Current Clinical Laboratory Challenges to Widespread Adoption of Phage Therapy in the United States
Abstract
1. Introduction
2. Existing Milestones in Phage Therapy
2.1. Phages Are Abundant
2.2. Successful Trials and Experience with Phage Therapy
2.3. Promising Results for Phage Therapy for Treating Resistant Bacteria
2.4. The Existence of a Variety of Laboratory Methods for Phage Selection
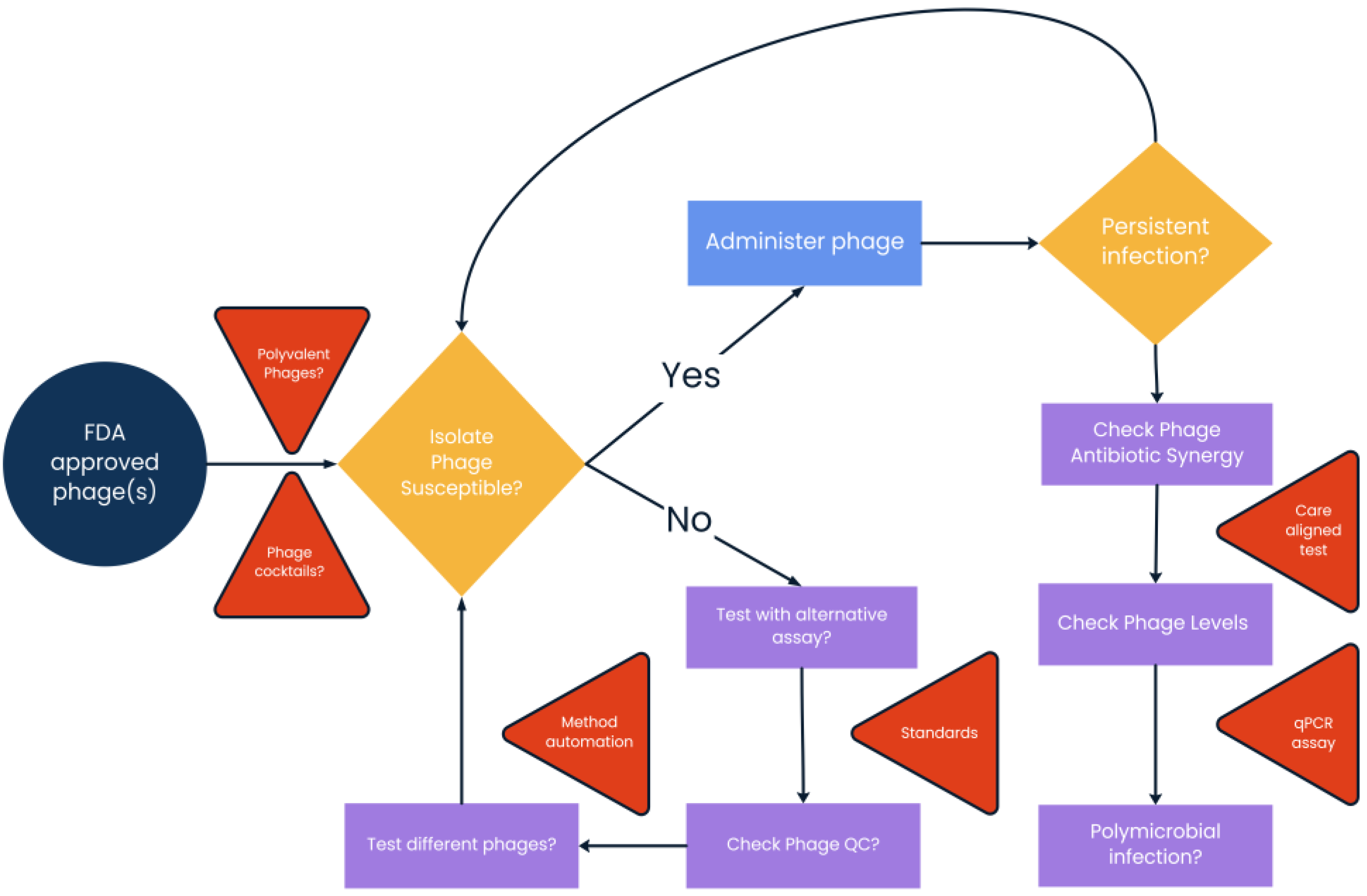
3. Methodology Challenges to Phage Testing in the Clinical Microbiology Laboratory
3.1. Analysis in Liquid Solution Compared to Solid Medium
3.2. Establishment of Quality Control Bacteria and Phages
3.3. The Need for a Standards Committee
3.4. The Need for Antibiotic Synergy Testing
4. Phage Therapy Treatment Differences That May Influence Laboratory Methodology
4.1. Cocktails vs. Individual Phages
4.2. Phage Therapy for Multispecies Infections
4.3. Assays for Phages Used to Treat Biofilms
4.4. Accounting for Phage Passaging
4.5. The Need for Lytic Phages
5. Organization and Regulatory Barriers to Widespread Phage Testing in Clinical Microbiology Laboratories
5.1. Automation and Personnel Issues
5.2. Concurrent Development of PK/PD and Anti-Phage Antibody Assays
5.3. Regulatory Hurdles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Twort, F.W. AN INVESTIGATION ON THE NATURE OF ULTRA-MICROSCOPIC VIRUSES. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- d’Hérelles, F. BACTERIOPHAGE: Sur un Microbe Invisible Antagoniste des Bacilles Dysentériques; Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences; Gauthier-Villars: Paris, France, 1917; Volume 165. [Google Scholar]
- Altamirano, F.L.G.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [PubMed]
- Chanishvili, N. Chapter 1—Phage Therapy—History from Twort and d’Herelle Through Soviet Experience to Current Approaches. In Advances in Virus Research; Łobocka, M., Szybalski, W., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 83, pp. 3–40. [Google Scholar]
- Marongiu, L.; Burkard, M.; Lauer, U.M.; Hoelzle, L.E.; Venturelli, S. Reassessment of Historical Clinical Trials Supports the Effectiveness of Phage Therapy. Clin. Microbiol. Rev. 2022, 35, e00062-22. [Google Scholar] [CrossRef] [PubMed]
- Otten, H. Domagk and the development of the sulphonamides. J. Antimicrob. Chemother. 1986, 17, 689–690. [Google Scholar] [CrossRef]
- Gaynes, R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Straub, M.E. Studies on Commercial Bacteriophage Products. JAMA J. Am. Med. Assoc. 1933, 100, 110–113. [Google Scholar] [CrossRef]
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef]
- Kirby, T. Nina Chanishvili—Keeping bacteriophages in the limelight. Lancet Infect. Dis. 2021, 21, 926. [Google Scholar] [CrossRef]
- Venturini, C.; Petrovic Fabijan, A.; Fajardo Lubian, A.; Barbirz, S.; Iredell, J. Biological foundations of successful bacteriophage therapy. EMBO Mol. Med. 2022, 14, e12435. [Google Scholar] [CrossRef]
- Brives, C.; Pourraz, J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Alas Portillo, M.; Gómez Rodríguez, I.M.; Gradmann, C.; Kirchhelle, C.; Leisner, J.J.; Martinenghi, L.D.; Paterson, E.L.; Santesmases, M.J.; Skender, B.; Vagneron, F. Paradoxes of the antibiotic pipeline. Humanit. Soc. Sci. Commun. 2024, 11, 678. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Soulsby, E.J. Resistance to antimicrobials in humans and animals. BMJ 2005, 331, 1219–1220. [Google Scholar] [CrossRef]
- Cobian Guemes, A.G.; Youle, M.; Cantu, V.A.; Felts, B.; Nulton, J.; Rohwer, F. Viruses as Winners in the Game of Life. Annu. Rev. Virol. 2016, 3, 197–214. [Google Scholar] [CrossRef]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Pride, D.T.; Salzman, J.; Haynes, M.; Rohwer, F.; Davis-Long, C.; White, R.A., 3rd; Loomer, P.; Armitage, G.C.; Relman, D.A. Evidence of a robust resident bacteriophage population revealed through analysis of the human salivary virome. ISME J. 2012, 6, 915–926. [Google Scholar] [CrossRef]
- Jurczak-Kurek, A.; Gasior, T.; Nejman-Falenczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef]
- Cobian Guemes, A.G.; Le, T.; Rojas, M.I.; Jacobson, N.E.; Villela, H.; McNair, K.; Hung, S.H.; Han, L.; Boling, L.; Octavio, J.C.; et al. Compounding Achromobacter Phages for Therapeutic Applications. Viruses 2023, 15, 1665. [Google Scholar] [CrossRef]
- Strancar, V.; Marusic, M.; Tusar, J.; Pracek, N.; Kolenc, M.; Suster, K.; Horvat, S.; Janez, N.; Peterka, M. Isolation and in vitro characterization of novel S. epidermidis phages for therapeutic applications. Front. Cell. Infect. Microbiol. 2023, 13, 1169135. [Google Scholar] [CrossRef]
- Lewis, R.; Clooney, A.G.; Stockdale, S.R.; Buttimer, C.; Draper, L.A.; Ross, R.P.; Hill, C. Isolation of a Novel Jumbo Bacteriophage Effective Against Klebsiella aerogenes. Front. Med. 2020, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, G.; Resch, G.; Rezai, M.S.; Nemati Hevelaee, E. Characterization and Lytic Activity of Isolated Escherichia coli Bacteriophages against Escherichia coli in vitro. Iran J. Med. Sci. 2020, 45, 298–303. [Google Scholar] [PubMed]
- Parfitt, T. Georgia: An unlikely stronghold for bacteriophage therapy. Lancet 2005, 365, 2166–2167. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef]
- Dabizheva, A.N.; Voroshilova, N.N.; Prisada, T.V.; Efimova, M.G. Phages Attack: A History of Bacteriophage Production and Therapeutic Use in Russia. Science First Hand 2017 [cited 46 N1]. Available online: https://scfh.ru/en/papers/phages-attack/ (accessed on 5 May 2025).
- Uchechukwu, C.F.; Shonekan, A. Current status of clinical trials for phage therapy. J. Med. Microbiol. 2024, 73, 001895. [Google Scholar] [CrossRef]
- Zaldastanishvili, E.; Leshkasheli, L.; Dadiani, M.; Nadareishvili, L.; Askilashvili, L.; Kvatadze, N.; Goderdzishvili, M.; Kutateladze, M.; Balarjishvili, N. Phage Therapy Experience at the Eliava Phage Therapy Center: Three Cases of Bacterial Persistence. Viruses 2021, 13, 1901. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzki, R.; Hoyle, N.; Zhvaniya, F.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Łobocka, M.; Borysowski, J.; Alavidze, Z.; Kutter, E.; Górski, A.; et al. Current Updates from the Long-Standing Phage Research Centers in Georgia, Poland, and Russia. In Bacteriophages: Biology, Technology, Therapy; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 921–951. [Google Scholar]
- Nir-Paz, R.; Onallah, H.; Dekel, M.; Gellman, Y.N.; Haze, A.; Ben-Ami, R.; Braunstein, R.; Hazan, R.; Dror, D.; Oster, Y.; et al. Randomized double-blind study on safety and tolerability of TP-102 phage cocktail in patients with infected and non-infected diabetic foot ulcers. Med 2025, 6, 100565. [Google Scholar] [CrossRef]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses 2023, 15, 1020. [Google Scholar] [CrossRef]
- Biosciences, L. A Study of LBP-EC01 in the Treatment of Acute Uncomplicated UTI Caused by Drug Resistant E. Coli (ELIMINATE Trial). Available online: https://clinicaltrials.gov/study/NCT05488340 (accessed on 22 April 2025).
- Abedon, S.T. Phage Companies. 2023. Available online: https://companies.phage.org/ (accessed on 22 April 2025).
- BiomX, Inc. BiomX Announces Closing of the Acquisition of Adaptive Phage Therapeutics and Concurrent $50 Million Financing; BiomX, Inc.: Gaithersburg, MD, USA, 2024. [Google Scholar]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Rajput, A.; Seif, Y.; Choudhary Kumari, S.; Dalldorf, C.; Poudel, S.; Monk Jonathan, M.; Palsson Bernhard, O. Pangenome Analytics Reveal Two-Component Systems as Conserved Targets in ESKAPEE Pathogens. mSystems 2021, 6, e00981-20. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, X.; Huang, J.; Zhu, X.; Han, G.; Wan, Y.; Xu, Y.; Luan, G.; Jia, X. Phage Selective Pressure Reduces Virulence of Hypervirulent Klebsiella pneumoniae Through Mutation of the wzc Gene. Front. Microbiol. 2021, 12, 739319. [Google Scholar] [CrossRef]
- Page, M.L. Phage Therapies for Superbug Infections are Being Tested in Belgium; New Scientist: London, UK, 2022. [Google Scholar]
- Naval Medical Research Center (NMRC). NMRC Maintains a Library of Diverse Bacteriophages to Fight Multidrug Resistant Infections; Naval Medical Research Center (NMRC): Silver Spring, MD, USA, 2019. [Google Scholar]
- Locus Biosciences. Locus Biosciences Announces $23.9 Million in Funding from BARDA to Support First Phase 2 Trial of CRISPR-Engineered Bacteriophage Therapy; Locus Biosciences: Morrisville, NC, USA, 2024. [Google Scholar]
- Center for Innovative Phage Applications and Therapeutics. MicrobiotiX Co., Ltd. and the Center for Innovative Phage Applications and Therapeutics (IPATH) Sign Collaborative Agreement to Improve Access to Phages for IPATH’s Phage Therapy Patients; Division of Infectious Diseases & Global Public Health, University of California San Diego: San Diego, CA, USA, 2022. [Google Scholar]
- Aslam, S.; Roach, D.; Nikolich, M.P.; Biswas, B.; Schooley, R.T.; Lilly-Bishop, K.A.; Rice, G.K.; Cer, R.Z.; Hamilton, T.; Henry, M.; et al. Pseudomonas aeruginosa ventricular assist device infections: Findings from ineffective phage therapies in five cases. Antimicrob. Agents Chemother. 2024, 68, e0172823. [Google Scholar] [CrossRef] [PubMed]
- Onallah, H.; Hazan, R.; Nir-Paz, R.; Brownstein, M.J.; Fackler, J.R.; Horne, B.; Hopkins, R.; Basu, S.; Yerushalmy, O.; Alkalay-Oren, S.; et al. Refractory Pseudomonas aeruginosa infections treated with phage PASA16: A compassionate use case series. Med 2023, 4, 600–611.e4. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Schooley, R.T. What’s Old Is New Again: Bacteriophage Therapy in the 21st Century. Antimicrob. Agents Chemother. 2019, 64, e01987-19. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S.; et al. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients with Drug-Resistant Mycobacterial Disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef]
- Hosseiniporgham, S.; Sechi, L.A. A Review on Mycobacteriophages: From Classification to Applications. Pathogens 2022, 11, 777. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Śliwka, P.; Ochocka, M.; Skaradzińska, A. Applications of bacteriophages against intracellular bacteria. Crit. Rev. Microbiol. 2022, 48, 222–239. [Google Scholar] [CrossRef]
- Glonti, T.; Pirnay, J.P. In Vitro Techniques and Measurements of Phage Characteristics That Are Important for Phage Therapy Success. Viruses 2022, 14, 1490. [Google Scholar] [CrossRef]
- Daubie, V.; Chalhoub, H.; Blasdel, B.; Dahma, H.; Merabishvili, M.; Glonti, T.; De Vos, N.; Quintens, J.; Pirnay, J.P.; Hallin, M.; et al. Determination of phage susceptibility as a clinical diagnostic tool: A routine perspective. Front. Cell. Infect. Microbiol. 2022, 12, 1000721. [Google Scholar] [CrossRef]
- Xie, Y.; Wahab, L.; Gill, J.J. Development and Validation of a Microtiter Plate-Based Assay for Determination of Bacteriophage Host Range and Virulence. Viruses 2018, 10, 189. [Google Scholar] [CrossRef]
- Yerushalmy, O.; Braunstein, R.; Alkalay-Oren, S.; Rimon, A.; Coppenhagn-Glazer, S.; Onallah, H.; Nir-Paz, R.; Hazan, R. Towards Standardization of Phage Susceptibility Testing: The Israeli Phage Therapy Center “Clinical Phage Microbiology”—A Pipeline Proposal. Clin. Infect. Dis. 2023, 77 (Suppl. S5), S337–S351. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E. Phage host range and efficiency of plating. Methods Mol. Biol. 2009, 501, 141–149. [Google Scholar]
- Khan Mirzaei, M.; Nilsson, A.S. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef] [PubMed]
- Cobian Guemes, A.G.; Ghatbale, P.; Blanc, A.N.; Morgan, C.J.; Garcia, A.; Leonard, J.; Huang, L.; Kovalick, G.; Proost, M.; Chiu, M.; et al. Jumbo phages are active against extensively drug-resistant eyedrop-associated Pseudomonas aeruginosa infections. Antimicrob. Agents Chemother. 2023, 67, e0065423. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.; Komarow, L.; Ellison, D.W.; Filippov, A.A.; Nikolich, M.P.; Fackler, J.R.; Lee, M.; Nair, A.; Agrawal, P.; Tamma, P.D.; et al. Interlaboratory comparison of Pseudomonas aeruginosa phage susceptibility testing. J. Clin. Microbiol. 2023, 61, e0061423. [Google Scholar] [CrossRef]
- Tamma, P.D.; Souli, M.; Billard, M.; Campbell, J.; Conrad, D.; Ellison, D.W.; Evans, B.; Evans, S.R.; Greenwood-Quaintance, K.E.; Filippov, A.A.; et al. Safety and microbiological activity of phage therapy in persons with cystic fibrosis colonized with Pseudomonas aeruginosa: Study protocol for a phase 1b/2, multicenter, randomized, double-blind, placebo-controlled trial. Trials 2022, 23, 1057. [Google Scholar] [CrossRef]
- Grossman, M.K.; Rankin, D.A.; Maloney, M.; Stanton, R.A.; Gable, P.; Stevens, V.A.; Ewing, T.; Saunders, K.; Kogut, S.; Nazarian, E.; et al. Extensively Drug-Resistant Pseudomonas aeruginosa Outbreak associated with Artificial Tears. Clin. Infect. Dis. 2024, 79, 6–14. [Google Scholar] [CrossRef]
- Sundermann, A.J.; Rangachar Srinivasa, V.; Mills, E.G.; Griffith, M.P.; Waggle, K.D.; Ayres, A.M.; Pless, L.; Snyder, G.M.; Harrison, L.H.; Van Tyne, D. Two Artificial Tears Outbreak-Associated Cases of Extensively Drug-Resistant Pseudomonas aeruginosa Detected Through Whole Genome Sequencing-Based Surveillance. J. Infect. Dis. 2024, 229, 517–521. [Google Scholar] [CrossRef]
- Chan, B.K.; Stanley, G.L.; Kortright, K.E.; Modak, M.; Ott, I.M.; Sun, Y.; Würstle, S.; Grun, C.; Kazmierczak, B.; Rajagopalan, G.; et al. Personalized Inhaled Bacteriophage Therapy Decreases Multidrug-Resistant Pseudomonas aeruginosa. MedRxiv 2023. [Google Scholar]
- Iyer, L.M.; Anantharaman, V.; Krishnan, A.; Burroughs, A.M.; Aravind, L. Jumbo Phages: A Comparative Genomic Overview of Core Functions and Adaptions for Biological Conflicts. Viruses 2021, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gao, M. Jumbo Bacteriophages: An Overview. Front. Microbiol. 2017, 8, 403. [Google Scholar] [CrossRef]
- Chaikeeratisak, V.; Nguyen, K.; Khanna, K.; Brilot, A.F.; Erb, M.L.; Coker, J.K.; Vavilina, A.; Newton, G.L.; Buschauer, R.; Pogliano, K.; et al. Assembly of a nucleus-like structure during viral replication in bacteria. Science 2017, 355, 194–197. [Google Scholar] [CrossRef]
- Malone, L.M.; Warring, S.L.; Jackson, S.A.; Warnecke, C.; Gardner, P.P.; Gumy, L.F.; Fineran, P.C. A jumbo phage that forms a nucleus-like structure evades CRISPR-Cas DNA targeting but is vulnerable to type III RNA-based immunity. Nat. Microbiol. 2020, 5, 48–55. [Google Scholar] [CrossRef]
- Costa, A.R.; van den Berg, D.F.; Esser, J.Q.; Muralidharan, A.; van den Bossche, H.; Bonilla, B.E.; van der Steen, B.A.; Haagsma, A.C.; Fluit, A.C.; Nobrega, F.L.; et al. Accumulation of defense systems in phage-resistant strains of Pseudomonas aeruginosa. Sci. Adv. 2024, 10, eadj0341. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, C.; Jourdan, J.; Roussel-Gaillard, T.; Medina, M.; Laurent, F. Phage susceptibility testing methods or ‘phagograms’: Where do we stand and where should we go? J. Antimicrob. Chemother. 2024, 79, 2742–2749. [Google Scholar] [CrossRef]
- Trofimova, E.; Jaschke, P.R. Plaque Size Tool: An automated plaque analysis tool for simplifying and standardising bacteriophage plaque morphology measurements. Virology 2021, 561, 1–5. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Kostoulias, X.; Subedi, D.; Korneev, D.; Peleg, A.Y.; Barr, J.J. Phage-antibiotic combination is a superior treatment against Acinetobacter baumannii in a preclinical study. EBioMedicine 2022, 80, 104045. [Google Scholar] [CrossRef]
- Khong, E.; Oh, J.J.; Jimenez, J.M.; Liu, R.; Dunham, S.; Monsibais, A.; Rhoads, A.; Ghatbale, P.; Garcia, A.; Cobian Guemes, A.G.; et al. A simple solid media assay for detection of synergy between bacteriophages and antibiotics. Microbiol. Spectr. 2024, 12, e0322123. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Wozniak, D.J. Phage Cocktail Development for Bacteriophage Therapy: Toward Improving Spectrum of Activity Breadth and Depth. Pharmaceuticals 2021, 14, 1019. [Google Scholar] [CrossRef] [PubMed]
- The EUCAST Subcommittee on Phage Susceptibility Testing. Available online: https://www.eucast.org/ast-of-phages (accessed on 25 October 2024).
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons Learned from the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef] [PubMed]
- Stellfox, M.E.; Fernandes, C.; Shields, R.K.; Haidar, G.; Hughes Kramer, K.; Dembinski, E.; Mangalea, M.R.; Arya, G.; Canfield, G.S.; Duerkop, B.A.; et al. Bacteriophage and antibiotic combination therapy for recurrent Enterococcus faecium bacteremia. mBio 2024, 15, e0339623. [Google Scholar] [CrossRef]
- Nikolic, I.; Vukovic, D.; Gavric, D.; Cvetanovic, J.; Aleksic Sabo, V.; Gostimirovic, S.; Narancic, J.; Knezevic, P. An Optimized Checkerboard Method for Phage-Antibiotic Synergy Detection. Viruses 2022, 14, 1542. [Google Scholar] [CrossRef]
- Gu Liu, C.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar] [CrossRef]
- Knezevic, P.; Curcin, S.; Aleksic, V.; Petrusic, M.; Vlaski, L. Phage-antibiotic synergism: A possible approach to combatting Pseudomonas aeruginosa. Res. Microbiol. 2013, 164, 55–60. [Google Scholar] [CrossRef]
- Neter, E. Inhibitory Effect of Sulfamido Compounds upon Development and Growth of Phage-Resistant Bacteria. Proc. Soc. Exp. Biol. Med. 1941, 47, 20–23. [Google Scholar] [CrossRef]
- Hagens, S.; Habel, A.; Bläsi, U. Augmentation of the antimicrobial efficacy of antibiotics by filamentous phage. Microb. Drug Resist. 2006, 12, 164–168. [Google Scholar] [CrossRef]
- Himmelweit, F. Combined Action of Penicillin and Bacteriophage on Staphylococci. Lancet 1945, 246, 104–105. [Google Scholar] [CrossRef]
- Diallo, K.; Dublanchet, A. Benefits of Combined Phage-Antibiotic Therapy for the Control of Antibiotic-Resistant Bacteria: A Literature Review. Antibiotics 2022, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Chen, Q.; Echterhof, A.; Pennetzdorfer, N.; McBride, R.C.; Banaei, N.; Burgener, E.B.; Milla, C.E.; Bollyky, P.L. A blueprint for broadly effective bacteriophage-antibiotic cocktails against bacterial infections. Nat. Commun. 2024, 15, 9987. [Google Scholar] [CrossRef] [PubMed]
- Kebriaei, R.; Lev, K.; Morrisette, T.; Stamper Kyle, C.; Abdul-Mutakabbir Jacinda, C.; Lehman Susan, M.; Morales, S.; Rybak Michael, J. Bacteriophage-Antibiotic Combination Strategy: An Alternative against Methicillin-Resistant Phenotypes of Staphylococcus aureus. Antimicrob. Agents Chemother. 2020, 64, e00461-20. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, B.; Galant, C.; Brichard, B.; Docquier, P.-L.; Djebara, S.; Pirnay, J.-P.; Van der Linden, D.; Merabishvili, M.; Chatzis, O. A Case of In Situ Phage Therapy against Staphylococcus aureus in a Bone Allograft Polymicrobial Biofilm Infection: Outcomes and Phage-Antibiotic Interactions. Viruses 2021, 13, 1898. [Google Scholar] [CrossRef]
- Ghatbale, P.; Sah, G.P.; Dunham, S.; Khong, E.; Blanc, A.; Monsibais, A.; Garcia, A.; Schooley, R.T.; Cobián Güemes, A.G.; Whiteson, K.; et al. In vitro resensitization of multidrug-resistant clinical isolates of Enterococcus faecium and E. faecalis through phage-antibiotic synergy. Antimicrob. Agents Chemother. 2024, 69, e0074024. [Google Scholar] [CrossRef]
- Loganathan, A.; Bozdogan, B.; Manohar, P.; Nachimuthu, R. Phage-antibiotic combinations in various treatment modalities to manage MRSA infections. Front. Pharmacol. 2024, 15, 1356179. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Zhang, P.; To, K.K.W.; Liu, Y.; Bai, C.; Leung, S.S.Y. Sequential treatment effects on phage–antibiotic synergistic application against multi-drug-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 2023, 62, 106951. [Google Scholar] [CrossRef]
- Ma, D.; Li, L.; Han, K.; Wang, L.; Cao, Y.; Zhou, Y.; Chen, H.; Wang, X. The antagonistic interactions between a polyvalent phage SaP7 and β-lactam antibiotics on combined therapies. Vet. Microbiol. 2022, 266, 109332. [Google Scholar] [CrossRef]
- Pacios, O.; Fernández-García, L.; Bleriot, I.; Blasco, L.; González-Bardanca, M.; López, M.; Fernández-Cuenca, F.; Oteo, J.; Pascual, Á.; Martínez-Martínez, L.; et al. Enhanced Antibacterial Activity of Repurposed Mitomycin C and Imipenem in Combination with the Lytic Phage vB_KpnM-VAC13 against Clinical Isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2021, 65, e0090021. [Google Scholar] [CrossRef]
- Shlezinger, M.; Coppenhagen-Glazer, S.; Gelman, D.; Beyth, N.; Hazan, R. Eradication of Vancomycin-Resistant Enterococci by Combining Phage and Vancomycin. Viruses 2019, 11, 954. [Google Scholar] [CrossRef]
- Molina, F.; Menor-Flores, M.; Fernandez, L.; Vega-Rodriguez, M.A.; Garcia, P. Systematic analysis of putative phage-phage interactions on minimum-sized phage cocktails. Sci. Rep. 2022, 12, 2458. [Google Scholar] [CrossRef]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am. J. Transplant. 2019, 19, 2631–2639. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Hajama, H.; Akong, K.; Jordan, M.; Stout, D.; Rowe, R.S.; Conrad, D.J.; Hingtgen, S.; Segall, A.M. Bacteriophage Therapy of Multidrug-resistant Achromobacter in an 11-Year-old Boy with Cystic Fibrosis Assessed by Metagenome Analysis. Pediatr. Infect. Dis. J. 2023, 42, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Moulton-Brown, C.E.; Friman, V.P. Rapid evolution of generalized resistance mechanisms can constrain the efficacy of phage-antibiotic treatments. Evol. Appl. 2018, 11, 1630–1641. [Google Scholar] [CrossRef]
- Gainey, A.B.; Burch, A.K.; Brownstein, M.J.; Brown, D.E.; Fackler, J.; Horne, B.; Biswas, B.; Bivens, B.N.; Malagon, F.; Daniels, R. Combining bacteriophages with cefiderocol and meropenem/vaborbactam to treat a pan-drug resistant Achromobacter species infection in a pediatric cystic fibrosis patient. Pediatr. Pulmonol. 2020, 55, 2990–2994. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Freeman, K.G.; Nguyen, J.A.; Bahadirli-Talbott, A.; Smith, B.E.; Wu, A.E.; Ong, A.S.; Lin, C.T.; Ruppel, L.C.; Parrish, N.M.; et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat. Med. 2021, 27, 1357–1361. [Google Scholar] [CrossRef]
- de Melo, A.G.; Morency, C.; Moineau, S. Virulence-associated factors as targets for phage infection. Curr. Opin. Microbiol. 2024, 79, 102471. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Turner, P.E. High-throughput discovery of phage receptors using transposon insertion sequencing of bacteria. Proc. Natl. Acad. Sci. USA 2020, 117, 18670–18679. [Google Scholar] [CrossRef] [PubMed]
- Gencay, Y.E.; Gambino, M.; Prussing, T.F.; Brondsted, L. The genera of bacteriophages and their receptors are the major determinants of host range. Environ. Microbiol. 2019, 21, 2095–2111. [Google Scholar] [CrossRef]
- Borin, J.M.; Lee, J.J.; Gerbino, K.R.; Meyer, J.R. Comparison of bacterial suppression by phage cocktails, dual-receptor generalists, and coevolutionarily trained phages. Evol. Appl. 2023, 16, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Wandro, S.; Ghatbale, P.; Attai, H.; Hendrickson, C.; Samillano, C.; Suh, J.; Dunham, S.J.B.; Pride, D.T.; Whiteson, K. Phage Cocktails Constrain the Growth of Enterococcus. mSystems 2022, 7, e0001922. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Ju, Z.; Huang, J.; Lin, C.; Wu, J.; Wu, Y.; Sun, S.; Wang, H.; Hao, G.; et al. Increased mutations in lipopolysaccharide biosynthetic genes cause time-dependent development of phage resistance in Salmonella. Antimicrob. Agents Chemother. 2024, 68, e0059423. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.A.; Kazakov, A.E.; Zhong, C.; Liu, H.; Kutter, E.; Lui, L.M.; Nielsen, T.N.; Carion, H.; Deutschbauer, A.M.; Mutalik, V.K.; et al. The genetic basis of phage susceptibility, cross-resistance and host-range in Salmonella. Microbiology 2021, 167, 001126. [Google Scholar] [CrossRef]
- Paule, A.; Frezza, D.; Edeas, M. Microbiota and Phage Therapy: Future Challenges in Medicine. Med. Sci. 2018, 6, 86. [Google Scholar] [CrossRef]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R.; et al. Successful Treatment of Antibiotic-resistant, Poly-microbial Bone Infection with Bacteriophages and Antibiotics Combination. Clin. Infect. Dis. 2019, 69, 2015–2018. [Google Scholar] [CrossRef]
- Alt, V.; Gessner, A.; Merabishvili, M.; Hitzenbichler, F.; Mannala, G.K.; Peterhoff, D.; Walter, N.; Pirnay, J.P.; Hiergeist, A.; Rupp, M. Case report: Local bacteriophage therapy for fracture-related infection with polymicrobial multi-resistant bacteria: Hydrogel application and postoperative phage analysis through metagenomic sequencing. Front. Med. 2024, 11, 1428432. [Google Scholar] [CrossRef]
- Onsea, J.; Soentjens, P.; Djebara, S.; Merabishvili, M.; Depypere, M.; Spriet, I.; De Munter, P.; Debaveye, Y.; Nijs, S.; Vanderschot, P.; et al. Bacteriophage Application for Difficult-To-Treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol. Viruses 2019, 11, 891. [Google Scholar] [CrossRef]
- Exarchos, V.; Tkhilaishvili, T.; Potapov, E.; Starck, C.; Trampuz, A.; Schoenrath, F. Successful bacteriophage treatment of infection involving cardiac implantable electronic device and aortic graft: A Trojan horse concept. EP Eur. 2020, 22, 597. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Malki, K.; Kula, A.; Bruder, K.; Sible, E.; Hatzopoulos, T.; Steidel, S.; Watkins, S.C.; Putonti, C. Bacteriophages isolated from Lake Michigan demonstrate broad host-range across several bacterial phyla. Virol. J. 2015, 12, 164. [Google Scholar] [CrossRef]
- Göller, P.C.; Elsener, T.; Lorgé, D.; Radulovic, N.; Bernardi, V.; Naumann, A.; Amri, N.; Khatchatourova, E.; Coutinho, F.H.; Loessner, M.J.; et al. Multi-species host range of staphylococcal phages isolated from wastewater. Nat. Commun. 2021, 12, 6965. [Google Scholar] [CrossRef]
- Kim, S.H.; Adeyemi, D.E.; Park, M.K. Characterization of a New and Efficient Polyvalent Phage Infecting E. coli O157:H7, Salmonella spp., and Shigella sonnei. Microorganisms 2021, 9, 2105. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Mallick, B.; Dutta, M.; Dutta, S. Isolation, characterization, and application of a novel polyvalent lytic phage STWB21 against typhoidal and nontyphoidal Salmonella spp. Front. Microbiol. 2022, 13, 980025. [Google Scholar] [CrossRef]
- Fong, K.; Wong, C.W.Y.; Wang, S.; Delaquis, P. How Broad Is Enough: The Host Range of Bacteriophages and Its Impact on the Agri-Food Sector. PHAGE 2021, 2, 83–91. [Google Scholar] [CrossRef]
- Yu, P.; Mathieu, J.; Li, M.; Dai, Z.; Alvarez, P.J.J. Isolation of Polyvalent Bacteriophages by Sequential Multiple-Host Approaches. Appl. Environ. Microbiol. 2016, 82, 808–815. [Google Scholar] [CrossRef]
- Jensen, E.C.; Schrader, H.S.; Rieland, B.; Thompson, T.L.; Lee, K.W.; Nickerson, K.W.; Kokjohn, T.A. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl. Env. Microbiol. 1998, 64, 575–580. [Google Scholar] [CrossRef]
- Federici, S.; Kredo-Russo, S.; Valdés-Mas, R.; Kviatcovsky, D.; Weinstock, E.; Matiuhin, Y.; Silberberg, Y.; Atarashi, K.; Furuichi, M.; Oka, A.; et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 2022, 185, 2879–2898.e24. [Google Scholar] [CrossRef]
- Yousif, A.; Jamal, M.A.; Raad, I. Biofilm-Based Central Line-Associated Bloodstream Infections. In Biofilm-Based Healthcare-Associated Infections: Volume I; Donelli, G., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 157–179. [Google Scholar]
- Hassan, A.Y.; Lin, J.T.; Ricker, N.; Anany, H. The Age of Phage: Friend or Foe in the New Dawn of Therapeutic and Biocontrol Applications? Pharmaceuticals 2021, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.J.; Rapp, E.M.; McKenzie, S.M.; Mazur, M.Z.; McHale, R.P.; Brasko, B.; Min, M.Y.; Burpo, F.J.; Barnhill, J.C. Disruption of Biofilm by Bacteriophages in Clinically Relevant Settings. Mil. Med. 2024, 189, e1294–e1302. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Shafique, M.; Alvi, I.A.; Abbas, Z.; ur Rehman, S. Assessment of biofilm removal capacity of a broad host range bacteriophage JHP against Pseudomonas aeruginosa. APMIS 2017, 125, 579–584. [Google Scholar] [CrossRef]
- El-Atrees, D.M.; El-Kased, R.F.; Abbas, A.M.; Yassien, M.A. Characterization and anti-biofilm activity of bacteriophages against urinary tract Enterococcus faecalis isolates. Sci. Rep. 2022, 12, 13048. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. npj Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Pallavali, R.R.; Degati, V.L.; Narala, V.R.; Velpula, K.K.; Yenugu, S.; Durbaka, V.R.P. Lytic Bacteriophages Against Bacterial Biofilms Formed by Multidrug-Resistant Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus Isolated from Burn Wounds. PHAGE 2021, 2, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Patel, R. Clinical phage microbiology: A narrative summary. Clin. Microbiol. Infect. 2023, 29, 710–713. [Google Scholar] [CrossRef]
- Skaradzińska, A.; Ochocka, M.; Śliwka, P.; Kuźmińska-Bajor, M.; Skaradziński, G.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U. Bacteriophage amplification—A comparison of selected methods. J. Virol. Methods 2020, 282, 113856. [Google Scholar] [CrossRef]
- Borin, J.M.; Avrani, S.; Barrick, J.E.; Petrie, K.L.; Meyer, J.R. Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2104592118. [Google Scholar] [CrossRef]
- Burrowes, B.H.; Molineux, I.J.; Fralick, J.A. Directed in Vitro Evolution of Therapeutic Bacteriophages: The Appelmans Protocol. Viruses 2019, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Pirnay, J.P.; De Vos, D. Guidelines to Compose an Ideal Bacteriophage Cocktail. Methods Mol. Biol. 2018, 1693, 99–110. [Google Scholar] [PubMed]
- Borin, J.M.; Lee, J.J.; Lucia-Sanz, A.; Gerbino, K.R.; Weitz, J.S.; Meyer, J.R. Rapid bacteria-phage coevolution drives the emergence of multiscale networks. Science 2023, 382, 674–678. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef]
- Grigson, S.R.; Giles, S.K.; Edwards, R.A.; Papudeshi, B. Knowing and Naming: Phage Annotation and Nomenclature for Phage Therapy. Clin. Infect. Dis. 2023, 77 (Suppl. S5), S352–S359. [Google Scholar] [CrossRef] [PubMed]
- McNair, K.; Zhou, C.; Dinsdale, E.A.; Souza, B.; Edwards, R.A. PHANOTATE: A novel approach to gene identification in phage genomes. Bioinformatics 2019, 35, 4537–4542. [Google Scholar] [CrossRef]
- Antonios, K.; Croxatto, A.; Culbreath, K. Current State of Laboratory Automation in Clinical Microbiology Laboratory. Clin. Chem. 2022, 68, 99–114. [Google Scholar] [CrossRef]
- Yu, H.-Y.E.; Lanzoni, H.; Steffen, T.; Derr, W.; Cannon, K.; Contreras, J.; Olson, J.E. Improving Laboratory Processes with Total Laboratory Automation. Lab. Med. 2019, 50, 96–102. [Google Scholar] [CrossRef]
- Trigueiro, G.; Oliveira, C.; Rodrigues, A.; Seabra, S.; Pinto, R.; Bala, Y.; Gutiérrez Granado, M.; Vallejo, S.; Gonzalez, V.; Cardoso, C. Conversion of a classical microbiology laboratory to a total automation laboratory enhanced by the application of lean principles. Microbiol. Spectr. 2024, 12, e02153-23. [Google Scholar] [CrossRef]
- Heggeness, M.L.; Roman, C.E.Z.; Ginther, D.K. Workforce Trends: The Future of Microbial Sciences; American Society for Microbiology: Bethesda, MD, USA, 2023. [Google Scholar]
- Cherkaoui, A.; Schrenzel, J. Total Laboratory Automation for Rapid Detection and Identification of Microorganisms and Their Antimicrobial Resistance Profiles. Front. Cell Infect. Microbiol. 2022, 12, 807668. [Google Scholar] [CrossRef]
- Snyder, J.W.; Thomson, G.K.; Heckman, S.; Jamros, K.; AbdelGhani, S.; Thomson, K.S. Automated preparation for identification and antimicrobial susceptibility testing: Evaluation of a research use only prototype, the BD Kiestra IdentifA/SusceptA system. Clin. Microbiol. Infect. 2021, 27, e781.e1–e783.e5. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; van den Broek, A.K.; de Vroom, S.L.; Prins, J.M.; Mathôt, R.A.A.; van Hest, R.M. Clinical Pharmacokinetics of Gentamicin in Various Patient Populations and Consequences for Optimal Dosing for Gram-Negative Infections: An Updated Review. Clin. Pharmacokinet. 2022, 61, 1075–1094. [Google Scholar] [CrossRef] [PubMed]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S.; et al. The monitoring of vancomycin: A systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef]
- McCreary, E.K.; Davis, M.R.; Narayanan, N.; Andes, D.R.; Cattaneo, D.; Christian, R.; Lewis, R.E.; Watt, K.M.; Wiederhold, N.P.; Johnson, M.D. Utility of triazole antifungal therapeutic drug monitoring: Insights from the Society of Infectious Diseases Pharmacists: Endorsed by the Mycoses Study Group Education and Research Consortium. Pharmacotherapy 2023, 43, 1043–1050. [Google Scholar] [CrossRef]
- Nang, S.C.; Lin, Y.-W.; Petrovic Fabijan, A.; Chang, R.Y.K.; Rao, G.G.; Iredell, J.; Chan, H.-K.; Li, J. Pharmacokinetics/pharmacodynamics of phage therapy: A major hurdle to clinical translation. Clin. Microbiol. Infect. 2023, 29, 702–709. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Kasprzak, H.; Letkiewicz, S.; Rogóż, P.; Żaczek, M.; Thomas, J.; Górski, A. Pharmacokinetic and Pharmacodynamic Obstacles for Phage Therapy from the Perspective of Clinical Practice. Clin. Infect. Dis. 2023, 77 (Suppl. S5), S395–S400. [Google Scholar] [CrossRef] [PubMed]
- Kłopot, A.; Zakrzewska, A.; Lecion, D.; Majewska, J.M.; Harhala, M.A.; Lahutta, K.; Kaźmierczak, Z.; Łaczmański, Ł.; Kłak, M.; Dąbrowska, K. Real-Time qPCR as a Method for Detection of Antibody-Neutralized Phage Particles. Front. Microbiol. 2017, 8, 2170. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.A.; Sharp, N.J.; Westwater, C. Phage-based platforms for the clinical detection of human bacterial pathogens. Bacteriophage 2012, 2, 105–283. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Z.; Gu, Y.; Huang, A.; Wang, J.; Hao, H. Phage Products for Fighting Antimicrobial Resistance. Microorganisms 2022, 10, 1324. [Google Scholar] [CrossRef]
- Marks, P.; Witten, C. Toward a new framework for the development of individualized therapies. Gene Ther. 2021, 28, 615–617. [Google Scholar] [CrossRef]
- Food and Drug Administrations. Human Gene Therapy Products Incorporating Human Genome Editing. In Guidance for Industry; Center for Biologics Evaluation and Research, Ed.; FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Food and Drug Administrations. Adaptive Designs for Clinical Trials of Drugs and Biologics. In Guidance for Industry; Center for Biologics Evaluation and Research, Ed.; FDA: Silver Spring, MD, USA, 2019. [Google Scholar]
- Palma, M.; Qi, B. Advancing Phage Therapy: A Comprehensive Review of the Safety, Efficacy, and Future Prospects for the Targeted Treatment of Bacterial Infections. Infect. Dis. Rep. 2024, 16, 1127–1181. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good Manufacturing Practice (GMP) Compliance for Phage Therapy Medicinal Products. Front. Microbiol. 2020, 11, 1161. [Google Scholar] [CrossRef]
- Henein, A. What are the limitations on the wider therapeutic use of phage? Bacteriophage 2013, 3, e24872. [Google Scholar] [CrossRef]
- Hopkins, C. We Have Arrived in the Post-Antibiotic Era’: WHO Warns of Too Few New Drugs for Deadly Superbugs; NBC News: New York, NY, USA, 2023. [Google Scholar]
| Center | Associated University or Hospital | Location | Website |
|---|---|---|---|
| Center for Innovative Phage Therapeutics and Applications (IPATH) | University of California, San Diego | San Diego, CA, USA | https://sites.medschool.ucsd.edu/som/medicine/divisions/idgph/research/center-innovative-phage-applications-and-therapeutics/Pages/default.aspx (accessed on 5 May 2025) |
| Center for Phage Technology (CPT) | Texas A&M University | College Station, TX, USA | https://cpt.tamu.edu/history-and-mandate/ (accessed on 5 May 2025) |
| TAILOR Labs | Baylor College of Medicine | Houston, TX, USA | https://www.bcm.edu/research/research-centers/tailor (accessed on 5 May 2025) |
| Center for Phage Research and Therapy at Yale | Yale University | New Haven, CT, USA | https://phage.yale.edu/ (accessed on 5 May 2025) |
| Pittsburgh Phage Program | Children’s Hospital of Pittsburgh | Pittsburgh, PA, USA | https://www.i4kids.org/research/pittsburgh-phage-progam-p3 (accessed on 5 May 2025) |
| Eliava Phage Therapy Center | Eliava Institute of Bacteriophages, Microbiology, and Virology | Tbilisi, Georgia | https://eptc.ge/ (accessed on 5 May 2025) |
| Phage Therapy Unit of the Medical Centre of the Institute of Immunology and Experimental Therapy PAS | Polish Academy of Sciences | Wroclaw, Poland | https://hirszfeld.pl/en/structure/iitd-pan-medical-center/phage-therapy-unit/ (accessed on 5 May 2025) |
| Israeli Phage Therapy Center of Hadassah | Hebrew University of Jerusalem and Ein Kerem Hospital | Jerusalem, Israel | https://phageil.com/the-israeli-phage-therapy-center/ (accessed on 5 May 2025) |
| Phage Therapy Institute at Waseda University | Waseda University | Tokyo, Japan | https://www.waseda.jp/inst/cro/en/institutes-list/phage-therapy-institute/ (accessed on 5 May 2025) |
| Phage Center at Queen Astrid Military Hospital | Queen Astrid military hospital | Brussels, Belgium | https://www.hopitalmilitaire.be/home_fr.php (accessed on 5 May 2025) |
| Monash Phage Foundry | Monash University | Melbourne, Australia | https://www.monash.edu/impact-amr/phage-therapy (accessed on 5 May 2025) |
| Shanghai Institute of Phage | Shanghai Public Health Clinical Center of Fudan University | Shanghai, China | |
| Phage Australia | The Westmead Institute for Medical Research | Westmead, New South Wales | https://www.phageaustralia.org/ (accessed on 5 May 2025) |
| Bacterium | Number of Requests | Number of Phages Found | Number of Cases Treated |
|---|---|---|---|
| Pseudomonas aeruginosa | 348 | 67 | 14 |
| Staphylococcus aureus | 175 | 7 | 4 |
| Escherichia coli | 131 | 17 | 5 |
| Klebsiella pneumoniae | 125 | 15 | 3 |
| Acinetobacter baumannii | 72 | 12 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kline, A.; Cobián Güemes, A.G.; Yore, J.; Ghose, C.; Van Tyne, D.; Whiteson, K.; Pride, D.T. Current Clinical Laboratory Challenges to Widespread Adoption of Phage Therapy in the United States. Antibiotics 2025, 14, 553. https://doi.org/10.3390/antibiotics14060553
Kline A, Cobián Güemes AG, Yore J, Ghose C, Van Tyne D, Whiteson K, Pride DT. Current Clinical Laboratory Challenges to Widespread Adoption of Phage Therapy in the United States. Antibiotics. 2025; 14(6):553. https://doi.org/10.3390/antibiotics14060553
Chicago/Turabian StyleKline, Ahnika, Ana G. Cobián Güemes, Jennifer Yore, Chandrabali Ghose, Daria Van Tyne, Katrine Whiteson, and David T. Pride. 2025. "Current Clinical Laboratory Challenges to Widespread Adoption of Phage Therapy in the United States" Antibiotics 14, no. 6: 553. https://doi.org/10.3390/antibiotics14060553
APA StyleKline, A., Cobián Güemes, A. G., Yore, J., Ghose, C., Van Tyne, D., Whiteson, K., & Pride, D. T. (2025). Current Clinical Laboratory Challenges to Widespread Adoption of Phage Therapy in the United States. Antibiotics, 14(6), 553. https://doi.org/10.3390/antibiotics14060553






