Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties
Abstract
1. Introduction
2. Mechanisms of Biofilm Formation and Virulence in Microbial Pathogens
2.1. Structural Complexity and Resistance Mechanisms
2.2. Virulence Factor Expression and Quorum Sensing
3. Plant Metabolites as Natural Antibiofilm and Virulence Agents
3.1. Alkaloids
3.2. Tannins
3.3. Flavonoids
3.4. Essential Oils
3.5. Terpenes
3.6. Phenolic Acids
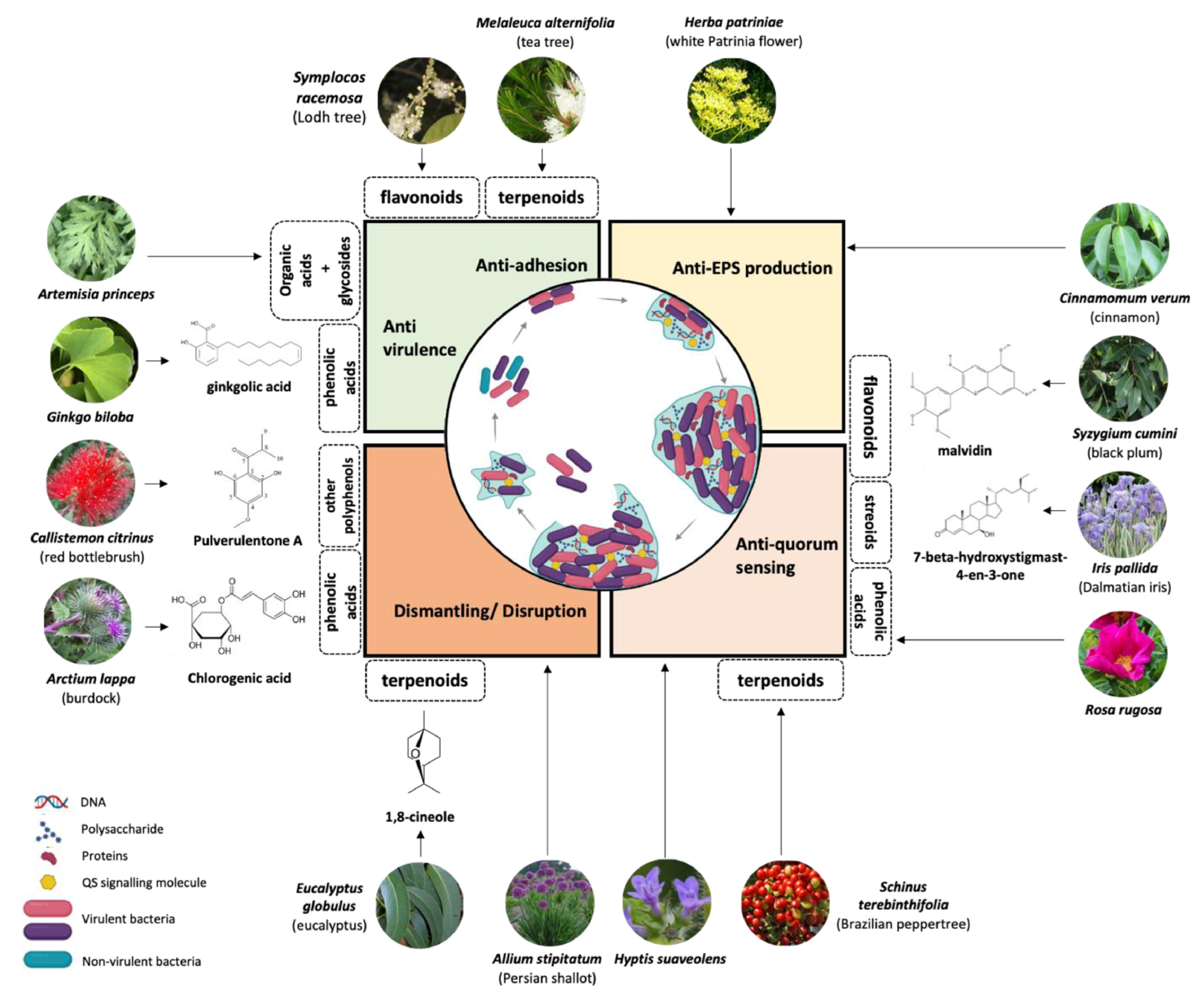
| Name of the Compound | Structure of Compounds | Source Plant Species | Target Microbes | Biofilm Inhibitory Concentration (µg/mL) | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| 14-Deoxy-11,12-didehydroandrographolide | 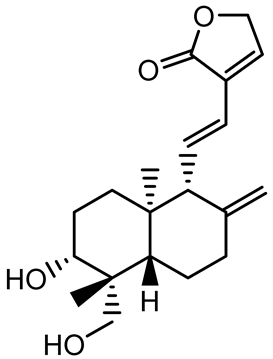 | Andrographis paniculata | Pseudomonas aeruginosa | 34.85 | Inhibits biofilm formation by targeting the quorum sensing pathway, leading to a reduction in extracellular polymeric substances, pyocyanin production, and extracellular protease synthesis. | [120] |
| Andrographolide, 14-deoxyandrographolide, 14-deoxy-12-hydroxyandrographolide, Neoandrographolide | 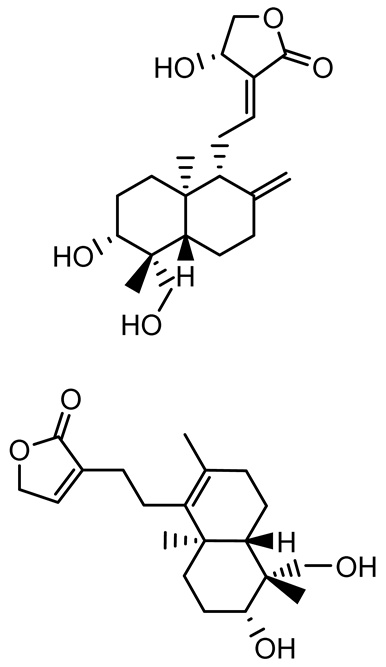 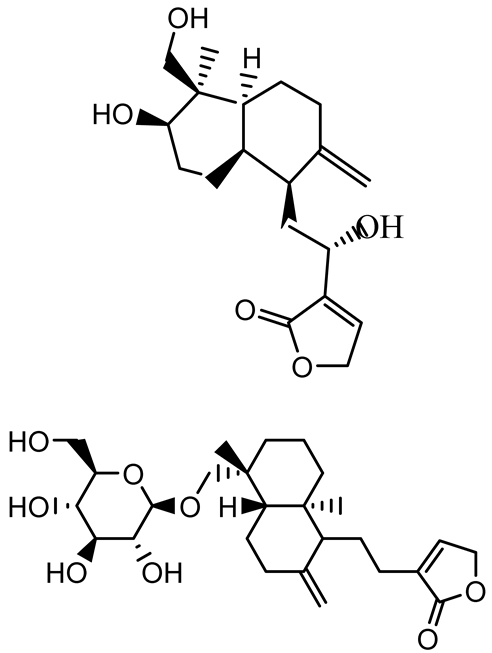 | P. aeruginosa PA22 and PA247 | 310–5000 | Exert their quorum quenching activity by downregulating lasR gene expression, thereby disrupting quorum sensing and leading to reduced biofilm formation, protease production, and swarming motility in P. aeruginosa. | [60] | |
| Andrographolide | 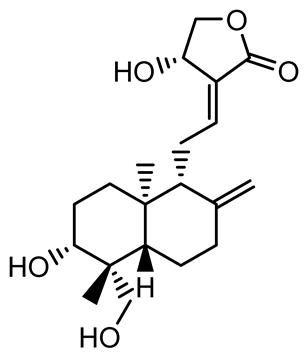 | Listeria monocytogenes 10403S | 125–1000 | Inhibits the Agr QS system by downregulating agrBDCA genes and P2 promoter activity, leading to reduced biofilm formation, virulence gene expression, hemolytic activity, and host cell invasion. | [121] | |
| Licochalcone A (LAA) | 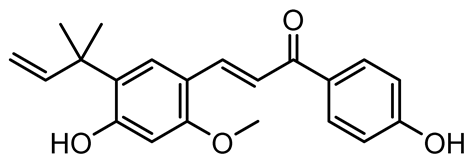 | Glycyrrhiza inflate | Salmonella typhimurium | 62.5 | Downregulates sdiA gene expression, disrupting AHLs and affecting the expression of QS-controlled virulence factors, thus decreasing motility, fimbria formation, bacterial invasion, biofilm production. | [122] |
| Epigallocatechin-3-gallate (EGCG) | 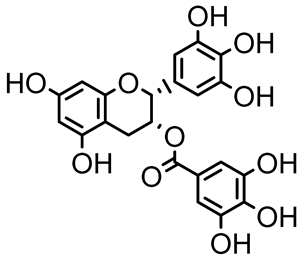 | Camellia sinensis | S. typhimurium | 3.125 | Downregulates luxS gene expression, affecting QS system and some other genes involved in virulence. | |
| Magnolol, Honokiol | 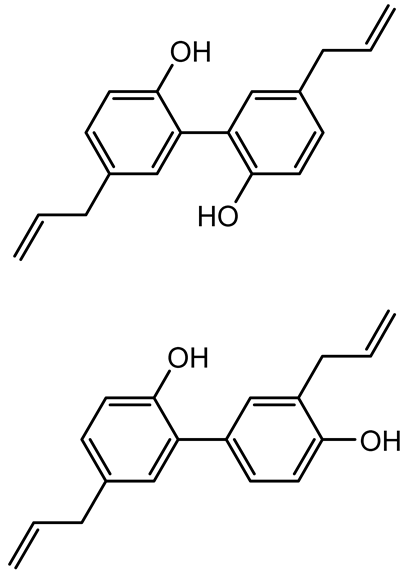 | Magnolia officinalis | Acinetobacter baumannii | - | Inhibit biofilm formation, disperse mature biofilms, suppress pellicle formation and surface motilities. | [123] |
| Verbascoside | 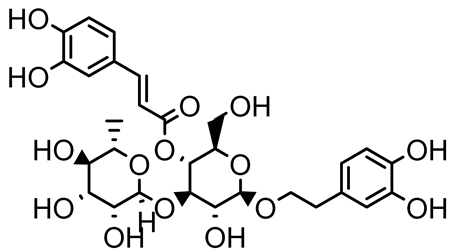 | Forsythia spp. | Staphylococcus aureus USA300 | ≥8 | Inhibits sortase A, blocking MSCRAMM anchoring to the cell wall; reduces adhesion, invasion, and biofilm formation. | [124] |
| Echinacoside | 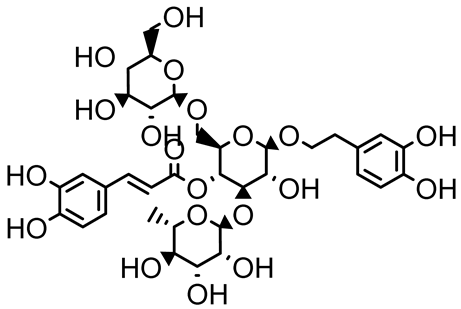 | Echinacea angustifolia | P. aeruginosa | 0.125–44 | Inhibits diguanylate cyclase SiaD, reducing intracellular c-di-GMP levels, thereby inhibiting autoaggregation and enhancing tobramycin efficacy against biofilm aggregates. | [125] |
| Scutellarein | 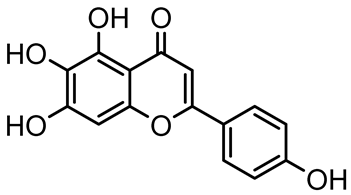 | Scutellaria baicalensis and Erigeron breviscapus | A. baumannii ATCC 17978 | 32–64 | Inhibits biofilm formation, motility, and bacterial persistence by targeting and inhibiting polyphosphate kinase 1 (PPK1). | [126] |
| Baicalin | 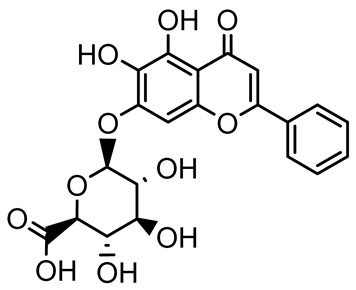 | Scutellaria baicalensis | P. aeruginosa | 64–256 | Inhibits quorum sensing by downregulating lasI/R, rhlI/R, pqsA/R genes; suppresses production of QS signals (3-oxo-C12-HSL, C4-HSL); reduces biofilm, virulence factors, and motility. | [127] |
| S. saprophyticus | 31.25–250 | Inhibits MsrA efflux pump; reduces ATP and pyruvate kinase activity; downregulates agrA, agrC, RNAIII, sarA; inhibits biofilm formation and quorum sensing system. | [128] | |||
| S. aureus 17546 | 32 and 64 | Inhibits quorum sensing by downregulating agrA, RNAIII, sarA, and ica genes; reduces virulence factors (SEA, hla); prevents and disrupts biofilm formation. | [129] | |||
| Wogonin | 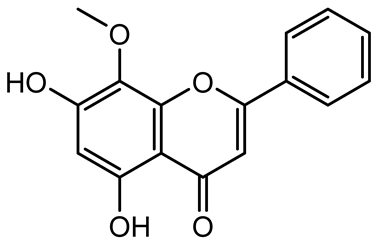 | Agrimonia pilosa | P. aeruginosa PAO1 | 15–30 | Inhibits the PQS quorum sensing system by targeting pqsA and pqsR genes; reduces PQS signal production; suppresses virulence factors; inhibits swimming, swarming, and twitching motility; attenuates biofilm formation and bacterial pathogenicity. | [130] |
| Vitexin | 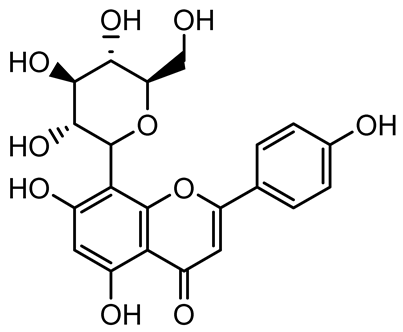 | Vitex peduncularis | S. aureus (MTCC 96) | 26–126 | Reduces cell surface hydrophobicity, membrane depolarization, and EPS production; downregulates biofilm genes (icaAB, dltA) and QS genes (agrAC). | [131] |
| P. aeruginosa (MTCC 2488) | 110 | Inhibits quorum sensing by targeting LuxR, LasA, LasI, and motility-related proteins (PilY1, PilT); reduces EPS, biofilm protein, pyocyanin, protease, LasA/B activity. | [132] | |||
| Morin |  | Fig, almond, guava | Methicillin-resistant S. aureus and Vancomycin-resistant S. aureus | 282 398 | Inhibits biofilm formation, disrupts established biofilms, reduces sliding motility, reduces EPS production, binds to SarA (global regulator), inhibiting its DNA-binding activity and thereby interfering with quorum sensing-regulated biofilm and virulence gene expression. | [133] |
| Naringin, Neohesperidin, Hesperidin | 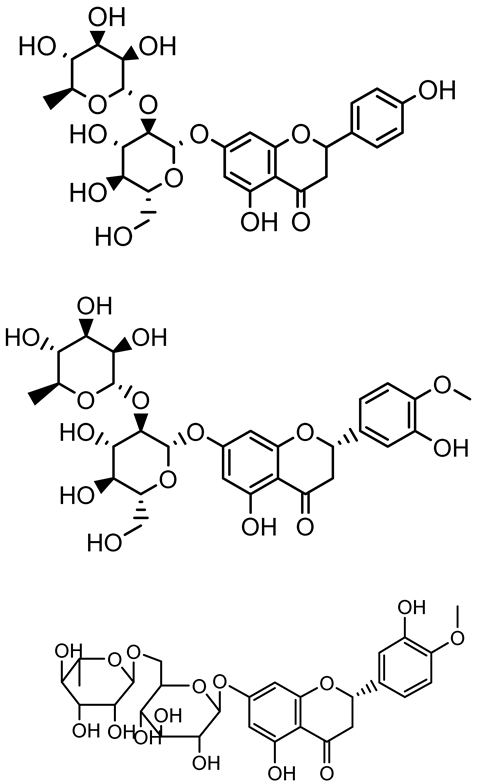 | Citrus | Yersinia enterocolitica | 100–400 | Inhibit quorum sensing by reducing AHL (3-oxo-C6-HSL and C6-HSL) production, inhibit biofilm formation, inhibit swimming motility, alter the expression of QS-related genes (yenR, fliA, flhDC). | [134] |
| Diosmin, myricetin, Neohesperidin | 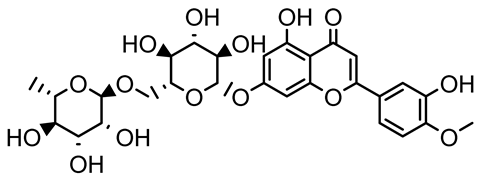 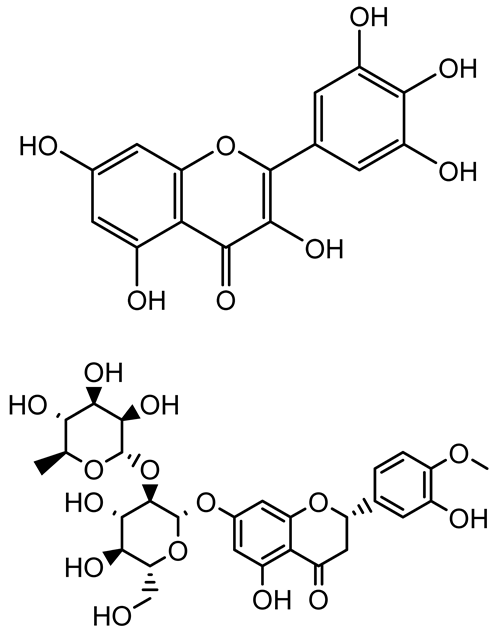 | Citrus fruits | P. aeruginosa | 50–400 | Inhibit biofilm formation, reduce EPS and eDNA production, interfere with quorum sensing by downregulating genes such as lasI, pvdS, and rhlC. | [135] |
| Glabridin | 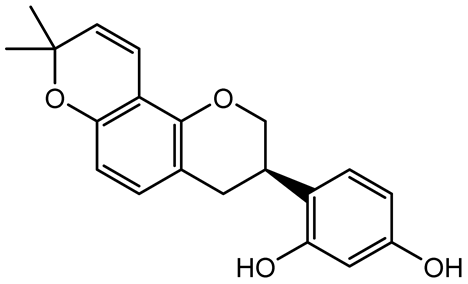 | Glycyrrhiza glabra | L. monocytogenes | 3.91–15.63 | Reduces motility and hemolytic activity, decreases intracellular survival, inhibits hly gene expression, induces ROS in macrophages without affecting biofilm formation. | [136] |
| β-Glycyrrhetinic acid (BGA) | 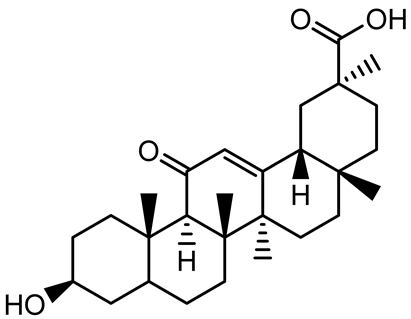 | Glycyrrhiza spp. | Streptococcus mutans, S. sobrinus, S. gordonii, Porphyromonas gingivalis | 128–256 | Inhibits bacterial growth, biofilm formation, and bacterial coaggregation; suppresses plaque biofilm maturation by affecting early colonizers and preventing P. gingivalis adhesion. | [137] |
| Glycyrrhizin | 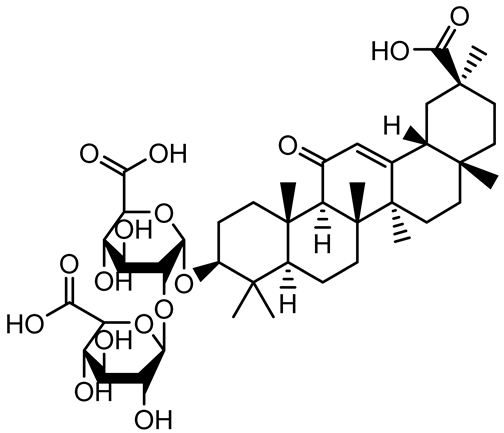 | Glycyrrhiza uralensis | P. plecoglossicida | 60–100 | Inhibits biofilm formation, increases bacterial membrane permeability, suppresses bacterial growth, enhances phagocytosis and bactericidal capacity of host immune cells. | [138] |
| Carnosic acid | 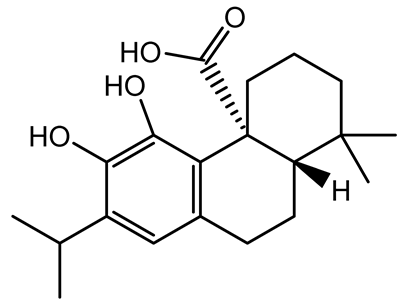 | Salvia rosmarinus | S. aureus | 50 | Inhibits quorum sensing by downregulating agrA and rnaIII genes, reduces virulence genes (hla, psmα), prevents biofilm formation, enhances intracellular killing by macrophages without bactericidal effects, directly binds AgrA DNA-binding site. | [139] |
| Ursolic acid | 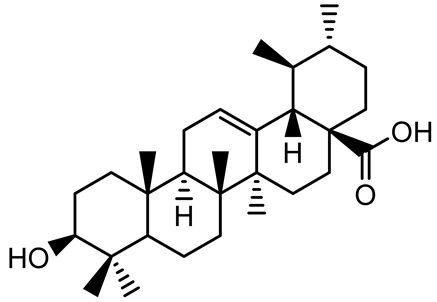 | Rosmarinus officinalis | S. aureus | 39 | Disrupts bacterial cell wall and membrane integrity, inhibits protein synthesis, reduces biofilm formation, induces intracellular ROS production, leading to bacterial death. | [140] |
| Betulinic acid | 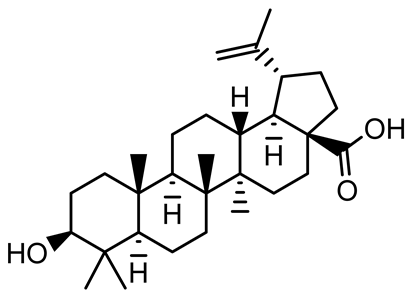 | Ludwigia grandiflora | S. aureus, Candida albicans | 25 | Disrupts cell membranes and surface hydrophobicity, reduces biofilm biomass and early adhesion, downregulates sasF (S. aureus) gene expression, inhibits yeast-to-hyphae transition in C. albicans without microbicidal effects. | [141] |
| Betulin, Betulinic acid | 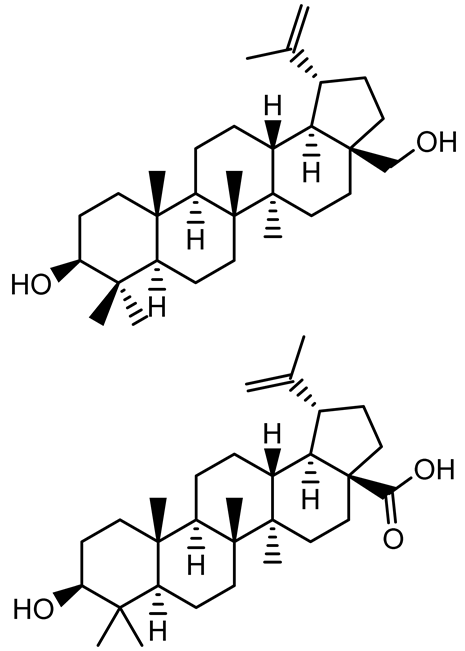 | Betula species (birch trees), Trochodendron aralioides, Ziziphus vulgaris var. spinosus | P. aeruginosa PAO1 | 125 | Inhibit quorum sensing by competitive binding to LasR and RhlR receptors; suppress virulence factors (pyocyanin, elastase, protease, rhamnolipid, chitinase); inhibit biofilm formation; reduce EPS, alginate, eDNA production, and surface hydrophobicity; impair motility. | [142] |
| 3,5-di-O-galloylquinic acid, myricetin | 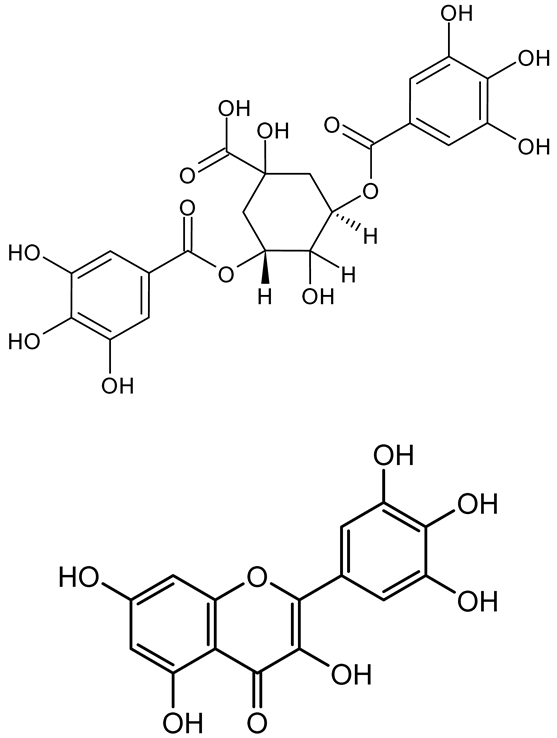 | Myrtus communis | Chromobacterium violaceum 6267, P. aeruginosa PAO1 | 31.25–125 | Inhibit quorum sensing by binding to QS receptor CviR; downregulate lasI, lasR, rhlI, rhlR, pqsA genes; inhibit biofilm formation, pyocyanin production, swarming motility, and protease activity. | [143] |
| Methyl gallate, Pyrogallol, Betulin, Epicatechin gallate, Dehydroabietic acid | 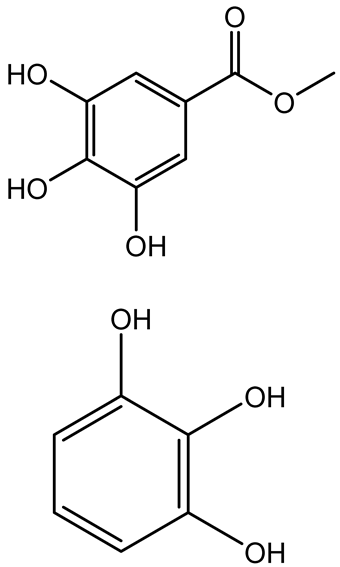 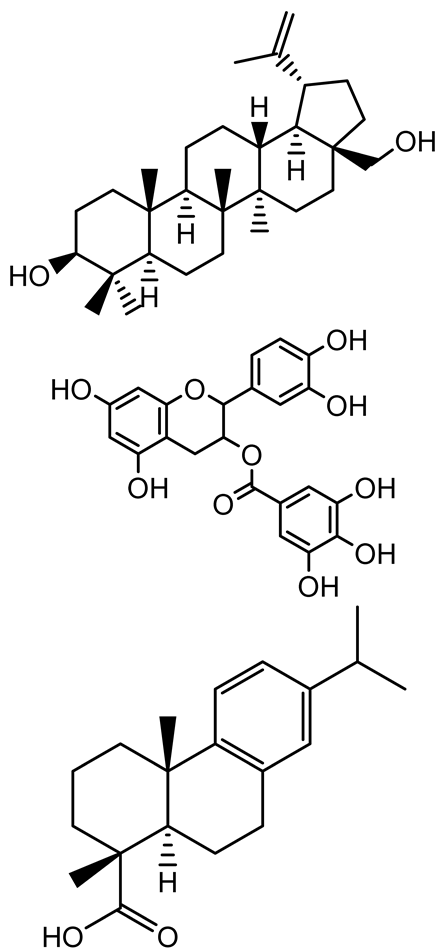 | Acacia nilotica pods | C. violaceum, P. aeruginosa, Serratia marcescens | 250–500 | Inhibit quorum sensing by binding QS receptors (LasI, LasR, RhlR, CviR) and biofilm proteins (PilY1, PilT); suppress violacein, pyocyanin, protease, swarming, and biofilm formation; promote oxidative stress that disrupts bacterial communication and biofilm stability. | [144] |
| Taxifolin, quercetin, Silybin, Silychristin | 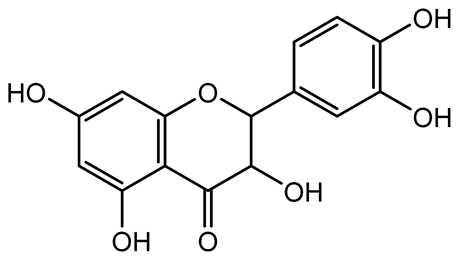  | Silybum marianum | Vibrio campbellii, S. aureus, P. aeruginosa | <10 µM | Inhibit AI-1 and AI-2 quorum sensing by interfering with bacterial communication signals, prevent early bacterial surface colonization (biofilm initiation), enhance antibiotic sensitization in resistant strains. | [145] |
| Tormentic acid, 23-Hydroxycorosolic acid |   | Sarcochlamys pulcherrima | S. aureus | 20 25 | Depolarize bacterial membrane, inhibit biofilm formation, reduce exopolysaccharide production, suppress motility and protease activity, downregulate virulence gene expression, bind strongly to biofilm and quorum sensing proteins (TarO for TA, AgrA for HCA). | [146] |
| Ellagic acid, gallic acid, Methyl gallate, Chlorogenic acid, naringenin, Apigenin |  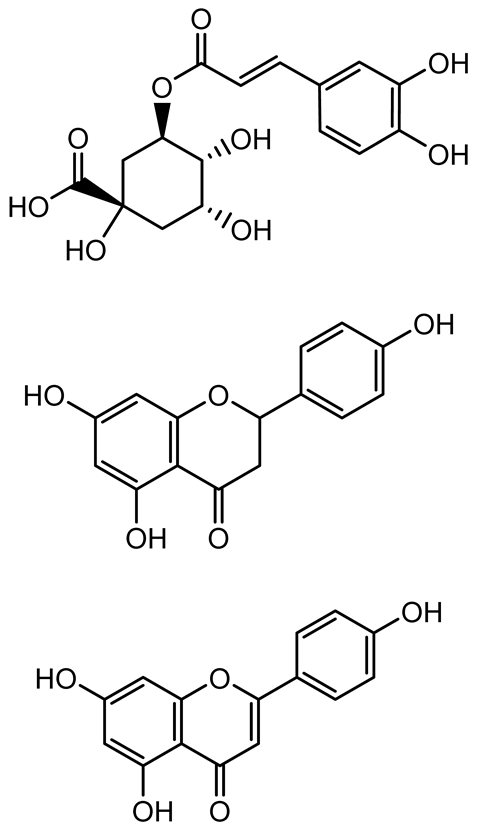 | Dioon spinulosum | P. aeruginosa, C. violaceum | 250–1000 | Inhibits quorum sensing by downregulating lasI, lasR, rhlI, rhlR genes; reduces EPS production, cell surface hydrophobicity, violacein production. | [147] |
| Alpha-copaene, Caryophyllene, Nerolidol | 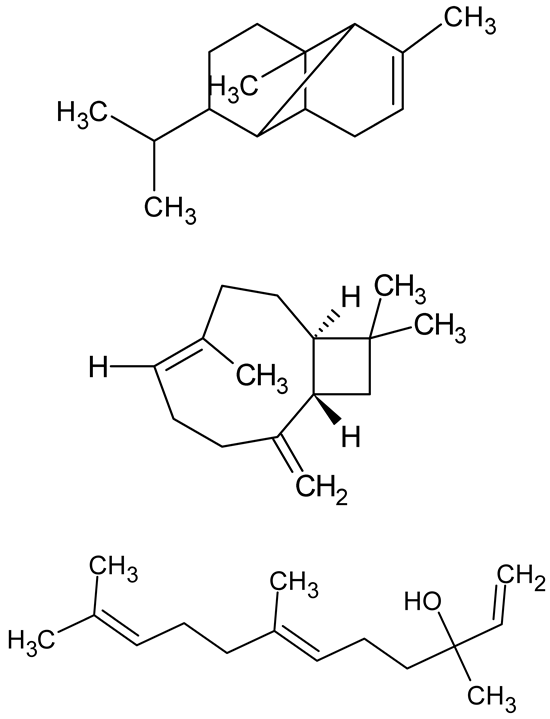 | Psidium guajava | C. violaceum 12742, P. aeruginosa PAO1 | 500–1000 | Inhibit quorum sensing by binding to QS receptors (RhlR, CviR’, LasI, LasR), suppress AHL production, reduce virulence factors (pyoverdin, pyocyanin, rhamnolipid), inhibit biofilm formation in a concentration-dependent manner. | [148] |
| Curcumin | 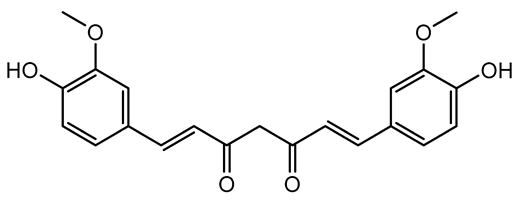 | Curcuma longa | Escherichia coli, P. aeruginosa PAO1, Proteus mirabilis, Serratia marcescens | 0.125–600 | Inhibits biofilm formation and disrupts mature biofilms by interfering with quorum sensing systems, reduces EPS and alginate production, and suppresses swimming and swarming motility. | [149] |
| Oleanolic aldehyde coumarate | 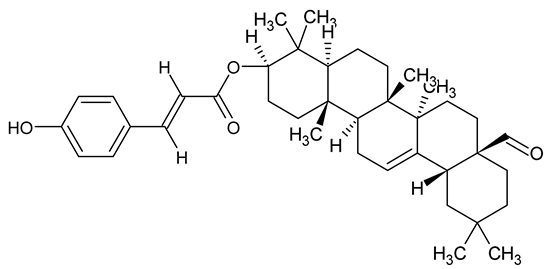 | Dalbergia trichocarpa | P. aeruginosa PAO1 | 117 | Inhibits quorum sensing systems (las and rhl), reduces AHL production, inhibits QS-regulated virulence factors, inhibits biofilm formation and maintenance, reduces extracellular polysaccharides, enhances antibiotic (tobramycin) activity against biofilm-encapsulated bacteria. | [150] |
| Apigenin, Acacetin, Genistein, Biochanin A, Daidzein | 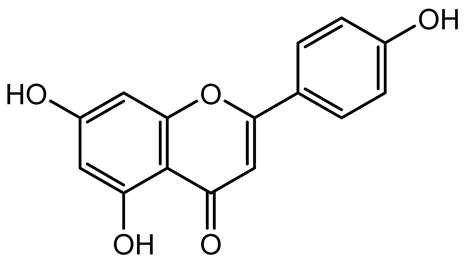 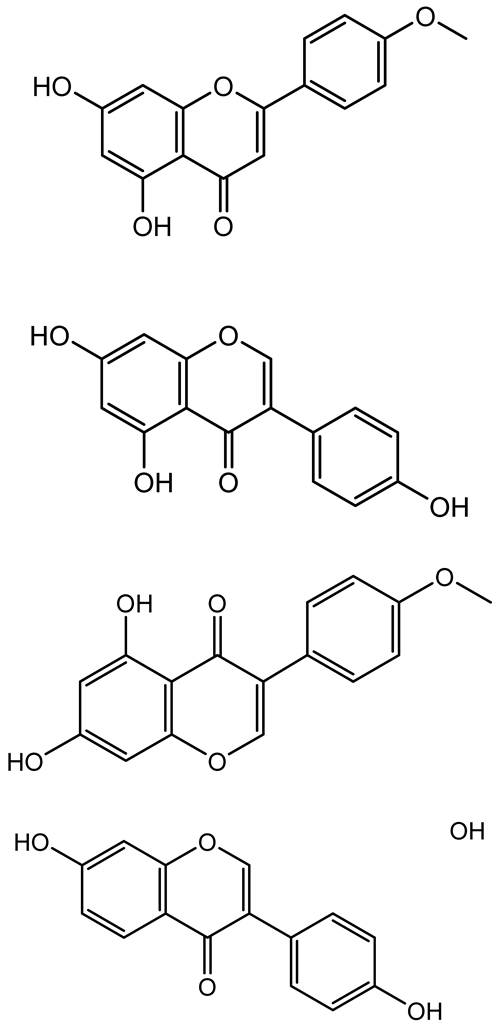 | Legumes | P. aeruginosa PAO1 | 0.4875–45 | Inhibit quorum sensing by downregulating lasI, lasR, rhlI, rhlR pathways; suppress virulence factors (biofilm, pyocyanin, pyoverdin, rhamnolipid, alginate, protease, exopolysaccharide); inhibit swimming and swarming motility. | [151] |
| Isoliquiritin, EGCG, Eugenol, Luteolin, Chrysin | 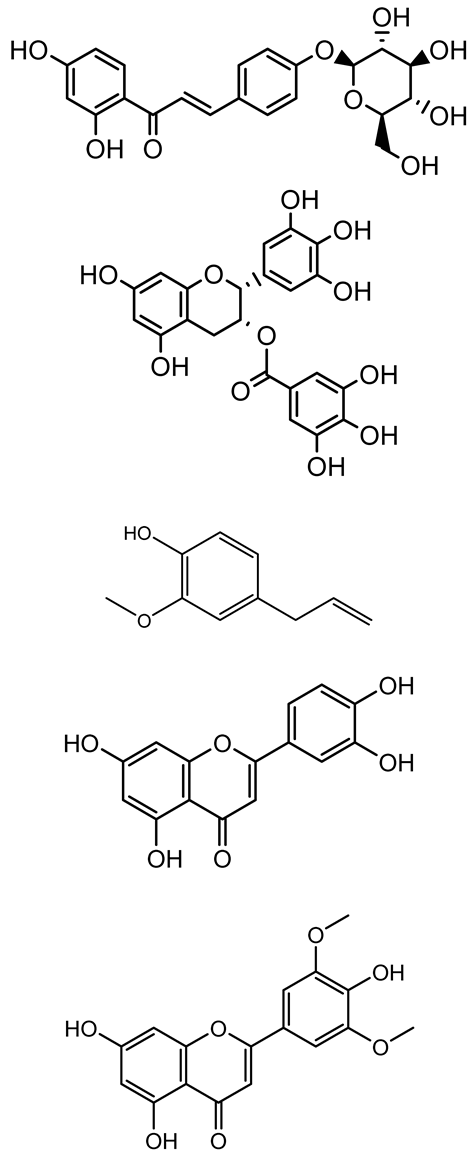 | Various plants | P. aeruginosa | 95–500 | Inhibit quorum sensing by targeting LasI, LasR, RhlI, and RhlR systems; inhibit biofilm formation; reduce exopolysaccharide production, aggregation, and hydrophobicity. | [152] |
4. Medicinal Plant Compound-Derived Nanoparticles with Antibiofilm and Virulence Properties
4.1. Nanomaterials for Effective Delivery of Plant Compounds
4.1.1. Lipid-Based Nanocarriers
4.1.2. Polymeric Nanoparticles
4.1.3. Metal Nanoparticles
5. Synergy of Plant Compounds with Nanocarriers
6. Exploring the Potential and Pitfalls of Medicinal Plant Compounds
6.1. Changes in Phytochemical Profiles
6.2. Concerns with Stability and Bioavailability
6.3. Profiles of Safety and Toxicology
6.4. Challenges in Regulation and Commercialization
7. Future Prospects and Emerging Directions
7.1. Advances in Omics and High-Throughput Screening Technologies
7.2. Targeted Therapies and Personalized Medicine
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EGCG | Epigallocatechin-3-gallate |
| Β | Beta |
| QS | Quorum sensing |
| EPS | Extracellular polymeric substance |
References
- EclinicalMedicine. Antimicrobial resistance: A top ten global public health threat. EClinicalMedicine 2021, 41, 101221. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Böhning, J.; Tarafder, A.K.; Bharat, T.A.M. The role of filamentous matrix molecules in shaping the architecture and emergent properties of bacterial biofilms. Biochem. J. 2024, 481, 245–263. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr. Med. Chem. 2015, 22, 2590–2614. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323. [Google Scholar] [CrossRef]
- Abdulbaqi, H.R.; Himratul-Aznita, W.H.; Baharuddin, N.A. Evaluation of Salvadora persica L. and green tea anti-plaque effect: A randomized controlled crossover clinical trial. BMC Complement. Altern. Med. 2016, 16, 493. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Shamim, A.; Ali, A.; Iqbal, Z.; Mirza, M.A.; Aqil, M.; Kawish, S.M.; Siddiqui, A.; Kumar, V.; Naseef, P.P.; Alshadidi, A.A.F.; et al. Natural Medicine a Promising Candidate in Combating Microbial Biofilm. Antibiotics 2023, 12, 299. [Google Scholar] [CrossRef]
- de Melo, A.L.F.; Rossato, L.; Barbosa, M.d.S.; Palozi, R.A.C.; Alfredo, T.M.; Antunes, K.A.; Eduvirgem, J.; Ribeiro, S.M.; Simionatto, S. From the environment to the hospital: How plants can help to fight bacteria biofilm. Microbiol. Res. 2022, 261, 127074. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Roy, R.; Monalisa, T.; Gianfranco, D.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action. Int. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E. Molecular aspects of the functioning of pathogenic bacteria biofilm based on quorum sensing (QS) signal-response system and innovative non-antibiotic strategies for their elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef]
- Zeng, X.; Zou, Y.; Zheng, J.; Qiu, S.; Liu, L.; Wei, C. Quorum sensing-mediated microbial interactions: Mechanisms, applications, challenges and perspectives. Microbiol. Res. 2023, 273, 127414. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hu, X.; Wang, Y. Alternatives to conventional antibiotic therapy: Potential therapeutic strategies of combating antimicrobial-resistance and biofilm-related infections. Mol. Biotechnol. 2021, 63, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Shi, J.; Fang, C.; Zeng, X.; Wu, Z.; Du, Q.; Tu, M.; Pan, D. Elimination of Pathogen Biofilms via Postbiotics from Lactic Acid Bacteria: A Promising Method in Food and Biomedicine. Microorganisms 2024, 12, 704. [Google Scholar] [CrossRef]
- Almatroudi, A. Investigating biofilms: Advanced methods for comprehending microbial behavior and antibiotic resistance. Front. Biosci.-Landmark 2024, 29, 133. [Google Scholar] [CrossRef]
- Joshi, S.; Lahiri, D.; Ray, R.R.; Davoodbasha, M. Microbial Biofilms: Challenges and Advances in Metabolomic Study; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar]
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, structure, and function of extracellular polymeric substances (EPS) of microbial biofilm in biological wastewater treatment systems: A review. Desalination Water Treat. 2016, 57, 16220–16237. [Google Scholar] [CrossRef]
- Billings, N.; Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The extracellular matrix Component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef]
- Zhao, K.; Tseng, B.S.; Beckerman, B.; Jin, F.; Gibiansky, M.L.; Harrison, J.J.; Luijten, E.; Parsek, M.R.; Wong, G.C.L. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature 2013, 497, 388–391. [Google Scholar] [CrossRef]
- Nett, J.E.; Sanchez, H.; Cain, M.T.; Andes, D.R. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 2010, 202, 171–175. [Google Scholar] [CrossRef]
- Soares, A.; Alexandre, K.; Etienne, M. Tolerance and persistence of Pseudomonas aeruginosa in biofilms exposed to antibiotics: Molecular mechanisms, antibiotic strategies and therapeutic perspectives. Front. Microbiol. 2020, 11, 2057. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Flint, S.; Li, Y.; Ou, K.; Yuan, L.; He, G.Q. Phenotypic and genetic heterogeneity within biofilms with particular emphasis on persistence and antimicrobial tolerance. Future Microbiol. 2017, 12, 1087–1107. [Google Scholar] [CrossRef]
- Almatroudi, A. Biofilm Resilience: Molecular Mechanisms Driving Antibiotic Resistance in Clinical Contexts. Biology 2025, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Azeem, K.; Fatima, S.; Ali, A.; Ubaid, A.; Husain, F.M.; Abid, M. Biochemistry of Bacterial Biofilm: Insights into Antibiotic Resistance Mechanisms and Therapeutic Intervention. Life 2025, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, C.; Grohmann, E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Brito, I.L. Examining horizontal gene transfer in microbial communities. Nat. Rev. Microbiol. 2021, 19, 442–453. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G. Detection and various environmental factors of antibiotic resistance gene horizontal transfer. Environ. Res. 2022, 212, 113267. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Pugazhendhi, A.S.; Wei, F.; Hughes, M.; Coathup, M. Bacterial adhesion, virulence, and biofilm formation. In Musculoskeletal Infection; Springer: Berlin/Heidelberg, Germany, 2022; pp. 19–64. [Google Scholar]
- Sarkar, P.; Issac, P.K.; Raju, S.V.; Elumalai, P.; Arshad, A.; Arockiaraj, J. Pathogenic bacterial toxins and virulence influences in cultivable fish. Aquac. Res. 2021, 52, 2361–2376. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Yu, C.; Li, J.; Zhou, X. Biofilm formation: Mechanistic insights and therapeutic targets. Mol. Biomed. 2023, 4, 49. [Google Scholar] [CrossRef]
- Milly, T.A.; Tal-Gan, Y. Targeting peptide-based quorum sensing systems for the treatment of gram-positive bacterial infections. Pept. Sci. 2023, 115, e24298. [Google Scholar] [CrossRef]
- Haque, S.; Yadav, D.K.; Bisht, S.C.; Yadav, N.; Singh, V.; Dubey, K.K.; Jawed, A.; Wahid, M.; Dar, S.A. Quorum sensing pathways in Gram-positive and-negative bacteria: Potential of their interruption in abating drug resistance. J. Chemother. 2019, 31, 161–187. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, F.; Li, S.; Yin, J.; Hu, L.; Zheng, S.; He, L.; Xia, H.; Liu, J.; Hu, W. The mechanisms of biofilm antibiotic resistance in chronic rhinosinusitis: A review. Medicine 2022, 101, e32168. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A. Combatting biofilm-mediated infections in clinical settings by targeting quorum sensing. Cell Surf. 2024, 12, 100133. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- Wagner, V.E.; Gillis, R.J.; Iglewski, B.H. Transcriptome analysis of quorum-sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine 2004, 22, S15–S20. [Google Scholar] [CrossRef]
- Duan, K.; Surette Michael, G. Environmental Regulation of Pseudomonas aeruginosa PAO1 Las and Rhl Quorum-Sensing Systems. J. Bacteriol. 2007, 189, 4827–4836. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, W.-J.; Bhatt, K.; Zhou, Z.; Huang, Y.; Zhang, L.-H.; Chen, S.; Wang, J. Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review. Front. Plant Sci. 2023, 13, 1063393. [Google Scholar] [CrossRef]
- Tu, Y.; Li, H.; Huo, J.; Gou, L.; Wen, X.; Yu, X.; Zhang, X.; Zeng, J.; Li, Y. Disrupting the bacterial language: Quorum quenching and its applications. Crit. Rev. Microbiol. 2025, 1–15. [Google Scholar] [CrossRef]
- Tan Lim, A.M.; Oyong, G.G.; Tan, M.C.S.; Chang Shen, C.; Ragasa, C.Y.; Cabrera, E.C. Quorum quenching activity of Andrographis paniculata (Burm f.) Nees andrographolide compounds on metallo-β-lactamase-producing clinical isolates of Pseudomonas aeruginosa PA22 and PA247 and their effect on lasR gene expression. Heliyon 2021, 7, e07002. [Google Scholar] [CrossRef]
- Khanashyam, A.C.; Shanker, M.A.; Thomas, P.E.; Babu, K.S.; Nirmal, N.P. Phytochemicals in biofilm inhibition. In Recent Frontiers of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 397–412. [Google Scholar]
- Zhang, M.; Han, W.; Gu, J.; Qiu, C.; Jiang, Q.; Dong, J.; Lei, L.; Li, F. Recent advances on the regulation of bacterial biofilm formation by herbal medicines. Front. Microbiol. 2022, 13, 1039297. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef]
- Budzyńska, A.; Wieckowska-Szakiel, M.; Sadowska, B.; Kalemba, D.; Rozalska, B. Antibiofilm activity of selected plant essential oils and their major components. Pol. J. Microbiol. 2011, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Teixeira, J.A.; Pereira, M.O.; Rocha, C.M.; Sousa, A.M. Evolving biofilm inhibition and eradication in clinical settings through plant-based antibiofilm agents. Phytomedicine 2023, 119, 154973. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Sarkar, A.; Mukherjee, S.; Das, A.; Banerjee, T.; Paul, P.; Bhowmik, R.; Karmakar, S.; Ghosh, N. The Potential of Phytomolecules in Countering Biofilm Formation and Quorum Sensing. Curr. Indian Sci. 2025, 3, e2210299X2355078. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Wang, C.; Zhu, J.; Wang, H.; Liao, L.; Zhao, Y.; Wang, X.; Yang, C.; He, Z. Plant-derived virulence arresting drugs as novel antimicrobial agents: Discovery, perspective, and challenges in clinical use. Phytother. Res. 2024, 38, 727–754. [Google Scholar] [CrossRef]
- Roy, M.; Adhikari, M.; Tiwary, B. Phytocompound-Mediated Inhibition of Quorum-Sensing Cascade in Pathogenic Bacteria. In Natural Products; CRC Press: Boca Raton, FL, USA, 2024; pp. 79–101. [Google Scholar]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Morkunas, B.; Gal, B.; Galloway, W.R.; Hodgkinson, J.T.; Ibbeson, B.M.; Tan, Y.S.; Welch, M.; Spring, D.R. Discovery of an inhibitor of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells. Beilstein J. Org. Chem. 2016, 12, 1428–1433. [Google Scholar] [CrossRef]
- Lee, J.; Attila, C.; Cirillo, S.L.G.; Cirillo, J.D.; Wood, T.K. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb. Biotechnol. 2009, 2, 75–90. [Google Scholar] [CrossRef]
- Zhou, J.W.; Luo, H.Z.; Jiang, H.; Jian, T.K.; Chen, Z.Q.; Jia, A.Q. Hordenine: A Novel Quorum Sensing Inhibitor and Antibiofilm Agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018, 66, 1620–1628. [Google Scholar] [CrossRef]
- Rudrapal, M.; Sarkar, B.; Deb, P.; Bendale, A.; Nagar, A. Addressing antimicrobial resistance by repurposing polyphenolic phytochemicals with novel antibacterial potential. In Polyphenols: Food Nutraceutical Nanotherapeutic Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 260–289. [Google Scholar]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Li, J.; Qin, G. New strategies for biocontrol of bacterial toxins and virulence: Focusing on quorum-sensing interference and biofilm inhibition. Toxins 2023, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Nouh, H.S.; El-Zawawy, N.A.; Halawa, M.; Shalamesh, E.M.; Ali, S.S.; Korbecka-Glinka, G.; Shala, A.Y.; El-Sapagh, S. Endophytic Penicillium oxalicum AUMC 14898 from Opuntia ficus-indica: A Novel Source of Tannic Acid Inhibiting Virulence and Quorum Sensing of Extensively Drug-Resistant Pseudomonas aeruginosa. Int. J. Mol. Sci. 2024, 25, 11115. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Ta, C.A.; Arnason, J.T. Mini Review of Phytochemicals and Plant Taxa with Activity as Microbial Biofilm and Quorum Sensing Inhibitors. Molecules 2015, 21, E29. [Google Scholar] [CrossRef]
- Vermote, A.; Brackman, G.; Risseeuw, M.D.; Vanhoutte, B.; Cos, P.; Van Hecke, K.; Breyne, K.; Meyer, E.; Coenye, T.; Van Calenbergh, S. Hamamelitannin Analogues that Modulate Quorum Sensing as Potentiators of Antibiotics against Staphylococcus aureus. Angew. Chem. Int. Ed. Engl. 2016, 55, 6551–6555. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Liu, Z.; Zhi, Y.; Mei, C.; Wang, H. Flavonoids as Promising Natural Compounds for Combating Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 2455. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing phytochemicals as anti-virulent agents to attenuate quorum sensing-regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2022, 15, 1695–1718. [Google Scholar] [CrossRef]
- Samrot, A.V.; Abubakar Mohamed, A.; Faradjeva, E.; Si Jie, L.; Hooi Sze, C.; Arif, A.; Chuan Sean, T.; Norbert Michael, E.; Yeok Mun, C.; Xiao, Q.N.; et al. Mechanisms and impact of biofilms and targeting of biofilms using bioactive compounds—A review. Medicina 2021, 57, 839. [Google Scholar] [CrossRef]
- Lal, A.F.; Singh, S.; Franco, F.C.; Bhatia, S. Potential of polyphenols in curbing quorum sensing and biofilm formation in Gram-negative pathogens. Asian Pac. J. Trop. Biomed. 2021, 11, 231–243. [Google Scholar] [CrossRef]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Dal Piaz, F. Interactions with microbial proteins driving the antibacterial activity of flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.X.; Thirapanmethee, K.; Audshasai, T.; Khuntayaporn, P.; Chomnawang, M.T. Insights into molecular mechanisms of phytochemicals in quorum sensing modulation for bacterial biofilm control. Arch. Microbiol. 2024, 206, 459. [Google Scholar] [CrossRef]
- Joseph, J.; Boby, S.; Muyyarikkandy, M.S. Phytochemicals: A Promising Strategy to Combat Biofilm-Associated Antimicrobial Resistance; IntechOpen: London, UK, 2025. [Google Scholar]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef]
- Pruteanu, M.; Hernández Lobato, J.I.; Stach, T.; Hengge, R. Common plant flavonoids prevent the assembly of amyloid curli fibres and can interfere with bacterial biofilm formation. Environ. Microbiol. 2020, 22, 5280–5299. [Google Scholar] [CrossRef]
- Imam, M.W.; Luqman, S. Unveiling the mechanism of essential oil action against skin pathogens: From ancient wisdom to modern science. Arch. Microbiol. 2024, 206, 347. [Google Scholar] [CrossRef]
- Rasheed, H.A.; Rehman, A.; Chen, X.; Aziz, T.; Al-Asmari, F.; Alhomrani, M.; Alamri, A.S.; Cui, H.; Lin, L. Unveiling the anti-listerial effect of Citrus bergamia essential oil: Mechanism of membrane disruption and anti-hemolytic activity. Food Biosci. 2024, 61, 104742. [Google Scholar] [CrossRef]
- Nasaj, M.; Chehelgerdi, M.; Asghari, B.; Ahmadieh-Yazdi, A.; Asgari, M.; Kabiri-Samani, S.; Sharifi, E.; Arabestani, M. Factors influencing the antimicrobial mechanism of chitosan action and its derivatives: A review. Int. J. Biol. Macromol. 2024, 277, 134321. [Google Scholar] [CrossRef]
- Maggio, F.; Rossi, C.; Serio, A.; Chaves-Lopez, C.; Casaccia, M.; Paparella, A. Anti-biofilm mechanisms of action of essential oils by targeting genes involved in quorum sensing, motility, adhesion, and virulence: A review. Int. J. Food Microbiol. 2025, 426, 110874. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2172–2191. [Google Scholar] [CrossRef]
- Millezi, A.F.; Piccoli, R.H.; Oliveira, J.M.; Pereira, M.O. Anti-biofim and antibacterial effect of essential oils and their major compounds. J. Essent. Oil Bear. Plants 2016, 19, 624–631. [Google Scholar] [CrossRef]
- Alibi, S.; Ben Selma, W.; Ramos-Vivas, J.; Smach, M.A.; Touati, R.; Boukadida, J.; Navas, J.; Ben Mansour, H. Anti-oxidant, antibacterial, anti-biofilm, and anti-quorum sensing activities of four essential oils against multidrug-resistant bacterial clinical isolates. Curr. Res. Transl. Med. 2020, 68, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lagha, R.; Ben Abdallah, F.; AL-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Ismaël, I.S.; Hosenally, M.; Zengin, G.; Mahomoodally, M.F. Essential oils from tropical medicinal herbs and food plants inhibit biofilm formation in vitro and are non-cytotoxic to human cells. 3 Biotech 2018, 8, 395. [Google Scholar] [CrossRef]
- Milho, C.; Silva, J.; Guimarães, R.; Ferreira, I.C.F.R.; Barros, L.; Alves, M.J. Antimicrobials from Medicinal Plants: An Emergent Strategy to Control Oral Biofilms. Appl. Sci. 2021, 11, 4020. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Lagha, R.; Gaber, A. Biofilm Inhibition and Eradication Properties of Medicinal Plant Essential Oils against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2020, 13, 369. [Google Scholar] [CrossRef]
- Guimarães, R.; Milho, C.; Liberal, Â.; Silva, J.; Fonseca, C.; Barbosa, A.; Ferreira, I.C.F.R.; Alves, M.J.; Barros, L. Antibiofilm Potential of Medicinal Plants against Candida spp. Oral Biofilms: A Review. Antibiotics 2021, 10, 1142. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants: From Farm to Pharmacy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 333–359. [Google Scholar] [CrossRef]
- Raut, J.S.; Shinde, R.; Chauhan, N.; Karuppayil, S.M. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 2013, 29, 87–96. [Google Scholar] [CrossRef]
- Braga, P.C.; Culici, M.; Alfieri, M.; Dal Sasso, M. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int. J. Antimicrob. Agents 2008, 31, 472–477. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Ngezahayo, J.; Pottier, L.; Ribeiro, S.O.; Souard, F.; Hari, L.; Stévigny, C.; Jaziri, M.E.; Duez, P. Terpenoids from Platostoma rotundifolium (Briq.) A. J. Paton Alter the Expression of Quorum Sensing-Related Virulence Factors and the Formation of Biofilm in Pseudomonas aeruginosa PAO1. Int. J. Mol. Sci. 2017, 18, 1270. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef] [PubMed]
- Maquera Huacho, P.M.; Esteban, R.H.; Tim, V.; Martine, P.; Elcio, M.J.; Madalena, P.S.D.; Teughels, W. Terpinen-4-ol and carvacrol affect multi-species biofilm composition. Biofouling 2019, 35, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of Carvacrol and Thymol in Reducing Biofilm Formation on Technical Surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Soni, K.A.; Oladunjoye, A.; Nannapaneni, R.; Schilling, M.W.; Silva, J.L.; Mikel, B.; Bailey, R.H. Inhibition and Inactivation of Salmonella typhimurium Biofilms from Polystyrene and Stainless Steel Surfaces by Essential Oils and Phenolic Constituent Carvacrol†. J. Food Prot. 2013, 76, 205–212. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Rupasinghe, H.P.V. Carvacrol inhibits Streptococcus pyogenes biofilms by suppressing the expression of genes associated with quorum-sensing and reducing cell surface hydrophobicity. Microb. Pathog. 2022, 169, 105684. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Ivanova, V.; Trendafilova, A.; Paunova-Krasteva, T. Anti-Biofilm and Anti-Quorum-Sensing Activity of Inula Extracts: A Strategy for Modulating Chromobacterium violaceum Virulence Factors. Pharmaceuticals 2024, 17, 573. [Google Scholar] [CrossRef]
- Wiart, C.; Kathirvalu, G.; Raju, C.S.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; Rajagopal, M.; Sathiya Seelan, J.S.; Rusdi, N.A.; Lanting, S.; et al. Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles. Molecules 2023, 28, 3873. [Google Scholar] [CrossRef]
- Juárez, C.; Contreras Rodolfo, G.; López Macrina, P.; Cazares Naybi, M.; Castillo Juárez, I. Phenolic Compounds with Anti-virulence Properties. In Phenolic Compounds-Biological Activity; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Li, Q.; Mao, S.; Wang, H.; Ye, X. The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors. Mar. Drugs 2022, 20, 488. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.; Misra, T.K.; Roy, D.N. In vitro anti-biofilm activity of 14-deoxy-11, 12-didehydroandrographolide from Andrographis paniculata against Pseudomonas aeruginosa. Braz. J. Microbiol. 2020, 51, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jiang, X.; Xu, X.; Jiang, C.; Kang, R.; Jiang, X. Andrographolide inhibits biofilm and virulence in Listeria monocytogenes as a quorum-sensing inhibitor. Molecules 2022, 27, 3234. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Saei, H.D.; Ahmadi, M.; Zahraei-Salehi, T. Anti-quorum sensing effects of licochalcone A and epigallocatechin-3-gallate against Salmonella typhimurium isolates from poultry sources. Vet. Res. Forum 2020, 11, 273–279. [Google Scholar]
- Khadke, S.K.; Lee, J.-H.; Woo, J.-T.; Lee, J. Inhibitory effects of honokiol and magnolol on biofilm formation by Acinetobacter baumannii. Biotechnol. Bioprocess Eng. 2019, 24, 359–365. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Zou, H.; Wang, Y.; Xu, Y.; Wang, L.; Wang, B.; Yan, M.; Leng, X. Unraveling the efficacy of verbascoside in thwarting MRSA pathogenicity by targeting sortase A. Appl. Microbiol. Biotechnol. 2024, 108, 360. [Google Scholar] [CrossRef]
- Cai, Y.-M.; Hong, F.; De Craemer, A.; Malone, J.G.; Crabbé, A.; Coenye, T. Echinacoside reduces intracellular c-di-GMP levels and potentiates tobramycin activity against Pseudomonas aeruginosa biofilm aggregates. npj Biofilms Microbiomes 2025, 11, 40. [Google Scholar] [CrossRef]
- Song, Y.; Lv, H.; Xu, L.; Liu, Z.; Wang, J.; Fang, T.; Deng, X.; Zhou, Y.; Li, D. In vitro and in vivo activities of scutellarein, a novel polyphosphate kinase 1 inhibitor against Acinetobacter baumannii infection. Microb. Cell Factories 2024, 23, 269. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, H.; Meng, J.; Qiao, M.; Du, H.; He, M.; Ming, K.; Liu, J.; Wang, D.; Wu, Y. Baicalin inhibits biofilm formation and the quorum-sensing system by regulating the MsrA drug efflux pump in Staphylococcus saprophyticus. Front. Microbiol. 2019, 10, 2800. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, Y.; Han, X.; Cai, X.; Yang, L.; Liu, C.; Shen, L. Inhibition of virulence factors and biofilm formation by wogonin attenuates pathogenicity of Pseudomonas aeruginosa PAO1 via targeting pqs quorum-sensing system. Int. J. Mol. Sci. 2021, 22, 12699. [Google Scholar] [CrossRef] [PubMed]
- Das, M.C.; Samaddar, S.; Jawed, J.J.; Ghosh, C.; Acharjee, S.; Sandhu, P.; Das, A.; Daware, A.V.; De, U.C.; Majumdar, S. Vitexin alters Staphylococcus aureus surface hydrophobicity to obstruct biofilm formation. Microbiol. Res. 2022, 263, 127126. [Google Scholar] [CrossRef] [PubMed]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef]
- Chemmugil, P.; Lakshmi, P.; Annamalai, A. Exploring Morin as an anti-quorum sensing agent (anti-QSA) against resistant strains of Staphylococcus aureus. Microb. Pathog. 2019, 127, 304–315. [Google Scholar] [CrossRef]
- Truchado, P.; Giménez-Bastida, J.-A.; Larrosa, M.; Castro-Ibáñez, I.; Espín, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Allende, A. Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J. Agric. Food Chem. 2012, 60, 8885–8894. [Google Scholar] [CrossRef]
- Carević, T.; Kolarević, S.; Kolarević, M.K.; Nestorović, N.; Novović, K.; Nikolić, B.; Ivanov, M. Citrus flavonoids diosmin, myricetin and neohesperidin as inhibitors of Pseudomonas aeruginosa: Evidence from antibiofilm, gene expression and in vivo analysis. Biomed. Pharmacother. 2024, 181, 117642. [Google Scholar] [CrossRef]
- Liao, C.; Yu, C.; Guo, J.; Guan, M. Subinhibitory concentrations of glabridin from Glycyrrhiza glabra L. reduce Listeria monocytogenes motility and hemolytic activity but do not exhibit antimicrobial activity. Front. Microbiol. 2024, 15, 1388388. [Google Scholar] [CrossRef]
- Dewake, N.; Ma, X.; Sato, K.; Nakatsu, S.; Yoshimura, K.; Eshita, Y.; Fujinaka, H.; Yano, Y.; Yoshinari, N.; Yoshida, A. Β-Glycyrrhetinic acid inhibits the bacterial growth and biofilm formation by supragingival plaque commensals. Microbiol. Immunol. 2021, 65, 343–351. [Google Scholar] [CrossRef]
- Tao, F.; Cao, J.-F.; Liu, Y.-J.; Chen, R.-Y.; Shi, J.-J.; Li, C.-Y.; Lu, J.-F.; Yang, G.-J.; Chen, J. Evaluation on the antibacterial activity of glycyrrhizin against Pseudomonas plecoglossicida in ayu fish (Plecoglossus altivelis). Aquaculture 2025, 595, 741520. [Google Scholar] [CrossRef]
- Iobbi, V.; Parisi, V.; Bernabè, G.; De Tommasi, N.; Bisio, A.; Brun, P. Anti-biofilm activity of carnosic acid from Salvia rosmarinus against methicillin-resistant Staphylococcus aureus. Plants 2023, 12, 3679. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Qin, P.; Cheng, X.; Wu, L.; Zhao, W.; Gao, W. Evaluation of the mechanistic basis for the antibacterial activity of ursolic acid against Staphylococcus aureus. Front. Microbiol. 2024, 15, 1389242. [Google Scholar] [CrossRef] [PubMed]
- Hamion, G.; Aucher, W.; Mercier, A.; Tewes, F.; Menard, M.; Bertaux, J.; Girardot, M.; Imbert, C. Insights into betulinic acid as a promising molecule to fight the interkingdom biofilm Staphylococcus aureus–Candida albicans. Int. J. Antimicrob. Agents 2024, 63, 107166. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb. Pathog. 2018, 118, 48–60. [Google Scholar] [CrossRef]
- Khadraoui, N.; Essid, R.; Damergi, B.; Fares, N.; Gharbi, D.; Forero, A.M.; Rodríguez, J.; Abid, G.; Kerekes, E.-B.; Limam, F. Myrtus communis leaf compounds as novel inhibitors of quorum sensing-regulated virulence factors and biofilm formation: In vitro and in silico investigations. Biofilm 2024, 8, 100205. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I. Anti-quorum sensing and biofilm inhibitory effect of some medicinal plants against gram-negative bacterial pathogens: In vitro and in silico investigations. Heliyon 2022, 8, e11113. [Google Scholar]
- Hurtová, M.; Káňová, K.; Dobiasová, S.; Holasová, K.; Čáková, D.; Hoang, L.; Biedermann, D.; Kuzma, M.; Cvačka, J.; Křen, V. Selectively halogenated flavonolignans—Preparation and antibacterial activity. Int. J. Mol. Sci. 2022, 23, 15121. [Google Scholar] [CrossRef]
- Ghosh, C.; Das, M.C.; Acharjee, S.; Bhattacharjee, S.; Sandhu, P.; Kumari, M.; Bhowmik, J.; Ghosh, R.; Banerjee, B.; De, U.C.; et al. Combating Staphylococcus aureus biofilm formation: The inhibitory potential of tormentic acid and 23-hydroxycorosolic acid. Arch. Microbiol. 2024, 206, 25. [Google Scholar] [CrossRef]
- Elekhnawy, E.; Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Alqarni, M.; Batiha, G.E.-S.; Obaidullah, A.J.; Fawzy, H.M. Histological assessment, anti-quorum sensing, and anti-biofilm activities of Dioon spinulosum extract: In vitro and in vivo approach. Sci. Rep. 2022, 12, 180. [Google Scholar] [CrossRef]
- Khan, M.A.; Celik, I.; Khan, H.M.; Shahid, M.; Shahzad, A.; Kumar, S.; Ahmed, B. Antibiofilm and anti-quorum sensing activity of Psidium guajava L. leaf extract: In vitro and in silico approach. PLoS ONE 2023, 18, e0295524. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Vandeputte, O.M.; Pottier, L.; Huet, J.; Rabemanantsoa, C.; Kiendrebeogo, M.; Andriantsimahavandy, A.; Rasamindrakotroka, A.; Stévigny, C.; Duez, P. Pseudomonas aeruginosa biofilm formation and persistence, along with the production of quorum sensing-dependent virulence factors, are disrupted by a triterpenoid coumarate ester isolated from Dalbergia trichocarpa, a tropical legume. PLoS ONE 2015, 10, e0132791. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Rajamuthu, T.P.; Sarkar, A.; Natrajan, P.; Krishnan, N.; Sakthivelu, M.; Velusamy, P.; Ramasamy, P.; Gopinath, S.C. N-acyl-homoserine lactone mediated virulence factor(s) of Pseudomonas aeruginosa inhibited by flavonoids and isoflavonoids. Process Biochem. 2022, 116, 84–93. [Google Scholar] [CrossRef]
- Fakhar, M.; Ahmed, M.; Sabri, A.N. Computational and experimental strategies for combating MBL P. aeruginosa (MBLPA) biofilms using phytochemicals: Targeting the quorum sensing network. Saudi J. Biol. Sci. 2024, 31, 104001. [Google Scholar] [CrossRef]
- Tungare, K.; Gupta, J.; Bhori, M.; Garse, S.; Kadam, A.; Jha, P.; Jobby, R.; Amanullah, M.; Vijayakumar, S. Nanomaterial in controlling biofilms and virulence of microbial pathogens. Microb. Pathog. 2024, 192, 106722. [Google Scholar] [CrossRef]
- Han, C.; Romero, N.; Fischer, S.; Dookran, J.; Berger, A.; Doiron, A.L. Recent developments in the use of nanoparticles for treatment of biofilms. Nanotechnol. Rev. 2017, 6, 383–404. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangku, D.; Hu, C.M.; Huang, C.M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. biofilms: A battle against another paradigm of antibiotic resistance. MedChemComm 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Jabber Al-Saady, M.A.A.; Aldujaili, N.H.; Rabeea Banoon, S.; Al-Abboodi, A. Antimicrobial properties of nanoparticles in biofilms. Bionatura 2022, 7, 1–9. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Fazly Bazzaz, B.S.; Mirhadi, E.; Tajani, A.S.; Khameneh, B. The role of nanotechnology in combating biofilm-based antibiotic resistance. J. Drug Deliv. Sci. Technol. 2020, 60, 101880. [Google Scholar] [CrossRef]
- Blecher, K.; Adnan, N.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.G.; Ansari, M.A.; Khan, H.M.; Jalal, M.; Mahdi, A.A.; Cameotra, S.S. Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Pseudomonas aeruginosa. J. Basic Microbiol. 2017, 57, 193–203. [Google Scholar] [CrossRef]
- Khan, F.; Kang, M.-G.; Jo, D.-M.; Chandika, P.; Jung, W.-K.; Kang, H.W.; Kim, Y.-M. Phloroglucinol-Gold and -Zinc Oxide Nanoparticles: Antibiofilm and Antivirulence Activities towards Pseudomonas aeruginosa PAO1. Mar. Drugs 2021, 19, 601. [Google Scholar] [CrossRef]
- Tabassum, N.; Jeong, G.-J.; Jo, D.-M.; Khan, F.; Kim, Y.-M. Attenuation of biofilm and virulence factors of Pseudomonas aeruginosa by tetramethylpyrazine-gold nanoparticles. Microb. Pathog. 2024, 191, 106658. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef]
- Das, P.; Ghosh, S.; Nayak, B. Phyto-fabricated Nanoparticles and Their Anti-biofilm Activity: Progress and Current Status. Front. Nanotechnol. 2021, 3, 739286. [Google Scholar] [CrossRef]
- Nwafor, I.R.; Alhassan, Y.; Udoh, J.I.; Odanibeh, D.; Oyaniyi, J.; Efoli-Bam, V.K.; Azubuike, E.O.; Ojobor, J.-F.C.; Nwokafor, C.V. Plant-derived Bioactive Compounds and Their Mechanistic Roles in Combating Microbial Biofilms. Microbiol. Res. J. Int. 2024, 34, 74–85. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, P.; Vasanthan, M. Lipid-based nanocarriers for enhanced delivery of plant-derived bioactive molecules: A comprehensive review. Ther. Deliv. 2024, 15, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Mustafa, M.A.; Kumar, S.; Gupta, P.; Pramanik, A.; Rizaev, J.A.; Shareef, H.K.; Alubiady, M.H.S.; Al-Abdeen, S.H.Z.; Shakier, H.G. Advancing therapeutic efficacy: Nanovesicular delivery systems for medicinal plant-based therapeutics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 7229–7254. [Google Scholar] [CrossRef]
- Gali, L.; Pirozzi, A.; Donsì, F. Biopolymer-and lipid-based carriers for the delivery of plant-based ingredients. Pharmaceutics 2023, 15, 927. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M. Lipid-based delivery systems for flavonoids and flavonolignans: Liposomes, nanoemulsions, and solid lipid nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef]
- Jain, V.; Kumar, H.; Chand, P.; Jain, S.; Preethi, S. Lipid-Based Nanocarriers as Drug Delivery System and Its Applications. In Nanopharmaceutical Advanced Delivery Systems; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 1–29. [Google Scholar]
- Tang, S.; Davoudi, Z.; Wang, G.; Xu, Z.; Rehman, T.; Prominski, A.; Tian, B.; Bratlie, K.M.; Peng, H.; Wang, Q. Soft materials as biological and artificial membranes. Chem. Soc. Rev. 2021, 50, 12679–12701. [Google Scholar] [CrossRef]
- Yang, L.; Lin, X.; Zhou, J.; Hou, S.; Fang, Y.; Bi, X.; Yang, L.; Li, L.; Fan, Y. Cell membrane-biomimetic coating via click-mediated liposome fusion for mitigating the foreign-body reaction. Biomaterials 2021, 271, 120768. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An emerging novel technology for improving the bioavailability of drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent advances in improving the bioavailability of hydrophobic/lipophilic drugs and their delivery via self-emulsifying formulations. Colloids Interfaces 2023, 7, 16. [Google Scholar] [CrossRef]
- Modi, D.; Jonnalagadda, S.; Campbell, G.A.; Dalwadi, G. Enhancing oil solubility of BCS class II drug phenytoin through hydrophobic ion pairing to enable high drug load in injectable nanoemulsion to prevent precipitation at physiological pH with a potential to prevent phlebitis. J. Pharm. Sci. 2023, 112, 2427–2443. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.F.; Tomé, C.; Oliveira, T.; Martin, V.; Santos, C.; Gonçalves, L.; Fernandes, M.H.; Gomes, P.S.; Ribeiro, I.A.C. Exploring the potential of chitosan-based particles as delivery-carriers for promising antimicrobial glycolipid biosurfactants. Carbohydr. Polym. 2021, 254, 117433. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, G.; Ferraro, E.; Puppi, D. Polymeric systems for the controlled release of flavonoids. Pharmaceutics 2023, 15, 628. [Google Scholar] [CrossRef]
- Gulati, S.; Ansari, N.; Moriya, Y.; Joshi, K.; Prasad, D.; Sajwan, G.; Shukla, S.; Kumar, S.; Varma, R.S. Nanobiopolymers in cancer therapeutics: Advancing targeted drug delivery through sustainable and controlled release mechanisms. J. Mater. Chem. B 2024, 12, 11887–11915. [Google Scholar] [CrossRef]
- Palani, N.; Vijayakumar, P.; Monisha, P.; Ayyadurai, S.; Rajadesingu, S. Electrospun nanofibers synthesized from polymers incorporated with bioactive compounds for wound healing. J. Nanobiotechnol. 2024, 22, 211. [Google Scholar] [CrossRef]
- Rodà, F.; Caraffi, R.; Picciolini, S.; Tosi, G.; Vandelli, M.A.; Ruozi, B.; Bedoni, M.; Ottonelli, I.; Duskey, J.T. Recent advances on surface-modified GBM targeted nanoparticles: Targeting strategies and surface characterization. Int. J. Mol. Sci. 2023, 24, 2496. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Sheibani, N.; Khataee, A. Smart active-targeting of lipid-polymer hybrid nanoparticles for therapeutic applications: Recent advances and challenges. Int. J. Biol. Macromol. 2022, 213, 166–194. [Google Scholar] [CrossRef]
- Shariati, A.; Chegini, Z.; Ghaznavi-Rad, E.; Zare, E.N.; Hosseini, S.M. PLGA-based nanoplatforms in drug delivery for inhibition and destruction of microbial biofilm. Front. Cell. Infect. Microbiol. 2022, 12, 926363. [Google Scholar] [CrossRef]
- Cetin, F.N.; Mignon, A.; Van Vlierberghe, S.; Kolouchova, K. Polymer-and Lipid-Based Nanostructures Serving Wound Healing Applications: A Review. Adv. Healthc. Mater. 2025, 14, 2402699. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D. Functional Nanogel Coatings as Antifouling and Antibacterial Surfaces; University of Groningen: Groningen, The Netherlands, 2021. [Google Scholar]
- Khalaf, M.M.; Gouda, M.; Abou Taleb, M.F.; Heakal, F.E.-T.; Abd El-Lateef, H.M. Fabrication of smart nanogel based on carrageenan and green coffee extract as a long-term antifouling agent to improve biofilm prevention in food production. Food Chem. 2024, 461, 140719. [Google Scholar] [CrossRef] [PubMed]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and gold nanoparticles for antimicrobial purposes against multi-drug resistance bacteria. Materials 2022, 15, 1799. [Google Scholar] [CrossRef]
- Karami, M.H.; Abdouss, M.; Maleki, B. The state of the art metal nanoparticles in drug delivery systems: A comprehensive review. Nanomed. J. 2024, 11, 222–249. [Google Scholar]
- Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and metal oxide nanoparticle as a novel antibiotic carrier for the direct delivery of antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, antibiofilm, antiquorum sensing, antimotility, and antioxidant activities of green fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis lichen aqueous extract against multi-drug-resistant bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, A.; Mitra, R.; Kundu, S.; Banerjee, P.; Chowdhury, A.A.; Ghosh, S. Escaping the ESKAPE pathogens: A review on antibiofilm potential of nanoparticles. Microb. Pathog. 2024, 194, 106842. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pandey, P.C.; Gupta, M.K.; Narayan, R.J. Nano–Bio Interaction and Antibacterial Mechanism of Engineered Metal Nanoparticles: Fundamentals and Current Understanding. J. Clust. Sci. 2025, 36, 5. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic effects between metal nanoparticles and commercial antimicrobial agents: A review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef] [PubMed]
- Ahamad Khan, M.; Lone, S.A.; Shahid, M.; Zeyad, M.T.; Syed, A.; Ehtram, A.; Elgorban, A.M.; Verma, M.; Danish, M. Phytogenically synthesized zinc oxide nanoparticles (ZnO-NPs) potentially inhibit the bacterial pathogens: In vitro studies. Toxics 2023, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S.-I. Metallic nanoparticles: A promising arsenal against antimicrobial resistance—Unraveling mechanisms and enhancing medication efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Bharti, S. Harnessing the potential of bimetallic nanoparticles: Exploring a novel approach to address antimicrobial resistance. World J. Microbiol. Biotechnol. 2024, 40, 89. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B. Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: A review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K. Nanotechnology in combating biofilm: A smart and promising therapeutic strategy. Front. Microbiol. 2023, 13, 1028086. [Google Scholar] [CrossRef]
- Bag, N.; Bardhan, S.; Roy, S.; Roy, J.; Mondal, D.; Guo, B.; Das, S. Nanoparticle-mediated stimulus-responsive antibacterial therapy. Biomater. Sci. 2023, 11, 1994–2019. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R.; Chen, J.; Kashi, P.A.; Mohammadi, A. Dispersion strategies of nanomaterials in polymeric inks for efficient 3D printing of soft and smart 3D structures: A systematic review. Adv. Colloid Interface Sci. 2024, 333, 103285. [Google Scholar] [CrossRef]
- Foroohimanjili, F.; Mirzaie, A.; Hamdi, S.M.M.; Noorbazargan, H.; Hedayati Ch, M.; Dolatabadi, A.; Rezaie, H.; Bishak, F.M. Antibacterial, antibiofilm, and antiquorum sensing activities of phytosynthesized silver nanoparticles fabricated from Mespilus germanica extract against multidrug resistance of Klebsiella pneumoniae clinical strains. J. Basic Microbiol. 2020, 60, 216–230. [Google Scholar] [CrossRef]
- Srinivasan, R.; Vigneshwari, L.; Rajavel, T.; Durgadevi, R.; Kannappan, A.; Balamurugan, K.; Pandima Devi, K.; Veera Ravi, A. Biogenic synthesis of silver nanoparticles using Piper betle aqueous extract and evaluation of its anti-quorum sensing and antibiofilm potential against uropathogens with cytotoxic effects: An in vitro and in vivo approach. Environ. Sci. Pollut. Res. 2018, 25, 10538–10554. [Google Scholar] [CrossRef] [PubMed]
- Qais, F.A.; Shafiq, A.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I. Green synthesis of silver nanoparticles using Carum copticum: Assessment of its quorum sensing and biofilm inhibitory potential against gram negative bacterial pathogens. Microb. Pathog. 2020, 144, 104172. [Google Scholar] [CrossRef] [PubMed]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alshamrani, M.; Ali, S.G.; Jamous, Y.F.; Alyahya, S.A.; Alhumaidi, M.S.; Altammar, K.A.; Alsalhi, A.; Khan, H.M. Crinum latifolium mediated biosynthesis of gold nanoparticles and their anticandidal, antibiofilm and antivirulence activity. J. Saudi Chem. Soc. 2023, 27, 101644. [Google Scholar] [CrossRef]
- Ali, S.G.; Jalal, M.; Ahmad, H.; Umar, K.; Ahmad, A.; Alshammari, M.B.; Khan, H.M. Biosynthesis of gold nanoparticles and its effect against Pseudomonas aeruginosa. Molecules 2022, 27, 8685. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Alotaibi, S.H. Biofabrication of gold nanoparticles using Capsicum annuum extract and its antiquorum sensing and antibiofilm activity against bacterial pathogens. ACS Omega 2021, 6, 16670–16682. [Google Scholar] [CrossRef]
- Kamli, M.R.; Malik, M.A.; Srivastava, V.; Sabir, J.S.; Mattar, E.H.; Ahmad, A. Biogenic ZnO nanoparticles synthesized from origanum vulgare abrogates quorum sensing and biofilm formation in opportunistic pathogen Chromobacterium violaceum. Pharmaceutics 2021, 13, 1743. [Google Scholar] [CrossRef]
- Husain, F.M.; Qais, F.A.; Ahmad, I.; Hakeem, M.J.; Baig, M.H.; Masood Khan, J.; Al-Shabib, N.A. Biosynthesized zinc oxide nanoparticles disrupt established biofilms of pathogenic bacteria. Appl. Sci. 2022, 12, 710. [Google Scholar] [CrossRef]
- Bai, B.; Saranya, S.; Dheepaasri, V.; Muniasamy, S.; Alharbi, N.S.; Selvaraj, B.; Undal, V.S.; Gnanamangai, B.M. Biosynthesized copper oxide nanoparticles (CuO NPs) enhances the anti-biofilm efficacy against K. pneumoniae and S. aureus. J. King Saud. Univ.-Sci. 2022, 34, 102120. [Google Scholar] [CrossRef]
- Ansari, M.A.; Al Dhneem, H.N.; Ali, S.G.; Jamous, Y.F.; Alomary, M.N.; Atwah, B.; Alhumaidi, M.S.; Hani, U.; Haider, N.; Asiri, S. Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications. Green Process. Synth. 2024, 13, 20230174. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N. Ultrasound assisted-phytofabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2405–2423. [Google Scholar] [CrossRef]
- Govindan, R.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K.; Muthuchamy, M.; Quero, F.; Mothana, R.A.; Noman, O.M.; Siddiqui, N.A.; Li, W.-J. Effective removal of biofilm formation in Acinetobacter baumannii using chitosan nanoparticles loaded plant essential oils. J. King Saud. Univ.-Sci. 2022, 34, 101845. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Eltarahony, M.; Hafez, E.E.; Bashir, S.I. Green fabrication of chitosan nanoparticles using Lavendula angustifolia, optimization, characterization and in-vitro antibiofilm activity. Sci. Rep. 2023, 13, 11127. [Google Scholar] [CrossRef]
- Salem, S.S.; Badawy, M.S.E.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green biosynthesis of selenium nanoparticles using orange peel waste: Characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef]
- Alomary, M.N.; Ansari, M.A. Proanthocyanin-capped biogenic TiO2 nanoparticles with enhanced penetration, antibacterial and ROS mediated inhibition of bacteria proliferation and biofilm formation: A comparative approach. Chem.–A Eur. J. 2021, 27, 5817–5829. [Google Scholar] [CrossRef]
- Younis, I.Y.; El-Hawary, S.S.; Eldahshan, O.A.; Abdel-Aziz, M.M.; Ali, Z.Y. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 2021, 11, 16868. [Google Scholar] [CrossRef]
- Uzair, B.; Akhtar, N.; Sajjad, S.; Bano, A.; Fasim, F.; Zafar, N.; Leghari, S.A.K. Targeting microbial biofilms: By Arctium lappa L. synthesised biocompatible CeO2-NPs encapsulated in nano-chitosan. IET Nanobiotechnol. 2020, 14, 217–223. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Qayyum, S.; Khan, A.U. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: A comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 2017, 103, 167–177. [Google Scholar] [CrossRef]
- Valookolaei, F.-S.G.; Sazegar, H.; Rouhi, L. Limonene encapsulated alginate/collagen as antibiofilm drug against Acinetobacter baumannii. BMC Biotechnol. 2024, 24, 86. [Google Scholar] [CrossRef]
- Iadnut, A.; Mamoon, K.; Thammasit, P.; Pawichai, S.; Tima, S.; Preechasuth, K.; Kaewkod, T.; Tragoolpua, Y.; Tragoolpua, K. In Vitro Antifungal and Antivirulence Activities of Biologically Synthesized Ethanolic Extract of Propolis-Loaded PLGA Nanoparticles against Candida albicans. Evid.-Based Complement. Altern. Med. 2019, 2019, 3715481. [Google Scholar] [CrossRef]
- Radmand, F.; Baseri, M.; Memar, M.Y.; Ebrahimi, A.; Hamishehkar, H.; Asnaashari, S.; Naseri, A.; Kouhsoltani, M. Anti-biofilm and anti-glucosyltransferase effects of nano liposomal plant extracts against Streptococcus mutans. Sci. Rep. 2024, 14, 27304. [Google Scholar] [CrossRef] [PubMed]
- Al-Azawi, M.T.; Hadi, S.; Mohammed, C.H. Synthesis of silica nanoparticles via green approach by using hot aqueous extract of Thuja orientalis leaf and their effect on biofilm formation. Iraqi J. Agric. Sci. 2019, 50, 245–255. [Google Scholar]
- Dohare, S.; Dubey, S.D.; Kalia, M.; Verma, P.; Pandey, H.; Singh, N.K.; Agarwal, V. Anti-biofilm activity of Eucalyptus globulus oil encapsulated silica nanoparticles against E. coli biofilm. Int. J. Pharm. Sci. Res. 2014, 5, 5011. [Google Scholar]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Li, P.-L.; Ji, P.-C.; Yin, K.-Y.; Tan, X.-J.; Chen, H.; Xing, X.-D.; Jia, A.-Q. Carbon quantum dots derived from resveratrol enhances anti-virulence activity against Pseudomonas aeruginosa. Surf. Interfaces 2024, 44, 103662. [Google Scholar] [CrossRef]
- Hamid, L.L.; Hassan, M.H.; Obaid, A.S. Allium sativum extract mediate the biosynthesis of palladium nanoparticles as potential nanodrug for combating multidrug-resistant bacteria and wound healing. Mater. Chem. Phys. 2024, 321, 129507. [Google Scholar] [CrossRef]
- Sonbol, H.; Ameen, F.; AlYahya, S.; Almansob, A.; Alwakeel, S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef]
- Krishnasamy, N.; Ramadoss, R.; Vemuri, S.; Sujai, G.N.S. Optimizing Desmostachya bipinnata-derived platinum nanoparticles for enhanced antibacterial and biofilm reduction. Microb. Pathog. 2024, 196, 107004. [Google Scholar] [CrossRef]
- Siddique, M.H.; Hayat, S.; Muzammil, S.; Ashraf, A.; Khan, A.M.; Ijaz, M.U.; Khurshid, M.; Afzal, M. Ecofriendly phytosynthesized zirconium oxide nanoparticles as antibiofilm and quorum quenching agents against Acinetobacter baumannii. Drug Dev. Ind. Pharm. 2022, 48, 502–509. [Google Scholar] [CrossRef]
- Suresh, P.; Doss, A.; Selvi, G.A.; Rani, T.K.P. Antibiofilm, antibacterial and antioxidant activity of biofabricated bimetallic (Ag-ZnO) nanoparticles from Elephantopus scaber L. Biomass Convers. Biorefinery 2024, 14, 20911–20921. [Google Scholar] [CrossRef]
- Nagarajan, P.; Subramaniyan, V.; Elavarasan, V.; Mohandoss, N.; Subramaniyan, P.; Vijayakumar, S. Biofabricated aluminium oxide nanoparticles derived from Citrus aurantium L.: Antimicrobial, anti-proliferation, and photocatalytic efficiencies. Sustainability 2023, 15, 1743. [Google Scholar] [CrossRef]
- Mohmand, N.Z.K.; Ullah, H.; Shah, Y.; Ahmad, S.; Hassan, A.; Ali, L.; Rahman, H.U. Exploration of Antibacterial Activity of Plant-Mediated Green Synthesis of Cadmium Sulfide Nanoparticles. Lett. Appl. NanoBioSci. 2024, 13, 74. [Google Scholar]
- Umesh, M.; Choudhury, D.D.; Shanmugam, S.; Ganesan, S.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Eggshells biowaste for hydroxyapatite green synthesis using extract piper betel leaf-Evaluation of antibacterial and antibiofilm activity. Environ. Res. 2021, 200, 111493. [Google Scholar] [CrossRef]
- do Nascimento Filho, C.A.; da Silva Jr, F.A.G.; da Costa, M.M.; de Oliveira, H.P. Antibacterial activity of molybdenum disulfide and green silver nanoparticles reduced by tea tree essential oil compounds. Res. Soc. Dev. 2024, 13, e6713545818. [Google Scholar] [CrossRef]
- Rashmi, B.; Harlapur, S.F.; Gurushantha, K.; Ravikumar, C.; Kumar, M.A.; Santosh, M.; Kumar, V.D.; Kumar, A.N.; Azad, A.K.; Murthy, H.A. Facile green synthesis of lanthanum oxide nanoparticles using Centella Asiatica and Tridax plants: Photocatalytic, electrochemical sensor and antimicrobial studies. Appl. Surf. Sci. Adv. 2022, 7, 100210. [Google Scholar] [CrossRef]
- Elkady, F.M.; Badr, B.M.; Saied, E.; Hashem, A.H.; Abdel-Maksoud, M.A.; Fatima, S.; Malik, A.; Aufy, M.; Hussein, A.M.; Abdulrahman, M.S. Green Biosynthesis of Bimetallic Copper Oxide-Selenium Nanoparticles Using Leaf Extract of Lagenaria Siceraria: Antibacterial, Anti-Virulence Activities Against Multidrug-Resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2025, 20, 4705–4727. [Google Scholar] [CrossRef]
- Mostafa, H.Y.; El-Sayyad, G.S.; Nada, H.G.; Ellethy, R.A.; Zaki, E. Promising antimicrobial and antibiofilm activities of Orobanche aegyptiaca extract-mediated bimetallic silver-selenium nanoparticles synthesis: Effect of UV-exposure, bacterial membrane leakage reaction mechanism, and kinetic study. Arch. Biochem. Biophys. 2023, 736, 109539. [Google Scholar] [CrossRef]
- Ameen, F.; Alown, F.; Dawoud, T.; Sharaf, A.; Sakayanathan, P.; Alyahya, S. Versatility of copper-iron bimetallic nanoparticles fabricated using Hibiscus rosa-sinensis flower phytochemicals: Various enzymes inhibition, antibiofilm effect, chromium reduction and dyes removal. Environ. Geochem. Health 2024, 46, 142. [Google Scholar] [CrossRef]
- Mohammed, N.M.S.; Idrees, S.A. Green synthesis of Co-Zn-Ni trimetallic oxide nanoparticles using Cicer Arietinum leaf extract and their antibiofilm activity: Experimental and computational study. Mater. Sci. Eng. B 2024, 310, 117681. [Google Scholar] [CrossRef]
- Arunachalam, K.; Krishnan, G.P.; Sethuraman, S.; Abraham, S.V.P.I.; Krishnan, S.T.; Venkateswar, A.; Arunkumar, J.; Shi, C.; MubarakAli, D. Exploring possible ways to enhance the potential and use of natural products through nanotechnology in the battle against biofilms of foodborne bacterial pathogens. Pathogens 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Habeeb Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Luzala, M.M.; Muanga, C.K.; Kyana, J.; Safari, J.B.; Zola, E.N.; Mbusa, G.V.; Nuapia, Y.B.; Liesse, J.-M.I.; Nkanga, C.I.; Krause, R.W. A critical review of the antimicrobial and antibiofilm activities of green-synthesized plant-based metallic nanoparticles. Nanomaterials 2022, 12, 1841. [Google Scholar] [PubMed]
- Samantaray, A.; Pradhan, D.; Nayak, N.R.; Chawla, S.; Behera, B.; Mohanty, L.; Bisoyi, S.K.; Gandhi, S. Nanoquercetin based nanoformulations for triple negative breast cancer therapy and its role in overcoming drug resistance. Discov. Oncol. 2024, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Bei, D.; Meng, J.; Youan, B.-B.C. Engineering nanomedicines for improved melanoma therapy: Progress and promises. Nanomedicine 2010, 5, 1385–1399. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Partoazar, A. Targeting bacterial biofilm-related genes with nanoparticle-based strategies. Front. Microbiol. 2024, 15, 1387114. [Google Scholar] [CrossRef]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics 2022, 12, 2383. [Google Scholar] [CrossRef]
- Varshan, G.A.; Namasivayam, S.K.R. A Critical Review on Sustainable Formulation of Anti-quorum Sensing Compounds Using Nanotechnology Principles Against Candida albicans. BioNanoScience 2025, 15, 161. [Google Scholar] [CrossRef]
- Ampomah-Wireko, M.; Luo, C.; Cao, Y.; Wang, H.; Nininahazwe, L.; Wu, C. Chemical probe of AHL modulators on quorum sensing in Gram-Negative Bacteria and as antiproliferative agents: A review. Eur. J. Med. Chem. 2021, 226, 113864. [Google Scholar] [CrossRef]
- Ratha, B.N.; Lahiri, D.; Ray, R.R. Inhibition of Biofilm Formation. In Biofilm-Mediated Diseases: Causes and Controls; Springer: Singapore, 2021; pp. 209–237. [Google Scholar]
- Blackman, L.D.; Qu, Y.; Cass, P.; Locock, K.E. Approaches for the inhibition and elimination of microbial biofilms using macromolecular agents. Chem. Soc. Rev. 2021, 50, 1587–1616. [Google Scholar] [CrossRef]
- Banerjee, A.; Chowdhury, P.; Bauri, K.; Saha, B.; De, P. Inhibition and eradication of bacterial biofilm using polymeric materials. Biomater. Sci. 2023, 11, 11–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Singh, M.R.; Pradhan, K.; Pradhan, M.; Yadav, K.; Chauhan, N.S.; Dwivedi, S.D.; Singh, D. Lipid-based particulate systems for delivery of plant actives and extracts: Extraction, prospective carriers, and safety issues. In Phytopharmaceuticals and Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2023; pp. 83–114. [Google Scholar]
- Palit, P.; Mandal, S.C. Climate change, geographical location, and other allied triggering factors modulate the standardization and characterization of traditional medicinal plants: A challenge and prospect for phyto-drug development. In Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Springer: Berlin/Heidelberg, Germany, 2021; pp. 359–369. [Google Scholar]
- Hurkul, M.M.; Cetinkaya, A.; Yayla, S.; Ozkan, S.A. Advanced sample preparation and chromatographic techniques for analyzing plant-based bioactive chemicals in nutraceuticals. J. Chromatogr. Open 2024, 5, 100131. [Google Scholar] [CrossRef]
- Zhou, R.; Su, W.H.; Zhang, G.F.; Zhang, Y.N.; Guo, X.R. Relationship between flavonoids and photoprotection in shade-developed Erigeron breviscapus transferred to sunlight. Photosynthetica 2016, 54, 201–209. [Google Scholar] [CrossRef]
- Xu, J.; Yu, Y.; Shi, R.; Xie, G.; Zhu, Y.; Wu, G.; Qin, M. Organ-Specific Metabolic Shifts of Flavonoids in Scutellaria baicalensis at Different Growth and Development Stages. Molecules 2018, 23, 428. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, C.; Huang, Y.; Yang, R.; Zeng, Q. Abiotic stress-induced expression of artemisinin biosynthesis genes in Artemisia annua L. Chin. J. Appl. Environ. Biol. 2008, 14, 1–5. [Google Scholar]
- Hosseini, M.S.; Samsampour, D.; Ebrahimi, M.; Abadía, J.; Khanahmadi, M. Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemistry 2018, 156, 124–134. [Google Scholar] [CrossRef]
- Hong, D.Y.; Lau, A.J.; Yeo, C.L.; Liu, X.K.; Yang, C.R.; Koh, H.L.; Hong, Y. Genetic diversity and variation of saponin contents in Panax notoginseng roots from a single farm. J. Agric. Food Chem. 2005, 53, 8460–8467. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Da Silva, R.F.; Carneiro, C.N.; de Sousa, C.B.d.C.; Gomez, F.J.; Espino, M.; Boiteux, J.; Fernández, M.d.l.Á.; Silva, M.F.; Dias, F.d.S. Sustainable extraction bioactive compounds procedures in medicinal plants based on the principles of green analytical chemistry: A review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern methods of pre-treatment of plant material for the extraction of bioactive compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- Ong, E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Govindarajan, R.; Tejas, V.; Pushpangadan, P. High-Performance Liquid Chromatography (HPLC) as a Tool for Standardization of Complex Herbal Drugs. J. AOAC Int. 2019, 102, 986–992. [Google Scholar] [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P.S. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustain. Food Technol. 2023, 1, 500–510. [Google Scholar] [CrossRef]
- Asiminicesei, D.-M.; Fertu, D.I.; Gavrilescu, M. Impact of heavy metal pollution in the environment on the metabolic profile of medicinal plants and their therapeutic potential. Plants 2024, 13, 913. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Kumar, A.; Kumar, V.; Sharma, A.K. Plant secondary metabolites: An introduction of their chemistry and biological significance with physicochemical aspect. In Plant Secondary Metabolites: Physico-Chemical Properties and Therapeutic Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–45. [Google Scholar]
- Sanjai, C.; Gaonkar, S.L.; Hakkimane, S.S. Harnessing Nature’s Toolbox: Naturally Derived Bioactive Compounds in Nanotechnology Enhanced Formulations. ACS Omega 2024, 9, 43302–43318. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative delivery systems loaded with plant bioactive ingredients: Formulation approaches and applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Cao, J.; Gao, M.; Wang, J.; Liu, Y.; Zhang, X.; Ping, Y.; Liu, J.; Chen, G.; Xu, D.; Huang, X. Construction of nano slow-release systems for antibacterial active substances and its applications: A comprehensive review. Front. Nutr. 2023, 10, 1109204. [Google Scholar] [CrossRef]
- Jităreanu, A.; Trifan, A.; Vieriu, M.; Caba, I.-C.; Mârțu, I.; Agoroaei, L. Current trends in toxicity assessment of herbal medicines: A narrative review. Processes 2022, 11, 83. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S. Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Oguntibeju, O.O. African herbal medicines: Adverse effects and cytotoxic potentials with different therapeutic applications. Int. J. Environ. Res. Public Health 2021, 18, 5988. [Google Scholar] [CrossRef]
- Unger, P.; Melzig, M.F. Comparative study of the cytotoxicity and genotoxicity of alpha- and Beta-asarone. Sci. Pharm. 2012, 80, 663–668. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Widelski, J. Alkaloids. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–198. [Google Scholar]
- Ibrahim, A.; Khalifa, S.I.; Khafagi, I.; Youssef, D.T.; Khan, S.; Mesbah, M.; Khan, I. Microbial metabolism of biologically active secondary metabolites from Nerium oleander L. Chem. Pharm. Bull. 2008, 56, 1253–1258. [Google Scholar] [CrossRef]
- Wasfi, I.A.; Zorob, O.; Al Katheeri, N.A.; Al Awadhi, A.M. A fatal case of oleandrin poisoning. Forensic Sci. Int. 2008, 179, e31–e36. [Google Scholar] [CrossRef]
- Ugwah-Oguejiofor, C.J.; Okoli, C.O.; Ugwah, M.O.; Umaru, M.L.; Ogbulie, C.S.; Mshelia, H.E.; Umar, M.; Njan, A.A. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon 2019, 5, e01179. [Google Scholar] [CrossRef]
- Al-Yahya, A.A.; Al-Majed, A.A.; Al-Bekairi, A.M.; Al-Shabanah, O.A.; Qureshi, S. Studies on the reproductive, cytological and biochemical toxicity of Ginkgo biloba in Swiss albino mice. J. Ethnopharmacol. 2006, 107, 222–228. [Google Scholar] [CrossRef]
- Bahmani, M.; Rafieian, M.; Baradaran, A.; Rafieian, S.; Rafieian-Kopaei, M. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J. Nephropathol. 2014, 3, 81–85. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Sharma, N. Precision nanotoxicology in drug development: Current trends and challenges in safety and toxicity implications of customized multifunctional nanocarriers for drug-delivery applications. Pharmaceutics 2022, 14, 2463. [Google Scholar] [CrossRef]
- Lv, Y.; Li, W.; Liao, W.; Jiang, H.; Liu, Y.; Cao, J.; Lu, W.; Feng, Y. Nano-drug delivery systems based on natural products. Int. J. Nanomed. 2024, 19, 541–569. [Google Scholar] [CrossRef]
- Hossain, C.M.; Gera, M.; Ali, K.A. Current status and challenges of herbal drug development and regulatory aspect: A global perspective. Asian J. Pharm. Clin. Res. 2022, 15, 31–41. [Google Scholar] [CrossRef]
- Oliveira da Silva, L.; Assunção Ferreira, M.R.; Lira Soares, L.A. Nanotechnology Formulations Designed with Herbal Extracts and Their Therapeutic Applications–A Review. Chem. Biodivers. 2023, 20, e202201241. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Anubhav, A.; Preety, G.; Smriti, O. Application of Nanotechnology for Herbal Medicine Development: A Review. Lett. Drug Des. Discov. 2024, 21, 1325–1333. [Google Scholar] [CrossRef]
- Shebley, M.; Sandhu, P.; Emami Riedmaier, A.; Jamei, M.; Narayanan, R.; Patel, A.; Peters, S.A.; Reddy, V.P.; Zheng, M.; de Zwart, L.; et al. Physiologically Based Pharmacokinetic Model Qualification and Reporting Procedures for Regulatory Submissions: A Consortium Perspective. Clin. Pharmacol. Ther. 2018, 104, 88–110. [Google Scholar] [CrossRef]
- Wadekar, R.; Mandal, S.C.; Patil, K. Intellectual Property Rights, Naturally Derived Bioactive Compounds, and Resource Conservation. In Role of Herbal Medicines: Management of Lifestyle Diseases; Springer: Berlin/Heidelberg, Germany, 2024; pp. 559–571. [Google Scholar]
- Raju, S.; Das, M. Medicinal plants industry in India: Challenges, opportunities and sustainability. Med. Plants—Int. J. Phytomedicines Relat. Ind. 2024, 16, 1–14. [Google Scholar] [CrossRef]
- Kapoor, R.K.; Hafeez, A.; Kumar, S.; Mishra, D.; Gupta, M.K. Intellectual property rights of medicinal products. In Medicinal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2025; pp. 327–341. [Google Scholar]
- Klymenko, I. Nagoya Protocol on Access and Benefit Sharing: An Effective International Instrument for Safeguarding the Indigenous Peoples’ Rights or Declarative Rhetoric of Double Standards? UiT Norges Arktiske Universitet: Tromsø, Norway, 2022. [Google Scholar]
- Indrayanto, G. Chapter Six-Regulation and standardization of herbal drugs: Current status, limitation, challenge’s and future prospective. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 49, pp. 153–199. [Google Scholar]
- Dubale, S.; Usure, R.E.; Mekasha, Y.T.; Hasen, G.; Hafiz, F.; Kebebe, D.; Suleman, S. Traditional herbal medicine legislative and regulatory framework: A cross-sectional quantitative study and archival review perspectives. Front. Pharmacol. 2025, 16, 1475297. [Google Scholar] [CrossRef]
- Elazzazy, A.M.; Baeshen, M.N.; Alasmi, K.M.; Alqurashi, S.I.; Desouky, S.E.; Khattab, S.M. Where Biology Meets Engineering: Scaling Up Microbial Nutraceuticals to Bridge Nutrition, Therapeutics, and Global Impact. Microorganisms 2025, 13, 566. [Google Scholar] [CrossRef]
- Saifi, M.; Ashrafi, K.; Qamar, F.; Abdin, M. Regulatory trends in engineering bioactive-phytocompounds. Plant Sci. 2024, 346, 112167. [Google Scholar] [CrossRef]
- Ayon, N.J. High-throughput screening of natural product and synthetic molecule libraries for antibacterial drug discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef]
- Balderas, I.; Olivares, J. Omics Technologies and Anti-Infective Drug Discovery. In Frontiers in Anti-Infective Drug Discovery: Volume 10; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 74–115. [Google Scholar]
- Pandita, D.; Pandita, A.; Wani, S.H.; Abdelmohsen, S.A.; Alyousef, H.A.; Abdelbacki, A.M.; Al-Yafrasi, M.A.; Al-Mana, F.A.; Elansary, H.O. Crosstalk of multi-omics platforms with plants of therapeutic importance. Cells 2021, 10, 1296. [Google Scholar] [CrossRef]
- Focht, P. Bioprospecting and Pathovar Profiling of Plant Pathogenic Bacteria Using Novel High Throughput Technology Platforms; Newcastle University: Newcastle upon Tyne, UK, 2023. [Google Scholar]
- Ogidi, O.I.; Emaikwu, N.G. Plant Phenolic Compound Isolation and Its Bioinformatics Approaches to Molecular Mechanisms in Antimicrobial Activities and Resistance. In Computational Approaches in Biotechnology and Bioinformatics; CRC Press: Boca Raton, FL, USA, 2024; pp. 188–215. [Google Scholar]
- Masucci, M.; Karlsson, C.; Blomqvist, L.; Ernberg, I. Bridging the divide: A review on the implementation of personalized cancer medicine. J. Pers. Med. 2024, 14, 561. [Google Scholar] [CrossRef]
- Dhoundiyal, S.; Srivastava, S.; Kumar, S.; Singh, G.; Ashique, S.; Pal, R.; Mishra, N.; Taghizadeh-Hesary, F. Radiopharmaceuticals: Navigating the frontier of precision medicine and therapeutic innovation. Eur. J. Med. Res. 2024, 29, 26. [Google Scholar] [CrossRef]
- Casotti, M.C.; Meira, D.D.; Zetum, A.S.S.; Campanharo, C.V.; da Silva, D.R.C.; Giacinti, G.M.; da Silva, I.M.; Moura, J.A.D.; Barbosa, K.R.M.; Altoé, L.S.C. Integrating frontiers: A holistic, quantum and evolutionary approach to conquering cancer through systems biology and multidisciplinary synergy. Front. Oncol. 2024, 14, 1419599. [Google Scholar] [CrossRef]
- Prajapati, V.; Verma, M.; Kumar, G.S.; Patel, J. High-Throughput Preclinical Models and Pharmacoproteomics. In Pharmacoproteomics: Recent Trends and Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 429–468. [Google Scholar]
- Aborode, A.T.; Awuah, W.A.; Mikhailova, T.; Abdul-Rahman, T.; Pavlock, S.; Kundu, M.; Yarlagadda, R.; Pustake, M.; Correia, I.F.d.S.; Mehmood, Q. OMICs technologies for natural compounds-based drug development. Curr. Top. Med. Chem. 2022, 22, 1751–1765. [Google Scholar] [CrossRef]
- Ahmad, M.; Tahir, M.; Hong, Z.; Zia, M.A.; Rafeeq, H.; Ahmad, M.S.; Rehman, S.u.; Sun, J. Plant and marine-derived natural products: Sustainable pathways for future drug discovery and therapeutic development. Front. Pharmacol. 2025, 15, 1497668. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B. Methods for Rational Design and Discovery of Multitarget Drugs. In Polypharmacology: Principles and Methodologies; Springer: Berlin/Heidelberg, Germany, 2022; pp. 781–814. [Google Scholar]
- Satrio, R.D.; Fendiyanto, M.H.; Miftahudin, M. Tools and techniques used at global scale through genomics, transcriptomics, proteomics, and metabolomics to investigate plant stress responses at the molecular level. In Molecular Dynamics of Plant Stress and Its Management; Springer: Berlin/Heidelberg, Germany, 2024; pp. 555–607. [Google Scholar]
- Saini, P.; Mishra, P. Revolution in Microbial Bioprospecting via the Development of Omics-Based Technologies. In Bioprospecting of Microbial Resources for Agriculture, Environment and Bio-Chemical Industry; Springer: Berlin/Heidelberg, Germany, 2024; pp. 171–191. [Google Scholar]
- Qureshi, R.; Zou, B.; Alam, T.; Wu, J.; Lee, V.H.; Yan, H. Computational methods for the analysis and prediction of egfr-mutated lung cancer drug resistance: Recent advances in drug design, challenges and future prospects. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 20, 238–255. [Google Scholar] [CrossRef]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef]
- Goonetilleke, E.C.; Huang, X. Targeting Bacterial RNA Polymerase: Harnessing Simulations and Machine Learning to Design Inhibitors for Drug-Resistant Pathogens. Biochemistry 2025, 64, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.K.; Keerthanah, S.; Swarna, S.; Gopinath, R. Innovative Approaches in Combating Antimicrobial Resistance: A Comprehensive Review. Int. J. Pharm. Clin. Res. 2024, 16, 245–254. [Google Scholar]
- Olatunji, A.O.; Olaboye, J.A.; Maha, C.C.; Kolawole, T.O.; Abdul, S. Next-Generation strategies to combat antimicrobial resistance: Integrating genomics, CRISPR, and novel therapeutics for effective treatment. Eng. Sci. Technol. J. 2024, 5, 2284–2303. [Google Scholar] [CrossRef]
- Shukla, V.; Singh, S.; Verma, S.; Verma, S.; Rizvi, A.A.; Abbas, M. Targeting the microbiome to improve human health with the approach of personalized medicine: Latest aspects and current updates. Clin. Nutr. ESPEN 2024, 63, 813–820. [Google Scholar] [CrossRef]
- Kamel, M.; Aleya, S.; Alsubih, M.; Aleya, L. Microbiome dynamics: A paradigm shift in combatting infectious diseases. J. Pers. Med. 2024, 14, 217. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The promise of nanotechnology in personalized medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Hamdy, N.M.; Basalious, E.B.; El-Sisi, M.G.; Nasr, M.; Kabel, A.M.; Nossier, E.S.; Abadi, A.H. Advancements in current one-size-fits-all therapies compared to future treatment innovations for better improved chemotherapeutic outcomes: A step-toward personalized medicine. Curr. Med. Res. Opin. 2024, 40, 1943–1961. [Google Scholar] [CrossRef]
- Carvalho, B.G.; Vit, F.F.; Carvalho, H.F.; Han, S.W.; de la Torre, L.G. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J. Mater. Chem. B 2021, 9, 1208–1237. [Google Scholar] [CrossRef]
- Shaikh, M.A.J.; Afzal, O.; Kazmi, I.; Alzarea, S.I.; Jafar, M.; Altamimi, A.S.A.; Jakhmola, V.; Anand, K.; Singh, S.K.; Dua, K. Harnessing chitosan-adorned liposomes for enhanced drug delivery in cancer. J. Drug Deliv. Sci. Technol. 2023, 85, 104619. [Google Scholar] [CrossRef]
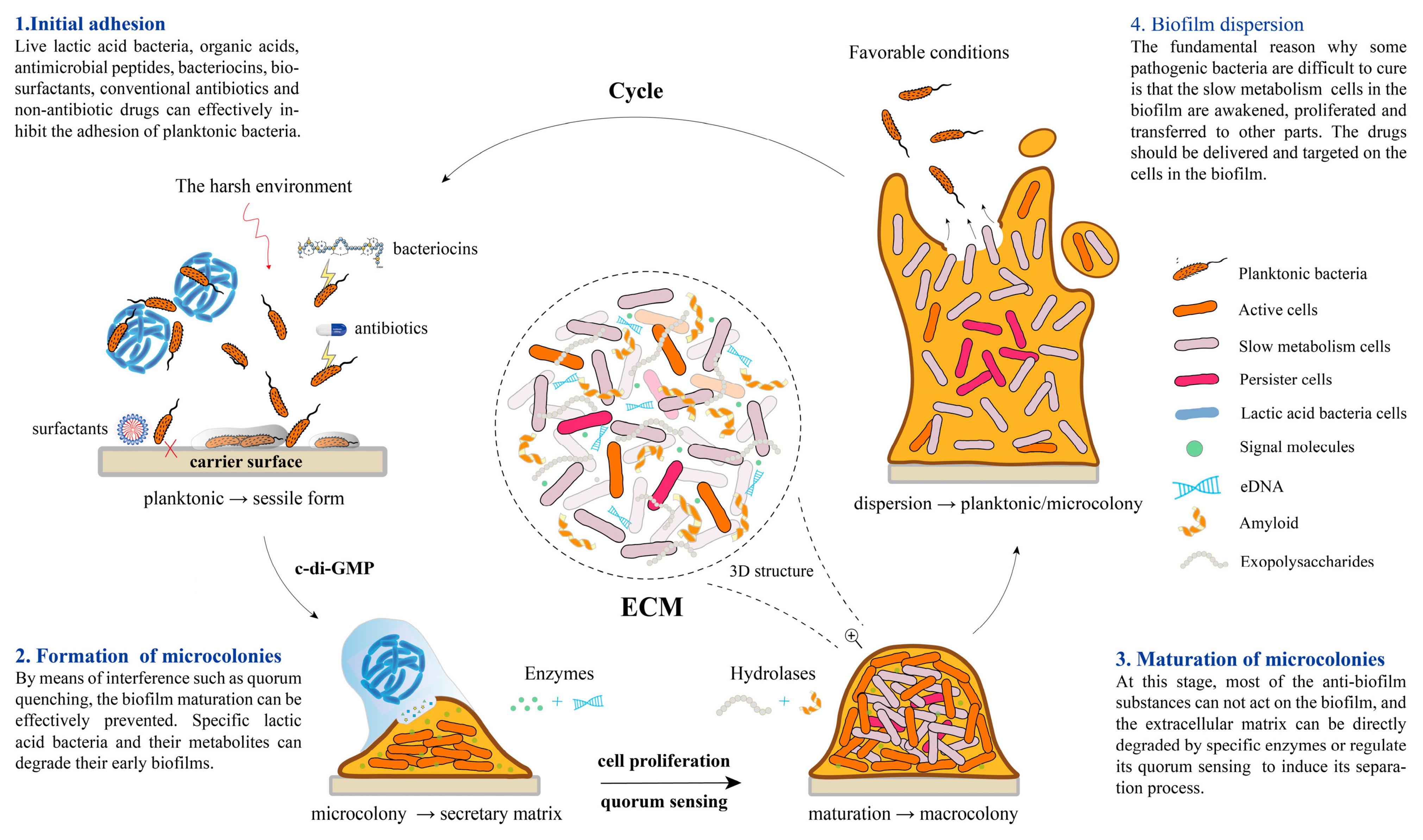
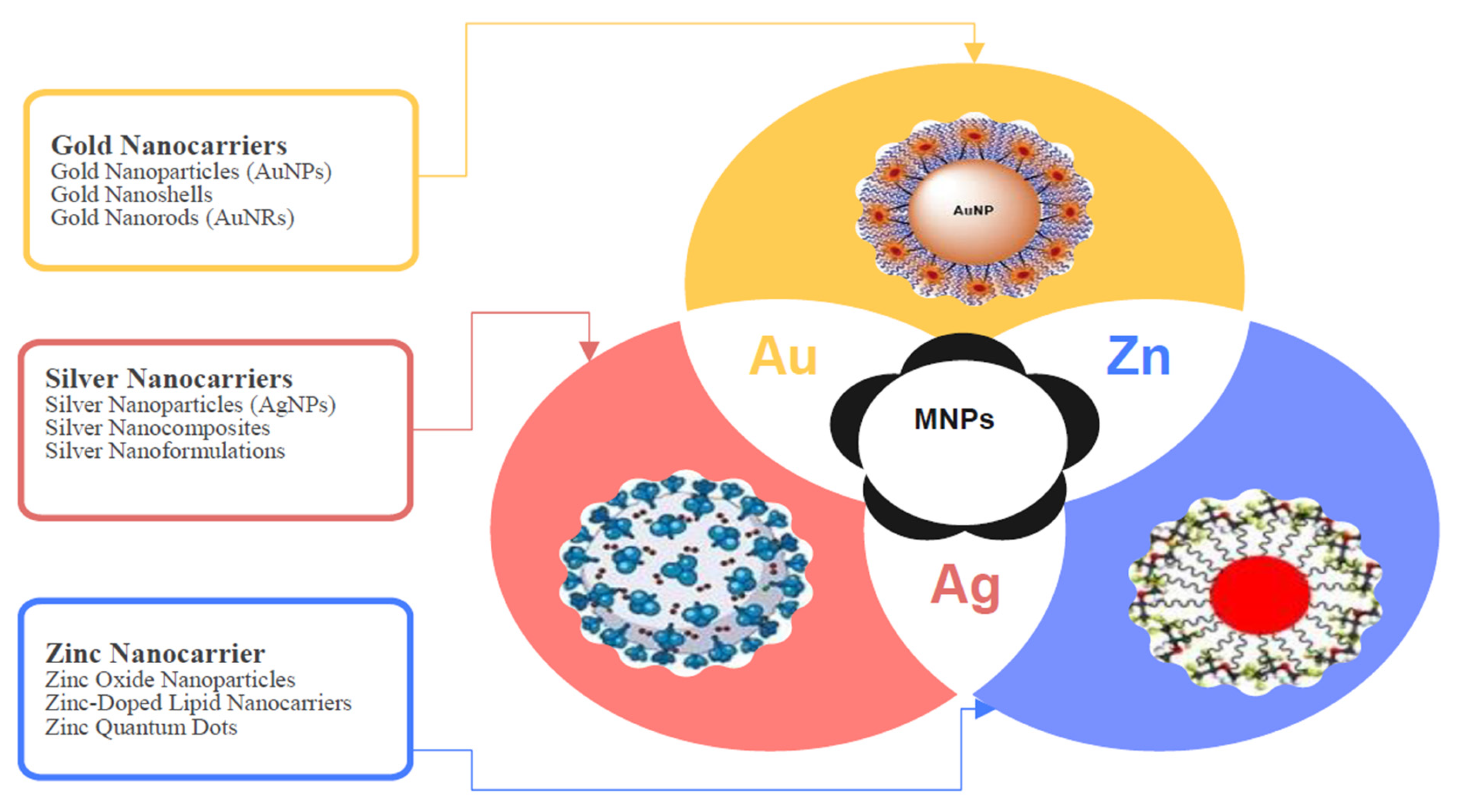
| Name of Nanoparticles | Plants and Their Parts Used for NP Synthesis | Biofilm Inhibitory Concentration (µg/mL) | Mechanisms of Action | Target Microorganisms | References |
|---|---|---|---|---|---|
| Silver NPs (AgNPs) | Mespilus germanica extract (phytosynthesis) | 1.95–100 (MIC values ranged from 6.25 to 100) | Disrupts bacterial membrane, produces reactive oxygen species (ROS), downregulates biofilm (mrkA) and quorum sensing (luxS) genes. | Klebsiella pneumoniae | [210] |
| Leaf extracts of Semecarpus anacardium, Glochidion lanceolarium, and Bridelia retusa | 10–100 | Inhibits exopolysaccharide (EPS) production, interferes with bacterial adhesion, disrupts biofilm matrix formation. | Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus | [164] | |
| Piper betle aqueous extract | 3–10 | Inhibits EPS production, reduces hydrophobicity, inhibits quorum sensing-regulated virulence factors (prodigiosin, protease), downregulates biofilm and motility genes (fimA, fimC, flhD, bsmB, flhB, rsbA). | Serratia marcescens, Proteus mirabilis | [211] | |
| Carum copticum aqueous extract | - | Inhibits quorum sensing-controlled virulence factors (violacein, pyocyanin, pyoverdin, protease, elastase, rhamnolipids, prodigiosin), disrupts swimming and swarming motility, inhibits EPS production and biofilm formation. | Chromobacterium violaceum, P. aeruginosa, S. marcescens | [212] | |
| Zingiber officinale (ginger) and Cinnamomum cassia (cinnamon) | Ginger: 15.6–62.5 Cinnamon: 156–1250 | Inhibits bacterial adhesion, disrupts biofilm matrix (EPS inhibition), reduces adherent cells on catheter surfaces, with ginger AgNPs also damaging intermolecular forces in biofilms. | Enterococcus faecalis and Enterococcus faecium | [213] | |
| Gold NPs (AuNPs) | Aqueous extract of Crinum latifolium leaves | 6.25–50 | Inhibits germ tube formation, suppresses biofilm matrix production, reduces secretion of hydrolytic enzymes (phospholipase, proteinase, esterase, lipase, hemolysin), disrupts cell wall and membrane integrity. | Candida spp. | [214] |
| Aqueous stem extract of Tinospora cordifolia | 1000–1800 | Inhibits pyocyanin production, reduces swarming and swimming motility, inhibits biofilm formation, reduces EPS secretion. | P. aeruginosa PAO1 | [215] | |
| Aqueous extract of Capsicum annuum | 25–200 | Inhibits quorum sensing-mediated virulence factors (pyocyanin, pyoverdin, protease, elastase, rhamnolipid), reduces swimming motility, inhibits EPS production, disrupts biofilm matrix. | P. aeruginosa PAO1, Serratia marcescens MTCC 97 | [216] | |
| Zinc oxide NPs (ZnO NPs) | Aqueous extract of Origanum vulgare leaves | 2–8 | Inhibits quorum sensing (violacein pigment), downregulates QS genes (cviL, vioA, vioB, vioD, vioE), inhibits EPS production, disrupts biofilm formation. | Chromobacterium violaceum ATCC12472 | [217] |
| Aqueous leaf extract of Cassia siamea | 0.5–20 | Inhibits quorum sensing (reduces pyocyanin, pyoverdine, exoprotease, elastase, rhamnolipid), impairs EPS production, inhibits swimming motility, reduces violacein production, disrupts biofilm matrix. | P. aeruginosa PAO1, C. violaceum MCC2290 | [202] | |
| Aqueous root extract of Plumbago zeylanica | 200–400 | Inhibits EPS production, disrupts bacterial adhesion, alters biofilm architecture, and eradicates established biofilms. | E. coli, P. aeruginosa, S. aureus | [218] | |
| Copper Oxide NPs (CuO NPs) | Aqueous leaf extract of Moringa oleifera | 1000 | Disrupts biofilm matrix, reduces EPS production, inhibits quorum sensing-regulated virulence factors, causes membrane damage and cell lysis. | Klebsiella pneumoniae, S. aureus, Acinetobacter baumannii | [219] |
| Polyherbal drug (Septilin) containing plant extracts | 1–2.5 | Inhibits biofilm formation, disrupts cell morphology, reduces EPS production, induces membrane damage. | P. aeruginosa, MRSA (S. aureus), C. albicans, E. coli | [220] | |
| Iron Oxide NPs (Fe₃O₄ NPs) | Ultrasound-assisted synthesis using aqueous leaf extract of Artemisia haussknechtii | 12.5–50 | Inhibits biofilm formation, reduces EPS secretion, disrupts biofilm architecture, decreases bacterial spreading ability. | MRSA (S. aureus), E. coli, S. marcescens | [221] |
| Chitosan NPs | Encapsulation of plant essential oils into chitosan nanoparticles | 25–150 | Inhibits EPS production, disrupts biofilm matrix, reduces metabolic activity, damages internal and external bacterial structures. | A. baumannii | [222] |
| Lavandula angustifolia (lavender) leaf extract | 10–1500 | Disrupts EPS production, reduces biofilm biomass and metabolic activity, alters biofilm morphology, decreases viability of biofilm-embedded cells. | P. aeruginosa, S. aureus, C. albicans | [223] | |
| Selenium NPs (SeNPs) | Orange peel waste extract | MIC: 62.5–250 | Inhibits biofilm formation, reduces biofilm biomass, disrupts surface adhesion of biofilm-forming cells. | P. aeruginosa PAO1, K. pneumoniae, E. coli, S. aureus | [224] |
| Titanium Dioxide NPs (TiO₂ NPs) | Grape seed extract rich in proanthocyanidins | - | Enhances intracellular uptake, induces intracellular ROS generation, inhibits planktonic cell proliferation, disrupts biofilm formation and biofilm matrix penetration. | P. aeruginosa, S. saprophyticus | [225] |
| Magnesium Oxide NPs (MgO NPs) | Flower extract of Rosa floribunda charisma | 7.81–31.25 | Disrupts biofilm formation, reduces bacterial growth, generates oxidative stress, inhibits bacterial membrane integrity. | S. epidermidis, Streptococcus pyogenes, P. aeruginosa | [226] |
| Cerium Oxide NPs (CeO₂ NPs) | Arctium lappa (burdock root) aqueous extract, encapsulated in nano-chitosan via sol–gel method | 10–18 | Inhibits biofilm formation by disrupting EPS production, induces oxidative stress (ROS generation), disrupts bacterial membrane integrity, reduces bacterial viability. | S. aureus, P. aeruginosa | [227] |
| Graphene Oxide–silver NPs (GO-Ag NPs) | Floral extract of Lagerstroemia speciosa (Banaba flower) | 47–94 | Inhibits biofilm formation, reduces EPS production, induces ROS generation, disrupts cell membrane integrity, downregulates biofilm-associated genes (vicR, spaP, comDE). | S. mutans, Enterobacter cloacae | [228] |
| Limonene-loaded alginate/collagen nanoparticles (LAC) | Encapsulation of limonene into alginate/collagen nanoparticles | 0.781–1.56 | Reduces biofilm formation by inhibiting OmpA and Bap biofilm gene expression, disrupts biofilm matrix integrity, and enhances ROS generation. | A. baumannii | [229] |
| PLGA NPs | Encapsulation of ethanolic extract of propolis (EEP) into poly(lactic-co-glycolic acid) (PLGA) nanoparticles via oil-in-water emulsion solvent evaporation method | 1.25–2.5 | Reduces adhesion, hyphal germination, and biofilm formation; downregulates virulence genes (ALS3, HWP1). | C. albicans | [230] |
| Liposomal NPs | Encapsulation of aqueous extract of Punica granatum into phospholipid-based nanoliposomes | 2–2048 | Inhibits biofilm formation, suppresses glucosyltransferase (GTF) activity, and reduces glucan production. | S. mutans | [231] |
| Silica NPs (SiO₂ NPs) | Hot aqueous leaf extract of Thuja orientalis | - | Inhibits biofilm, disrupts bacterial adhesion, interaction with bacterial DNA, and membrane. | S. aureus, E. coli | [232] |
| Silica nanoparticles (SNPs) loaded with Eucalyptus globulus oil | Synthesis of silica nanoparticles using the sol–gel method; eucalyptus oil encapsulated into SNPs | 50 | Inhibits biofilm formation by disrupting biofilm matrix, enhances oil penetration into biofilm, yet no direct virulence gene or quorum sensing suppression has been studied. | E. coli ATCC 25922 | [233] |
| Nickel Oxide NPs (NiO NPs) | Eucalyptus globulus leaf extract | 0.8–1.6 | Inhibits biofilm formation, increases cell membrane permeability, disrupts cell morphology, generates ROS, damages DNA and proteins. | P. aeruginosa, E. coli, S. aureus | [234] |
| Carbon Quantum Dots (CQDs) | CQDs are synthesized from resveratrol (a phenolic compound from fruits) | 100 | Inhibits chemotaxis, biofilm formation, elastase, pyocyanin, rhamnolipid production; disrupts the pqs quorum sensing system (downregulates virulence genes, reduces PQS signaling). | P. aeruginosa PAO1 | [235] |
| Palladium NPs (PdNPs) | Aqueous extract of Allium sativum (garlic) | 3.125–50 | Disrupts bacterial membrane integrity, inhibits biofilm formation, enhances antibiotic efficacy, promotes wound healing in vivo. | S. aureus, P. aeruginosa | [236] |
| Padina boryana extract | 31.25–125 | Induces ROS generation, disrupts membrane integrity, inhibits biofilm formation, reduces CFU counts. | S. aureus, E. fergusonii, A. pittii, P. aeruginosa, A. enteropelogenes, Proteus mirabilis | [237] | |
| Platinum NPs (PtNPs) | Aqueous extract of Desmostachya bipinnata | - | Disrupts biofilm matrix, inhibits bacterial adhesion, reduces plaque formation, exerts strong antibacterial activity against Gram-positive and Gram-negative bacteria. | S. aureus, E. coli | [238] |
| Zirconium Oxide NPs (ZrO2 NPs) | Aqueous ginger (Zingiber officinale) extract | 5000–50,000 | Disrupts membrane integrity (leakage of proteins, DNA, sugars), induces ROS generation, inhibits biofilm formation and quorum quenching via interaction with biofilm-associated proteins. | A. baumannii | [239] |
| Ag-ZnO NPs | Aqueous leaf extract of Elephantopus scaber | 0.125 | Disrupts bacterial growth and inhibits biofilm formation; antioxidant properties also confirmed, with possible ROS-mediated mechanism inferred. | S. aureus, Bacillus subtilis | [240] |
| Aluminum Oxide NPs (Al₂O₃ NPs) | Citrus aurantium (bitter orange) peel extract | - | Antimicrobial action disrupts microbial growth; antioxidant and anti-proliferative activities noted. | P. aeruginosa, S. aureus, S. epidermidis, Klebsiella pneumoniae, C. albicans, A. niger | [241] |
| Cadmium Sulfide NPs (CdS NPs) | Green synthesis using Coronopus didymus ethanolic extract | 30,000–50,000 | Disrupts bacterial membranes; ROS generation leading to DNA, RNA, and protein synthesis inhibition; cell lysis through interaction with thiol groups. | E. coli, K. pneumoniae, S. aureus | [242] |
| Piper betel leaf extract-coated hydroxyapatite nanoparticles (PBL-HAp) | Using hydroxyapatite (HAp) derived from eggshells, coated with Piper betel leaf extract using the microwave conversion method | - | Inhibits bacterial growth, inhibits biofilm formation, disrupts biofilm matrix integrity. | S. aureus, E. coli, V. harveyi, P. aeruginosa | [243] |
| AgNPs combined with molybdenum disulfide (AgNPs/MoS₂ Nanocomposite) | Tea tree essential oil (Melaleuca alternifolia); combined with exfoliated molybdenum disulfide nanosheets (MoS₂) | - | Disrupts bacterial membranes, induces DNA leakage, generates ROS, inhibits biofilm formation (99%). | S. aureus, E. coli | [244] |
| Lanthanum Oxide NPs (La₂O₃ NPs) | Combustion synthesis using Centella asiatica and Tridax plant leaf powders | 50,000–100,000 | Antibacterial action via cell membrane damage, moderate antifungal activity, photocatalytic ROS generation. | S. aureus, E. coli, C. albicans, A. fumigatus | [245] |
| CuO-Se Bimetallic NPs | Lagenaria siceraria leaf extract | 7.8–250 | Disrupts membrane and ROS generation; inhibits pyocyanin, protease, and pyoverdine production; inhibits biofilm formation via quorum sensing interference. | P. aeruginosa | [246] |
| Ag-Se Bimetallic NPs | Orobanche aegyptiaca extract with guar gum stabilizer | 1000 | Disrupts bacterial and fungal membranes, causes leakage of cytoplasmic content, inhibits biofilm formation, enhances ROS-mediated killing under UV exposure. | S. aureus, E. coli, P. aeruginosa, C. albicans | [247] |
| Cu-Fe Bimetallic NPs | Hibiscus rosa-sinensis flower extract | 100 | Disrupts biofilm network structure, induces bacterial cell death, inhibits biofilm formation. | S. mutans | [248] |
| Co-Zn-Ni Trimetallic Oxide NPs | Cicer arietinum leaf extract | - | Inhibits biofilm formation and binds to DNA Gyrase and surface protein G, suggesting anti-replication and anti-adhesion virulence inhibition. | S. aureus | [249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulat, M.; Banicod, R.J.S.; Tabassum, N.; Javaid, A.; Karthikeyan, A.; Jeong, G.-J.; Kim, Y.-M.; Jung, W.-K.; Khan, F. Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties. Antibiotics 2025, 14, 555. https://doi.org/10.3390/antibiotics14060555
Mulat M, Banicod RJS, Tabassum N, Javaid A, Karthikeyan A, Jeong G-J, Kim Y-M, Jung W-K, Khan F. Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties. Antibiotics. 2025; 14(6):555. https://doi.org/10.3390/antibiotics14060555
Chicago/Turabian StyleMulat, Mulugeta, Riza Jane S. Banicod, Nazia Tabassum, Aqib Javaid, Abirami Karthikeyan, Geum-Jae Jeong, Young-Mog Kim, Won-Kyo Jung, and Fazlurrahman Khan. 2025. "Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties" Antibiotics 14, no. 6: 555. https://doi.org/10.3390/antibiotics14060555
APA StyleMulat, M., Banicod, R. J. S., Tabassum, N., Javaid, A., Karthikeyan, A., Jeong, G.-J., Kim, Y.-M., Jung, W.-K., & Khan, F. (2025). Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties. Antibiotics, 14(6), 555. https://doi.org/10.3390/antibiotics14060555









