acute Cholangitis Patients with Malignant and Benign Biliary Obstructions: A Comparative Study. Medicina 2023, 59, 721. [Google Scholar] [CrossRef]
- Miutescu, B.; Vuletici, D.; Burciu, C.; Bende, F.; Ratiu, I.; Moga, T.; Gadour, E.; Bratosin, F.; Tummala, D.; Sandru, V.; et al. Comparative Analysis of Antibiotic Resistance in Acute Cholangitis Patients with Stent Placement and Sphincterotomy Interventions. Life 2023, 13, 2205. [Google Scholar] [CrossRef]
- Miutescu, B.; Vuletici, D.; Burciu, C.; Bende, F.; Ratiu, I.; Moga, T.; Gadour, E.; Reddy, S.; Sandru, V.; Balan, G.; et al. Comparative Analysis of Microbial Species and Multidrug Resistance Patterns in Acute Cholangitis Patients with Cholecystectomy: A Single-Center Study. Diseases 2024, 12, 19. [Google Scholar] [CrossRef]
- Melzer, M.; Toner, R.; Lacey, S.; Bettany, E.; Rait, G. Biliary tract infection and bacteraemia: Presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad. Med. J. 2007, 83, 773–776. [Google Scholar] [CrossRef]
- Kasparian, K.; Christou, C.D.; Petidis, K.; Doumas, M.; Giouleme, O. Short vs. long-course antibiotic therapy in adults with acute cholangitis: A systematic review, meta-analysis, and evidence quality assessment. World J. Gastroenterol. 2023, 29, 3027–3039. [Google Scholar] [CrossRef]
- Englesbe, M.J.; Dawes, L.G. Resistant pathogens in biliary obstruction: Importance of cultures to guide antibiotic therapy. HPB 2005, 7, 144–148. [Google Scholar] [CrossRef]
- Schneider, J.; De Waha, P.; Hapfelmeier, A.; Feihl, S.; Rommler, F.; Schlag, C.; Algul, H.; Schmid, R.M.; Wantia, N.; Huber, W.; et al. Risk factors for increased antimicrobial resistance: A retrospective analysis of 309 acute cholangitis episodes. J. Antimicrob. Chemother. 2014, 69, 519–525. [Google Scholar] [CrossRef]
- Miura, F.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Pitt, H.A.; Gomi, H.; Solomkin, J.S.; Schlossberg, D.; Han, H.; et al. Tokyo Guidelines 2018: Initial management of acute biliary infection and flowchart for acute cholangitis. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 31–40. [Google Scholar] [CrossRef]
- Gromski, M.A.; Gutta, A.; Lehman, G.A.; Tong, Y.; Fogel, E.L.; Watkins, J.L.; Easler, J.J.; Bick, B.L.; McHenry, L.; Beeler, C.; et al. Microbiology of bile aspirates obtained at ERCP in patients with suspected acute cholangitis. Endoscopy 2022, 54, 1045–1052. [Google Scholar] [CrossRef]
- Lee, C.C.; Chang, I.J.; Lai, Y.C.; Chen, S.Y.; Chen, S.C. Epidemiology and Prognostic Determinants of Patients with Bacteremic Cholecystitis or Cholangitis. Off. J. Am. Coll. Gastroenterol. 2007, 102, 563–569. [Google Scholar] [CrossRef]
- Mosler, P. Diagnosis and Management of Acute Cholangitis. Curr. Gastroenterol. Rep. 2011, 13, 166–172. [Google Scholar] [CrossRef]
- Gomi, H.; Solomkin, J.S.; Schlossberg, D.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Ukai, T.; Endo, I.; Iwashita, Y.; Hibi, T.; et al. Tokyo Guidelines 2018: Antimicrobial therapy for acute cholangitis and cholecystitis. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 3–16. [Google Scholar] [CrossRef]
- Kaya, M. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. WJG 2012, 18, 3585. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, S.; Bai, X.; Song, J.; Fan, Q.; Chen, J. A Retrospective Study on Bile Culture and Antibiotic Susceptibility Patterns of Patients with Biliary Tract Infections. Muzammil, S., editor. Evid.-Based Complement. Altern. Med. 2022, 2022, 9255444. [Google Scholar]
- Otani, T.; Ichiba, T.; Seo, K.; Naito, H. Blood cultures should be collected for acute cholangitis regardless of severity. J. Infect. Chemother. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance [Internet]; World Health Organization: Geneva, Switzerland, 2015; 28p, Available online: https://iris.who.int/handle/10665/193736 (accessed on 19 January 2025).
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019 [Internet]; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA, 2019. Available online: https://stacks.cdc.gov/view/cdc/82532 (accessed on 19 January 2025).
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; 1p.
- Reuken, P.A.; Torres, D.; Baier, M.; Löffler, B.; Lübbert, C.; Lippmann, N.; Stallmach, A.; Bruns, T. Risk Factors for Multi-Drug Resistant Pathogens and Failure of Empiric First-Line Therapy in Acute Cholangitis. Galdiero, M., editor. PLoS ONE 2017, 12, e0169900. [Google Scholar]
- Kwon, J.S.; Han, J.; Kim, T.W.; Oh, J.-H.; Kwon, H.H.; Jung, J.T.; Kwon, J.G.; Kim, E.Y.; Kim, H.G. Changes in Causative Pathogens of Acute Cholangitis and Their Antimicrobial Susceptibility over a Period of 6 Years. Korean J. Gastroenterol. 2014, 63, 299. [Google Scholar] [CrossRef]
- Rahbe, E.; Watier, L.; Guillemot, D.; Glaser, P.; Opatowski, L. Determinants of worldwide antibiotic resistance dynamics across drug-bacterium pairs: A multivariable spatial-temporal analysis using ATLAS. Lancet Planet. Health 2023, 7, e547–e557. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. IDR 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- World Health Organization. Strengthening Romania’s Health System to Address Antimicrobial Resistance; WHO Regional Office: Copenhagen, Denmark, 2020. [Google Scholar]
- Rafila, A.; Streinu-Cercel, A.; Pistol, A.; Florea, D.; Valeriu, G. National Action Plan on Antimicrobial Resistance in Romania. 2021. Available online: https://mateibals.ro/wp-content/uploads/2022/05/3_plan_actiune.pdf (accessed on 19 January 2025).
- Hîncu, S.; Apetroaei, M.M.; Negulescu, M.C.; Blidaru, A.; Ghica, M.; Udeanu, D.I. Implementation of an Antibiotic Restriction Formulary and the Impact on Consumption in a Romanian Hospital: A Three-Year Retrospective Study. Farmacia 2025, 73, 376–392. [Google Scholar]
- Maugat, S.; Berger-Carbonne, A.; Nion-Huang, M.; Ben Hmidene, G.; Cavalié, P. Antibiotic resistance prevention: A “One Health” Approach [Internet]. 2022. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/resistance-aux-antibiotiques/documents/rapport-synthese/prevention-de-la-resistance-aux-antibiotiques-une-demarche-une-seule-sante (accessed on 19 January 2025).
- French Ministry of Solidarity and Health. 2022–2025 National Strategy for Preventing Infections and Antibiotic Resistance [Internet]. 2022. Available online: https://www.who.int/publications/m/item/france-national-strategy-for-preventing-infections-and-antibiotic-resistance (accessed on 19 January 2025).
- Pulcini, C.; Berger-Carbonne, A.; Coignard, B.; Salomon, J. National Antibiotic Resistance Strategy for Human Health in France. China CDC Wkly. 2022, 4, 1097–1100. [Google Scholar] [CrossRef]
- Kruis, T.; Güse-Jaschuck, S.; Siegmund, B.; Adam, T.; Epple, H.-J. Use of microbiological and patient data for choice of empirical antibiotic therapy in acute cholangitis. BMC Gastroenterol. 2020, 20, 65. [Google Scholar] [CrossRef]
- Reiter, F.P.; Obermeier, W.; Jung, J.; Denk, G.; Mahajan, U.M.; De Toni, E.N.; Schirra, J.; Mayerle, J.; Schulz, C. Prevalence, Resistance Rates, and Risk Factors of Pathogens in Routine Bile Cultures Obtained during Endoscopic Retrograde Cholangiography. Dig. Dis. 2021, 39, 42–51. [Google Scholar] [CrossRef]
- Shafagh, S.; Rohani, S.H.; Hajian, A. Biliary infection; distribution of species and antibiogram study. Ann. Med. Surg. 2021, 70, 102822. [Google Scholar] [CrossRef]
- Suh, S.-W.; Choi, Y.S.; Choi, S.-H.; Do, J.H.; Oh, H.-C.; Kim, H.J.; Lee, S.E. Antibiotic selection based on microbiology and resistance profiles of bile from gallbladder of patients with acute cholecystitis. Sci. Rep. 2021, 11, 2969. [Google Scholar] [CrossRef]
- Krupik, Y.; Ariam, E.; Cohen, D.L.; Bermont, A.; Vosko, S.; Shirin, H.; Matalon, S. Do the Results of Bile Cultures Affect the Outcomes of Patients with Mild-to-Moderate Ascending Cholangitis? A Single Center Prospective Study. Diagnostics 2025, 15, 695. [Google Scholar] [CrossRef]
- Rehman, S.; Ahmed, S.; Metry, M.; Canelo, R. Significance of Bile Culture and Biliary Tract Pathology in Determining Severity of Cholangitis: Review of Current Literature. Ann. Emerg. Surg. 2017, 2, 1009. Available online: https://www.jscimedcentral.com/jounal-article-info/Annals-of-Emergency-Surgery/Significance-of-Bile-Culture--and-Biliary-Tract-Pathology--in-Determining-Severity-of--Cholangitis%3B-Review-of-Current--Literature-2897 (accessed on 19 January 2025).
- Stathopoulos, P.; Lerner, P.; Astheimer, P.; Breitling, L.P.; Zumblick, M.; Pararas, M.; Lohoff, M.; Gress, T.M.; Denzer, U.W. Endoscopic retrograde cholangiopancreatography-obtained bile culture in acute cholangitis: Retrospective analysis of bile cultures and risk factors in a tertiary care center. J. Gastroenterol. Hepatol. 2024, 39, 935–941. [Google Scholar] [CrossRef]
- Jahn, M.; Özçürümez, M.K.; Dolff, S.; Rohn, H.; Heider, D.; Dechêne, A.; Canbay, A.; Rath, P.M.; Katsounas, A. A Multipathogen Bile Sample-based PCR Assay Can Guide Empirical Antimicrobial Strategies in Cholestatic Liver Diseases. J. Clin. Transl. Hepatol. 2022, 10, 788–795. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Cao, J.; Chen, Y.; Yu, J.; Sun, N. Identification and characterization of bile microbiota in patients with biliary obstructive diseases using next-generation sequencing of 16S rRNA and ITS. Front. Cell. Infect. Microbiol. 2025, 15, 1575824. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Zhao, M.; Pan, X. Clinical and biochemical factors for bacteria in bile among patients with acute cholangitis. Eur. J. Gastroenterol. Hepatol. 2025, 37, 33–38. [Google Scholar] [CrossRef]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Wakabayashi, G.; Kozaka, K.; Endo, I.; Deziel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef]
- Marchetti, B.; Boustière, C.; Chapuis, C.; Systchenko, R.; Arpurt, J.-P.; Barrioz, T.; Barthet, M.; Cellier, C.; Croguennec, B.; Dalbiès, P.-A.; et al. La désinfection du matériel en endoscopie digestive. Acta Endosc. 2007, 37, 699–704. [Google Scholar] [CrossRef]
- Beilenhoff, U.; Biering, H.; Blum, R.; Brljak, J.; Cimbro, M.; Dumonceau, J.-M.; Hassan, C.; Jung, M.; Neumann, C.; Pietsch, M.; et al. Prevention of multidrug-resistant infections from contaminated duodenoscopes: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA). Endoscopy 2017, 49, 1098–1106. [Google Scholar] [CrossRef]
- OLYMPUS EVIS EXERA III TJF-Q190V Duodenoscope Reprocessiing Manual. [Internet]. Available online: https://www.olympus-europa.com/medical/en/Contact-and-Support (accessed on 19 January 2025).
- Société Française d’Hygiène Hospitalière (SF2H). Guide de Bonnes Pratiques de Traitement des Dispositifs Médicaux Réutilisables; SF2H: Paris, France, 2022; Available online: https://www.sf2h.net/k-stock/data/uploads/2022/11/Guide_DM_22_SF2H.pdf (accessed on 19 January 2025).
- Nass, K.J.; Zwager, L.W.; Van Der Vlugt, M.; Dekker, E.; Bossuyt, P.M.M.; Ravindran, S.; Thomas-Gibson, S.; Fockens, P. Novel classification for adverse events in GI endoscopy: The AGREE classification. Gastrointest. Endosc. 2022, 95, 1078–1085.e8. [Google Scholar] [CrossRef]

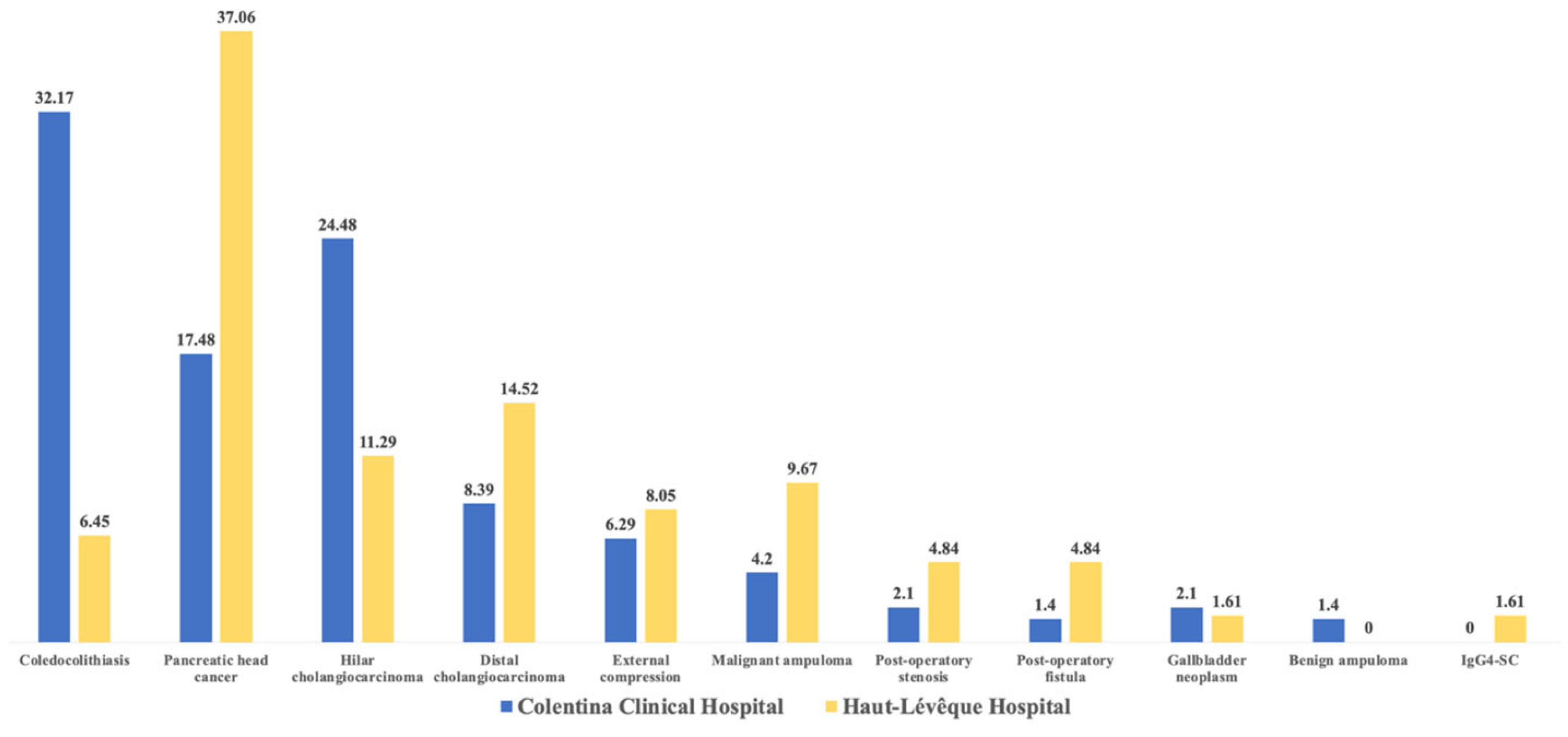
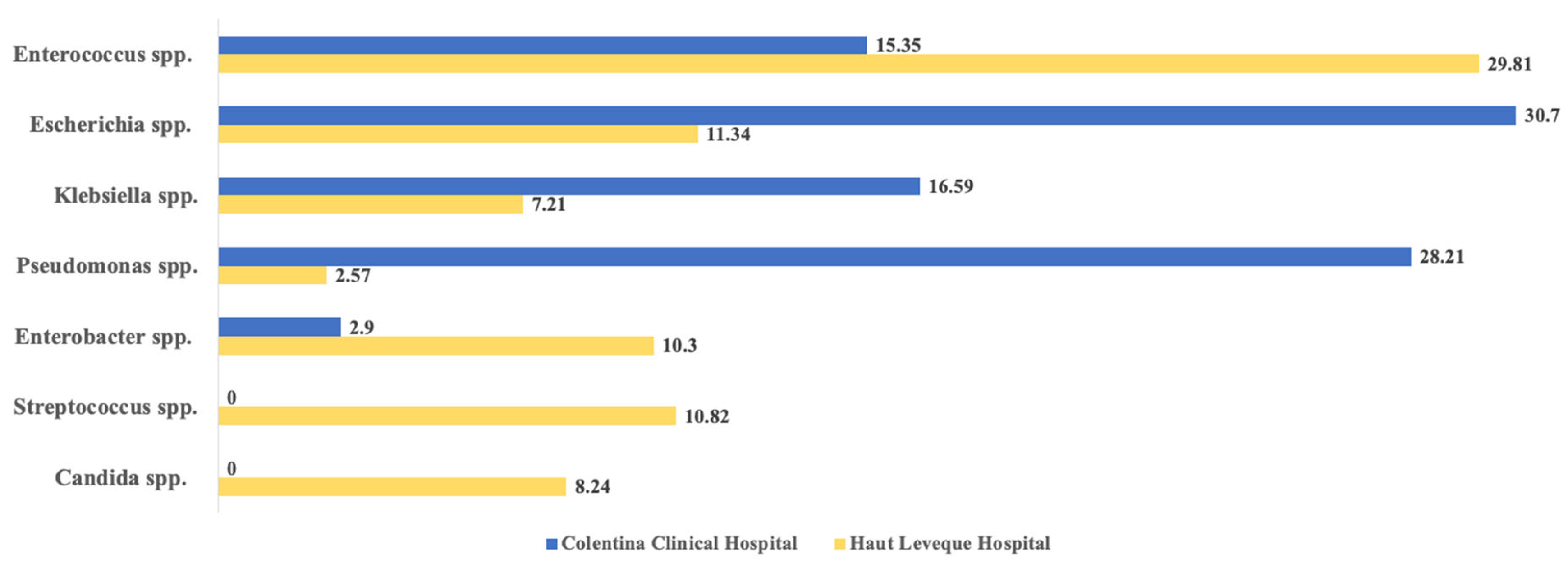
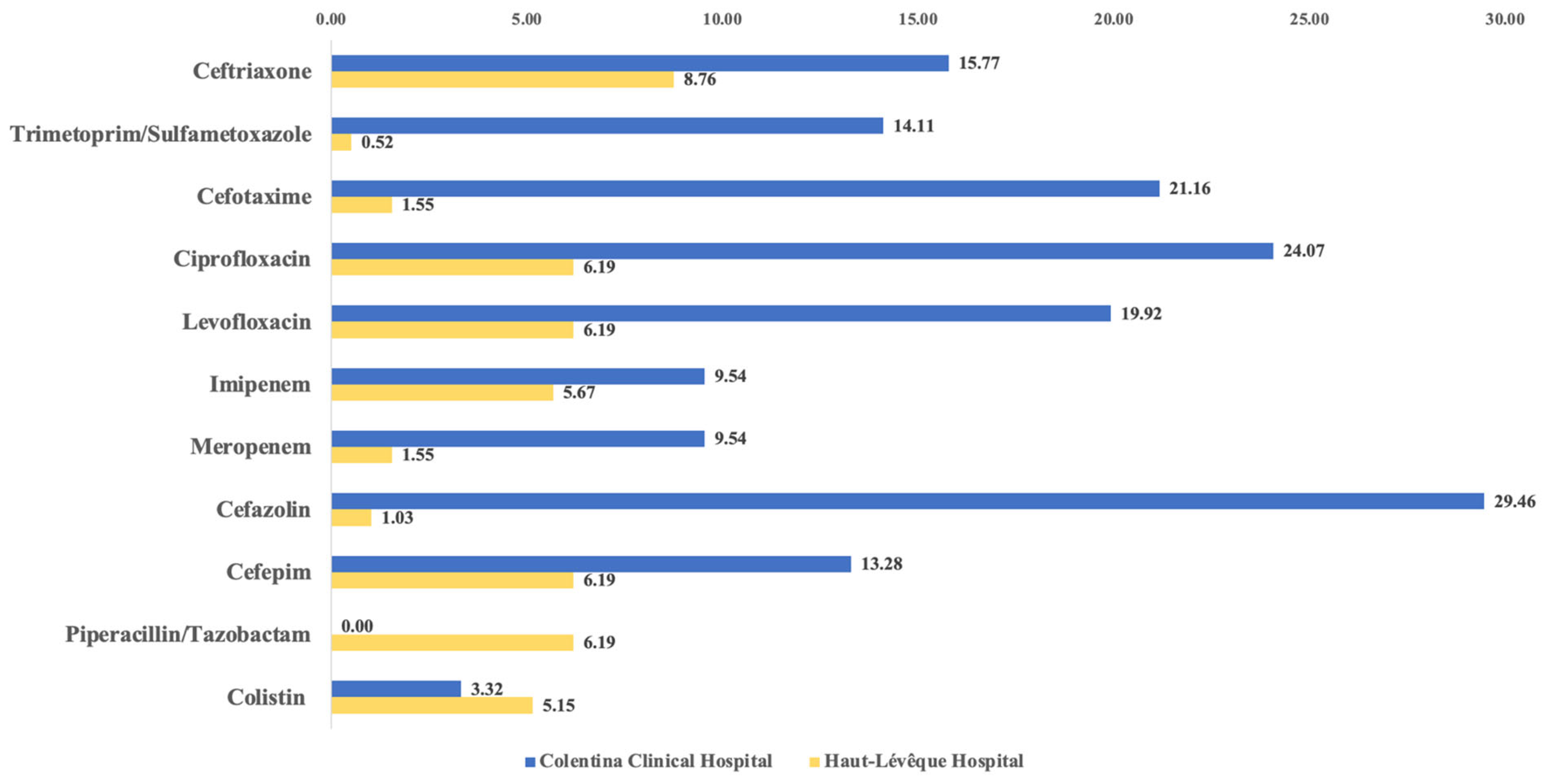
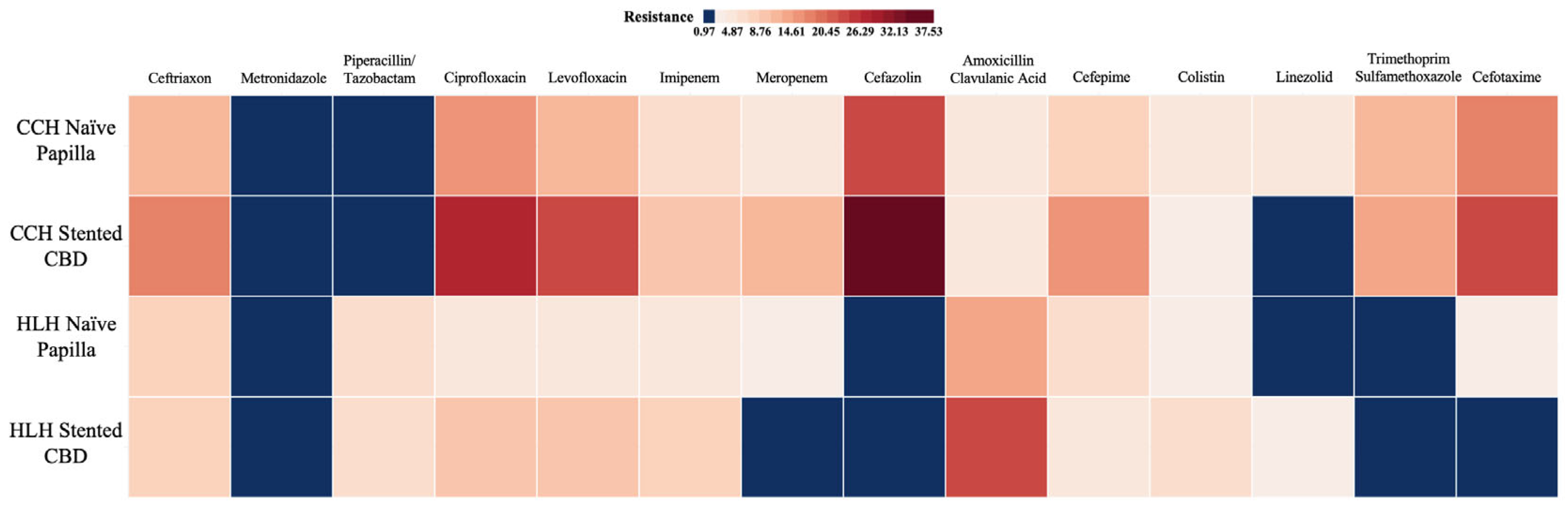
| Variables | Colentina Clinical Hp. | Haut-Lévêque Hp. | Comparison Between Totals (p-Value) | ||||
|---|---|---|---|---|---|---|---|
| Total (n = 143) | Naive Papilla (n = 66) | Stented CBD (n = 77) | Total (n = 62) | Naive Papilla (n = 41) | Stented CBD (n = 21) | ||
| Male sex (%) | 83 (58.04) | 38 (57.57) | 45 (58.44) | 40 (64.51) | 23 (56.09) | 17 (80.95) | 0.5355 |
| Mean age (SD) | 68.49 (10.40) | 67.71 (10.49) | 69.15 (10.34) | 70.34 (9.02) | 70.24 (9.52) | 70.58 (8.16) | 0.7243 |
| Malignant Stenosis (%) | 83 (58.04%) | 7 (10.60) | 60 (77.92) | 47 (75.80) | 31 (75.60) | 16 (76.19) | 0.0179 |
| Cholecystectomy (%) | 41 | 14 | 27 | 27 | 18 | 9 | 0.0521 |
| Episodes of AC | |||||||
| Variables | Colentina Clinical Hp. | Haut-Lévêque Hp. | |||||
| Total n = 189 | Naive Papilla n = 86 | Stent N = 103 | Total N = 67 | Naive Papilla N = 42 | Stent N = 25 | ||
| Hospitalization days. (mean) | 5.64 | 5.59 | 5.67 | 3.35 | 3.38 | 3.32 | <0.0001 |
| Complications. yes | 57 (30.15%) | 26 (30.23%) | 31 (30.09%) | 13 (19.40%) | 7 (16.66%) | 6 (24%) | 0.1107 |
| Tokyo Severity score | |||||||
| I | 140 (74.07%) | 68 (79.06%) | 72 (69.90%) | 45 (67.16%) | 28 (66.66%) | 17 (68%) | 0.3406 |
| II | 33 (17.46%) | 14 (16.27%) | 19 (18.44%) | 15 (22.38%) | 10 (23.80%) | 5 (20%) | 0.3686 |
| III | 16 (8.46%) | 4 (4.65%) | 12 (11.65%) | 7 (10.44%) | 4 (9.52%) | 3 (12%) | 0.6237 |
| Need for reintervention in the same hospitalization | 22 (11.64%) | 9 (10.46%) | 13 (12.62%) | 2 (2.98%) | 1 (2.38%) | 1 (4%) | 0.0484 |
| ICU admission | 6 (3.17%) | 1 (1.16%) | 5 (4.85%) | 4 (5.97%) | 2 (4.76%) | 2 (8%) | 0.2941 |
| Need for readmission < 72 h after initial discharge | 4 (2.11%) | 1 (1.16%) | 3 (2.91%) | 0 | 0 | 0 | 0.5755 |
| Initial neg culture in previous episode | 44 | 2 | 42 | No data | Not possible | ||
| Positive/Negative blood cultures | 19/18 | 10/7 | 9/11 | 22/9 | 14/4 | 8/5 | |
| WBC (mean value) (/mm3) | 12,388 | 12,100 | 12,630 | 11,861 | 11,519 | 12,437 | >0.9999 |
| TB (mean value) (mg/dL) | 8.18 | 9.39 | 7.17 | 7.07 | 6.92 | 7.31 | >0.9999 |
| CRP (mean value) | 91.94 | 74.51 | 106.51 | 85.87 | 83.26 | 90.24 | 0.8821 |
| CCH | HLH | Comparison Between Totals (p Value) | |||||
|---|---|---|---|---|---|---|---|
| Variables | Total (n = 190) | Naive Papilla (n = 78) | Stented CBD (n = 112) | Total (n = 67) | Naive Papilla (n = 42) | Stented CBD (n = 25) | |
| 1 bact | 142 | 63 | 79 | 18 | 8 | 10 | <0.0001 |
| 2 bact | 45 | 14 | 31 | 13 | 10 | 3 | 0.5026 |
| 3 bact | 3 | 1 | 2 | 12 | 5 | 7 | <0.0001 |
| ≥4 bact | 0 | 0 | 0 | 24 | 19 | 5 | <0.0001 |
| Causative Agents | Colentina Clinical Hp. | Haut-Lévêque Hp. | Comparison Between Totals (p Value) | ||||
|---|---|---|---|---|---|---|---|
| Total (n = 241) | Naive Papilla (n = 94) | Stented CBD (n = 147) | Total (n = 194) | Naive Papilla (n = 135) | Stented CBD (n = 59) | ||
| Enterococcus spp. | 37 (15.35%) | 14 (14.89%) | 23 (15.64%) | 58 (29.81%) | 40 (29.62%) | 18 (30.50%) | 0.0003 |
| Escherichia spp. | 74 (30.70%) | 24 (25.53%) | 50 (34.01%) | 22 (11.34%) | 17 (12.59%) | 5 (8.47%) | <0.0001 |
| Klebsiella spp. | 40 (16.59%) | 11 (11.70%) | 29 (19.72%) | 14 (7.21%) | 11 (8.14%) | 3 (5.08%) | 0.0033 |
| Enterobacter spp. | 7 (2.90%) | 5 (5.32%) | 2 (1.36%) | 20 (10.30%) | 12 (8.88%) | 8 (13.55%) | 0.0022 |
| Streptococcus spp. | 0 (0%) | 0 (0.0%) | 0 (0.0%) | 21 (10.82%) | 12 (8.88%) | 9 (15.25%) | <0.0001 |
| Candida spp. | 0 (0%) | 0 (0.0%) | 0 (0.0%) | 16 (8.54%) | 12 (8.88%) | 4 (6.77%) | <0.0001 |
| Pseudomonas spp. | 68 (28.21%) | 33 (35.10%) | 35 (23.80%) | 5 (2.57%) | 4 (2.96%) | 1 (1.69%) | <0.0001 |
| CCH (n = 241) | HLH (n = 194) | p-Value | |
|---|---|---|---|
| Ceftriaxone | 38 (15.77%) | 17 (8.76%) | 0.030 |
| Piperacillin/Tazobactam | 0 (0.00%) | 12 (6.19%) | <0.0001 |
| Ciprofloxacin | 58 (24.07%) | 12 (6.19%) | <0.0001 |
| Levofloxacin | 48 (19.92%) | 12 (6.19%) | <0.0001 |
| Meropenem | 23 (9.54%) | 3 (1.55%) | 0.0003 |
| Cefazolin | 71 (29.46%) | 2 (1.03%) | <0.0001 |
| Amoxicillin/Clavulanic Acid | 11 (4.56%) | 42 (21.65%) | <0.0001 |
| Colistin | 8 (3.32%) | 10 (5.15%) | 0.346 |
| Linezolid | 6 (2.49%) | 3 (1.55%) | 0.737 |
| Trimethoprim/Sulfamethoxazole | 34 (14.11%) | 1 (0.52%) | <0.0001 |
| Cefotaxime | 51 (21.16%) | 3 (1.55%) | <0.0001 |
| Colentina Clinical Hospital (n = 241) | Haut-Lévêque Hospital (n = 194) | p-Value N-CCH vs. N-HLH | p-Value S-CCH vs. S-HLH | |||||
|---|---|---|---|---|---|---|---|---|
| Naïve Papilla (n = 94) | Stented CBD (n = 147) | p-Value N-CCH vs. S-CCH | Naïve Papilla (n = 136) | Stented CBD (n = 58) | p-Value N-HLH vs. S-HLH | |||
| Ceftriaxone | 12 (12.77%) | 26 (17.69%) | 0.366 | 12 (8.82%) | 5 (8.62%) | >0.9999 | 0.383 | 0.130 |
| Piperacillin/Tazobactam | 0 (0.0%) | 0 (0.0%) | >0.9999 | 8 (5.88%) | 4 (6.9%) | 0.753 | 0.022 | 0.0059 |
| Ciprofloxacin | 16 (17.02%) | 42 (28.57%) | 0.045 | 6 (4.41%) | 6 (10.34%) | 0.188 | 0.002 | 0.0056 |
| Levofloxacin | 11 (11.7%) | 37 (25.17%) | 0.012 | 6 (4.41%) | 6 (10.34%) | 0.188 | 0.043 | 0.021 |
| Imipenem | 7 (7.45%) | 16 (10.88%) | 0.501 | 6 (4.41%) | 5 (8.62%) | 0.309 | 0.389 | 0.799 |
| Meropenem | 5 (5.32%) | 18 (12.24%) | 0.113 | 3 (2.21%) | 0 (0.0%) | 0.555 | 0.276 | 0.002 |
| Cefazolin | 35 (37.23%) | 36 (24.49%) | 0.042 | 2 (1.47%) | 0 (0.0%) | >0.9999 | >0.9999 | >0.9999 |
| Amoxicillin/Clavulanic Acid | 4 (4.26%) | 7 (4.76%) | >0.9999 | 33 (24.26%) | 9 (15.52%) | 0.189 | >0.9999 | 0.017 |
| Cefepime | 8 (8.51%) | 24 (16.33%) | 0.118 | 9 (6.62%) | 3 (5.17%) | >0.9999 | 0.615 | 0.038 |
| Colistin | 5 (5.32%) | 3 (2.04%) | 0.267 | 8 (5.88%) | 2 (3.45%) | 0.726 | >0.9999 | 0.622 |
| Linezolid | 5 (5.32%) | 1 (0.68%) | 0.034 | 3 (2.21%) | 0 (0.0%) | 0.555 | 0.276 | >0.9999 |
| Trimethoprim/Sulfamethoxazole | 12 (12.77%) | 22 (14.97%) | 0.706 | 0 (0.0%) | 1 (1.72%) | 0.298 | >0.9999 | 0.0056 |
| Cefotaxime | 23 (24.47%) | 28 (19.05%) | 0.335 | 3 (2.21%) | 0 (0.0%) | 0.555 | >0.9999 | >0.9999 |
| MDR Agents | Colentina Clinical Hp. | Haut-Lévêque Hp. | ||||
|---|---|---|---|---|---|---|
| Total 51 | Naive Papilla 11 | Stent 40 | Total 15 | Naive Papilla 7 | Stent 8 | |
| Extended-spectrum beta-lactamase (ESBL) | 28 | 4 | 24 | 6 | 4 | 2 |
| Carbapenemase-producing Enterobacterales (CPE) | 7 | 1 | 6 | 0 | 0 | 0 |
| Carbapenemase-producing Enterobacterales New Delhi metallo-β-lactamase (CPE-NDM) | 2 | 0 | 2 | 0 | 0 | 0 |
| Vancomycin-resistant Enterococci (VRE) | 5 | 3 | 2 | 0 | 0 | 0 |
| Macrolide–lincosamide–streptogramin B antibiotics resistant (MLS) | 0 | 0 | 0 | 8 | 2 | 6 |
| High level aminoglycoside resistant Enterococci (HLAR) | 7 | 2 | 5 | 0 | 0 | 0 |
| Others * | 2 | 1 | 1 | 1 | 1 | 0 |
| Antibiotics Used as First-line Treatment | Colentina Clinical Hp. | Haut-Lévêque Hp. | ||||
|---|---|---|---|---|---|---|
| Total | Naïve Papilla | Stented CBD | Total | Naive Papilla | Stented CBD | |
| Ceftriaxone + Metronidazole | 121 | 43 | 78 | 2 | 0 | 2 |
| Ceftriaxone | 22 | 9 | 13 | 0 | 0 | 0 |
| Ciprofloxacin | 13 | 9 | 4 | 0 | 0 | 0 |
| Meropenem | 9 | 2 | 7 | 2 | 2 | 0 |
| Amoxicillin/Clavulanic Acid | 5 | 4 | 1 | 9 | 5 | 4 |
| Piperacillin/Tazobactam | 0 | 0 | 0 | 30 | 19 | 11 |
| Variables | Odds Ratio (Lower CI–Upper CI) | p-Value | Odds Ratio (Lower CI–Upper CI) | p-Value |
|---|---|---|---|---|
| CCH | HLH | |||
| Age > 75 years old | 1.232 (0.642–2.356) | 0.531 | 1.057 (0.34–3.292) | 0.923 |
| Male sex | 1.137 (0.606–2.136) | 0.689 | 0.643 (0.194–2.132) | 0.471 |
| Polymicrobial bile cultures | 1.786 (0.954–3.343) | 0.700 | 1.356 (0.167–11.01) | 0.776 |
| Positive blood culture | 2.646 (0.212–1.967) | 0.042 | 0.231 (0.029–1.814) | 0.163 |
| Complications | 0.678 (0.331–1.388) | 0.288 | 3.148 (0.98–10.116) | 0.054 |
| Malignant stenosis | 1.664 (0.815–3.397) | 0.162 | 0.537 (0.139–2.071) | 0.367 |
| Resistance to ≥2 Fluoroquinolones | 6.25 (2.817–13.869) | <0.001 | 19.444 (4.752–79.566) | <0.001 |
| Resistance to Carbapenems | 4.45 (1.803–10.982) | 0.001 | Not significant | |
| Resistance to ≥2 III/IV Cephalosporins | 6.009 (3.084–11.708) | <0.001 | 3.107 (0.013–9.533) | 0.047 |
| Resistance to Piperacillin/Tazobactam | 1.985 (0.894–4.405) | 0.092 | 3.99 (1.225–12.994) | 0.022 |
| Resistance to Amoxicillin/Clavulanic Acid | 2.213 (0.622–7.876) | 0.22 | 3.508 (1.136–10.831) | 0.029 |
| Hospital stay | 0.969 (0.881–1.065) | 0.51 | 1.24 (0.939–1.636) | 0.129 |
| ICU admission | 0.61 (0.072–5.185) | 0.651 | Not significant | |
| Need for reintervention | 0.87 (0.311–2.432) | 0.79 | Not significant | |
| Stent placement | 2.847 (1.377–5.888) | 0.005 | 2.462 (0.823–7.366) | 0.107 |
| Tokyo score of III | 0.861 (0.277–2.682) | 0.059 | Not significant | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, M.-A.; Găman, M.-A.; Diaconu, C.C.; Berger, A.; Zerbib, F.; Mateescu, R.B. Microbial Profile and Antibiotic Resistance Patterns in Bile Aspirates from Patients with Acute Cholangitis: A Multicenter International Study. Antibiotics 2025, 14, 679. https://doi.org/10.3390/antibiotics14070679
Cozma M-A, Găman M-A, Diaconu CC, Berger A, Zerbib F, Mateescu RB. Microbial Profile and Antibiotic Resistance Patterns in Bile Aspirates from Patients with Acute Cholangitis: A Multicenter International Study. Antibiotics. 2025; 14(7):679. https://doi.org/10.3390/antibiotics14070679
Chicago/Turabian StyleCozma, Matei-Alexandru, Mihnea-Alexandru Găman, Camelia Cristina Diaconu, Arthur Berger, Frank Zerbib, and Radu Bogdan Mateescu. 2025. "Microbial Profile and Antibiotic Resistance Patterns in Bile Aspirates from Patients with Acute Cholangitis: A Multicenter International Study" Antibiotics 14, no. 7: 679. https://doi.org/10.3390/antibiotics14070679
APA StyleCozma, M.-A., Găman, M.-A., Diaconu, C. C., Berger, A., Zerbib, F., & Mateescu, R. B. (2025). Microbial Profile and Antibiotic Resistance Patterns in Bile Aspirates from Patients with Acute Cholangitis: A Multicenter International Study. Antibiotics, 14(7), 679. https://doi.org/10.3390/antibiotics14070679








