Epoxy-Functionalized Isatin Derivative: Synthesis, Computational Evaluation, and Antibacterial Analysis
Abstract
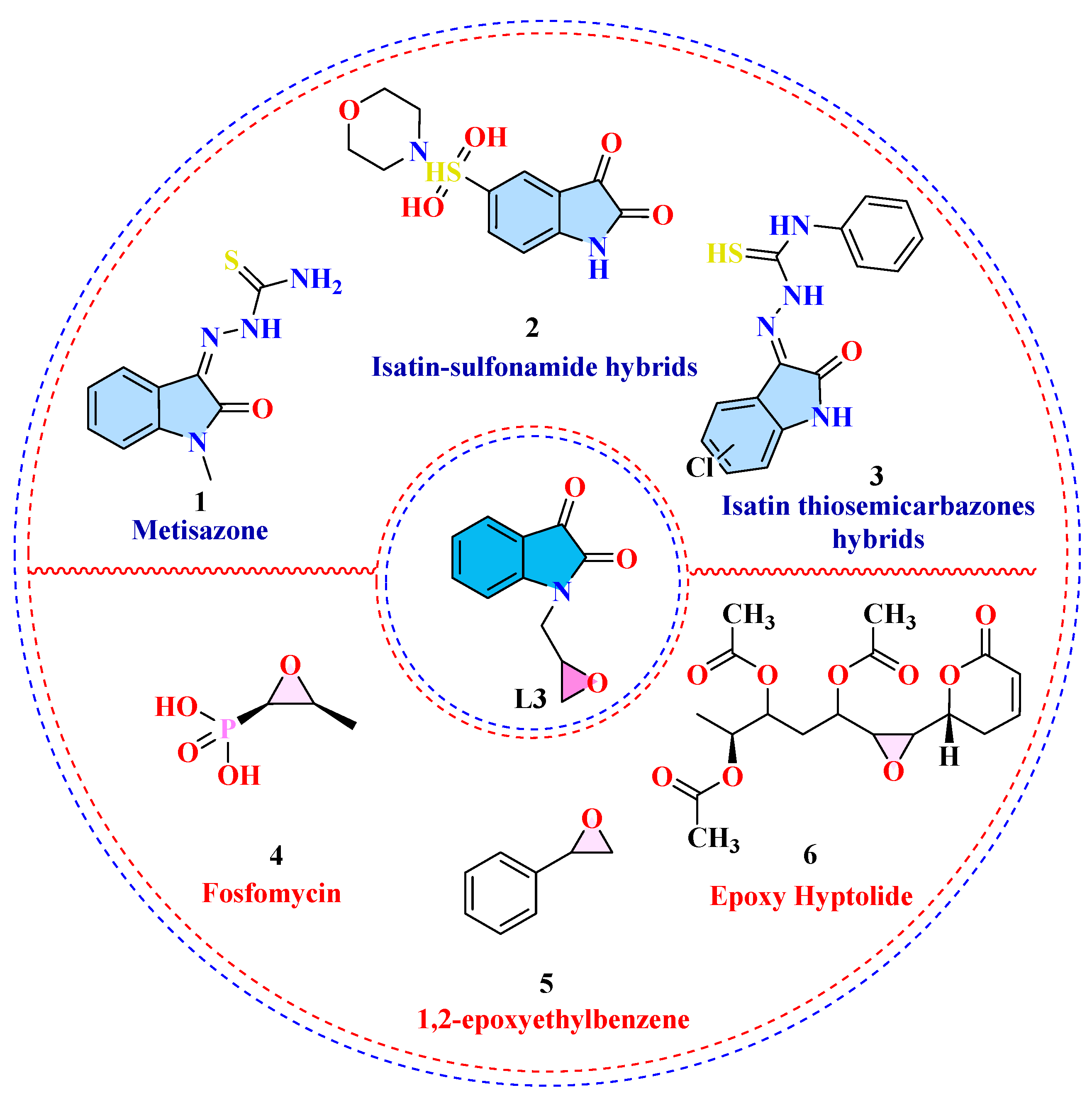
1. Introduction
2. Results
2.1. Chemistry
2.2. Characterization Details
Epoxy-Functionalized Isatin Derivative (1-(Oxiran-2-ylmethyl)indoline-2,3-dione; L3)
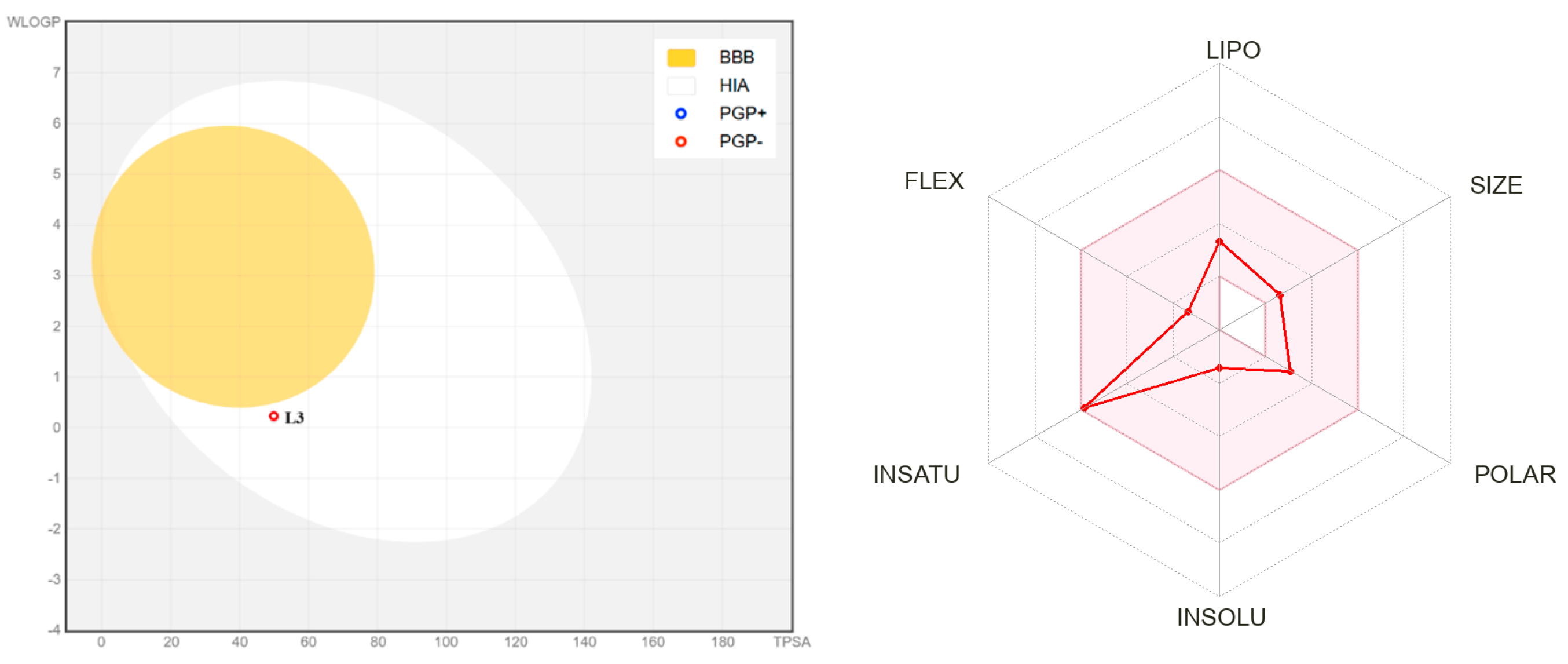
2.3. In Silico Evaluation of Drug-Likeness and ADMET Parameters
2.3.1. Physiological Properties
2.3.2. ADMET Properties
2.3.3. Toxicity Profile and Cosmetic Risk Assessment
2.4. Brain or Intestinal Estimated (BOILED–Egg) Analysis
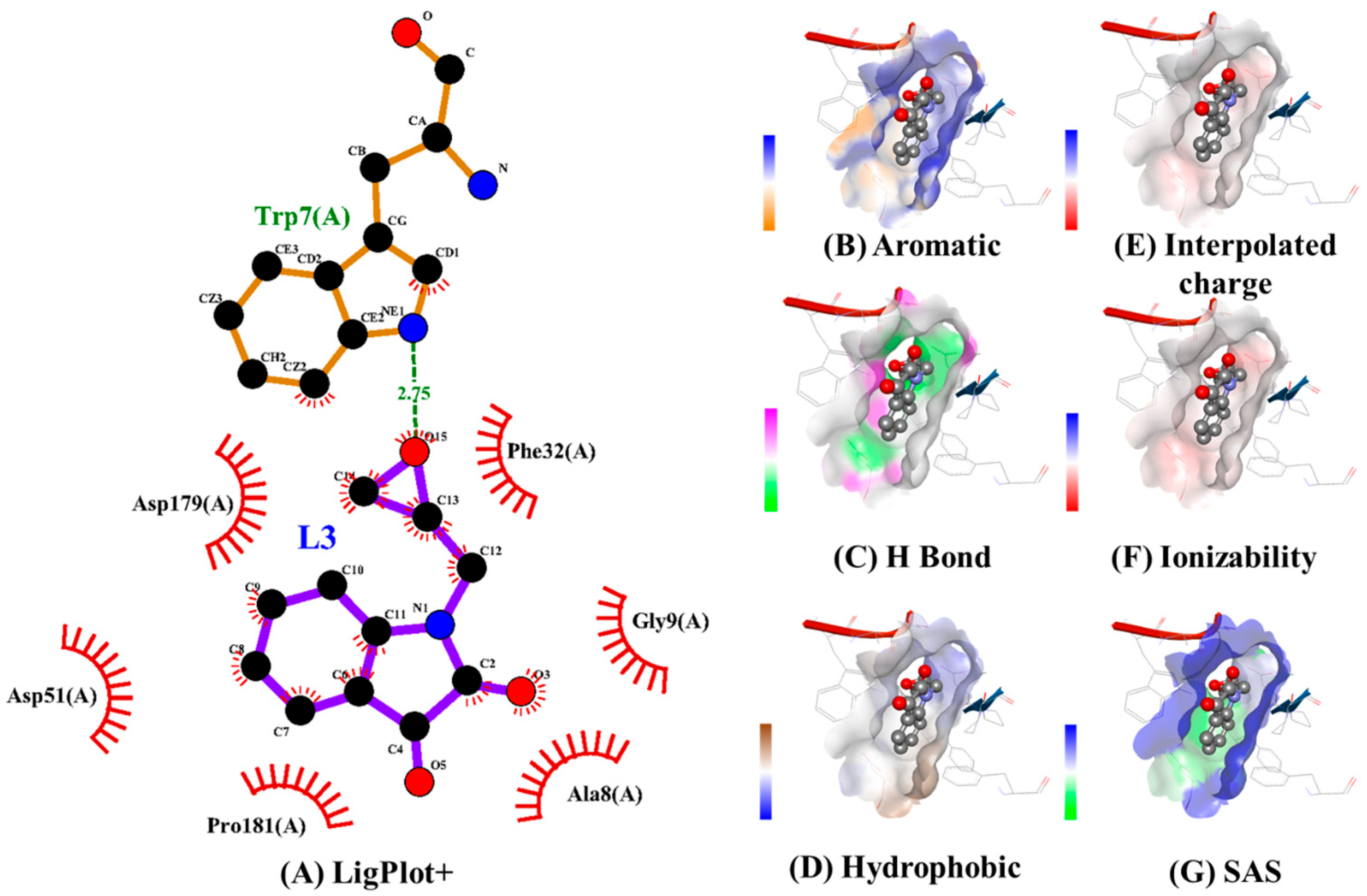
2.5. Molecular Docking Analysis
2.6. In Vitro Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Method for Synthesis of the Epoxy-Functionalized Isatin Derivative (L3)
4.2. In Silico Methodology
4.2.1. Drug-Likeness and ADMET Parameters
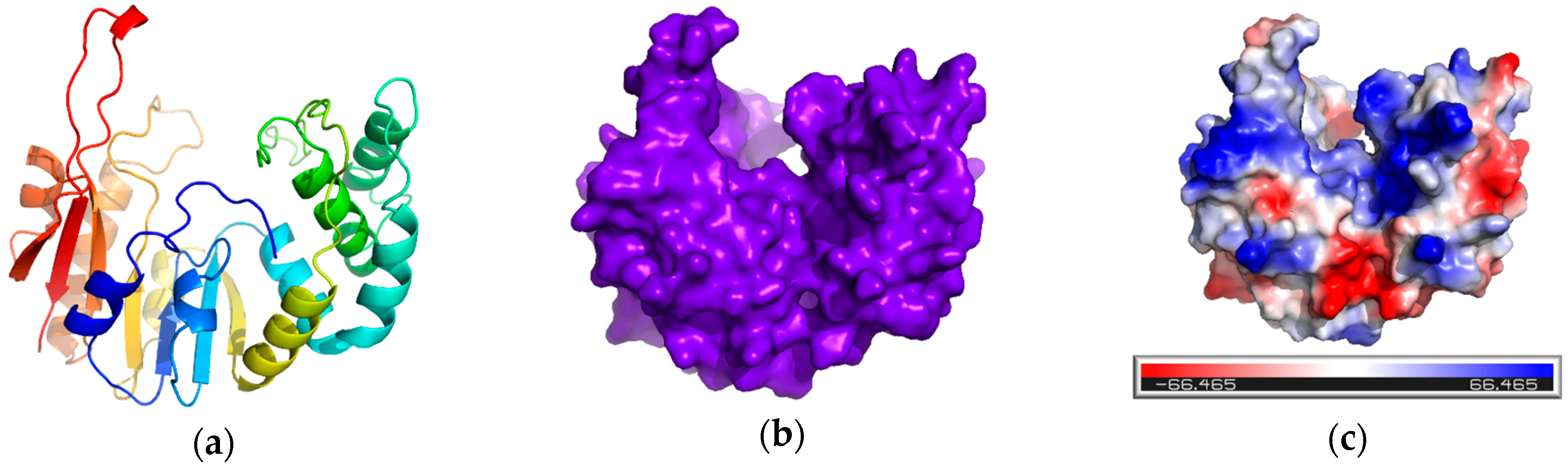
4.2.2. Sequence Retrieval and Homology Modelling
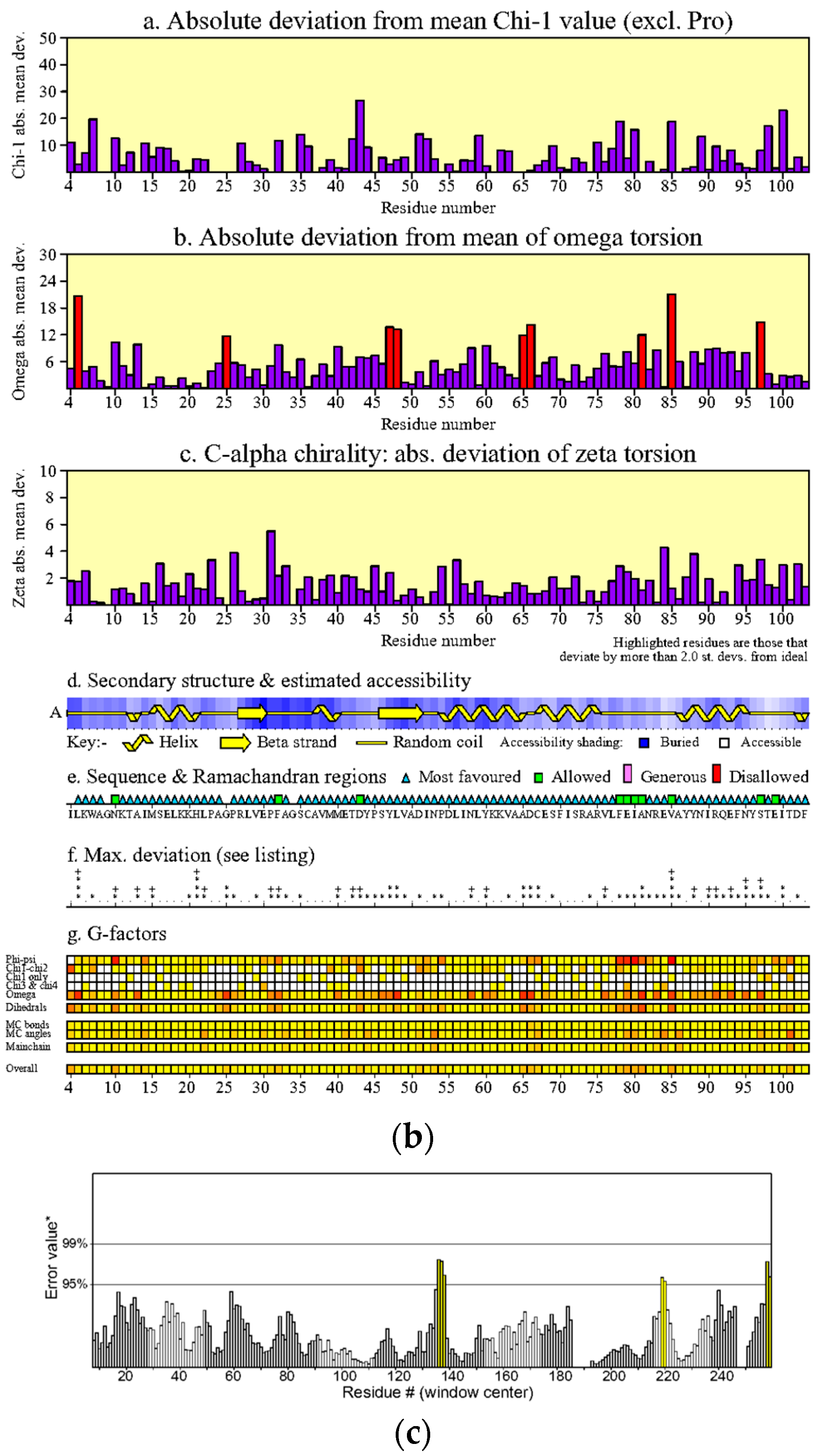
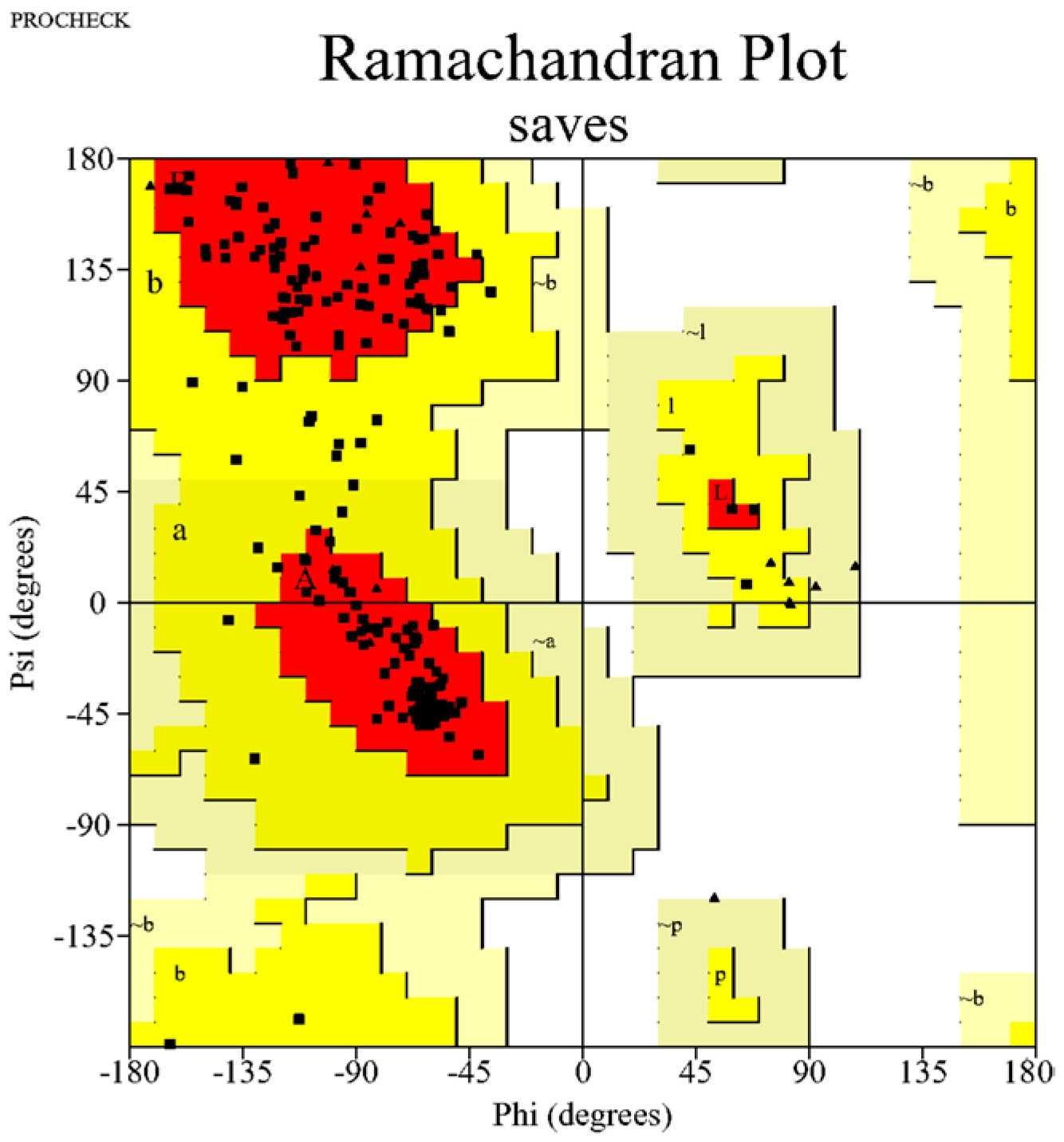
4.2.3. Validation of Modelled Structure
4.2.4. Active Site Prediction
4.3. Antibacterial Method
4.3.1. Determination of the Minimum Inhibitory Concentration
4.3.2. Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alara, J.A.; Alara, O.R. An Overview of the Global Alarming Increase of Multiple Drug Resistant: A Major Challenge in Clinical Diagnosis. Infect. Disord.-Drug Targets 2024, 24, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Fusco, P.; Morrone, H.L.; Trecarichi, E.M.; Torti, C. New Advances in Management and Treatment of Multidrug-Resistant Klebsiella pneumoniae. Expert Rev. Anti. Infect. Ther. 2023, 21, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Konwar, A.N.; Hazarika, S.N.; Bharadwaj, P.; Thakur, D. Emerging Non-Traditional Approaches to Combat Antibiotic Resistance. Curr. Microbiol. 2022, 79, 330. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; Parasannanavar, D.J.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic Non-Antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front. Microbiol. 2021, 12, 609459. [Google Scholar] [CrossRef]
- Tarín-Pelló, A.; Suay-García, B.; Pérez-Gracia, M.T. Antibiotic Resistant Bacteria: Current Situation and Treatment Options to Accelerate the Development of a New Antimicrobial Arsenal. Expert Rev. Anti. Infect. Ther. 2022, 20, 1095–1108. [Google Scholar] [CrossRef]
- Pradeep, S.D.; Mohanan, P.V. Advancements in Schiff Bases of 1H-Indole-2,3dione: A Versatile Hetero-Cyclic Compound in Pharmacological Field. Mini-Rev. Org. Chem. 2023, 20, 45–54. [Google Scholar] [CrossRef]
- Nath, P.; Mukherjee, A.; Mukherjee, S.; Banerjee, S.; Das, S.; Banerjee, S. Isatin: A Scaffold with Immense Biodiversity. Mini-Rev. Med. Chem. 2020, 21, 1096–1112. [Google Scholar] [CrossRef]
- Shu, V.A.; Eni, D.B.; Ntie-Kang, F. A Survey of Isatin Hybrids and Their Biological Properties. Mol. Divers. 2024, 29, 1737–1760. [Google Scholar] [CrossRef]
- Gandhi, P.V.; Burande, S.R.; Charde, M.S.; Chakole, R.D. A review on isatin and its derivatives: Synthesis, reactions and applications. J. Adv. Sci. Res. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top. Curr. Chem. 2021, 379, 34. [Google Scholar] [CrossRef] [PubMed]
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, M.I.; Adnan, M.; et al. Therapeutic Outcomes of Isatin and Its Derivatives against Multiple Diseases: Recent Developments in Drug Discovery. Pharmaceuticals 2022, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Dureja, D.; Pasricha, R.; Saini, P.D.K.; Bhalla, A. Recent Progress in Synthetic Strategies for Novel β-Lactams Linked with Five-Membered Heterocycles (N/O/S): Advances in Medicinal Chemistry (2020–2025). Med. Chem. Res. 2025, 34, 1145–1176. [Google Scholar] [CrossRef]
- Grenier, D.; Audebert, S.; Preto, J.; Guichou, J.F.; Krimm, I. Linkers in Fragment-Based Drug Design: An Overview of the Literature. Expert Opin. Drug Discov. 2023, 18, 987–1009. [Google Scholar] [CrossRef]
- Varun; Sonam; Kakkar, R. Isatin and Its Derivatives: A Survey of Recent Syntheses, Reactions, and Applications. MedChemComm 2019, 10, 351–368. [Google Scholar] [CrossRef]
- Singh, G.S.; Desta, Z.Y. Isatins as Privileged Molecules in Design and Synthesis of Spiro-Fused Cyclic Frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef]
- Kilbile, J.T.; Tamboli, Y.; Gadekar, S.S.; Islam, I.; Supuran, C.T.; Sapkal, S.B. An Insight into the Biological Activity and Structure-Based Drug Design Attributes of Sulfonylpiperazine Derivatives. J. Mol. Struct. 2023, 1278, 134971. [Google Scholar] [CrossRef]
- Ganesh, M.; Suraj, S. Expeditious Entry into Carbocyclic and Heterocyclic Spirooxindoles. Org. Biomol. Chem. 2022, 20, 5651–5693. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Sakla, A.P.; Laxmikeshav, K.; Tokala, R. Reliability of Click Chemistry on Drug Discovery: A Personal Account. Chem. Rec. 2020, 20, 253–272. [Google Scholar] [CrossRef]
- Sakla, A.P.; Kansal, P.; Shankaraiah, N. Syntheses and Reactivity of Spiro-Epoxy/Aziridine Oxindole Cores: Developments in the Past Decade. Org. Biomol. Chem. 2020, 18, 8572–8596. [Google Scholar] [CrossRef]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The Application of Isatin-Based Multicomponent-Reactions in the Quest for New Bioactive and Druglike Molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A. A Brief Review Depending on the Chemistry of Isatin in Single Pot Technique towards the Construction of Significant and Valuable Heterocyclic Scaffolds. Lett. Org. Chem. 2024, 21, 929–957. [Google Scholar] [CrossRef]
- Thibodeaux, C.J.; Chang, W.C.; Liu, H.W. Enzymatic Chemistry of Cyclopropane, Epoxide, and Aziridine Biosynthesis. Chem. Rev. 2012, 112, 1681–1709. [Google Scholar] [CrossRef]
- Schaich, K.M. Epoxides: An Underestimated Lipid Oxidation Product. Free Radic. Res. 2024, 58, 517–564. [Google Scholar] [CrossRef] [PubMed]
- Repetto, E.; Manzano, V.E.; Varela, O.; Uhrig, M.L. Synthesis and Reactivity of Carbohydrates Containing 3-Membered Heterocycles, Key Precursors of Modified Sugars and Mimetics. Eur. J. Org. Chem. 2024, 27, e202400488. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Didiuk, M.T. Structural Alerts, Reactive Metabolites, and Protein Covalent Binding: How Reliable Are These Attributes as Predictors of Drug Toxicity? Chem. Biodivers. 2009, 6, 2115–2137. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Obach, R.S.; Maurer, T.S. Mechanism-Based Inactivation of Cytochrome P450 Enzymes: Chemical Mechanisms, Structure-Activity Relationships and Relationship to Clinical Drug-Drug Interactions and Idiosyncratic Adverse Drug Reactions. Curr. Drug Metab. 2007, 8, 407–447. [Google Scholar] [CrossRef]
- Gideon, D.A.; Annadurai, P.; Nirusimhan, V.; Parashar, A.; James, J.; Dhayabaran, V.V. Evaluation of the Anticancer Activities of Isatin-Based Derivatives. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2022; pp. 1–25. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Omar, M.M.A.; Moustafa, B.S.; AbdEl-Halim, H.F.; Farag, N.A. Spectroscopic Investigation, Thermal, Molecular Structure, Antimicrobial and Anticancer Activity with Modelling Studies of Some Metal Complexes Derived from Isatin Schiff Base Ligand. Inorg. Chem. Commun. 2022, 141, 109606. [Google Scholar] [CrossRef]
- Brandão, P.M.d.C.G.; Piñeiro Gómez, M. Sustainable Catalytic Synthesis of Novel Bioactive Oxindole Derivatives. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2021. [Google Scholar]
- Elsaman, T.; Mohamed, M.S.; Eltayib, E.M.; Abdel-aziz, H.A.; Abdalla, A.E.; Munir, M.U.; Mohamed, M.A. Isatin Derivatives as Broad-Spectrum Antiviral Agents: The Current Landscape. Med. Chem. Res. 2022, 31, 244–273. [Google Scholar] [CrossRef]
- Nisha; Mehra, V.; Hopper, M.; Patel, N.; Hall, D.; Wrischnik, L.A.; Land, K.M.; Kumar, V. Design and Synthesis of β-Amino Alcohol Based β-Lactam-Isatin Chimeras and Preliminary Analysis of In Vitro Activity against the Protozoal Pathogen Trichomonas vaginalis. MedChemComm 2013, 4, 1018–1024. [Google Scholar] [CrossRef]
- Nisha; Gut, J.; Rosenthal, P.J.; Kumar, V. β-Amino-Alcohol Tethered 4-Aminoquinoline-Isatin Conjugates: Synthesis and Antimalarial Evaluation. Eur. J. Med. Chem. 2014, 84, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Zhang, P.L.; Tangadanchu, V.K.R.; Li, S.; Zhou, C.H. Novel Metronidazole-Derived Three-Component Hybrids as Promising Broad-Spectrum Agents to Combat Oppressive Bacterial Resistance. Bioorg. Chem. 2022, 122, 105718. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Zhao, W.H.; Tangadanchu, V.K.R.; Meng, J.P.; Zhou, C.H. Discovery of Novel Phenylhydrazone-Based Oxindole-Thiolazoles as Potent Antibacterial Agents toward Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 239, 114521. [Google Scholar] [CrossRef]
- Abatematteo, F.S.; Niso, M.; Contino, M.; Leopoldo, M.; Abate, C. Multi-Target Directed Ligands (MTDLs) Binding the σ1 Receptor as Promising Therapeutics: State of the Art and Perspectives. Int. J. Mol. Sci. 2021, 22, 6359. [Google Scholar] [CrossRef]
- Makhoba, X.H.; Viegas, C.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des. Devel. Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef]
- Ma, H.; Huang, B.; Zhang, Y. Recent Advances in Multitarget-Directed Ligands Targeting G-Protein-Coupled Receptors. Drug Discov. Today 2020, 25, 1682–1692. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive Survey on the Expediated Anti-COVID-19 Options Enabled by Metal Complexes—Tasks and Trials. Molecules 2023, 28, 3354. [Google Scholar] [CrossRef]
- Iqbal, N.; Ahmad, F.; Jamil, I.; Imran Khan, M.; Shanableh, A.; Naz, A.; Farooq, N.; Taj, M.B.; Lashari, M.H.; Manzoor, S. A Review on SARS CoV-2: History, Origin, Morphology, and the Predicted Strategy to Control the Pandemic through Metals and Metal-Based Compounds. ChemSci Adv. 2024, 1, 226–253. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, D.; Hu, G. The Antibacterial Activity of Isatin Hybrids. Curr. Top. Med. Chem. 2021, 22, 25–40. [Google Scholar] [CrossRef]
- Thanh, N.D.; Giang, N.T.K.; Toan, V.N.; Van, H.T.K.; Hai, D.S.; Tri, N.M.; Toan, D.N. Substituted Isatin-Thiosemicarbazones Containing d-Galactose Moiety: Synthesis, Antimicrobial Inhibition Assay and Molecular Simulation Study. Chem. Pap. 2023, 77, 7813–7834. [Google Scholar] [CrossRef]
- Bhattacharjee, M.K. Antibiotics That Inhibit Cell Wall Synthesis. In Chemistry of Antibiotics and Related Drugs; Springer: Cham, Switzerland, 2022; pp. 55–107. [Google Scholar] [CrossRef]
- dos Santos, C.; dos Santos, L.S.; Franco, O.L. Fosfomycin and Nitrofurantoin: Classic Antibiotics and Perspectives. J. Antibiot. 2021, 74, 547–558. [Google Scholar] [CrossRef]
- Refai, M.Y.; Elazzazy, A.M.; Desouky, S.E.; Abu-Elghait, M.; Fayed, E.A.; Alajel, S.M.; Alajlan, A.A.; Albureikan, M.O.; Nakayama, J. Interception of Epoxide Ring to Quorum Sensing System in Enterococcus faecalis and Staphylococcus aureus. AMB Express 2023, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.; Oliveira, A.S.; Carvalho, M.T.B.; Barreto, A.S.; Quintans, J.; de, S.S.; Quintans Júnior, L.J.; Barreto, R.d.S.S. H. pectinata (L.) Poit—Traditional Uses, Phytochemistry and Biological-Pharmacological Activities in Preclinical Studies: A Systematic Review. J. Ethnopharmacol. 2024, 333, 118478. [Google Scholar] [CrossRef] [PubMed]
- Cahyono, B.; Suzery, M.; Amalina, N.D.; Wahyudi; Bima, D.N. Synthesis and Antibacterial Activity of Epoxide from Hyptolide (Hyptis pectinata (L.) Poit) against Gram-Positive and Gram-Negative Bacteria. J. Appl. Pharm. Sci. 2020, 10, 013–022. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, A.S.; Abdelgawad, M.A.; Mathew, B. Exploration of the Detailed Structure–Activity Relationships of Isatin and Their Isomers As Monoamine Oxidase Inhibitors. ACS Omega 2022, 7, 16244–16259. [Google Scholar] [CrossRef]
- Thanikachalam, P.V.; Maurya, R.K.; Garg, V.; Monga, V. An Insight into the Medicinal Perspective of Synthetic Analogs of Indole: A Review. Eur. J. Med. Chem. 2019, 180, 562–612. [Google Scholar] [CrossRef]
- ul Firdaus, J.; Siddiqui, N.; Alam, O.; Manaithiya, A.; Chandra, K. Pyrazole Scaffold-Based Derivatives: A Glimpse of α-Glucosidase Inhibitory Activity, SAR, and Route of Synthesis. Arch. Pharm. 2023, 356, 2200421. [Google Scholar] [CrossRef]
- Mushtaq, A.; Asif, R.; Humayun, W.A.; Naseer, M.M. Novel Isatin–Triazole Based Thiosemicarbazones as Potential Anticancer Agents: Synthesis, DFT and Molecular Docking Studies. RSC Adv. 2024, 14, 14051–14067. [Google Scholar] [CrossRef]
- Rohilla, S.; Goyal, G.; Berwal, P.; Mathur, N. A Review on Indole-Triazole Molecular Hybrids as a Leading Edge in Drug Discovery: Current Landscape and Future Perspectives. Curr. Top. Med. Chem. 2024, 24, 1557–1588. [Google Scholar] [CrossRef]
- Shahjahan, S.; Naraharisetti, L.T.; Begum, A.; Yakkala, P.A.; Lakshmi Soukya, P.S.; Godugu, C.; Begum, S.A.; Kamal, A. Oxindoline Containing Thiazolidine-4-One Tethered Triazoles Act as Antimitotic Agents by Targeting Microtubule Dynamics. ChemistrySelect 2024, 9, e202400539. [Google Scholar] [CrossRef]
- Manakkadan, V.; Haribabu, J.; Palakkeezhillam, V.N.V.; Rasin, P.; Mandal, M.; Kumar, V.S.; Bhuvanesh, N.; Udayabhaskar, R.; Sreekanth, A. Synthesis and Characterization of N4-Substituted Thiosemicarbazones: DNA/BSA Binding, Molecular Docking, Anticancer Activity, ADME Study and Computational Investigations. J. Mol. Struct. 2023, 1285, 135494. [Google Scholar] [CrossRef]
- Batool, Z.; Ullah, S.; Khan, A.; Mali, S.N.; Gurav, S.S.; Jawarkar, R.D.; Alshammari, A.; Albekairi, N.A.; Al-Harrasi, A.; Shafiq, Z. Design, Synthesis, QSAR Modelling and Molecular Dynamic Simulations of N-Tosyl-Indole Hybrid Thiosemicarbazones as Competitive Tyrosinase Inhibitors. Sci. Rep. 2024, 14, 25754. [Google Scholar] [CrossRef] [PubMed]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Ranson, M.; Skropeta, D. Cytotoxic and Anticancer Activities of Isatin and Its Derivatives: A Comprehensive Review from 2000–2008. Anticancer Agents Med. Chem. 2012, 9, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical-Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Khan, H.; Azad, I.; Arif, Z.; Nasibullah, M.; Khan, F.; Arshad, M. Computational and Biological Insights for Anti-Cancer Effects of Karanjin through AR/ER Modulation, ROS Generation and Cell Cycle Arrest in Prostate Cancer. J. Tradit. Complement. Med. 2025, in press. [Google Scholar] [CrossRef]
- Azad, I.; Khan, T.; Ahmad, N.; Khan, A.R.; Akhter, Y. Updates on Drug Designing Approach through Computational Strategies: A Review. Futur. Sci. OA 2023, 9, FSO862. [Google Scholar] [CrossRef]
- Azad, I.; Azad, I. Molecular Docking in the Study of Ligand-Protein Recognition: An Overview. In Molecular Docking—Recent Advances; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, X.H.; Cheng, W.; Peng, Y.F.; Yu, Q.M.; Tan, X.D. Identification of Novel Inhibitors of S-Adenosyl-L-Homocysteine Hydrolase via Structure-Based Virtual Screening and Molecular Dynamics Simulations. J. Mol. Model. 2022, 28, 336. [Google Scholar] [CrossRef]
- Ito, H.; Monobe, K.; Okubo, S.; Aoki, S. Identification of Novel Antimicrobial Compounds Targeting Mycobacterium Tuberculosis S-Adenosyl-L-Homocysteine Hydrolase Using Dual Hierarchical In Silico Structure-Based Drug Screening. Molecules 2024, 29, 1303. [Google Scholar] [CrossRef]
- Shah, S.S.T.H.; Naeem, I.; Akram, F.; Akhtar, M.T.; Noor, F. In-Silico Study of an Inhibitor of S-Adenosyl-L-Homocysteine Hydrolase (SAHH) of Naegleria Fowleri Using Molecular Docking, Density Functional Theory (DFT), and Molecular Dynamics (MD) Simulation. Mol. Biotechnol. 2025, 1–18. [Google Scholar] [CrossRef]
- Singh, D.B.; Dwivedi, S. Computational Screening and ADMET-Based Study for Targeting Plasmodium S-Adenosyl-l-Homocysteine Hydrolase: Top Scoring Inhibitors. Netw. Model. Anal. Health Inform. Bioinform. 2019, 8, 4. [Google Scholar] [CrossRef]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef] [PubMed]
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. In Silico ADME Profiling of Salubrinal and Its Analogues. Futur. Pharmacol. 2022, 2, 160–197. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, Z.; Wang, Y.; Chen, L.; Lou, C.; Yang, C.; Li, W.; Liu, G.; Tang, Y. AdmetSAR3.0: A Comprehensive Platform for Exploration, Prediction and Optimization of Chemical ADMET Properties. Nucleic Acids Res. 2024, 52, W432–W438. [Google Scholar] [CrossRef]
- Jurowski, K.; Niżnik, Ł.; Frydrych, A.; Kobylarz, D.; Noga, M.; Krośniak, A.; Fijałkowska, O.; Świdniak, A.; Ahuja, V. Toxicological Profile of Acovenoside A as an Active Pharmaceutical Ingredient—Prediction of Missing Key Toxicological Endpoints Using In Silico Toxicology Methodology. Chem. Biol. Interact. 2025, 408, 111404. [Google Scholar] [CrossRef]
- Tiwari, S.; Nagarajan, K.; Gupta, A. SwissADME: An In-Silico ADME Examination Tool. In Emerging Trends in IoT and Computing Technologies; Routledge: London, UK, 2023; pp. 219–225. [Google Scholar] [CrossRef]
- Kececi Sarıkaya, M.; Sahin Yaglioglu, A.; Ceylan, M.; Yırtıcı, Ü. Novel Tricyclo [4.2.1]Nonane and Bicyclo[2.2.1]Heptane Derivatives: Synthesis, in Vitro Biological Activities and in Silico Studies. Chem. Biodivers. 2025, 22, e202401980. [Google Scholar] [CrossRef]
- Alsedfy, M.Y.; Ebnalwaled, A.A.; Moustafa, M.; Said, A.H. Investigating the Binding Affinity, Molecular Dynamics, and ADMET Properties of Curcumin-IONPs as a Mucoadhesive Bioavailable Oral Treatment for Iron Deficiency Anemia. Sci. Rep. 2024, 14, 22027. [Google Scholar] [CrossRef]
- Attaullah, H.M.; Ejaz, S.A.; Channar, P.A.; Saeed, A.; Ujan, R.; Zargar, S.; Channar, S.A.; Sahito, R.; Wani, T.A.; Abbas, Q. Exploration of Newly Synthesized Azo-Thiohydantoins as the Potential Alkaline Phosphatase Inhibitors via Advanced Biochemical Characterization and Molecular Modeling Approaches. BMC Chem. 2024, 18, 47. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A. Computational Strategies and Tools for Protein Tertiary Structure Prediction. In Basic Biotechniques for Bioprocess and Bioentrepreneurship; Academic Press: Cambridge, MA, USA, 2023; pp. 225–242. [Google Scholar] [CrossRef]
- Silva, M.A.; do Nascimento Júnior, J.C.; Thomaz, D.V.; Maia, R.T.; Costa Amador, V.; Tommaso, G.; Coelho, G.D. Comparative Homology of Pleurotus ostreatus Laccase Enzyme: Swiss Model or Modeller? J. Biomol. Struct. Dyn. 2023, 41, 8927–8940. [Google Scholar] [CrossRef]
- Robin, X.; Waterhouse, A.M.; Bienert, S.; Studer, G.; Alexander, L.T.; Tauriello, G.; Schwede, T.; Pereira, J. The SWISS-MODEL Repository of 3D Protein Structures and Models. In Open Access Databases and Datasets for Drug Discovery; Wiley-VCH: Weinheim, Germany, 2023; pp. 175–199. [Google Scholar] [CrossRef]
- Azad, I.; Khan, T.; Ahmad, R.; Kamal, A.; Khan, A.R.; Nasibullah, M. A Simplistic Approach for Preparation of Alkylidenemalononitrile Derivatives: Characterization, In Silico Studies, Quantum Chemical Evaluation, Molecular Docking, and In Vitro Biological Activity Evaluation. J. Mol. Struct. 2021, 1228, 129451. [Google Scholar] [CrossRef]
- Kumar, S. Molecular Modeling and Identification of Substrate Binding Site of Orphan Human Cytochrome P450 4F22. Bioinformation 2011, 7, 207. [Google Scholar] [CrossRef][Green Version]
- Horton, J.R.; Zhang, X.; Blumenthal, R.M.; Cheng, X. Structures of Escherichia coli DNA Adenine Methyltransferase (Dam) in Complex with a Non-GATC Sequence: Potential Implications for Methylation-Independent Transcriptional Repression. Nucleic Acids Res. 2015, 43, 4296–4308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, F.; Wu, X.; Chen, W.S.; Yan, F.; Li, W. Computer-Assisted Discovery and Evaluation of Potential Ribosomal Protein S6 Kinase Beta 2 Inhibitors. Comput. Biol. Med. 2024, 172, 108204. [Google Scholar] [CrossRef] [PubMed]
- Goswami, V.; Patel, D.; Rohit, S.; Chaube, U.; Patel, B. Homology Modeling, Binding Site Identification, Molecular Docking and Molecular Dynamics Simulation Study of Emerging and Promising Drug Target of Wnt Signaling—Human Porcupine Enzyme. Results Chem. 2024, 7, 101482. [Google Scholar] [CrossRef]
- Sawal, H.A.; Nighat, S.; Safdar, T.; Anees, L. Comparative In Silico Analysis and Functional Characterization of TANK-Binding Kinase 1–Binding Protein 1. Bioinform. Biol. Insights 2023, 17, 11779322231164828. [Google Scholar] [CrossRef]
- Rehman, H.M.; Naz, M.; Ghous, A.G.; Malik, M.; Ahmad, S.; Bashir, H. Computational Design and Evaluation of a Novel Temporin 1CEa-IL24 Fusion Protein for Anti-Tumor Potential. Biomed. Res. Ther. 2025, 12, 7138–7152. [Google Scholar] [CrossRef]
- Golichenari, B.; Heiat, M.; Rezaei, E.; Ramshini, A.; Sahebkar, A.; Gholipour, N. Compromising the Immunogenicity of Diphtheria Toxin-Based Immunotoxins through Epitope Engineering: An In Silico Approach. J. Pharmacol. Toxicol. Methods 2025, 131, 107571. [Google Scholar] [CrossRef]
- Amidzadeh, Z.; Rismani, E.; Shokrgozar, M.A.; Rahimi, H.; Golkar, M. In Silico Design of Fusion Keratinocyte Growth Factor Containing Collagen-Binding Domain for Tissue Engineering Application. J. Mol. Graph. Model. 2023, 118, 108351. [Google Scholar] [CrossRef]
- Singh, S.; Qureshi, I.A. Identification of Potent Inhibitors against Chorismate Synthase of Toxoplasma gondii Using Molecular Dynamics Simulations. J. Mol. Graph. Model. 2022, 114, 108183. [Google Scholar] [CrossRef]
- Francisco, S.; Lamacchia, L.; Turco, A.; Ermondi, G.; Caron, G.; Rossi Sebastiano, M. Restoring Adapter Protein Complex 4 Function with Small Molecules: An In Silico Approach to Spastic Paraplegia 50. Protein Sci. 2025, 34, e70006. [Google Scholar] [CrossRef]
- Kabiri, M.; Hajizade, M.S.; Zarei, M.; Eskandari, S.; Sakhteman, A.; Khoshneviszadeh, M. A Repurposing Pipeline to Candidate-Suitable Inhibitors of Tyrosinase: Computational and Bioassay Studies. Chem. Biodivers. 2024, 21, e202401035. [Google Scholar] [CrossRef]
- Behera, M.; Singh, G.; De, S.; Ghorai, S.M. Molecular Docking and Molecular Dynamics Simulation Insight of Amidase_2 Endolysin Domain as an Antifungal Enzyme. Chem. Africa 2024, 8, 487–503. [Google Scholar] [CrossRef]
- Ismail, C.M.K.H.; Abdul Hamid, A.A.; Abdul Rashid, N.N.; Lestari, W.; Mokhtar, K.I.; Mustafa Alahmad, B.E.; Abd Razak, M.R.M.; Ismail, A. An Ensemble Docking-Based Virtual Screening and Molecular Dynamics Simulation of Phytochemical Compounds from Malaysian Kelulut Honey (KH) against SARS-CoV-2 Target Enzyme, Human Angiotensin-Converting Enzyme 2 (ACE-2). J. Biomol. Struct. Dyn. 2024, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of Diffusion and Dilution Methods to Determine the Antibacterial Activity of Plant Extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Liu, C.Y.; Huang, Y.T.; Liao, C.H.; Teng, L.J.; Turnidge, J.D.; Hsueh, P.R. Antimicrobial Susceptibilities of Commonly Encountered Bacterial Isolates to Fosfomycin Determined by Agar Dilution and Disk Diffusion Methods. Antimicrob. Agents Chemother. 2011, 55, 4295–4301. [Google Scholar] [CrossRef]
- Hassan, F.; Nasibullah, M.; Ahmad, N.; Kamal, A.; Mohammad, S.; Rizvi, D.; Khan, S.; Rahman Khan, A. Synthesis, Characterization and Physicochemical Analysis of Some Mannofuranoside Derivatives with Potent Antimicrobial Activity. Orient. J. Chem. 2017, 33, 2731–2741. [Google Scholar] [CrossRef]
- D’Este, F.; Benincasa, M.; Cannone, G.; Furlan, M.; Scarsini, M.; Volpatti, D.; Gennaro, R.; Tossi, A.; Skerlavaj, B.; Scocchi, M. Antimicrobial and Host Cell-Directed Activities of Gly/Ser-Rich Peptides from Salmonid Cathelicidins. Fish Shellfish Immunol. 2016, 59, 456–468. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, K.; Ahmad, A.; Raish, M.; Jan, B.L.; Khan, A.; Khan, M.S. Biogenic Pentagonal Silver Nanoparticles for Safer and More Effective Antibacterial Therapeutics. Int. J. Nanomed. 2018, 13, 7789–7799. [Google Scholar] [CrossRef]
- Tang, Y.; Lai, W.; Zhang, J.; Tang, D. Competitive Photometric and Visual ELISA for Aflatoxin B1 Based on the Inhibition of the Oxidation of ABTS. Microchim. Acta 2017, 184, 2387–2394. [Google Scholar] [CrossRef]
- Max-Harry, I.M.; Counts, G.P.; Nunemaker, C.S.; Whitticar, N.B. A Trypan Blue-Based ELISA Practice Test to Evaluate Trainee Performance. J. Chem. Educ. 2023, 100, 2379–2386. [Google Scholar] [CrossRef]
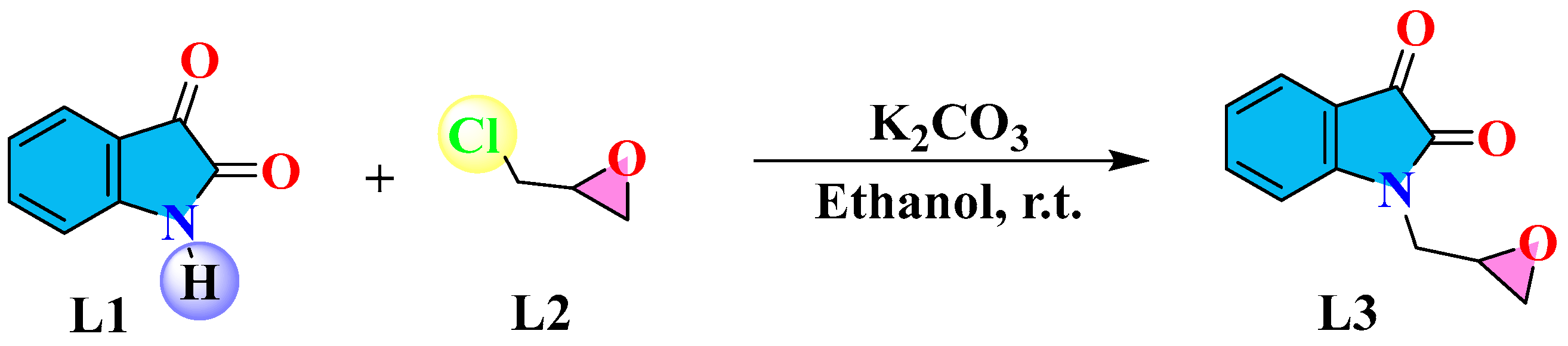



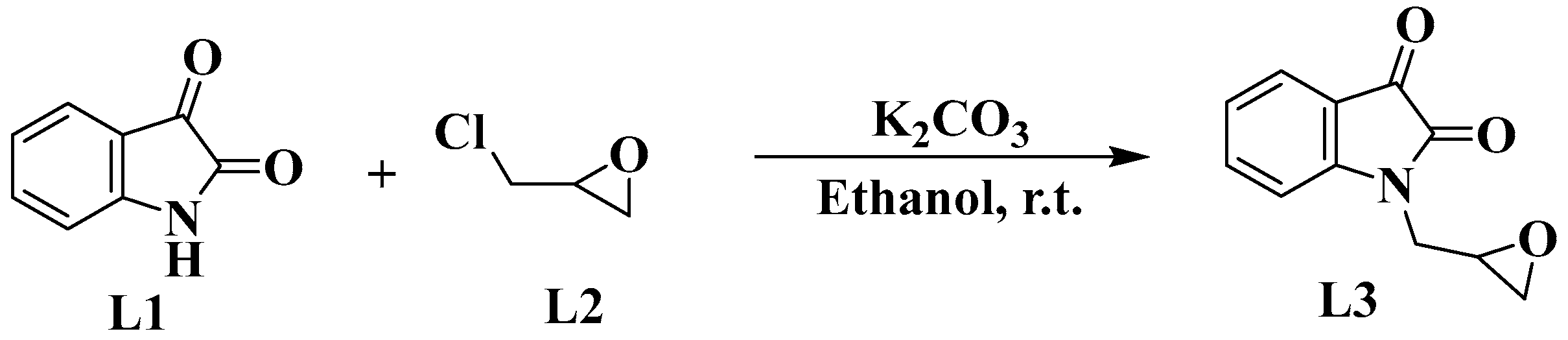

| Physicochemical Property | ||
|---|---|---|
| Parameters | L3 |  |
| Molecular Weight (MW) | 203.197 | |
| Number of atoms (nAtom) | 15 | |
| Number of heteroatoms (nHet) | 4 | |
| Number of rings (nRing) | 3 | |
| Number of rotatable bonds (nRot) | 2 | |
| Hydrogen bond acceptors (HBA) | 3 | |
| Hydrogen bond donors (HBD) | 0 | |
| Topological polar surface area (TPSA) | 49.91 | |
| Lipophilicity (logP) | 0.615 | |
| Medicinal Chemistry | |
|---|---|
| Parameters | L3 |
| Quantitative estimate of drug-likeness (QED) | 0.52 |
| Lipinski Rule (Pfizer’s Rule of Five/Rule of Five/RO5) | Accept |
| GSK Rule (GlaxoSmithKline rule/Veber rule) | Accept |
| Absorption | |
| Parameters | L3 |
| Logarithm of aqueous solubility (logS) | −2.418 |
| Logarithm of the partition coefficient (logP) | 0.680 |
| Acid dissociation constant (Acidic pKa) | 6.170 |
| Base dissociation constant (Basic pKa) | 1.360 |
| Human colon carcinoma cell line (Caco-2) | 0.942 |
| Human Intestinal Absorption (HIA) | 0.981 |
| Madin-Darby Canine Kidney cells (MDCK) | 0.889 |
| Fraction of drug absorbed at 50% concentration (F50%) | 0.962 |
| Distribution | |
| Blood-Brain Barrier (BBB) | 0.967 |
| OATP2B1 inhibitor | 0.055 |
| OCT1 inhibitor | 0.121 |
| OCT2 inhibitor | 0.050 |
| Breast Cancer Resistance Protein (BCRP) inhibitor | 0.073 |
| Bile Salt Export Pump (BSEP) inhibitor | 0.063 |
| Multidrug And Toxin Extrusion Protein 1 (MATE1) inhibitor | 0.051 |
| Permeability Glycoprotein (Pgp) inhibitor | 0.031 |
| Permeability Glycoprotein (Pgp) substrate | 0.034 |
| Plasma Protein Binding (PPB) | 0.412 |
| Metabolism | |
| CYP1A2 inhibitor | 0.786 |
| CYP3A4 inhibitor | 0.006 |
| CYP2B6 inhibitor | 0.235 |
| CYP2C9 inhibitor | 0.131 |
| CYP2C19 inhibitor | 0.209 |
| CYP2D6 inhibitor | 0.008 |
| CYP1A2 substrate | 0.775 |
| CYP3A4 substrate | 0.510 |
| CYP2B6 substrate | 0.649 |
| CYP2C9 substrate | 0.535 |
| CYP2C19 substrate | 0.668 |
| CYP2D6 substrate | 0.275 |
| Human Liver Microsomes (HLM) | 0.118 |
| Rat Liver Microsomes (RLM) | 0.268 |
| UDP-Glucuronosyltransferase (UGT) substrate | 0.154 |
| Excretion | |
| Plasma Clearance (CLp) | 0.787 |
| Renal Clearance (CLr) | 0.267 |
| Half-Life (T1/2) | −0.434 |
| Mean Residence Time (MRT) | −0.399 |
| Human Health Toxicity | |
| Parameters | L3 |
| Organ toxicity | |
| Neurotoxicity | −2.844 |
| Drug-Induced Liver Injury (DILI) | 0.930 |
| human ether-a-go-go related gene (hERG) 1 µM | 0.006 |
| human ether-a-go-go related gene (hERG) 1–10 µM | 0.004 |
| Respiratory toxicity | 0.312 |
| Nephrotoxicity | 0.838 |
| Toxicity endpoint | |
| Ames’s mutagenesis | 0.934 |
| Mouse carcinogenicity | 0.775 |
| Rat carcinogenicity | 0.794 |
| Rat carcinogenicity | 0.932 |
| Rodents’ carcinogenicity | 0.884 |
| Micronucleus | 0.933 |
| Acute oral toxicity | 0.893 |
| Endocrine disruption | |
| Androgen Receptor (AR) | 0.044 |
| Estrogen Receptor (ER) | 0.008 |
| Aromatase | 0.046 |
| ATPase Family AAA Domain-Containing Protein 5 (ATAD5) | 0.019 |
| Heat Shock Element (HSE) | 0.037 |
| Tumor Protein p53 (p53) | 0.088 |
| Peroxisome Proliferator-Activated Receptor (PPAR) | 0.030 |
| Matrix Metalloproteinases (MMP) | 0.057 |
| Thyroid Hormone Receptor (TR) | 0.023 |
| Glucocorticoid Receptor (GR) | 0.058 |
| Ecological Risk Assessment | |
| Terrestrial organisms | |
| Honeybee toxicity | 0.266 |
| Aquatic organisms | |
| Fish toxicity | 0.492 |
| Fathead minnow toxicity | 0.424 |
| Bluegill sunfish toxicity | 0.595 |
| Rainbow trout toxicity | 0.649 |
| Sheepshead minnow toxicity | 0.451 |
| Other | |
| Bioconcentration Factor (BCF) | 0.012 |
| Biodegradability | 0.235 |
| Cosmetic Risk Assessment | ||
|---|---|---|
| Parameters | L3 | 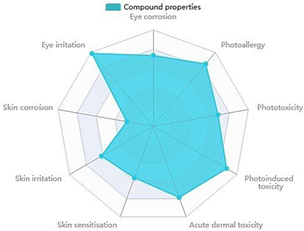 |
| Eye corrosion | 0.735 | |
| Eye irritation | 0.985 | |
| Skin corrosion | 0.277 | |
| Skin irritation | 0.618 | |
| Skin sensitization | 0.568 | |
| Acute dermal toxicity | 0.786 | |
| Photoinduced toxicity | 0.876 | |
| Phototoxicity | 0.681 | |
| Photoallergy | 0.845 | |
| Grid Size (Å) | Grid Coordinates (Å) | BE (kcal/mol) | Kd (µM) | Most Interacting Amino Acids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | AD4 | Vina | VDW | H-Bond | π-π | |
| 56 | 64 | 56 | −5.09 | 0.63 | 92.86 | −4.29 | −6.4 | 712.71 | TRP7, ALA8, GLY9, PHE32, GLY34, ASP179, TYR182, PHE194 | - | PRO181 |
| 30 | 30 | 30 | −5.09 | 0.63 | 92.86 | −4.85 | −6.4 | 280.35 | GLY9, ASP51, ASP179 | TRP7, PHE32 | ALA8, PRO180, PRO181, PHE194 |
| 30 | 30 | 30 | −2.79 | 0.63 | 94.50 | −4.51 | −6.4 | 494.15 | PHE32, ASP179, PRO180, TYR182 | TRP7, GLY9 | ALA8, PRO181 |
| 30 | 30 | 30 | −4.99 | −1.45 | 95.57 | −4.51 | −6.4 | 494.15 | PHE32, ASP179, PRO180, TYR182 | TRP7, GLY9 | ALA8, PRO181 |
| Microbial Strains | MIC Value of L3 | MIC of Gentamycin | SD | ER. BAR |
|---|---|---|---|---|
| B. cereus | 156.250 | 80 | ±0.519 | 0.416 |
| B. pumilus | 156.250 | 80 | ±0.527 | 0.419 |
| K. pneumoniae | 93.750 | 80 | ±0.650 | 0.465 |
| E. coli | 137.500 | 80 | ±0.548 | 0.427 |
| Score | Draggability | Alpha Spheres | Volume | Total SASA | Polar SASA | Alpha SASA | 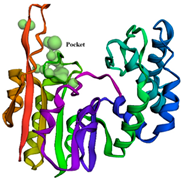 | |
| I | 0.985 | 0.985 | 117 | 1293 | 123.153 | 85.717 | 37.436 | |
| II | 0.554 | 0.036 | 48 | 443.142 | 45.001 | 45.001 | 0.000 | |
| III | 0.511 | 0.064 | 42 | 447.774 | 28.403 | 23.572 | 4.831 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, D.; Azad, I.; Khan, M.A.; Husain, Z.; Kamal, A.; Sheikh, S.Y.; Alotibi, I.; Ahmad, V.; Hassan, F. Epoxy-Functionalized Isatin Derivative: Synthesis, Computational Evaluation, and Antibacterial Analysis. Antibiotics 2025, 14, 595. https://doi.org/10.3390/antibiotics14060595
Shukla D, Azad I, Khan MA, Husain Z, Kamal A, Sheikh SY, Alotibi I, Ahmad V, Hassan F. Epoxy-Functionalized Isatin Derivative: Synthesis, Computational Evaluation, and Antibacterial Analysis. Antibiotics. 2025; 14(6):595. https://doi.org/10.3390/antibiotics14060595
Chicago/Turabian StyleShukla, Deepanjali, Iqbal Azad, Mohd Arsh Khan, Ziaul Husain, Azhar Kamal, Sabahat Yasmeen Sheikh, Ibrahim Alotibi, Varish Ahmad, and Firoj Hassan. 2025. "Epoxy-Functionalized Isatin Derivative: Synthesis, Computational Evaluation, and Antibacterial Analysis" Antibiotics 14, no. 6: 595. https://doi.org/10.3390/antibiotics14060595
APA StyleShukla, D., Azad, I., Khan, M. A., Husain, Z., Kamal, A., Sheikh, S. Y., Alotibi, I., Ahmad, V., & Hassan, F. (2025). Epoxy-Functionalized Isatin Derivative: Synthesis, Computational Evaluation, and Antibacterial Analysis. Antibiotics, 14(6), 595. https://doi.org/10.3390/antibiotics14060595






