Outbreak of NDM-5-Producing Proteus mirabilis During the COVID-19 Pandemic in an Argentine Hospital
Abstract
1. Introduction
2. Results
2.1. Epidemiological and Clinical Characteristics of the P. mirabilis Outbreak
2.2. Antimicrobial Susceptibility Testing (AST) and Carbapenemase Detection
2.3. Clonal Relationship
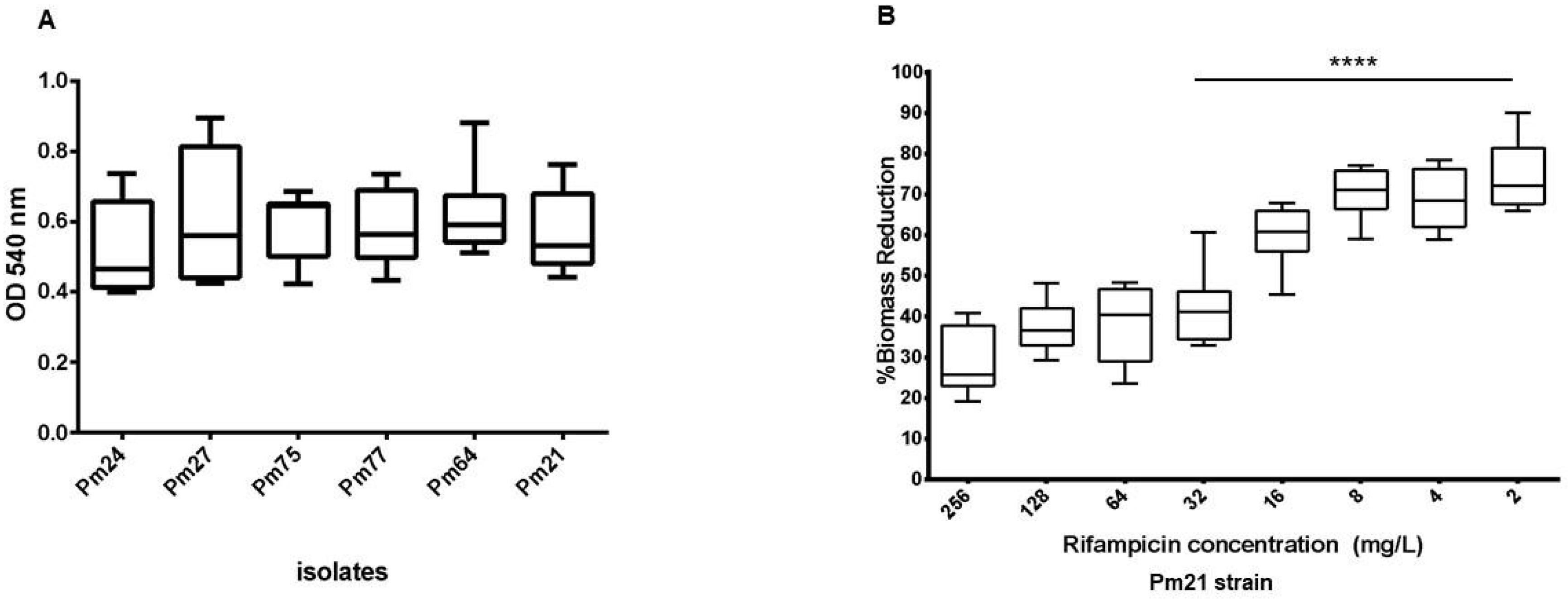
2.4. Biofilm Formation and Substrate-Specific Growth
2.5. Impact of Rifampicin on Established Biofilms
2.6. Genome Analysis
2.7. Genetic Analysis of Resistance Marker Environments
2.8. Phylogenetic Tree
3. Discussion
4. Materials and Methods
4.1. Hospital Setting and Bacterial Isolates
4.2. Bacterial Identification and Antimicrobial Susceptibility Testing (AST)
4.3. Resistance Mechanisms
4.4. Molecular Typing
4.5. Biofilm Formation Assays
4.6. Biofilm Antimicrobial Susceptibility
4.7. Statistical Analysis
4.8. Whole Genome Sequencing Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMK | amikacin |
| AST | antimicrobial susceptibility testing |
| AZT | aztreonam |
| CAZ | ceftazidime |
| CPE | carbapenemase-producing Enterobacteriaceae |
| CFU | colony forming unit |
| CIP | ciprofloxacin |
| CLSI | Clinical and Laboratory Standards Institute |
| CTX | cefotaxime |
| CV | crystal violet |
| EDTA | ethylenediaminetetraacetic Acid |
| ERIC | Enterobacterial Repetitive Intergenic Consensus |
| ESBL | extended spectrum β-lactamase |
| FEP | cefepime |
| GEN | gentamicin |
| GI | genomic island |
| IPM | Imipenem |
| LB | lysogeny broth |
| MALDI-TOF MS | matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MB | minimum biofilm MIC (assumed: MIC-b) |
| MBL | metallo-β-lactamase |
| MDR | multidrug resistant |
| MEM | meropenem |
| MIC | minimum inhibitory concentration |
| MIC-b | minimum inhibitory concentration in the biofilm plate |
| MLST | multilocus sequence typing |
| MRC | minimum regrowth concentration |
| OD | optical density |
| PBA | phenylboronic acid |
| PBS | phosphate-buffered saline |
| PTZ | piperacillin/tazobactam |
| REP-PCR | repetitive extragenic palindromic PCR |
| ST | sequence type |
| TMS | trimethoprim/sulfamethoxazole |
| TSA | tryptic soy agar |
| TSB | tryptic soy broth |
| WGS | whole genome sequencing |
| cgMLST | core genome multilocus sequence typing |
References
- Zhu, X.; Zhang, Y.; Shen, Z.; Xia, L.; Wang, J.; Zhao, L.; Wang, K.; Wang, W.; Hao, Z.; Liu, Z. Characterization of NDM-1-Producing Carbapenemase in Proteus Mirabilis among Broilers in China. Microorganisms 2021, 9, 2443. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, D.; Jia, W.; Ma, J.; Zhao, X. Study of the Molecular Characteristics and Homology of Carbapenem-Resistant Proteus Mirabilis by Whole Genome Sequencing. J. Med. Microbiol. 2023, 72, 001648. [Google Scholar] [CrossRef]
- Bitar, I.; Mattioni Marchetti, V.; Mercato, A.; Nucleo, E.; Anesi, A.; Bracco, S.; Rognoni, V.; Hrabak, J.; Migliavacca, R. Complete Genome and Plasmids Sequences of a Clinical Proteus Mirabilis Isolate Producing Plasmid Mediated NDM-1 From Italy. Microorganisms 2020, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Coretti, L.; Savio, V.; Buommino, E.; Lembo, F.; Donnarumma, G. Biofilm Formation and Immunomodulatory Activity of Proteus Mirabilis Clinically Isolated Strains. Int. J. Mol. Sci. 2017, 18, 414. [Google Scholar] [CrossRef]
- Wilks, S.A.; Fader, M.J.; Keevil, C.W. Novel Insights into the Proteus Mirabilis Crystalline Biofilm Using Real-Time Imaging. PLoS ONE 2015, 10, e0141711. [Google Scholar] [CrossRef]
- Ficik, J.; Andrezál, M.; Drahovská, H.; Böhmer, M.; Szemes, T.; Liptáková, A.; Slobodníková, L. Carbapenem-Resistant Klebsiella Pneumoniae in COVID-19 Era—Challenges and Solutions. Antibiotics 2023, 12, 1285. [Google Scholar] [CrossRef]
- Li, Y.; Yin, M.; Fang, C.; Fu, Y.; Dai, X.; Zeng, W.; Zhang, L. Genetic Analysis of Resistance and Virulence Characteristics of Clinical Multidrug-Resistant Proteus Mirabilis Isolates. Front. Cell. Infect. Microbiol. 2023, 13, 1229194. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhang, L.; Moran, R.A.; Xu, Q.; Sun, L.; van Schaik, W.; Yu, Y. Cointegration as a Mechanism for the Evolution of a KPC-Producing Multidrug Resistance Plasmid in Proteus Mirabilis. Emerg. Microbes Infect. 2020, 9, 1206–1218. [Google Scholar] [CrossRef]
- He, J.; Sun, L.; Zhang, L.; Leptihn, S.; Yu, Y.; Hua, X. A Novel SXT/R391 Integrative and Conjugative Element Carries Two Copies of the blaNDM-1 Gene in Proteus Mirabilis. mSphere 2021, 6, e0058821. [Google Scholar] [CrossRef]
- Fritzenwanker, M.; Falgenhauer, J.; Hain, T.; Imirzalioglu, C.; Chakraborty, T.; Yao, Y. The Detection of Extensively Drug-Resistant Proteus Mirabilis Strains Harboring Both VIM-4 and VIM-75 Metallo-β-Lactamases from Patients in Germany. Microorganisms 2025, 13, 266. [Google Scholar] [CrossRef]
- Costa, A.; Figueroa-Espinosa, R.; Gaudenzi, F.; Lincopan, N.; Fuga, B.; Ghiglione, B.; Gutkind, G.; Di Conza, J. Co-Occurrence of NDM-5 and RmtB in a Clinical Isolate of Escherichia Coli Belonging to CC354 in Latin America. Front. Cell. Infect. Microbiol. 2021, 11, 654852. [Google Scholar] [CrossRef] [PubMed]
- González-Espinosa, F.; Di Pilato, V.; Calabrese, L.; Costa, E.; Costa, A.; Gutkind, G.; Cejas, D.; Radice, M. Integral Genomic Description of BlaNDM-5-Harbouring Plasmids Recovered from Enterobacterales in Argentina. J. Glob. Antimicrob. Resist. 2024, 39, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Bleichenbacher, S.; Stevens, M.J.A.; Zurfluh, K.; Perreten, V.; Endimiani, A.; Stephan, R.; Nüesch-Inderbinen, M. Environmental Dissemination of Carbapenemase-Producing Enterobacteriaceae in Rivers in Switzerland. Environ. Pollut. 2020, 265, 115081. [Google Scholar] [CrossRef]
- Yang, L.; He, H.; Chen, Q.; Wang, K.; Lin, Y.; Li, P.; Li, J.; Liu, X.; Jia, L.; Song, H.; et al. Nosocomial Outbreak of Carbapenemase-Producing Proteus Mirabilis With Two Novel Salmonella Genomic Island 1 Variants Carrying Different blaNDM–1 Gene Copies in China. Front. Microbiol. 2022, 12, 800938. [Google Scholar] [CrossRef]
- Protonotariou, E.; Poulou, A.; Politi, L.; Meletis, G.; Chatzopoulou, F.; Malousi, A.; Metallidis, S.; Tsakris, A.; Skoura, L. Clonal Outbreak Caused by VIM-4-Producing Proteus Mirabilis in a Greek Tertiary-Care Hospital. Int. J. Antimicrob. Agents 2020, 56, 106060. [Google Scholar] [CrossRef]
- Nakano, R.; Nakano, A.; Abe, M.; Inoue, M.; Okamoto, R. Regional Outbreak of CTX-M-2 β-Lactamase-Producing Proteus Mirabilis in Japan. J. Med. Microbiol. 2012, 61, 1727–1735. [Google Scholar] [CrossRef]
- Cremet, L.; Bemer, P.; Rome, J.; Juvin, M.-E.; Navas, D.; Bourigault, C.; Guillouzouic, A.; Caroff, N.; Lepelletier, D.; Asseray, N.; et al. Outbreak Caused by Proteus Mirabilis Isolates Producing Weakly Expressed TEM-Derived Extended-Spectrum β-Lactamase in Spinal Cord Injury Patients with Recurrent Bacteriuria. Scand. J. Infect. Dis. 2011, 43, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Gaind, R.; Kothari, C.; Sehgal, R.; Shamweel, A.; Thukral, S.S.; Chellani, H.K. VEB-1 Extended-Spectrum β-Lactamase-Producing Multidrug-Resistant Proteus Mirabilis Sepsis Outbreak in a Neonatal Intensive Care Unit in India: Clinical and Diagnostic Implications. JMM Case Rep. 2016, 3, e005056. [Google Scholar] [CrossRef]
- Sun, L.; Xu, J.; He, F. Genomic Characterisation of a Proteus mirabilis Clinical Isolate from China Carrying blaNDM-5 on an IncX3 Plasmid. J. Glob. Antimicrob. Resist. 2019, 19, 317–319. [Google Scholar] [CrossRef]
- Valentin, T.; Feierl, G.; Masoud-Landgraf, L.; Kohek, P.; Luxner, J.; Zarfel, G. Proteus mirabilis Harboring Carbapenemase NDM-5 and ESBL VEB-6 Detected in Austria. Diagn. Microbiol. Infect. Dis. 2018, 91, 284–286. [Google Scholar] [CrossRef]
- Shaaban, M.; Elshaer, S.L.; Abd El-Rahman, O.A. Prevalence of Extended-Spectrum β-Lactamases, AmpC, and Carbapenemases in Proteus mirabilis Clinical Isolates. BMC Microbiol. 2022, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Rödel, J.; Mellmann, A.; Stein, C.; Alexi, M.; Kipp, F.; Edel, B.; Dawczynski, K.; Brandt, C.; Seidel, L.; Pfister, W.; et al. Use of MALDI-TOF Mass Spectrometry to Detect Nosocomial Outbreaks of Serratia Marcescens and Citrobacter Freundii. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 581–591. [Google Scholar] [CrossRef] [PubMed]
- De Florio, L.; Riva, E.; Giona, A.; Dedej, E.; Fogolari, M.; Cella, E.; Spoto, S.; Lai, A.; Zehender, G.; Ciccozzi, M.; et al. MALDI-TOF MS Identification and Clustering Applied to Enterobacter Species in Nosocomial Setting. Front. Microbiol. 2018, 9, 1885. [Google Scholar] [CrossRef]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus Mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Bouhrour, N.; Nibbering, P.H.; Bendali, F. Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens. Pathogens 2024, 13, 393. [Google Scholar] [CrossRef]
- Nwabor, L.C.; Chukamnerd, A.; Nwabor, O.F.; Pomwised, R.; Voravuthikunchai, S.P.; Chusri, S. Rifampicin Enhanced Carbapenem Activity with Improved Antibacterial Effects and Eradicates Established Acinetobacter Baumannii Biofilms. Pharmaceuticals 2023, 16, 477. [Google Scholar] [CrossRef] [PubMed]
- Armengol, E.; Kragh, K.N.; Tolker-Nielsen, T.; Sierra, J.M.; Higazy, D.; Ciofu, O.; Viñas, M.; Høiby, N. Colistin Enhances Rifampicin’s Antimicrobial Action in Colistin-Resistant Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 2023, 67, e01641-22. [Google Scholar] [CrossRef]
- Ferreira, L.; Pos, E.; Nogueira, D.R.; Ferreira, F.P.; Sousa, R.; Abreu, M.A. Antibiotics with Antibiofilm Activity—Rifampicin and Beyond. Front. Microbiol. 2024, 15, 1435720. [Google Scholar] [CrossRef]
- Marchetti, V.M.; Bitar, I.; Mercato, A.; Nucleo, E.; Bonomini, A.; Pedroni, P.; Hrabak, J.; Migliavacca, R. Complete Nucleotide Sequence of Plasmids of Two Escherichia Coli Strains Carrying blaNDM–5 and blaNDM–5 and blaOXA–181 From the Same Patient. Front. Microbiol. 2020, 10, 3095. [Google Scholar] [CrossRef]
- Hornsey, M.; Phee, L.; Wareham, D.W. A Novel Variant, NDM-5, of the New Delhi Metallo-β-Lactamase in a Multidrug-Resistant Escherichia Coli ST648 Isolate Recovered from a Patient in the United Kingdom. Antimicrob. Agents Chemother. 2011, 55, 5952–5954. [Google Scholar] [CrossRef]
- Sugawara, Y.; Akeda, Y.; Hagiya, H.; Sakamoto, N.; Takeuchi, D.; Shanmugakani, R.K.; Motooka, D.; Nishi, I.; Zin, K.N.; Aye, M.M.; et al. Spreading Patterns of NDM-Producing Enterobacteriaceae in Clinical and Environmental Settings in Yangon, Myanmar. Antimicrob. Agents Chemother. 2019, 63, e01924-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, K.; Liu, Y.; Xiao, X.; Wang, Z.; Li, R. Characterization of TMexCD3-TOprJ3, an RND-Type Efflux System Conferring Resistance to Tigecycline in Proteus Mirabilis, and Its Associated Integrative Conjugative Element. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Chen, S.L.; Kang, Y.T.; Liang, Y.H.; Qiu, X.T.; Li, Z.J. A Core Genome Multilocus Sequence Typing Scheme for Proteus Mirabilis. Biomed. Environ. Sci. 2023, 36, 343–352. [Google Scholar] [CrossRef]
- CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100 Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.
- Cordeiro-Moura, J.R.; Fehlberg, L.C.C.; Nodari, C.S.; de Matos, A.P.; de Alves, V.O.; Cayô, R.; Gales, A.C. Performance of Distinct Phenotypic Methods for Carbapenemase Detection: The Influence of Culture Media. Diagn. Microbiol. Infect. Dis. 2020, 96, 114912. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.E.; Redondo, L.M.; Figueroa Espinosa, R.A.; Cejas, D.; Gutkind, G.O.; Chacana, P.A.; Di Conza, J.A.; Fernández Miyakawa, M.E. Simultaneous Carriage of Mcr-1 and Other Antimicrobial Resistance Determinants in Escherichia Coli From Poultry. Front. Microbiol. 2018, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, M.L.; Liebrenz, K.I.; Méndez, E.d.l.A.; Di Conza, J.A. Molecular Epidemiology of Cefotaxime-Resistant but Ceftazidime-Susceptible Enterobacterales and Evaluation of the in Vitro Bactericidal Activity of Ceftazidime and Cefepime. Braz. J. Microbiol. 2021, 52, 1853–1863. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, R. Distribution of Repetitive DNA Sequences in Eubacteria and Application to Finerpriting of Bacterial Enomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef]
- Passerini de Rossi, B.; García, C.; Calenda, M.; Vay, C.; Franco, M. Activity of Levofloxacin and Ciprofloxacin on Biofilms and Planktonic Cells of Stenotrophomonas Maltophilia Isolates from Patients with Device-Associated Infections. Int. J. Antimicrob. Agents 2009, 34, 260–264. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Passerini de Rossi, B.; Feldman, L.; Pineda, M.S.; Vay, C.; Franco, M. Comparative in Vitro Efficacies of Ethanol-, EDTA- and Levofloxacin-Based Catheter Lock Solutions on Eradication of Stenotrophomonas Maltophilia Biofilms. J. Med. Microbiol. 2012, 61, 1248–1253. [Google Scholar] [CrossRef]
- Cernohorská, L.; Votava, M. Determination of Minimal Regrowth Concentration (MRC) in Clinical Isolates of Various Biofilm-Forming Bacteria. Folia Microbiol. 2004, 49, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and Standardized Annotation of Bacterial Genomes via Alignment-Free Sequence Identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- Bharat, A.; Petkau, A.; Avery, B.P.; Chen, J.C.; Folster, J.P.; Carson, C.A.; Kearney, A.; Nadon, C.; Mabon, P.; Thiessen, J.; et al. Correlation between Phenotypic and In Silico Detection of Antimicrobial Resistance in Salmonella Enterica in Canada Using Staramr. Microorganisms 2022, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Galata, V.; Fehlmann, T.; Backes, C.; Keller, A. PLSDB: A Resource of Complete Bacterial Plasmids. Nucleic Acids Res. 2019, 47, D195–D202. [Google Scholar] [CrossRef]
- Molano, L.-A.G.; Hirsch, P.; Hannig, M.; Müller, R.; Keller, A. The PLSDB 2025 Update: Enhanced Annotations and Improved Functionality for Comprehensive Plasmid Research. Nucleic Acids Res. 2025, 53, D189–D196. [Google Scholar] [CrossRef]
- Schmartz, G.P.; Hartung, A.; Hirsch, P.; Kern, F.; Fehlmann, T.; Müller, R.; Keller, A. PLSDB: Advancing a Comprehensive Database of Bacterial Plasmids. Nucleic Acids Res. 2022, 50, D273–D278. [Google Scholar] [CrossRef]
- Siebor, E.; Neuwirth, C. Proteus Genomic Island 1 (PGI1), a New Resistance Genomic Island from Two Proteus Mirabilis French Clinical Isolates. J. Antimicrob. Chemother. 2014, 69, 3216–3220. [Google Scholar] [CrossRef]
- Seemann, T. Snippy: Rapid Haploid Variant Calling and Core Genome Alignment. Available online: https://github.com/tseemann/snippy (accessed on 1 March 2025).
- Croucher, N.J.; Page, A.J.; Connor, T.R.; Delaney, A.J.; Keane, J.A.; Bentley, S.D.; Parkhill, J.; Harris, S.R. Rapid Phylogenetic Analysis of Large Samples of Recombinant Bacterial Whole Genome Sequences Using Gubbins. Nucleic Acids Res. 2015, 43, e15. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-Sites: Rapid Efficient Extraction of SNPs from Multi-FASTA Alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Argimón, S.; Abudahab, K.; Goater, R.J.E.; Fedosejev, A.; Bhai, J.; Glasner, C.; Feil, E.J.; Holden, M.T.G.; Yeats, C.A.; Grundmann, H.; et al. Microreact: Visualizing and Sharing Data for Genomic Epidemiology and Phylogeography. Microb. Genom. 2016, 2, e000093. [Google Scholar] [CrossRef] [PubMed]

| Patient | Age | Sex | COVID Status | Sample Origin * | Date # | Sample Source (n) |
|---|---|---|---|---|---|---|
| 1 | 61 | M | positive | ICU | 29 September 2020 | blood culture (1), urine (1) |
| 2 | 42 | M | positive | MCU | 1 October 2020 | catheter (2), tracheal aspirate (2) |
| 3 | 52 | M | unknown | ICU | 8 October 2020 | catheter (1) |
| 4 | 45 | M | positive | ICU | 20 October 2020 | urine (1) |
| 5 | 51 | M | positive | ICU | 23 October 2020 | urine (1), tracheal aspirate (1) |
| 6 | 37 | F | negative | ICU | 27 October 2020 | catheter (1), blood culture (1) |
| 7 | 60 | M | positive | ICU | 30 October 2020 | blood culture (2) |
| 8 | 46 | M | positive | ICU | 7 November 2020 | catheter (1) |
| 9 | 53 | F | positive | ICU | 8 November 2020 | blood culture (2), tracheal aspirate (2) |
| 10 | 57 | M | positive | ICU | 11 November 2020 | blood culture (1), tracheal aspirate (1), urine (1) |
| 11 | 45 | M | negative | MCU | 22 November 2020 | urine (1), blood culture (1), catheter (1) |
| 12 | 66 | F | negative | GER | 25 November 2020 | blood culture (1) |
| 13 | 47 | F | unknown | MCU | 25 November 2020 | blood culture (1) |
| 14 | 50 | F | unknown | ICU | 27 November 2020 | blood culture (1) |
| 15 | 53 | F | negative | GS | 2 December 2020 | tracheal aspirate (1), miscellaneous (1) |
| 16 | 51 | M | negative | MCU | 5 December 2020 | blood culture (1) |
| 17 | 55 | F | unknown | MCU | 6 December 2020 | miscellaneous (1) |
| 18 | 45 | M | positive | MCU | 20 December 2020 | blood culture (1) |
| 19 | 57 | M | unknown | GER | 7 January 2021 | blood culture (1), urine (2) |
| 20 | 65 | F | negative | ICU | 13 January 2021 | tracheal aspirate (1) |
| 21 | 60 | M | negative | MCU | 17 January 2021 | urine (1) |
| 22 | 43 | M | negative | ICU | 21 January 2021 | urine (1) |
| 23 | 48 | F | positive | ICU | 6 February 2021 | blood culture (1), catheter (1) |
| 24 | 90 | M | negative | MCU | 9 February 2021 | miscellaneous (1) |
| 25 | 45 | M | unknown | ICU | 10 February 2021 | urine (1) |
| 26 | 50 | M | unknown | ICU | 13 February 2021 | blood culture (4), retroculture (1), catheter (1) |
| 27 | 60 | F | unknown | ICU | 5 March 2021 | blood culture (2) |
| 28 | 71 | M | unknown | MCU | 10 March 2021 | urine (1) |
| 29 | 45 | M | positive | ICU | 12 March 2021 | catheter (2) |
| 30 | 44 | F | negative | GS | 24 March 2021 | blood culture (3), retroculture (2) |
| 31 | 54 | M | positive | ICU | 27 March 2021 | tracheal aspirate (1), blood culture (2), urine (2) |
| 32 | 57 | M | positive | ICU | 14 April 2021 | tracheal aspirate (1) |
| 33 | 64 | M | negative | ICU | 17 April 2021 | blood culture (2), tracheal aspirate (1) |
| 34 | 34 | F | positive | ICU | 18 April 2021 | tracheal aspirate (1) |
| 35 | 56 | F | unknown | ICU | 24 April 2021 | blood culture (2), retroculture (2) |
| 36 | 54 | M | positive | MCU | 26 April 2021 | blood culture (2) |
| 37 | 52 | M | positive | ICU | 27 April 2021 | tracheal aspirate (1) |
| 38 | 55 | F | positive | ICU | 27 April 2021 | tracheal aspirate (1) |
| 39 | 40 | M | positive | ICU | 29 April 2021 | tracheal aspirate (1) |
| 40 | 51 | M | positive | ICU | 30 April 2021 | blood culture (2), urine (2) |
| Proteus mirabilis Pm21 Assembly Metrics | ||||
|---|---|---|---|---|
| Characteristics | Details | |||
| Genome size (bp) | 4,265,609 | |||
| % GC content | 39.07 | |||
| N50 (bp) | 140,806 | |||
| Resistome | ||||
| Gene | Predicted Phenotype | %Identity | %Coverage | HSP Length/Total Length |
| aac(3)-IId | gentamicin | 99.88 | 100 | 861/861 |
| aac(3)-IV | gentamicin, tobramycin | 100 | 100 | 777/777 |
| aadA1 | streptomycin | 100 | 100 | 789/789 |
| aadA2 | streptomycin | 99.88 | 97.92 | 802/819 |
| aadA5 | streptomycin | 99.87 | 100 | 789/789 |
| aph(3″)-Ib | streptomycin | 100 | 100 | 804/804 |
| aph(3′)-Ia | kanamycin | 100 | 100 | 816/816 |
| aph(4)-Ia | hygromycin | 100 | 100 | 1026/1026 |
| aph(6)-Id | kanamycin | 100 | 100 | 837/837 |
| blaCTX-M-15 | ampicillin, ceftriaxone | 100 | 100 | 876/876 |
| blaNDM-5 | ampicillin, amoxicillin/clavulanic acid, cefoxitin, ceftriaxone, meropenem | 100 | 100 | 813/813 |
| blaOXA-1 | ampicillin | 100 | 100 | 831/831 |
| blaOXA-2 | ampicillin | 100 | 100 | 828/828 |
| blaTEM-1B | ampicillin | 100 | 100 | 861/861 |
| cat | chloramphenicol | 98.17 | 100.15 | 655/654 |
| catA1 | chloramphenicol | 99.85 | 100 | 660/660 |
| catB3 | chloramphenicol | 100 | 69.83 | 442/633 |
| dfrA1 | trimethoprim | 100 | 100 | 474/474 |
| dfrA17-like * | trimethoprim | 100 | 86.92 | 412/474 |
| dfrA32-like * | trimethoprim | 100 | 86.92 | 412/474 |
| ere(A) | erythromycin | 99.84 | 100 | 1221/1221 |
| qacEdelta1 | resistance to antiseptics | 100 | 84.68 | 282/333 |
| rmtB | amikacin, gentamicin, kanamycin, streptomycin | 100 | 100 | 756/756 |
| sul1 | sulfisoxazole | 100 | 100 | 840/840 |
| sul2 | sulfisoxazole | 100 | 100 | 816/816 |
| tet(C) | tetracycline | 99.66 | 100 | 1191/1191 |
| tet(J) | tetracycline | 99.08 | 100 | 1197/1197 |
| Plasmids | IncQ1 | 100 | 65.83 | 524/796 |
| GenBank accession number | Sequence Read Archive submission: PRJNA1236692. | |||
| Genome assembly | https://ri.conicet.gov.ar/handle/11336/252337 (accessed on 25 May 2025) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiglione, B.; Rodriguez, A.P.; Haim, M.S.; Friedman, L.E.; Lincopan, N.; Ochiuzzi, M.E.; Di Conza, J.A. Outbreak of NDM-5-Producing Proteus mirabilis During the COVID-19 Pandemic in an Argentine Hospital. Antibiotics 2025, 14, 557. https://doi.org/10.3390/antibiotics14060557
Ghiglione B, Rodriguez AP, Haim MS, Friedman LE, Lincopan N, Ochiuzzi ME, Di Conza JA. Outbreak of NDM-5-Producing Proteus mirabilis During the COVID-19 Pandemic in an Argentine Hospital. Antibiotics. 2025; 14(6):557. https://doi.org/10.3390/antibiotics14060557
Chicago/Turabian StyleGhiglione, Barbara, Ana Paula Rodriguez, María Sol Haim, Laura Esther Friedman, Nilton Lincopan, María Eugenia Ochiuzzi, and José Alejandro Di Conza. 2025. "Outbreak of NDM-5-Producing Proteus mirabilis During the COVID-19 Pandemic in an Argentine Hospital" Antibiotics 14, no. 6: 557. https://doi.org/10.3390/antibiotics14060557
APA StyleGhiglione, B., Rodriguez, A. P., Haim, M. S., Friedman, L. E., Lincopan, N., Ochiuzzi, M. E., & Di Conza, J. A. (2025). Outbreak of NDM-5-Producing Proteus mirabilis During the COVID-19 Pandemic in an Argentine Hospital. Antibiotics, 14(6), 557. https://doi.org/10.3390/antibiotics14060557








