Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement
Abstract
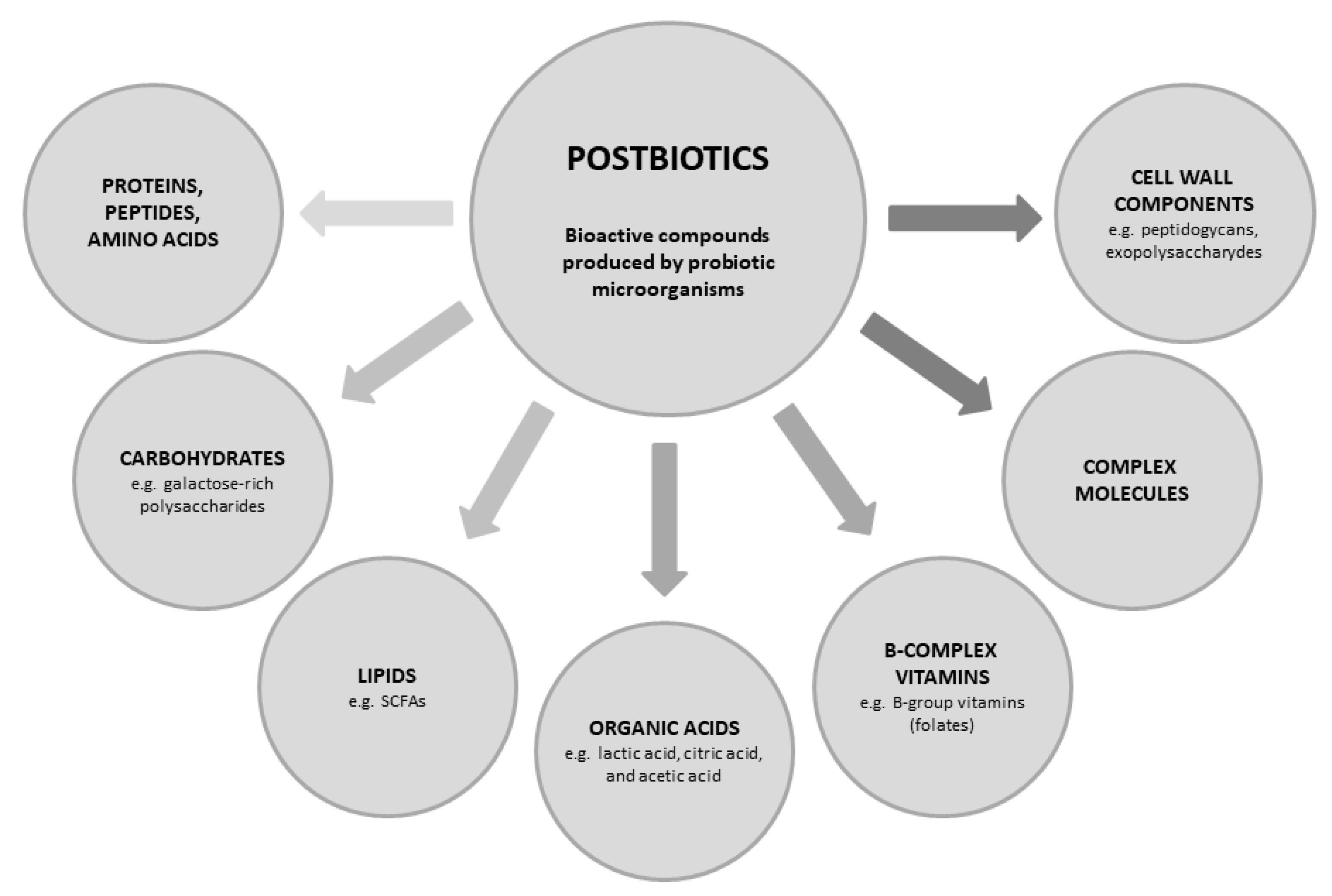
1. Introduction
2. Postbiotic Mechanisms of Action
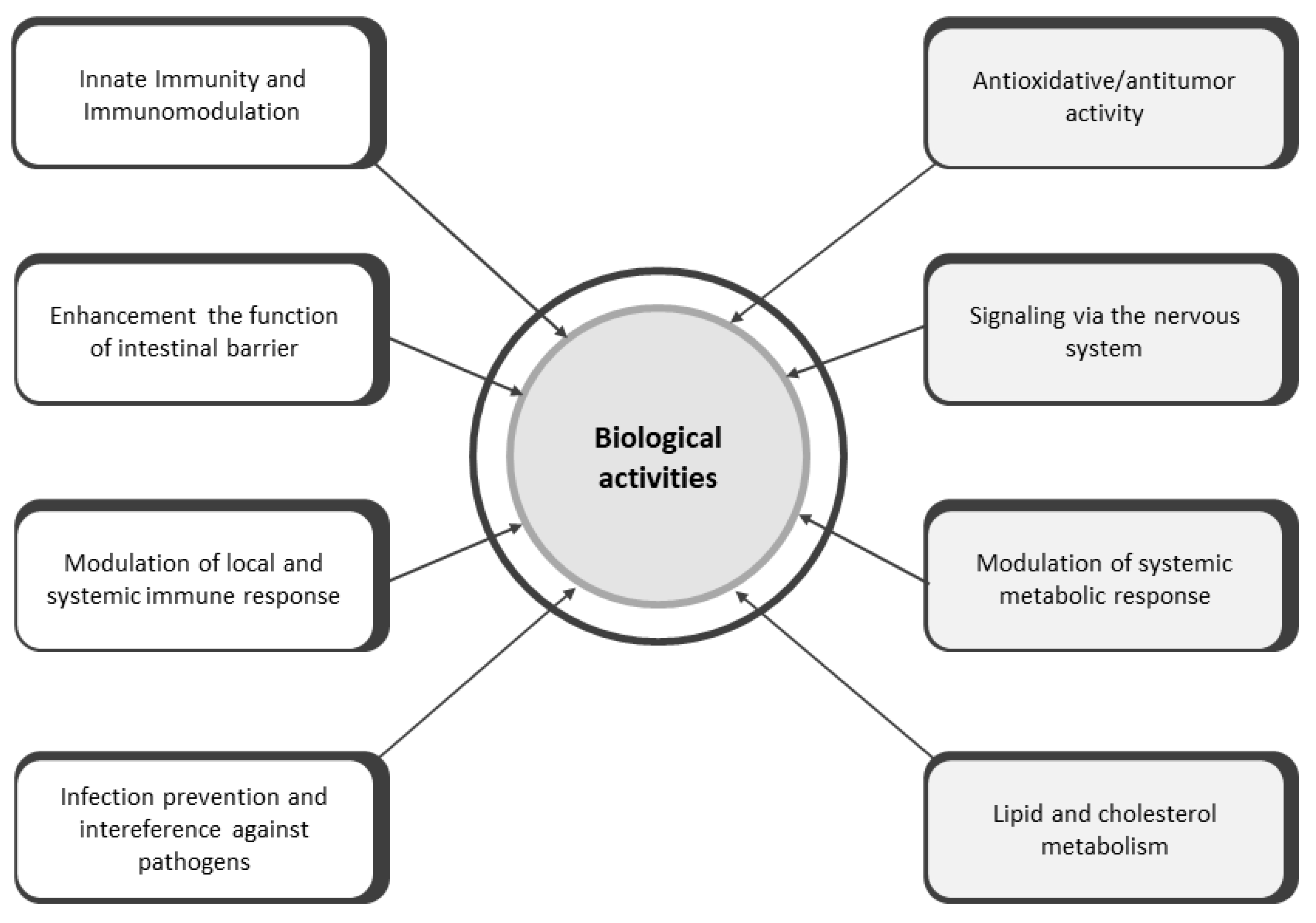
3. Biological Activities of Postbiotics
3.1. Anticancer Activity
3.2. Antioxidant Activity
3.3. Antiviral Potential
3.4. Anti-Inflammatory Potential
4. Antimicrobial Role of Postbiotics
5. Postbiotics in Food Application
6. Future Perspectives of Postbiotics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saettone, V.; Biasato, I.; Radice, E.; Schiavone, A.; Bergero, D.; Meineri, G. State-of-the-art of the nutritional alternatives to the use of antibiotics in humans and monogastric animals. Animals 2020, 10, 2199. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, R.; Tomičić, Z.; Nićetin, M.; Knežević, V.; Kocić-Tanackov, S.; Raspor, P. Food grade disinfectants based on hydrogen peroxide/peracetic acid and sodium hypochlorite interfere with the adhesion of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Listeria monocytogenes to stainless steel of differing surface roughness. Biofouling 2023, 39, 990–1003. [Google Scholar]
- Rabetafika, H.N.; Razafindralambo, A.; Ebenso, B.; Razafindralambo, H.L. Probiotics as antibiotic alternatives for human and animal applications. Encyclopedia 2023, 3, 561–581. [Google Scholar] [CrossRef]
- Tomičić, R.; Čebela, M.; Tomičić, Z.; Čabarkapa, I.; Kocić-Tanackov, S.; Raspor, P. ZnO nanoparticles enhance the efficiency of sodium hypochlorite disinfectant in reducing the adhesion of pathogenic bacteria to stainless steel surfaces. Food Microbiol. 2025, 129, 104760. [Google Scholar] [CrossRef]
- Tomičić, Z.; Šarić, L.; Tomičić, R. Novel insights in the application of probiotic yeast Saccharomyces boulardii in dairy products and health promotion. Foods 2024, 13, 2866. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, R.; Tomičić, Z.; Raspor, P. Influence of culture conditions on co-aggregation of probiotic yeast Saccharomyces boulardii with Candida spp. and their auto-aggregation. Folia Microbiol. 2022, 67, 507–515. [Google Scholar] [CrossRef]
- Aghebati-Maleki, L.; Hasannezhad, P.; Abbasi, A.; Khani, N. Antibacterial, antiviral, antioxidant, and anticancer activities of postbiotics: A review of mechanisms and therapeutic perspectives. Biointerface Res. Appl. Chem. 2022, 12, 2629–2645. [Google Scholar]
- Kumar, A.; Green, K.M.; Rawat, M.A. Comprehensive overview of postbiotics with a special focus on discovery techniques and clinical applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current trends in food and pharmaceutical industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Mishra, B.; Mishra, A.K.; Mohanta, Y.K.; Yadavalli, R.; Agrawal, D.C.; Reddy, H.R.; Gorrepati, R.; Reddy, C.N.; Mandal, S.K.; Shamim, M.Z.; et al. Postbiotics: The new horizons of microbial functional bioactive compounds in food preservation and security. Food Prod. Process. Nutr. 2024, 6, 28. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Lin, W.Y.; Kuo, Y.W.; Chen, C.W.; Hsu, Y.C.; Huang, Y.F.; Hsu, C.H.; Lin, J.H.; Lin, C.H.; Lin, C.C.; Yi, T.H.; et al. The function of mixed postbiotic PE0401 in improving intestinal health via elevating anti-inflammation, anti-oxidation, epithelial tight junction gene expression and promoting beneficial bacteria growth. J. Pure Appl. Microbiol. 2022, 16, 1771–1782. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Kim, J.H. Postbiotics: Functional food materials and therapeutic agents for cancer, diabetes, and inflammatory diseases. Foods 2023, 13, 89. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The current and future perspectives of postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Sidhu, A.; Ma, Z.; McClain, C.; Feng, W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 32–41. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, K.; Qi, W.; Zhou, Y.; Hong, T.; Xiong, T.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 enhances colonic mucosal homeostasis by controlling epithelial cell differentiation and c-Jun/Muc2 signaling. J. Agric. Food Chem. 2019, 67, 9831–9839. [Google Scholar] [CrossRef] [PubMed]
- Sudaarsan, A.S.K.; Ghosh, A.R. Appraisal of postbiotics in cancer therapy. Front. Pharmacol. 2024, 15, 1436021. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate properties in immune-related diseases: Friend or foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Assisi, R.F.; Group, G.S. Combined butyric acid/mesalazine treatment in ulcerative colitis with mild-moderate activity. Results of a multicentre pilot study. Minerva Gastroenterol. Dietol. 2008, 54, 231–238. [Google Scholar] [PubMed]
- Magnusson, M.K.; Isaksson, S.; Öhman, L. The anti-inflammatory immune regulation induced by butyrate is impaired in inflamed intestinal mucosa from patients with ulcerative colitis. Inflammation 2020, 43, 507–517. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Ventura, I.; Chomon-García, M.; Tomás-Aguirre, F.; Palau-Ferré, A.; Legidos-García, M.E.; Murillo-Llorente, M.T.; Pérez-Bermejo, M. Therapeutic and immunologic effects of short-chain fatty acids in inflammatory bowel disease: A systematic review. Int. J. Mol. Sci. 2024, 25, 10879. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic production: Harnessing the power of microbial metabolites for health applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and their health modulatory biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in human health: A narrative review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Nozari, S.; Faridvand, Y.; Etesami, A.; Ahmad Khan Beiki, M.; Miresmaeili Mazrakhondi, S.A.; Abdolalizadeh, J. Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco-2 cell line. Lett. Appl. Microbiol. 2019, 69, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, N.; Asqardokht-Aliabadi, A.; Sarabi-Aghdam, V.; Hashemi, N.; Dogahi, P.R.; Sarraf-Ov, N.; Homayouni-Rad, A. Antioxidant properties of postbiotics: An overview on the analysis and evaluation methods. Probiotics Antimicrob. Proteins 2025, 17, 606–624. [Google Scholar] [CrossRef]
- Riane, K.; Sifour, M.; Ouled-Haddar, H.; Idoui, T.; Bounar, S.; Boussebt, S. Probiotic properties and antioxidant efficiency of Lactobacillus plantarum 15 isolated from milk. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 516–520. [Google Scholar] [CrossRef]
- Chang, H.M.; Foo, H.L.; Loh, T.C.; Lim, E.T.C.; Abdul Mutalib, N.E. Comparative studies of inhibitory and antioxidant activities, and organic acids compositions of postbiotics produced by probiotic Lactiplantibacillus plantarum strains isolated from Malaysian foods. Front. Sci. 2021, 7, 602280. [Google Scholar] [CrossRef]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, 1087–1096. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef]
- Ooi, M.F.; Mazlan, N.; Foo, H.L.; Loh, T.C.; Mohammad, R.; Rahim, R.A.; Ariff, A. Effects of carbon and nitrogen sources on bacteriocin-inhibitory activity of postbiotic metabolites produced by Lactobacillus plantarum I-UL4. Malays. J. Microbiol. 2015, 1192, 176–184. [Google Scholar]
- Sunmola, A.A.; Ogbole, O.O.; Faleye, T.O.C.; Adetoye, A.; Adeniji, J.A.; Ayeni, F.A. Antiviral potentials of Lactobacillus plantarum, Lactobacillus amylovorus, and Enterococcus hirae against selected Enterovirus. Folia Microbiol. 2019, 64, 257–264. [Google Scholar] [CrossRef]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramon, D.; et al. Heat-treated Bifidobacterium longum CECT-7347: A whole-cell postbiotic with antioxidant, anti-Inflammatory, and gut-barrier protection properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef]
- Markowiak-Kopec, P.; Slizewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Erginkaya, Z.; Ünal, E.; Kalkan, S. Microbial Metabolites as Biological Control Agents in Food Safety. Agric. Food Sci. Biol. Environ. Sci. 2014, 225–259. [Google Scholar]
- Isaac-Bamgboye, F.J.; Mgbechidinma, C.L.; Onyeaka, H.; Isaac-Bamgboye, I.T.; Chukwugozie, D.C. Exploring the potential of postbiotics for food safety and human health improvement. J. Nutr. Metab. 2024, 6, 1868161. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Gilani, N.; Abbasi, A.; Khani, N. Postbiotics, as dynamic biomolecules, and their promising role in promoting food safety. Biointerface Res. Appl. Chem. 2021, 11, 14529–14544. [Google Scholar]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Hooi Ling, F.; Teck Chwen, L.; May Foong, O.; Anjas Asmara, S. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23. [Google Scholar] [CrossRef]
- Tong, Y.; Abbas, Z.; Zhang, J.; Wang, J.; Zhou, Y.; Si, D.; Wei, X.; Zhang, R. Antimicrobial activity and mechanism of novel postbiotics against foodborne pathogens. LWT 2025, 217, 117464. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Wang, Y.; Huang, Y.; Li, P.; Li, P. Lactobacillus plantarum LPL-1, a bacteriocin producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT-Food Sci. Technol. 2020, 128, 109385. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Desbois, A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent. Pat. Anti-Infect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef]
- Mali, J.K.; Sutar, Y.B.; Pahelkar, A.R.; Verma, P.M.; Telvekar, V.N. Novel fatty acid-thiadiazole derivatives as potential antimycobacterial agents. Chem. Biol. Drug Des. 2020, 95, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Almahbashi, A.; Gunes Altuntas, E. From preparation to Bioactivity: A comparative study on preparation methods and characterization of postbiotics. Food Sci. Nutr. 2025, 13, 70294. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, S.; Yao, K.; Liu, D.; Peng, X.; Huang, J.; Huang, Y.; Li, L. Physicochemical, microbiological, rheological, and sensory properties of yoghurts with new polysaccharide extracts from Lactarius volemus Fr. using three probiotics. Int. J. Dairy Technol. 2020, 73, 168–181. [Google Scholar] [CrossRef]
- Rather, I.A.; Seo, B.; Kumar, V.R.; Choi, U.H.; Choi, K.H.; Lim, J.; Park, Y.H. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML 007 and its application as a food preservative. Lett. Appl. Microbiol. 2013, 57, 69–76. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D.G. Bacteriocins and their food applications. Compr. Rev. Food Sci. Food Saf. 2003, 2, 82–100. [Google Scholar]
- Moradi, M.; Mardani, K.; Tajik, H. Characterization and application of postbiotics of Lactobacillus spp. on Listeria monocytogenes in vitro and in food models. LWT-Food Sci. Technol. 2019, 111, 457–464. [Google Scholar] [CrossRef]
- Hamad, G.M.; Abdelmotilib, N.M.; Darwish, A.M.; Zeitoun, A.M. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe 2020, 62, 102181. [Google Scholar] [CrossRef]
- George-Okafor, U.; Ozoani, U.; Tasie, F.; Mba-Omeje, K. The efficacy of cell-free supernatants from Lactobacillus plantarum Cs and Lactobacillus acidophilus ATCC 314 for the preservation of home-processed tomato-paste. Sci. Afr. 2020, 8, e00395. [Google Scholar] [CrossRef]
- Torino, M.I.; de Valdez, G.F.; Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front. Microbiol. 2015, 6, 834. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.S.; Guruprasath, N.; Adeyeye, S.A.O.; Sankarganesh, P.; Kumar, A.G.; Sivapriya, T.A. Critical analysis of postbiotics: Exploring their potential impact on the health and food industries. J. Pure Appl. Microbiol. 2023, 17, 2041–2059. [Google Scholar] [CrossRef]
- OmerOglou, E.; Karaca, B.; Kibar, H.; Haliscelik, O.; Kiran, F. The role of microbiota-derived postbiotic mediators on biofilm formation and quorum sensing-mediated virulence of Streptococcus mutans: A perspective on preventing dental caries. Microb. Pathog. 2022, 164, 105390. [Google Scholar] [CrossRef] [PubMed]
- Lalezadeh, A.; Fadaee, M.; Saedi, S.; Nezhadi, J.; Ozma, J.A.; Admadi, S.; Mobaseri, M.; Kafil, H.S. A critical review on the potential of inactivated bacteria in counteracting human pathogens. Curr. Microbiol. 2025, 82, 295. [Google Scholar] [CrossRef]
- Koohestani, M.; Moradi, M.; Tajik, H.; Badali, A. Effects of cell-free supernatant of Lactobacillus acidophilus LA5 and Lactobacillus casei 431 against planktonic form and biofilm of Staphylococcus aureus. Vet. Res. Forum 2018, 9, 301–306. [Google Scholar]
- Hosseini, S.A.; Abbasi, A.; Sabahi, S.; Khani, N. Application of postbiotics produced by lactic acid bacteria in the development of active food packaging. Biointerface Res. Appl. Chem. 2022, 12, 6164–6183. [Google Scholar]
- Sharafi, H.; Divsalar, E.; Rezaei, Z.; Liu, S.Q.; Moradi, M. The potential of postbiotics as a novel approach in food packaging and biopreservation: A systematic review of the latest developments. Crit. Rev. Food Sci. Nutr. 2023, 64, 12524–12554. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Boyte, M.E.; Smith, C.L.; Ouwehand, A.C.; Paraskevakos, G.; Younes, J.A. Commercial and regulatory frameworks for postbiotics: An industry-oriented scientific perspective for non-viable microbial ingredients conferring beneficial physiological effects. Trends Food Sci. Technol. 2025, 163, 105130. [Google Scholar] [CrossRef]
| Postbiotic | Food | Benefits |
|---|---|---|
| Polysaccharide extracts from Lactarius volemus Fr. | Yogurts | Improvement in water-retention capacity and reduction in pH; extended shelf life [55] |
| Supernatant from Lactobacillus plantarum YML007 | Soybeans | Extended shelf life [56] |
| Nisin | Dairy products, infant formula, canned soups | Acts as a preservative [14,57] |
| Pirrolo [1,2-a] and pyrazine-1,4-dione from Lactobacillus salivarious | Ground meat and whole milk | Antibiofilm activity against Listeria monocytogenes [58] |
| Cell-free supernatants from Lactobacillus rhamnosus EMCC 1105 | Poultry meat | Inhibition of Clostridium perfringens [59] |
| Bacteriocins from Bifidobacterium lactis Bb-12 | Minced meat | Inhibition of Aeromonas and Pseudomonas spp., extended shelf life [9] |
| Cell-free supernatants from Lactobacillus plantarum Cs and Lactobacillus acidophilus ATCC 314 | Tomato paste | Inhibition of Staphylococcus aureus, Escherichia coli, Aspergillus niger and Aspergillus flavus; extend shelf life [60] |
| Exopolysaccharide from Lactobacillus rhamnosus | Cheddar cheese | Improvement of product performance [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomičić, Z.; Šarić, L.; Tomičić, R. Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement. Antibiotics 2025, 14, 674. https://doi.org/10.3390/antibiotics14070674
Tomičić Z, Šarić L, Tomičić R. Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement. Antibiotics. 2025; 14(7):674. https://doi.org/10.3390/antibiotics14070674
Chicago/Turabian StyleTomičić, Zorica, Ljubiša Šarić, and Ružica Tomičić. 2025. "Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement" Antibiotics 14, no. 7: 674. https://doi.org/10.3390/antibiotics14070674
APA StyleTomičić, Z., Šarić, L., & Tomičić, R. (2025). Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement. Antibiotics, 14(7), 674. https://doi.org/10.3390/antibiotics14070674








