Genotypic and Phenotypic Methods in the Detection of MDR-TB and Evolution to XDR-TB
Abstract
1. Introduction
2. Results
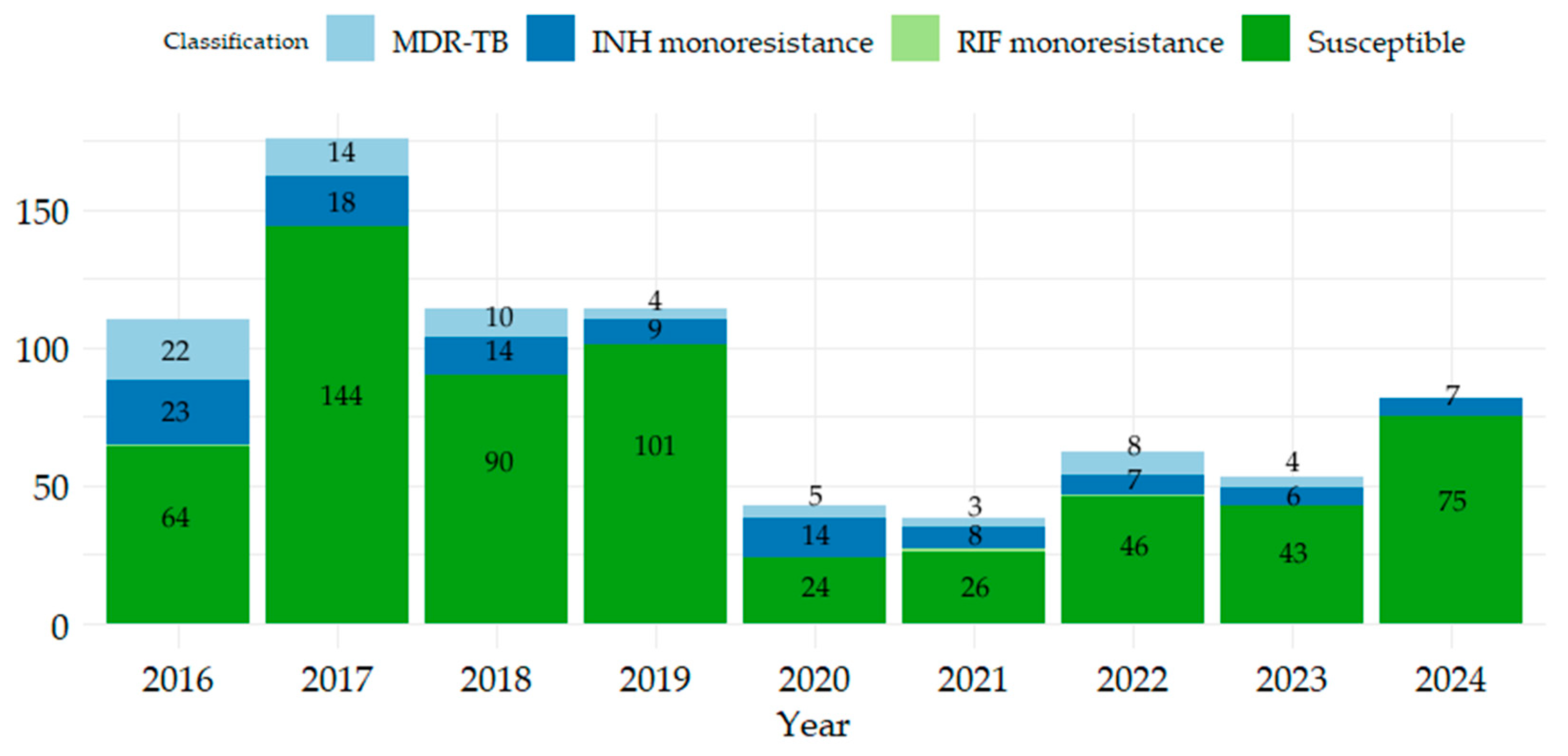
2.1. Description of Patients by Gender, Age Range, Product, and Patient Category According to Resistance/Sensitivity to RIF and INH
2.2. Comparison of Phenotypic, Genotypic Methods
2.3. Analysis of Mutations Associated with Resistance to RIF and INH
2.4. Phenotypic Resistance to First-Line and Second-Line Drugs
3. Discussion
4. Materials and Methods
4.1. Data Collection
- New case: Patients who have never been treated for TB or who have received anti-TB drugs for less than a month.
- Relapse case: Patients previously declared “cured” or “treatment completed” who develop active TB again, bacteriologically confirmed by positive culture for M. tuberculosis. Relapses are considered if the recurrence of the disease is diagnosed at least 4 months after completion of previous treatment.
- Chronic case: Patients who have completed a full course of treatment, including relapse, but who continue to be positive for M. tuberculosis on bacteriological culture. Persistent disease is confirmed when the culture remains positive at least 12 months after the initiation of the first treatment, indicating failure of bacteriological cure.
4.2. Drug Susceptibility Testing (Laboratory Procedure)
4.2.1. Löwenstein–Jensen Solid Media Sensitivity Test Method
4.2.2. GenoType MTBDRplus
4.3. Data Analysis
4.4. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDQ | Bedaquiline |
| CAP | Capreomycin |
| CFZ | Clofazimine |
| DLM | Delamanid |
| DR-TB | Drug-resistant tuberculosis |
| DST | Drug susceptibility tests |
| EMB | Ethambutol |
| ETH | Ethionamide |
| GenoType MTBDRplus | LPA GenoType MTBDRplus molecular method |
| Hr-TB | Isoniazid-resistant TB |
| INH | Isoniazid |
| KM | Kanamycin |
| L-J solid-medium | Löwenstein–Jensen solid-medium method () |
| LPA | Line probe assay |
| LVX | Levofloxacin |
| LZD | Linezolid |
| MDR-TB | Multidrug-resistant tuberculosis |
| MFX | Moxifloxacin |
| MGIT | BACTEC MGIT 960 |
| OFL | Ofloxacin |
| PCR | Polymerase Chain Reaction |
| pre-XDR-TB | Pre-extensive drug-resistant TB |
| RDR | Resistance Determining Region |
| RIF | Rifampicin |
| RR-TB | Rifampicin-resistant tuberculosis |
| STR | Streptomycin |
| TB | Tuberculosis |
| WGS | Whole genome sequencing |
| WHO | World Health Organization |
| XDR-TB | Extensively drug-resistant tuberculosis |
References
- WHO. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023; p. 75. Available online: https://iris.who.int/ (accessed on 24 February 2025).
- Shrivas, A.; Singh, S.; Singh, J.; Shankar, P.; Soni, P.; Rufai, S.B.; Maurya, A.; Purwar, S. Discordance in genotypic and phenotypic drug susceptibility results: Time to reconsider critical concentration of rifampicin. Microbiol. Spectr. 2025, 13, e02236-24. [Google Scholar] [CrossRef] [PubMed]
- WHO. Annual Report of Tuberculosis; Annual Global TB Report of WHO; World Health Organization: Geneva, Switzerland, 2022; Volume 8, pp. 1–68. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 25 February 2025).
- Tuberculosis Surveillance and Monitoring in Europe 2025–2023 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2025-2023-data (accessed on 10 July 2025).
- Cheng, S.; Hide, M.; Pheng, S.H.; Kerléguer, A.; Delvallez, G.; Sam, S.; Mao, T.E.; Nguyen, T.V.A.; Bañuls, A.L. Resistance to Second-Line Anti-TB Drugs in Cambodia: A Phenotypic and Genetic Study. Infect. Drug Resist. 2021, 14, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Dheda, K.; Chesov, D.; Mandalakas, A.M.; Udwadia, Z.; Horsburgh, C.R., Jr. Management of drug-resistant tuberculosis. Lancet 2019, 394, 953–966. [Google Scholar] [CrossRef] [PubMed]
- WHO. Drug-Resistant TB Treatment. Available online: https://www.who.int/publications/i/item/9789240063129 (accessed on 4 February 2025).
- WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment—Drug-Resistant Tuberculosis Treatment, 2022 Update; World Health Organization: Geneva, Switzerland, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK588564/ (accessed on 25 February 2025).
- Viney, K.; Linh, N.N.; Gegia, M.; Zignol, M.; Glaziou, P.; Ismail, N.; Kasaeva, T.; Mirzayev, F. New definitions of pre-extensively and extensively drug-resistant tuberculosis: Update from the World Health Organization. Eur. Respir. J. 2021, 57, 2100361. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Qiang, R.; Fang, L.; Peng, X.; Zhang, H.; Cheng, H. Global trends in the incidence rates of MDR and XDR tuberculosis: Findings from the global burden of disease study 2019. Front. Pharmacol. 2023, 14, 1156249. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Perumal, R.; Ngema, S.L.; Shunmugam, L.; Somboro, A.M. Rapid Diagnosis of Drug-Resistant Tuberculosis-Opportunities and Challenges. Pathogens 2023, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Timișoara Regional Public Health Center; National Center for Health Assessment and Promotion; National Institute of Public Health. Situation Analysis—National Communicable Diseases Awareness Month Campaign: Tuberculosis, HIV, Hepatitis; Region I; National Institute of Public Health: Bucharest, Romania, 2020. Available online: https://www.dsptimis.ro/promovare/bt_20_analiza.pdf (accessed on 24 February 2025).
- Rahman, S.M.M.; Nasrin, R.; Rahman, A.; Ahmed, S.; Khatun, R.; Uddin, M.K.M.; Rahman, M.M.; Banu, S. Performance of GenoType MTBDRsl assay for detection of second-line drugs and ethambutol resistance directly from sputum specimens of MDR-TB patients in Bangladesh. PLoS ONE 2021, 16, e0261329. [Google Scholar] [CrossRef] [PubMed]
- Heyckendorf, J.; Andres, S.; Köser, C.U.; Olaru, I.D.; Schön, T.; Sturegård, E.; Beckert, P.; Schleusener, V.; Kohl, T.A.; Hillemann, D.; et al. What Is Resistance? Impact of Phenotypic versus Molecular Drug Resistance Testing on Therapy for Multi- and Extensively Drug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e01550-17. [Google Scholar] [CrossRef] [PubMed]
- Sadovska, D.; Nodieva, A.; Pole, I.; Vīksna, A.; Ķimsis, J.; Ozere, I.; Norvaiša, I.; Bogdanova, I.; Bandere, D.; Ranka, R. Discordance Between Phenotypic and WGS-Based Drug Susceptibility Testing Results for Some Anti-Tuberculosis Drugs: A Snapshot Study of Paired Mycobacterium tuberculosis Isolates with Small Genetic Distance. Infect. Drug Resist. 2024, 17, 3289–3307. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Anthwal, D.; Kumar, G.; Sah, G.; Salfinger, M.; Choudhury, S.; Arora, J.; Bhalla, M.; Myneedu, V.P.; Sarin, R.; et al. Genotypic characterization of ‘inferred’ rifampin mutations in GenoType MTBDRplus assay and its association with phenotypic susceptibility testing of Mycobacterium tuberculosis. Diagn. Microbiol. Infect. Dis. 2020, 96, 114995. [Google Scholar] [CrossRef] [PubMed]
- Bokop, C.; Faye, L.M.; Apalata, T. Analysis of Discordance between Genotypic and Phenotypic Assays for Rifampicin-Resistant Mycobacterium tuberculosis Isolated from Healthcare Facilities in Mthatha. Pathogens 2023, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.S.; Zhang, X.D.; Yan, J.W.; Huang, T.T.; Liu, Z.Z.; Li, Z.K.; Wang, L.; Li, F. Identification of Mycobacterium tuberculosis Resistance to Common Antibiotics: An Overview of Current Methods and Techniques. Infect. Drug Resist. 2024, 17, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.; Wegayehu, T.; Wondale, B.; Gebresilase, T.T.; Gebreyohannes, T.; Tekola, A.; Alemu, M.; Neway, S.; Adnew, B.; Nassir, M.; et al. Phenotypic and genotypic drug susceptibility patterns of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in Central and Southern Ethiopia. PLoS ONE 2023, 18, e0285063. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, H.; Jia, X.; Wang, X.; Xu, P. Mechanisms and detection methods of Mycobacterium tuberculosis rifampicin resistance: The phenomenon of drug resistance is complex. Tuberculosis 2021, 128, 102083. [Google Scholar] [CrossRef] [PubMed]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb. Perspect Med. 2015, 5, a017863. [Google Scholar] [CrossRef] [PubMed]
- WHO. Catalogue of Mutations in Mycobacterium Tuberculosis Complex and Their Association with Drug Resistance, 2nd ed.; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240082410 (accessed on 4 February 2025).
- Lv, H.; Zhang, X.; Zhang, X.; Bai, J.; You, S.; Li, X.; Li, S.; Wang, Y.; Zhang, W.; Xu, Y. Global prevalence and burden of multidrug-resistant tuberculosis from 1990 to 2019. BMC Infect. Dis. 2024, 24, 243. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, A.; Augustynowicz-Kopeć, E.; Garnczarek, J.; Halicka, A.; Koszela, M.; Litwiniuk, W.; Maj, D.; Mazur, I.; Niestrój-Ostrowska, J.; Podlasin, R.; et al. The impact of the war in Ukraine on the prevalence of MDR/RR-TB in Poland. IJTLD Open 2025, 2, 6–12. [Google Scholar] [CrossRef] [PubMed]
- WHO. Tuberculosis Resurges as Top Infectious Disease Killer. Available online: https://www.who.int/news/item/29-10-2024-tuberculosis-resurges-as-top-infectious-disease-killer (accessed on 31 December 2024).
- Huyen, M.N.; Tiemersma, E.W.; Lan, N.T.; Cobelens, F.G.; Dung, N.H.; Sy, D.N.; Buu, T.N.; Kremer, K.; Hang, P.T.; Caws, M.; et al. Validation of the GenoType® MTBDRplus assay for diagnosis of multidrug resistant tuberculosis in South Vietnam. BMC Infect. Dis. 2010, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Aainouss, A.; Momen, G.; Chaoui, I.; Lamaammal, A.; Chetioui, F.; Messaoudi, M.; Mouslim, J.; Khyatti, M.; El Messaoudi, M.D. Genotype MTBDRplus Assay Detection of Rifampicin and Isoniazid Resistant Mycobacterium tuberculosis in Morocco. Acta Sci. Microbiol. 2024, 7, 75–82. [Google Scholar] [CrossRef]
- Hussain, S.; Sultan, S.; Riaz, S.; Hussain, H.; Javed, H.; Mazhar, R. Synergy of Xpert (MTB/RIF) and Line probe assay for detection of rifampicin resistant strains of Mycobacterium tuberculosis. J. Infect. Dev. Ctries 2024, 18, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Ish, P. Tuberculosis with discordant drug resistance patterns- A diagnostic dilemma. Indian J. Tuberc. 2022, 69, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Yakrus, M.A.; Driscoll, J.; Lentz, A.J.; Sikes, D.; Hartline, D.; Metchock, B.; Starks, A.M. Concordance between molecular and phenotypic testing of Mycobacterium tuberculosis complex isolates for resistance to rifampin and isoniazid in the United States. J. Clin. Microbiol. 2014, 52, 1932–1937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yusoof, K.A.; García, J.I.; Schami, A.; Garcia-Vilanova, A.; Kelley, H.V.; Wang, S.H.; Rendon, A.; Restrepo, B.I.; Yotebieng, M.; Torrelles, J.B. Tuberculosis Phenotypic and Genotypic Drug Susceptibility Testing and Immunodiagnostics: A Review. Front. Immunol. 2022, 13, 870768. [Google Scholar] [CrossRef] [PubMed]
- Motta, I.; Boeree, M.; Chesov, D.; Dheda, K.; Günther, G.; Horsburgh, C.R., Jr.; Kherabi, Y.; Lange, C.; Lienhardt, C.; McIlleron, H.M.; et al. Recent advances in the treatment of tuberculosis. Clin. Microbiol. Infect. 2024, 30, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Rivière, E.; Whitfield, M.G.; Nelen, J.; Heupink, T.H.; Van Rie, A. Identifying isoniazid resistance markers to guide inclusion of high-dose isoniazid in tuberculosis treatment regimens. Clin. Microbiol. Infect. 2020, 26, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Catanzaro, D.; Catanzaro, A.; Rodwell, T.C. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: A systematic review. PLoS ONE 2015, 10, e0119628. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Jacobs, W.R., Jr. Resistance to Isoniazid and Ethionamide in Mycobacterium tuberculosis: Genes, Mutations, and Causalities. Microbiol. Spectr. 2014, 2, MGM2-0014-2013. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Sharma, R.; Kodan, P.; Soneja, M.; Jorwal, P.; Nischal, N.; Biswas, A.; Sarin, S.; Ramachandran, R.; Wig, N. Diagnostic Evaluation of Non-Interpretable Results Associated with rpoB Gene in Genotype MTBDRplus Ver 2.0. Tuberc. Respir. Dis. 2020, 83, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Raikwar, V.; Vashishtha, R. Mutation Profile Assessment of rpoB, KatG and inhA Genes in Rifampicin and Isoniazid Resistant Mycobacterium tuberculosis in India. JSRG 2020, 9, 556–559. [Google Scholar] [CrossRef]
- Van Deun, A.; Aung, K.J.; Bola, V.; Lebeke, R.; Hossain, M.A.; de Rijk, W.B.; Rigouts, L.; Gumusboga, A.; Torrea, G.; de Jong, B.C. Rifampin drug resistance tests for tuberculosis: Challenging the gold standard. J. Clin. Microbiol. 2013, 51, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Sirgel, F.A.; Warren, R.M.; Böttger, E.C.; Klopper, M.; Victor, T.C.; van Helden, P.D. The rationale for using rifabutin in the treatment of MDR and XDR tuberculosis outbreaks. PLoS ONE 2013, 8, e59414. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, F.B.; Guthrie, J.L.; Neemuchwala, A.; Lastovetska, O.; Melano, R.G.; Mehaffy, C. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J. Clin. Microbiol. 2014, 52, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.; Halse, T.A.; Kohlerschmidt, D.; Lapierre, P.; Modestil, H.A.; Kearns, C.H.; Dworkin, F.F.; Rakeman, J.L.; Escuyer, V.; Musser, K.A. Low-Level Rifampin Resistance and rpoB Mutations in Mycobacterium tuberculosis: An Analysis of Whole-Genome Sequencing and Drug Susceptibility Test Data in New York. J. Clin. Microbiol. 2021, 59, e01885-20. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Tan, Y.; Li, Z.; Tian, X.; Du, C.; Li, H.; Li, G.; Yao, X.; Wang, Z.; Xu, Y.; et al. Highly Sensitive Detection of Isoniazid Heteroresistance in Mycobacterium tuberculosis by DeepMelt Assay. J. Clin. Microbiol. 2018, 56, e01239-17. [Google Scholar] [CrossRef] [PubMed]
- Köser, C.U.; Georghiou, S.B.; Schön, T.; Salfinger, M. On the Consequences of Poorly Defined Breakpoints for Rifampin Susceptibility Testing of Mycobacterium tuberculosis Complex. J. Clin. Microbiol. 2021, 59, e02328-20. [Google Scholar] [CrossRef] [PubMed]
- Pontali, E.; Raviglione, M. Updated treatment guidelines for drug-resistant TB: How safe are clofazimine-based regimens? IJTLD Open 2024, 1, 486–489. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Handbook on Tuberculosis Laboratory Diagnostic Methods in the European Union. Updated 2022. Available online: https://www.ecdc.europa.eu (accessed on 10 July 2025).

| Category | RIF-R and INH-R | RIF-R and INH-S | RIF-S and INH-R | RMP-S and INH-S | Category Subtotal | |
|---|---|---|---|---|---|---|
| Gender | F | 15 | - | 29 | 210 | 254 |
| M | 55 | 3 | 77 | 403 | 538 | |
| Age | (0,20] | 2 | - | 5 | 40 | 47 |
| (20,40] | 24 | 1 | 26 | 155 | 206 | |
| (40,60] | 36 | 1 | 46 | 248 | 331 | |
| (60,80] | 8 | 1 | 25 | 141 | 175 | |
| (80,100] | - | - | 4 | 29 | 33 | |
| Sample | Respiratory | 69 | 3 | 100 | 575 | 747 |
| Non-respiratory | 1 | - | 6 | 38 | 45 | |
| Patient category | New case | 36 | 2 | 76 | 559 | 673 |
| Recurrence case | 10 | 1 | 21 | 52 | 84 | |
| Chronic case | 24 | - | 9 | 2 | 35 | |
| Total | 70 | 3 | 106 | 613 | 792 | |
| DST-RIF | SE (95% CI) | SP (95% CI) | A (95% CI) | PPV (95% CI) | NPV (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| GenoType MTBDplus | R | S | Total | 94.74% (0.7397, 0.9987) | 99.39% (0.9824, 0.9987) | 99.22% (0.9802, 0.9979) | 85.71% (0.6366, 0.9695) | 99.80% (0.9887, 0.9999) |
| detected | 18 | 3 | 21 | |||||
| undetected | 1 | 491 | 492 | |||||
| Total | 19 | 494 | 513 | |||||
| DST-INH | ||||||||
| GenoType MTBDplus | R | S | Total | 95.16% (0.8650, 0.9899) | 99.56% (0.9841, 0.9995) | 99.03% (0.9774, 0.9968) | 96.72% (0.8865, 0.9960) | 99.34% (0.9807, 0.9986) |
| detected | 59 | 2 | 61 | |||||
| undetected | 3 | 449 | 452 | |||||
| Total | 62 | 451 | 513 | |||||
| Gene/Mutation | New Cases (%) | Recurrent Cases (%) | Chronic Cases (%) | |
|---|---|---|---|---|
| rpoB | rpoBMUT1 (D516V) | 1 (0.21%) | - | 1 (14.28%) |
| rpoBMUT2B (H526D) | - | 1 (2.12%) | - | |
| rpoBMUT3 (S531L) | 6 (1.30%) | 1 (2.12%) | 3 (42.85%) | |
| rpoBWT4 (del 518) | 4 (0.87%) | - | 2 (28.57%) | |
| rpoB/WT4 | - | 1 (2.12%) | - | |
| katG | katG (Ser315Thr) | 36 (7.84%) | 8 (17.02%) | 6 (85.71%) |
| inh A | 7 (1.52%) | - | - | |
| katG/inhA | 2 (0.43%) | - | - | |
| Total | 459 | 47 | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaporojan, N.; Hodișan, R.; Pantiș, C.; Csep, A.N.; Zaporojan, C.; Zaha, D.C. Genotypic and Phenotypic Methods in the Detection of MDR-TB and Evolution to XDR-TB. Antibiotics 2025, 14, 732. https://doi.org/10.3390/antibiotics14070732
Zaporojan N, Hodișan R, Pantiș C, Csep AN, Zaporojan C, Zaha DC. Genotypic and Phenotypic Methods in the Detection of MDR-TB and Evolution to XDR-TB. Antibiotics. 2025; 14(7):732. https://doi.org/10.3390/antibiotics14070732
Chicago/Turabian StyleZaporojan, Natalia, Ramona Hodișan, Carmen Pantiș, Andrei Nicolae Csep, Claudiu Zaporojan, and Dana Carmen Zaha. 2025. "Genotypic and Phenotypic Methods in the Detection of MDR-TB and Evolution to XDR-TB" Antibiotics 14, no. 7: 732. https://doi.org/10.3390/antibiotics14070732
APA StyleZaporojan, N., Hodișan, R., Pantiș, C., Csep, A. N., Zaporojan, C., & Zaha, D. C. (2025). Genotypic and Phenotypic Methods in the Detection of MDR-TB and Evolution to XDR-TB. Antibiotics, 14(7), 732. https://doi.org/10.3390/antibiotics14070732







