Antimicrobial Susceptibility Testing of the Combination of Aztreonam and Avibactam in NDM-Producing Enterobacterales: A Comparative Evaluation Using the CLSI and EUCAST Methods
Abstract
1. Introduction
2. Results
2.1. Comparison with Broth Disk Elution Test
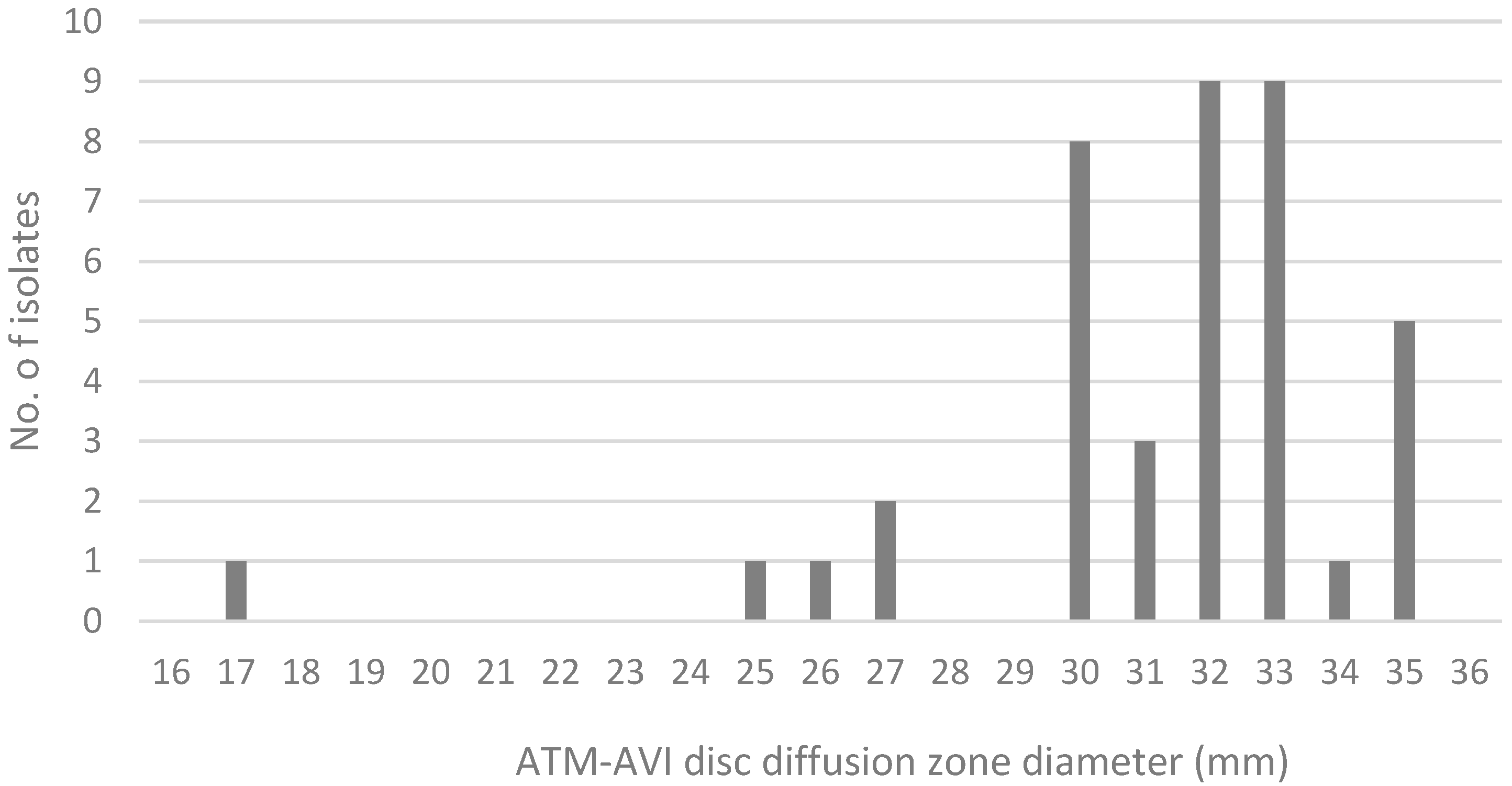
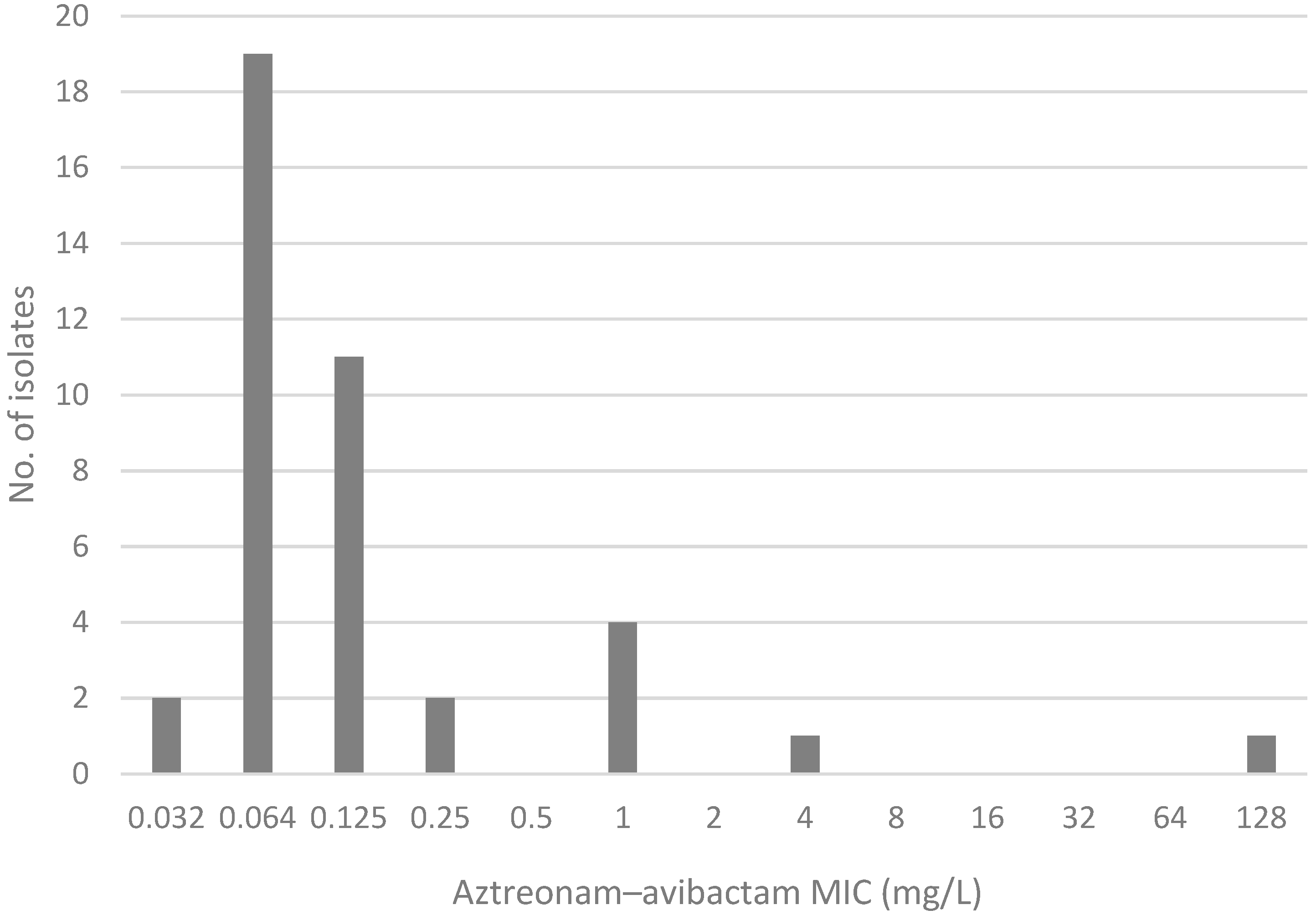
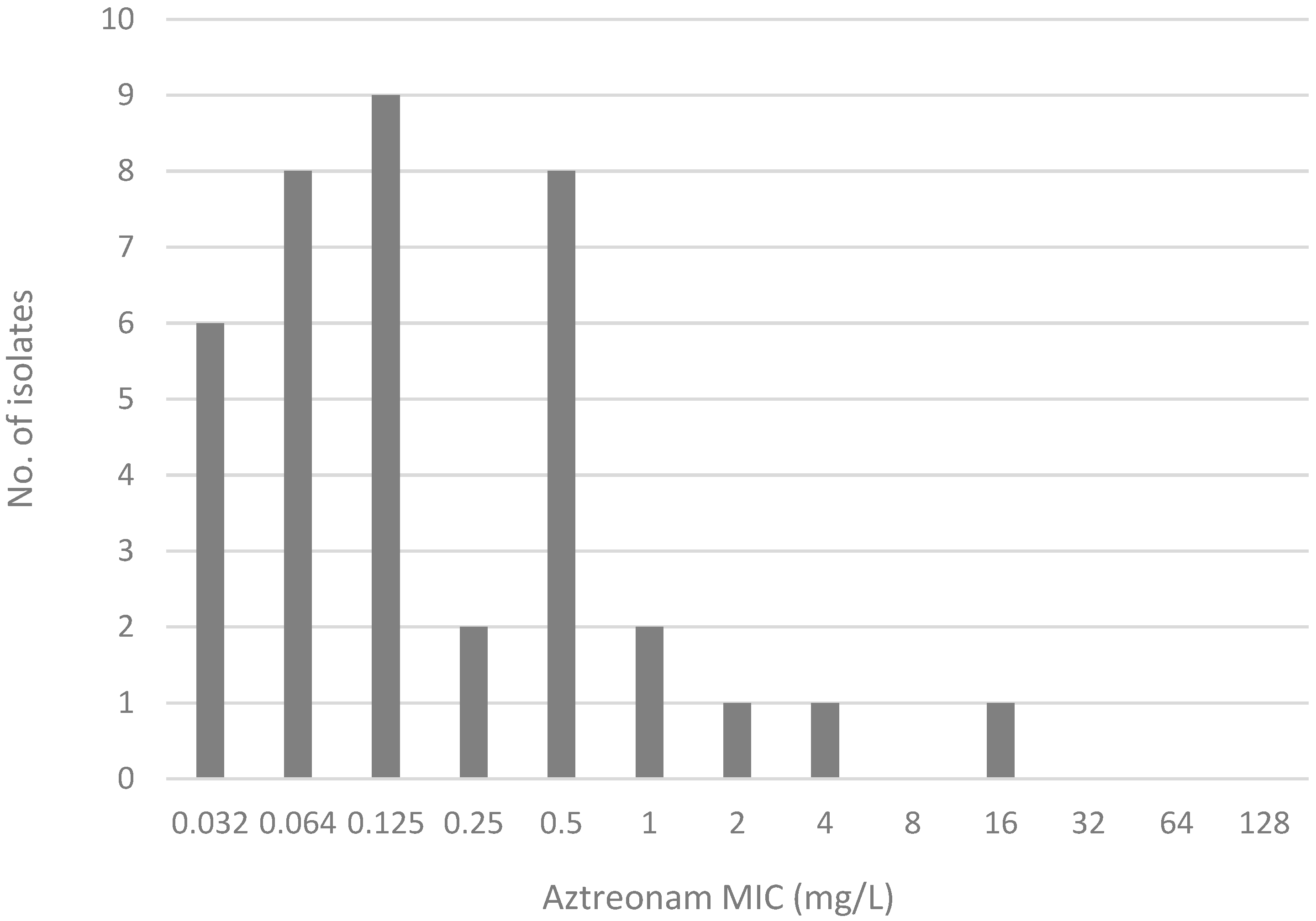
2.2. Comparison of MIC Distribution for AZA Disk Diffusion Test, AZA MIC Test Strip, and Gradient MIC Test Strips
3. Discussion
3.1. Clinical Isolates
3.2. Comparison of Methods for Routine Laboratory Use
3.3. Clinical Application for Guiding Antibiotic Use
3.4. Limitations and Future Directions
4. Materials and Methods
4.1. Strain Collection
4.2. The CLSI Aztreonam Plus Ceftazidime–Avibactam Broth Disk Elution Method (BDE)
4.3. Disk Diffusion Method for Aztreonam–Avibactam
4.4. MIC Test Strip Method for Aztreonam–Avibactam
4.5. Gradient Strip Stacking Method
4.6. Statistical Analysis
- CA refers to strains where the evaluated method yielded an AST result with the same categorical interpretation as the standard of comparison. CA ≥ 90% is considered acceptable.
- VME refers to strains tested resistant by the standard of comparison but tested susceptible by the method under evaluation. VME < 3% is considered acceptable.
- ME refers to strains tested susceptible by the standard of comparison but tested resistant by the method under evaluation. ME < 3% is considered acceptable.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Bacterial Priority Pathogens List, 2024; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Sader, H.S.; Mendes, R.E.; Carvalhaes, C.G.; Kimbrough, J.H.; Castanheira, M. Changing Epidemiology of Carbapenemases Among Carbapenem-Resistant Enterobacterales from United States Hospitals and the Activity of Aztreonam-Avibactam Against Contemporary Enterobacterales (2019-2021). Open Forum Infect. Dis. 2023, 10, ofad046. [Google Scholar] [CrossRef] [PubMed]
- Sabour, S.; Huang, J.Y.; Bhatnagar, A.; Gilbert, S.E.; Karlsson, M.; Lonsway, D.; Lutgring, J.D.; Rasheed, J.K.; Halpin, A.L.; Stanton, R.A.; et al. Detection and Characterization of Targeted Carbapenem-Resistant Health Care-Associated Threats: Findings from the Antibiotic Resistance Laboratory Network, 2017 to 2019. Antimicrob. Agents Chemother. 2021, 65, e0110521. [Google Scholar] [CrossRef] [PubMed]
- Farhat, N.; Khan, A.U. Evolving trends of New Delhi Metallo-betalactamse (NDM) variants: A threat to antimicrobial resistance. Infect. Genet. Evol. 2020, 86, 104588. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.O.; Chan, E.; Chung, P.H.; Tang, A.; Wei, W.I.; Zhu, C.; Riley, S.; Ip, M. Prevalence and associated factors for carriage of Enterobacteriaceae producing ESBLs or carbapenemase and methicillin-resistant Staphylococcus aureus in Hong Kong community. J. Infect. 2020, 81, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Bostanshirin, N.; Hajikhani, B.; Yaslianifard, S.; van Belkum, A.; Goudarzi, M.; Hashemi, A.; Darban-Sarokhalil, D.; Dadashi, M. Global genotype distribution of human clinical isolates of New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae; A systematic review. J. Glob. Antimicrob. Resist. 2020, 23, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Sapugahawatte, D.N.; Li, C.; Zhu, C.; Dharmaratne, P.; Wong, K.T.; Lo, N.; Ip, M. Prevalence and Characteristics of Extended-Spectrum-beta-Lactamase-Producing and Carbapenemase-Producing Enterobacteriaceae from Freshwater Fish and Pork in Wet Markets of Hong Kong. mSphere 2020, 5, 2. [Google Scholar] [CrossRef]
- Zhu, C.; Li, C.; Lai, C.K.C.; Ng, R.; Chau, K.Y.; Wong, K.T.; Lo, N.W.S.; Barua, N.; Yang, Y.; Liyanapathirana, V.; et al. Longitudinal Genomic Characterization of Carbapenemase-producing Enterobacteriaceae (CPE) Reveals Changing Pattern of CPE Isolated in Hong Kong Hospitals. Int. J. Antimicrob. Agents 2021, 58, 106430. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tangden, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef] [PubMed]
- Davido, B.; Fellous, L.; Lawrence, C.; Maxime, V.; Rottman, M.; Dinh, A. Ceftazidime-Avibactam and Aztreonam, an Interesting Strategy To Overcome beta-Lactam Resistance Conferred by Metallo-beta-Lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Erickson, S.G.; Pettaway, C.; Arias, C.A.; Miller, W.R.; Bhatti, M.M. Evaluation of Susceptibility Testing Methods for Aztreonam and Ceftazidime-Avibactam Combination Therapy on Extensively Drug-Resistant Gram-Negative Organisms. Antimicrob. Agents Chemother. 2021, 65, e0084621. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Brzeska-Trafny, I.; Maxfield, R.; Almeida, M.; Gilchrist, M.; Gonzalo, X.; Moore, L.S.; Donaldson, H.; Davies, F. A practical laboratory method to determine ceftazidime-avibactam-aztreonam synergy in patients with New Delhi metallo-beta-lactamase (NDM)-producing Enterobacterales infection. J. Glob. Antimicrob. Resist. 2022, 29, 558–562. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M100—Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2024. [Google Scholar]
- Harris, H.; Tao, L.; Jacobs, E.B.; Bergman, Y.; Adebayo, A.; Tekle, T.; Lewis, S.; Dahlquist, A.; Abbey, T.C.; Wenzler, E.; et al. Multicenter Evaluation of an MIC-Based Aztreonam and Ceftazidime-Avibactam Broth Disk Elution Test. J. Clin. Microbiol. 2023, 61, e0164722. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Addendum (May 2024) to EUCAST Breakpoint Tables v. 14.0; EUCAST: Växjö, Sweden, 2024. [Google Scholar]
- Deckers, C.; Bélik, F.; Denis, O.; Bogaerts, P.; Montesinos, I.; Berhin, C.; Bouchahrouf, W.; Hoebeke, M.; Evrard, S.; Gilliard, N.; et al. Comparison of testing methods assessing the in vitro efficacy of the combination of aztreonam with avibactam on multidrug-resistant Gram-negative bacilli. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, M.; Dauwalder, O.; Dortet, L. Comparison of ETEST® superposition method and the MTS™ Aztreonam-avibactam strip with the reference method for aztreonam/avibactam susceptibility testing. J. Antimicrob. Chemother. 2024, 79, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Emilie, C.M.; Alice, C.M.; Marine, G.; Farfour, E.; Pourbaix, A.; Dortet, L.; Lucie, L.; Marc, V. Evaluation of the MTS™ aztreonam-avibactam strip (Liofilchem) on New Delhimetallo-β-lactamase-producing Enterobacterales. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Verschelden, G.; Noeparast, M.; Stoefs, A.; Van Honacker, E.; Vandoorslaer, K.; Vandervore, L.; Olbrecht, M.; Van Damme, K.; Demuyser, T.; Piérard, D.; et al. Aztreonam-avibactam synergy, a validation and comparison of diagnostic tools. Front. Microbiol. 2023, 14, 1322180. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.; Juhas, M.; Poirel, L.; Nordmann, P. Genetic Features Leading to Reduced Susceptibility to Aztreonam-Avibactam among Metallo-β-Lactamase-Producing Escherichia coli Isolates. Antimicrob. Agents Chemother. 2020, 64, e01659-20. [Google Scholar] [CrossRef] [PubMed]
- Lemon, J.K.; Jankowsi-Romano, C.; Duong, S.; Juretschko, S.; Streva, V.A. Evaluation of gradient strip diffusion for susceptibility testing of aztreonam-avibactam in metallo-β-lactamase-producing Enterobacterales. J. Clin. Microbiol. 2024, 62, e0064924. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Yang, X.; Yang, Y.; Guo, Y.; Yin, D.; Ding, L.; Wu, S.; Zhu, D.; Hu, F. Assessment of Ceftazidime-Avibactam 30/20-μg Disk, Etest versus Broth Microdilution Results When Tested against Enterobacterales Clinical Isolates. Microbiol. Spectr. 2022, 10, e0109221. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K.; Development, C.M.; CLSI Methods Development and Standardization Working Group of the Subcommittee on Antimicrobial Susceptibility Testing. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed]
| AZA Disk Diffusion | AZA MIC Test Strip | Gradient Strip Stacking | |
|---|---|---|---|
| CA | 100% | 100% | 100% |
| VME | 0% | 0% | 0% |
| ME | 0% | 0% | 0% |
| ATM MIC (µg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.016 | 0.032 | 0.064 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ||
| Aztreonam–avibactam MIC (µg/mL) | 0.032 | 1 | 1 | |||||||||
| 0.064 | 4 | 6 | 5 | 3 | 1 | |||||||
| 0.125 | 1 | 1 | 2 | 3 | 2 | 1 | 1 | |||||
| 0.25 | 1 | 1 | ||||||||||
| 0.5 | ||||||||||||
| 1 | 3 | 1 | ||||||||||
| 2 | ||||||||||||
| 4 | 1 | |||||||||||
| 8 | ||||||||||||
| 16 | ||||||||||||
| 32 | ||||||||||||
| 64 | ||||||||||||
| 128 | 1 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, L.M.-W.; Lok, D.Y.L.; Wong, R.C.W.; Lee, A.L.-H.; Cheung, I.Y.-Y.; Lai, C.K.-C.; Chow, V.C.Y. Antimicrobial Susceptibility Testing of the Combination of Aztreonam and Avibactam in NDM-Producing Enterobacterales: A Comparative Evaluation Using the CLSI and EUCAST Methods. Antibiotics 2025, 14, 675. https://doi.org/10.3390/antibiotics14070675
Chan LM-W, Lok DYL, Wong RCW, Lee AL-H, Cheung IY-Y, Lai CK-C, Chow VCY. Antimicrobial Susceptibility Testing of the Combination of Aztreonam and Avibactam in NDM-Producing Enterobacterales: A Comparative Evaluation Using the CLSI and EUCAST Methods. Antibiotics. 2025; 14(7):675. https://doi.org/10.3390/antibiotics14070675
Chicago/Turabian StyleChan, Linda Mei-Wah, Doris Yui Ling Lok, River Chun Wai Wong, Alfred Lok-Hang Lee, Ingrid Yu-Ying Cheung, Christopher Koon-Chi Lai, and Viola C. Y. Chow. 2025. "Antimicrobial Susceptibility Testing of the Combination of Aztreonam and Avibactam in NDM-Producing Enterobacterales: A Comparative Evaluation Using the CLSI and EUCAST Methods" Antibiotics 14, no. 7: 675. https://doi.org/10.3390/antibiotics14070675
APA StyleChan, L. M.-W., Lok, D. Y. L., Wong, R. C. W., Lee, A. L.-H., Cheung, I. Y.-Y., Lai, C. K.-C., & Chow, V. C. Y. (2025). Antimicrobial Susceptibility Testing of the Combination of Aztreonam and Avibactam in NDM-Producing Enterobacterales: A Comparative Evaluation Using the CLSI and EUCAST Methods. Antibiotics, 14(7), 675. https://doi.org/10.3390/antibiotics14070675







