Development of Chrome-Doped Hydroxyapatite in a PVA Matrix Enriched with Amoxicillin for Biomedical Applications
Abstract
1. Introduction
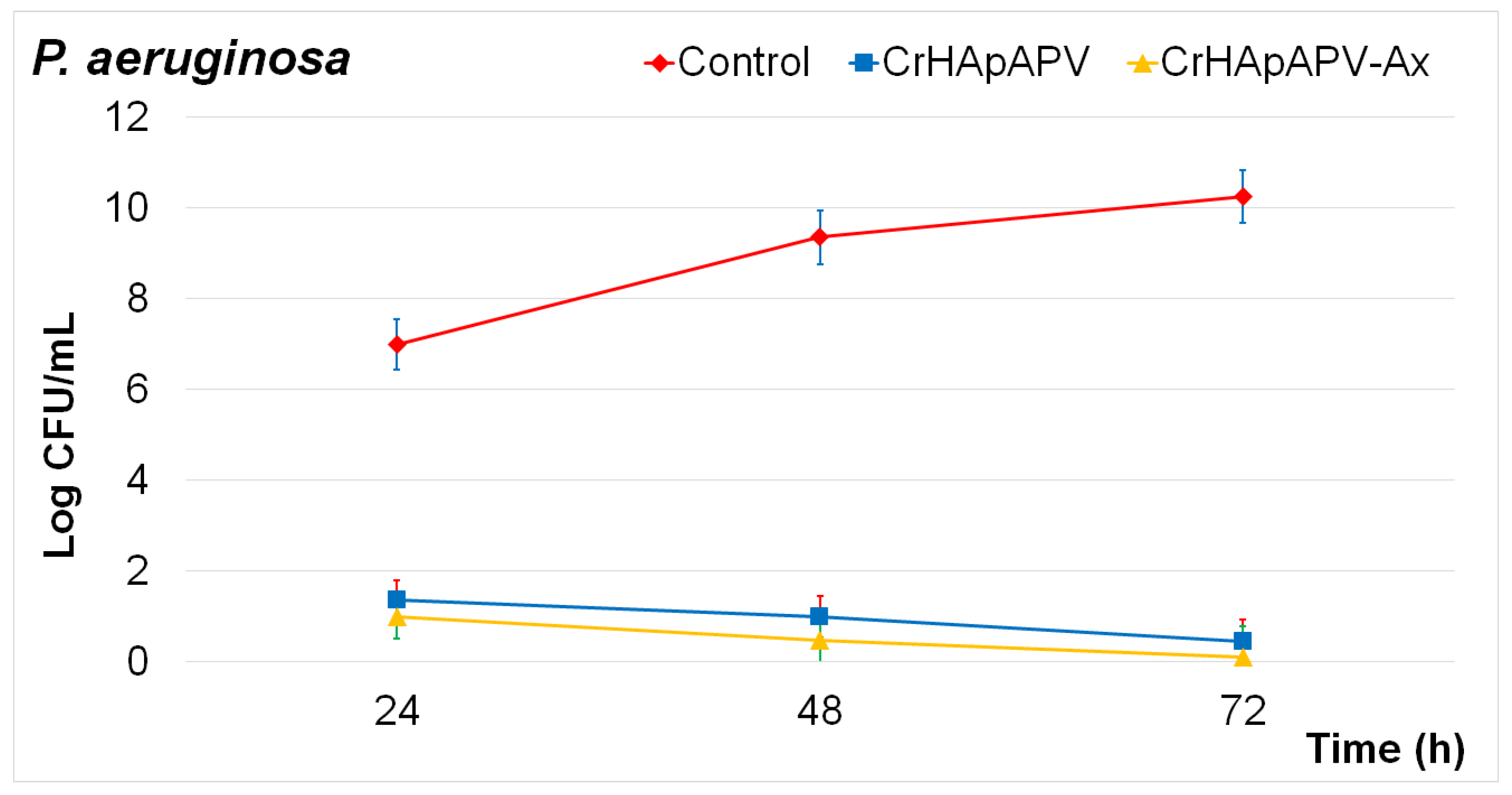
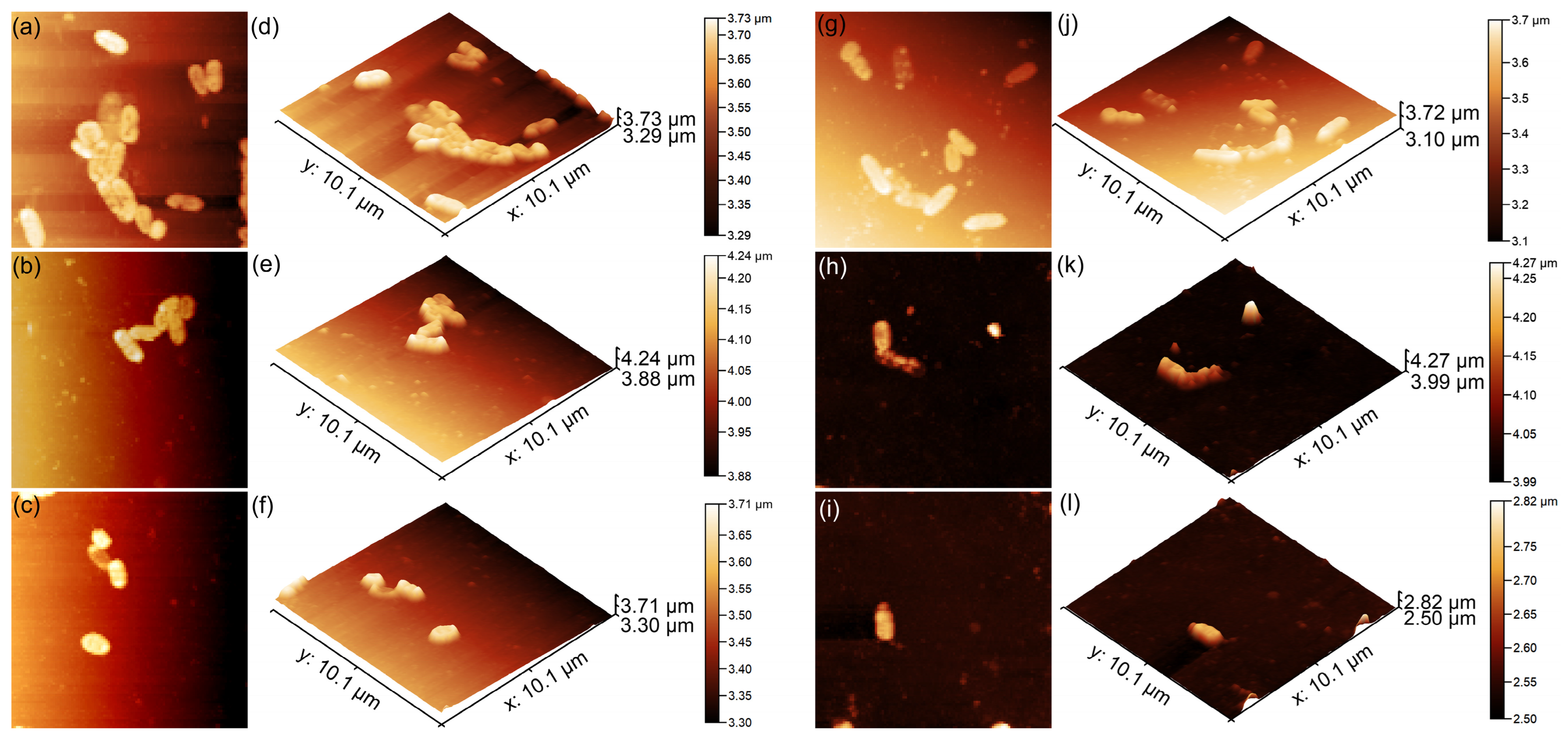
2. Results
3. Materials and Methods
3.1. Materials
3.2. Development CrHApAPV and CrHApAPV-Ax Coatings
3.3. Characterization Methods
3.4. Biological Assays
3.4.1. Biocompatibility Studies
3.4.2. Antibacterial Assay
3.4.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2009, 6, 1465–1485. [Google Scholar] [CrossRef]
- Lima, T.A.R.M.; Brito, N.S.; Peixoto, J.A.; Valerio, M.E.G. The incorporation of chromium (III) into hydroxyapatite crystals. Mater. Lett. 2015, 140, 187–191. [Google Scholar] [CrossRef]
- Elliot, J.C. Structure and Chemistry of the Apatite and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Predoi, S.A.; Chifiriuc, M.C.; Raaen, S.; Badea, M.L.; Rokosz, K. Impact of Gamma Irradiation on the Properties of Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Materials 2022, 15, 5372. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Gautam, S.; Sonowal, L.; Bhinder, S.S.; Ghosh, S.; Pati, F. Enhancing bone implants: Magnesium-doped hydroxyapatite for stronger, bioactive, and biocompatible applications. ACS Appl. Bio Mater. 2024, 7, 2272–2282. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef]
- Wakamura, M.; Kandori, K.; Ishikawa, T. Surface composition of calcium hydroxyapatite modified with metal ions. Colloids Surf. A 1998, 142, 107–116. [Google Scholar] [CrossRef]
- Vega-Zerpa, M.F.; Briceño, S.; Bahamonde-Duarte, J.; Vizuete, K.; Debut, A.; Uribe, R.; Borrero-González, L.J.; González, G. Optical and structural properties of Europium-doped hydroxyapatite. Ceram. Int. 2024; in press. [Google Scholar] [CrossRef]
- Liu, M.; Shu, M.; Yan, J.; Liu, X.; Wang, R.; Hou, Z.; Lin, J. Luminescent net-like inorganic scaffolds with europium-doped hydroxyapatite for enhanced bone reconstruction. Nanoscale 2021, 13, 1181–1194. [Google Scholar] [CrossRef]
- Lytle, C.M.; Lytle, F.W.; Yang, G.N.; Qian, J.H.; Hansen, D.; Zayed, A.; Terry, N. Reduction of Cr(VI) to Cr(III) by wetland plants: Potential for in situ heavy metal detoxification. Environ. Sci. Technol. 1998, 32, 3087–3093. [Google Scholar] [CrossRef]
- Ciupina, V.; Albu, M.; Caraiane, A.; Porosnicu, C.; Staicub, C.; Dinca, P.; Nicolescu, V.; Manu, R. Synthesis and characterization of some Cr-Zr and Cr-Zr-O nanostructures. J. Ovonic Res. 2023, 19, 439. [Google Scholar] [CrossRef]
- Levina, A.; Mulyani, I.; Lay, P.A. Redox Chemistry and Biological Activities of Chromium(III) Complexes; University of Sydney: Sydney, Australia, 2006. [Google Scholar]
- Stipniece, L.; Salma-Ancane, K.; Rjabovs, V.; Juhnevica, I.; Turks, M.; Narkevica, I.; Berzina-Cimdina, L. Development of functionalized hydroxyapatite/poly (vinyl alcohol) composites. J. Cryst. Growth 2016, 444, 14–20. [Google Scholar] [CrossRef]
- A Singh, A. Hydroxyapatite, a biomaterial: Its chemical synthesis, characterization and study of biocompatibility prepared from shell of garden snail helix aspersa. Bull. Mater. Sci. 2012, 35, 1031–1038. [Google Scholar] [CrossRef]
- Hench, L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Minaychev, V.V.; Teterina, A.Y.; Smirnova, P.V.; Menshikh, K.A.; Senotov, A.S.; Kobyakova, M.I.; Smirnov, I.V.; Pyatina, K.V.; Krasnov, K.S.; Fadeev, R.S.; et al. Composite Remineralization of Bone-Collagen Matrices by Low-Temperature Ceramics and Serum Albumin: A New Approach to the Creation of Highly Effective Osteoplastic Materials. J. Funct. Biomater. 2024, 15, 27. [Google Scholar] [CrossRef]
- Sözügeçer, S.; Bayramgil, N.P. Preparation and characterization of polyacrylic acid-hydroxyapatite nanocomposite by microwave-assisted synthesis method. Heliyon 2021, 7, e07226. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, A. Development and Characterization of Polylactic Acid/Chitosan Based Polymeric Bio-Nanocomposites Reinforced with Hydroxyapatite and Aluminum Oxide Bifiller for Biomedical Application. J. Inorg. Organomet. Polym. 2025, 1–27. [Google Scholar] [CrossRef]
- Feldman, D. Poly(Vinyl Alcohol) Recent Contributions to Engineering and Medicine. J. Compos. Sci. 2020, 4, 175. [Google Scholar] [CrossRef]
- Tsetsekou, A.; Brasinika, D.; Vaou, V.; Chatzitheodoridis, E. On the synthesis of tailored biomimetic hydroxyapatite nanoplatelets through a bioinspired approach in the presence of collagen or chitosan and L-arginine. Mater. Sci. Eng. C 2014, 43, 555–565. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Dinga, B.; Kimura, E.; Satoa, T.; Fujita, S.; Shiratori, S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2005, 45, 1895–1902. [Google Scholar] [CrossRef]
- Behmagham, F.; Dhiaa, S.M.; Hussein, A.H.A.; Radi, U.K.; Mushtaq, H.; Idan, A.H.; Vessally, E. Hydroxyapatite–polymer nanocomposites for drug delivery applications: A mini review. Eur. J. Med. Chem. Rep. 2024, 12, 100231. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kang, S.I.; Kwon, O.W.; Lee, S.G.; Lee, Y.R.; Min, B.G.; Han, S.S.; Noh, S.K.; Lyoo, W.S. Preparation of hydroxyapatite/poly(vinyl alcohol) composite film with uniformly dispersed hydroxyapatite particles using citric acid. J. Appl. Polym. Sci. 2007, 104, 3240–3244. [Google Scholar] [CrossRef]
- Jalageri, M.B.; Mohan Kumar, G.C. Hydroxyapatite Reinforced Polyvinyl Alcohol/Polyvinyl Pyrrolidone Based Hydrogel for Cartilage Replacement. Gels 2022, 8, 555. [Google Scholar] [CrossRef]
- Djošić, M.; Janković, A.; Stevanović, M.; Stojanović, J.; Vukašinović-Sekulić, M.; Kojić, V.; Mišković-Stanković, V. Hydroxyapatite/poly(vinyl alcohol)/chitosan coating with gentamicin for orthopedic implants. Mater. Chem. Phys. 2023, 303, 127766. [Google Scholar] [CrossRef]
- Huang, C.-C. Design and Characterization of a Bioinspired Polyvinyl Alcohol Matrix with Structural Foam-Wall Microarchitectures for Potential Tissue Engineering Applications. Polymers 2022, 14, 1585. [Google Scholar] [CrossRef]
- Hussain, R.; Tabassum, S.; Gilani, M.A.; Ahmed, E.; Sharif, A.; Manzoor, F.; Shah, A.T.; Asif, A.; Sharif, F.; Iqbal, F.; et al. In situ synthesis of mesoporous polyvinyl alcohol/hydroxyapatite composites for better biomedical coating adhesion. Appl. Surf. Sci. 2016, 364, 117–123. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Predoi, D.; Iconaru, S.L.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Ghegoiu, L.; Bleotu, C.; Predoi, M.V. Chrome Doped Hydroxyapatite Enriched with Amoxicillin Layers for Biomedical Applications. Coatings 2025, 15, 233. [Google Scholar] [CrossRef]
- Rohn, C.L. Analytical Polymer Rheology; Hanser Publishers: Cincinnati, OH, USA, 1995; p. 203. [Google Scholar]
- Available online: https://www.malvernpanalytical.com (accessed on 27 April 2025).
- Gafurov, M.; Biktagirov, T.; Mamin, G.; Orlinskii, S. A DFT, X-and W-band EPR and ENDOR study of nitrogen-centered species in (nano) hydroxyapatite. Appl. Magn. Reson. 2014, 45, 1189–1203. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Mamin, G.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Combination of EPR measurements and DFT calculations to study nitrate impurities in the carbonated nanohydroxyapatite. J. Phys. Chem. A 2014, 118, 1519–1526. [Google Scholar] [CrossRef]
- Brouers, F.; Ramsamugh, A. Percolation and anomalous conduction on fractals in fluid-saturated porous media. J. Phys. C Solid State Phys. 1988, 21, 1839. [Google Scholar] [CrossRef]
- Wei, J.; Xue, Q. Effects of synthetic additives on the friction and wear properties of a Cr2O3 coating. Wear 1994, 176, 213–216. [Google Scholar] [CrossRef]
- Luan, Z.; Zhang, M.; Chen, K. The investigation for the deactivation mechanism of Cu-Cr impregnated carbon by XPS. Carbon 1993, 31, 1179–1184. [Google Scholar] [CrossRef]
- Ikemoto, I.; Ishii, K.; Kinoshita, S.; Kuroda, H.; Franco, M.A.; Thomas, J.M. X-ray photoelectron spectroscopic studies of CrO₂ and some related chromium compounds. J. Solid State Chem. 1976, 17, 425–430. [Google Scholar] [CrossRef]
- Halada, G.P.; Clayton, C.R. Photoreduction of hexavalent chromium during X-ray photoelectron spectroscopy analysis of electrochemical and thermal films. J. Electrochem. Soc. 2006, 138, 2921. [Google Scholar] [CrossRef]
- Sleigh, C.; Pijpers, A.P.; Jaspers, A.; Coussens, B.; Meier, R.J. On the determination of atomic charge via ESCA including application to organometallics. J. Electron Spectrosc. Relat. Phenom. 1996, 77, 41–57. [Google Scholar] [CrossRef]
- Murugesan, M.; Song, J.; Zhu, W.; Zhou, H.; Zhang, J.; Meena, P.; Yuan, A. Polymer-Assisted Synthesis and Applications of Hydroxyapatite (HAp) Anchored Nitrogen-Doped 3D Graphene Foam-Based Nanostructured Ceramic Framework. RSC Adv. 2020, 10, 17918–17928. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A Highly Efficient Polysulfide Mediator for Lithium–Sulfur Batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef]
- Saman, N.; Johari, K.; Kong, H.; Mohtar, S.S.; Hassan, O.; Ali, N.; Mat, H. Enhanced Elemental Mercury Removal by Facile Sulfurization of Agrowaste Chars. Chem. Eng. Res. Des. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, T.S.; Venkataprasanna, K.S.; Niranjan, R.; Kaushik, M.; Samal, D.B.; Venkatasubbu, G.D. PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl. Surf. Sci. 2019, 495, 143543. [Google Scholar] [CrossRef]
- Asy-Syifa, N.; Waresindo, W.X.; Edikresnha, D.; Suciati, T.; Khairurrijal, K. The Study of the Swelling Degree of the PVA Hydrogel with varying concentrations of PVA. J. Phys. Conf. Ser. 2022, 2243, 012053. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Kolaja Dobra, J.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Supova, M.; Zaloudkova, M.; Babuska, V. In Situ Hydroxyapatite Synthesis Enhances Biocompatibility of PVA/HA Hydrogels. Int. J. Mol. Sci. 2021, 22, 9335. [Google Scholar] [CrossRef]
- Cimpeanu, C.; Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Rokosz, K.; Predoi, M.V.; Raaen, S.; Badea, M.L. Development of Novel Biocomposites with Antimicrobial-Activity-Based Magnesium-Doped Hydroxyapatite with Amoxicillin. Antibiotics 2024, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Okur, H.E. Rietveld Refinement-Based Structural Analysis of Biogenic Hydroxyapatite and Its PVA Composite for Dye Removal. Mater. Today Commun. 2025, 43, 111723. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nate, M.M.; Kurup, M.B.; Bambole, V.A.; Sabharwal, S. Effect of γ-radiation on the structure and morphology of polyvinyl alcohol films. Nucl. Instrum. Methods Phys. Res. B 2005, 237, 585–592. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar]
- Peppas, N.A.; Mikos, A.G. Preparation methods and structure of hydrogels. In Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1986; Volume 1, pp. 1–27. [Google Scholar]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1451–1457. [Google Scholar] [CrossRef]
- ISO 10993–1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- Bandgar, S.S.; Yadav, H.M.; Shirguppikar, S.S.; Shinde, M.A.; Shejawal, R.V.; Kolekar, T.V.; Bamane, S.R. Enhanced Hemolytic Biocompatibility of Hydroxyapatite by Chromium (Cr3+) Doping in Hydroxyapatite Nanoparticles Synthesized by Solution Combustion Method. J. Korean Ceram. Soc. 2017, 54, 158–166. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Ţălu, Ş.; Predoi, S.A.; Buton, N.; Ramos, G.Q.; da Fonseca Filho, H.D.; Matos, R.S. Synthesis, Characterization, and Antifungal Properties of Chrome-Doped Hydroxyapatite Thin Films. Mater. Chem. Phys. 2024, 324, 129690. [Google Scholar] [CrossRef]
- Fu, J.; Liang, X.; Chen, Y.; Tang, L.; Zhang, Q.H.; Dong, Q. Oxidative Stress as a Component of Chromium-Induced Cytotoxicity in Rat Calvarial Osteoblasts. Cell Biol. Toxicol. 2008, 24, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhang, L.; Wang, G.; Zhao, Z.; Peng, S.; Shuai, C. 3D Printed Zn-doped Mesoporous Silica-incorporated Poly-L-lactic Acid Scaffolds for Bone Repair. Int. J. Bioprint. 2021, 7, 346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, J.; Xu, Y.; Qian, S.; Wang, B.; Liu, F.; Liu, X. Biological Behavior of Osteoblast-like Cells on Titania and Zirconia Films Deposited by Cathodic Arc Deposition. Biointerphases 2012, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, B.; Mai, S.; Wu, X.; Zhang, H.; Qiao, W.; Luo, X.; Chen, Z. Effects of Fluoridation of Porcine Hydroxyapatite on Osteoblastic Activity of Human MG63 Cells. Sci. Technol. Adv. Mater. 2015, 16, 035006. [Google Scholar] [CrossRef]
- Keremidarska, M.; Radeva, E.; Eleršič, K.; Iglič, A.; Pramatarova, L.; Krasteva, N. Plasma Deposited Composite Coatings to Control Biological Response of Osteoblast-like MG-63 Cells. J. Phys. Conf. Ser. 2014, 558, 012057. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 Human Osteosarcoma Cell Lines Representative Models of the Osteoblastic Phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- American Type Culture Collection (ATCC). MG-63 [ATCC® CRL-1427™]. Available online: https://www.atcc.org/products/crl-1427 (accessed on 5 December 2024).
- Chatree, K.; Sriboonaied, P.; Phetkong, C.; Wattananit, W.; Chanchao, C.; Charoenpanich, A. Distinctions in Bone Matrix Nanostructure, Composition, and Formation between Osteoblast-like Cells, MG-63, and Human Mesenchymal Stem Cells, UE7T-13. Heliyon 2023, 9, e15556. [Google Scholar] [CrossRef]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of Osteosarcoma Cell Lines MG-63, Saos-2 and U-2 OS in Comparison to Human Osteoblasts. Anticancer. Res. 2004, 24, 3743–3748. [Google Scholar] [PubMed]
- Yang, J.-Y.; Ting, Y.-C.; Lai, J.-Y.; Liu, H.-L.; Fang, H.-W.; Tsai, W.-B. Quantitative Analysis of Osteoblast-like Cells (MG63) Morphology on Nanogrooved Substrata with Various Groove and Ridge Dimensions. J. Biomed. Mater. Res. A 2009, 90, 629–640. [Google Scholar] [CrossRef]
- Docheva, D.; Padula, D.; Popov, C.; Mutschler, W.; Clausen-Schaumann, H.; Schieker, M. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J. Cell. Mol. Med. 2008, 12, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.B.; Ting, Y.C.; Yang, J.Y.; Lai, J.Y.; Liu, H.L. Fibronectin modulates the morphology of osteoblast-like cells (MG-63) on nano-grooved substrates. J. Mater. Sci. Mater. Med. 2009, 20, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Price, N.; Bendall, S.P.; Frondoza, C.; Jinnah, R.H.; Hungerford, D.S. Human osteoblast-like cells (MG63) proliferate on a bioactive glass surface. J. Biomed. Mater. Res. 1997, 37, 394–400. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Speert, D.P. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and impact on treatment. Drug Resist. Updates 2000, 3, 247–255. [Google Scholar] [CrossRef]
- Poole, K. Resistance to β-lactam antibiotics in Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2004, 10, 16–22. [Google Scholar]
- Livermore, D.M. Multiple Mechanisms of Antimicrobial Resistance in Pseudomonas aeruginosa: Our Worst Nightmare? Clin. Infect. Dis. 2002, 34, 634–640. [Google Scholar] [CrossRef]
- Demurtas, M.; Perry, C.C. Facile one-pot synthesis of amoxicillin-coated gold nanoparticles and their antimicrobial activity. Gold Bull. 2014, 47, 103–107. [Google Scholar] [CrossRef]
- Todd, P.A.; Benfield, P. Amoxicillin/clavulanic acid. An update of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1990, 39, 264–307. [Google Scholar] [CrossRef]
- Bankole, O.M.; Ojubola, K.I.; Adanlawo, O.S.; Adesina, A.O.; Lawal, I.O.; Ogunlaja, A.S.; Achadu, O.J. Amoxicillin Encapsulation on Alginate/Magnetite Composite and Its Antimicrobial Properties Against Gram-Negative and Positive Microbes. BioNanoScience 2022, 12, 1136–1149. [Google Scholar] [CrossRef]
- Weber, D.J.; Tolkoff-Rubin, N.E.; Rubin, R.H. Amoxicillin and Potassium Clavulanate: An Antibiotic Combination Mechanism of Action, Pharmacokinetics, Antimicrobial Spectrum, Clinical Efficacy and Adverse Effects. Pharmacotherapy 1984, 4, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Güncüm, E.; Işıklan, N.; Anlaş, C.; Ünal, N.; Bulut, E.; Bakırel, T. Development and characterization of polymeric-based nanoparticles for sustained release of amoxicillin—An antimicrobial drug. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Elabbasy, M.T.; Algahtani, F.D.; Alshammari, H.F.; Kolsi, L.; Dkhil, M.A.; Abd El-Rahman, G.I.; El-Morsy, M.A.; Menazea, A.A. Improvement of mechanical and antibacterial features of hydroxyapatite/chromium oxide/graphene oxide nanocomposite for biomedical utilizations. Surf. Coat. Technol. 2022, 440, 128476. [Google Scholar] [CrossRef]
- Li, Y.; Ho, J.; Ooi, C.P. Antibacterial efficacy and cytotoxicity studies of copper (II) and titanium (IV) substituted hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2010, 30, 1137–1144. [Google Scholar] [CrossRef]
- Nickens, K.P.; Patierno, S.R.; Ceryak, S. Chromium Genotoxicity: A Double-Edged Sword. Chem. Biol. Interact. 2010, 188, 276–288. [Google Scholar] [CrossRef]
- Cohen, M.D.; Sisco, M.; Prophete, C.; Yoshida, K.; Chen, L.C.; Zelikoff, J.T.; Ghio, A.J. Effects of metal compounds with distinct physicochemical properties on iron homeostasis and antibacterial activity in the lungs: Chromium and vanadium. Inhal. Toxicol. 2010, 22, 169–178. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; Ruiz de Larramendi, I.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Kim, E.S.; Ahn, E.H.; Dvir, T.; Kim, D.H. Emerging nanotechnology approaches in tissue engineering and regenerative medicine. Int. J. Nanomed. 2014, 9 (Suppl. 1), 1–5. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Ghegoiu, L.; Buton, N.; Motelica-Heino, M. Copper doped hydroxyapatite nanocomposite thin films: Synthesis, physico-chemical and biological evaluation. Biometals 2024, 37, 1487–1500. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Predoi, M.V.; Chapon, P.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Motelica-Heino, M.; Predoi, D. Investigation of Spin Coating Cerium-Doped Hydroxyapatite Thin Films with Antifungal Properties. Coatings 2021, 11, 464. [Google Scholar] [CrossRef]
- CasaXPS: Processing Software for XPS, AES, SIMS and More. Available online: www.casaxps.com (accessed on 18 November 2024).
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20, Version 3.4. 2003. Available online: https://srdata.nist.gov/xps (accessed on 10 January 2024).
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 30 November 2024).
- ImageJ. Available online: http://imagej.nih.gov/ij (accessed on 10 January 2025).
- Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Motelica-Heino, M.; Guegan, R.; Bleotu, C. Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications. Coatings 2022, 12, 341. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Predoi, D.; Trușcă, R.-D.; Prodan, A.M.; Groza, A.; Chifiriuc, M.C.; Beuran, M. Fabrication of Novel Chitosan–Hydroxyapatite Nanostructured Coatings for Biomedical Applications. Coatings 2021, 11, 1561. [Google Scholar] [CrossRef]
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/54431.html (accessed on 10 January 2025).

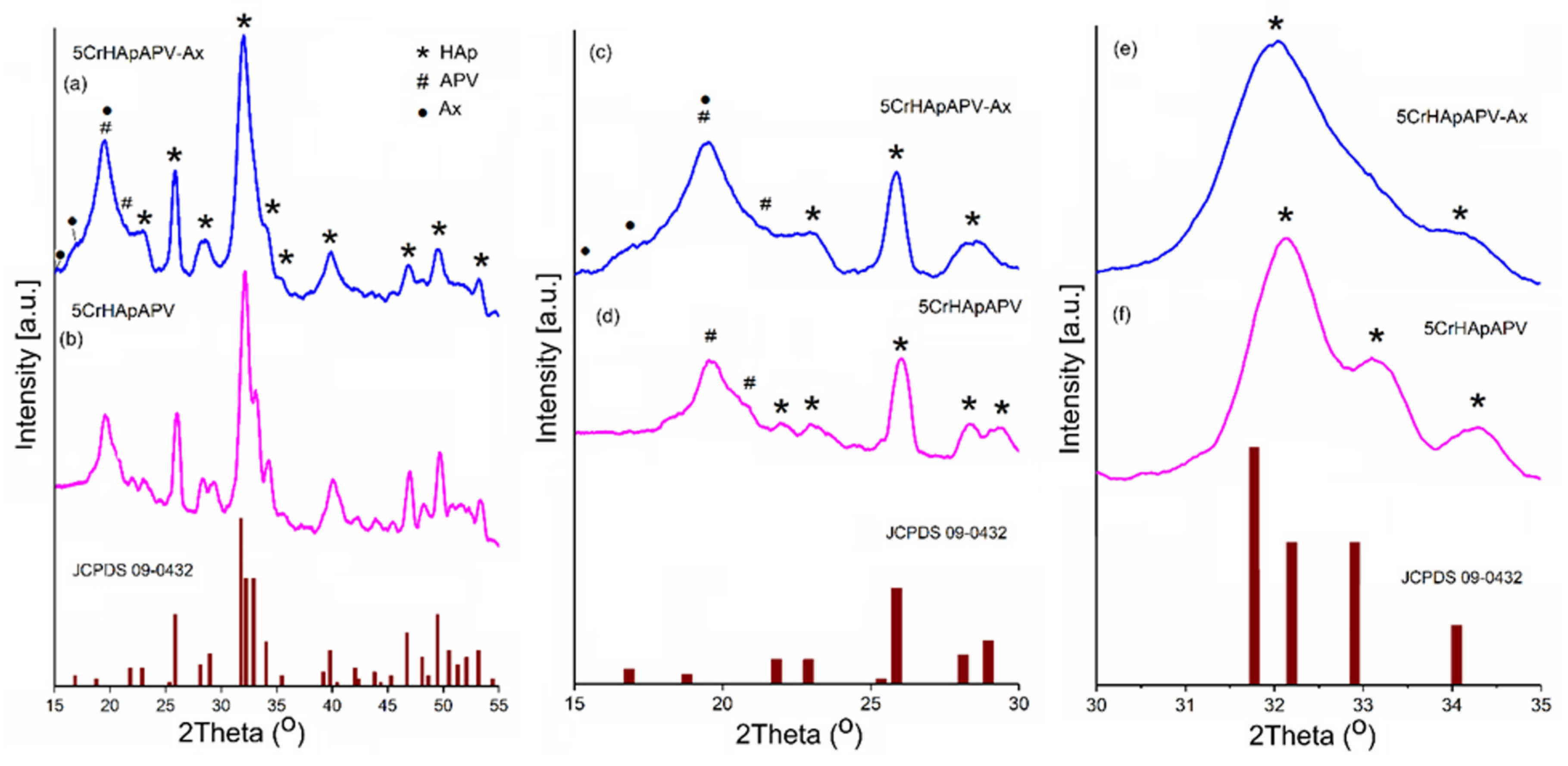
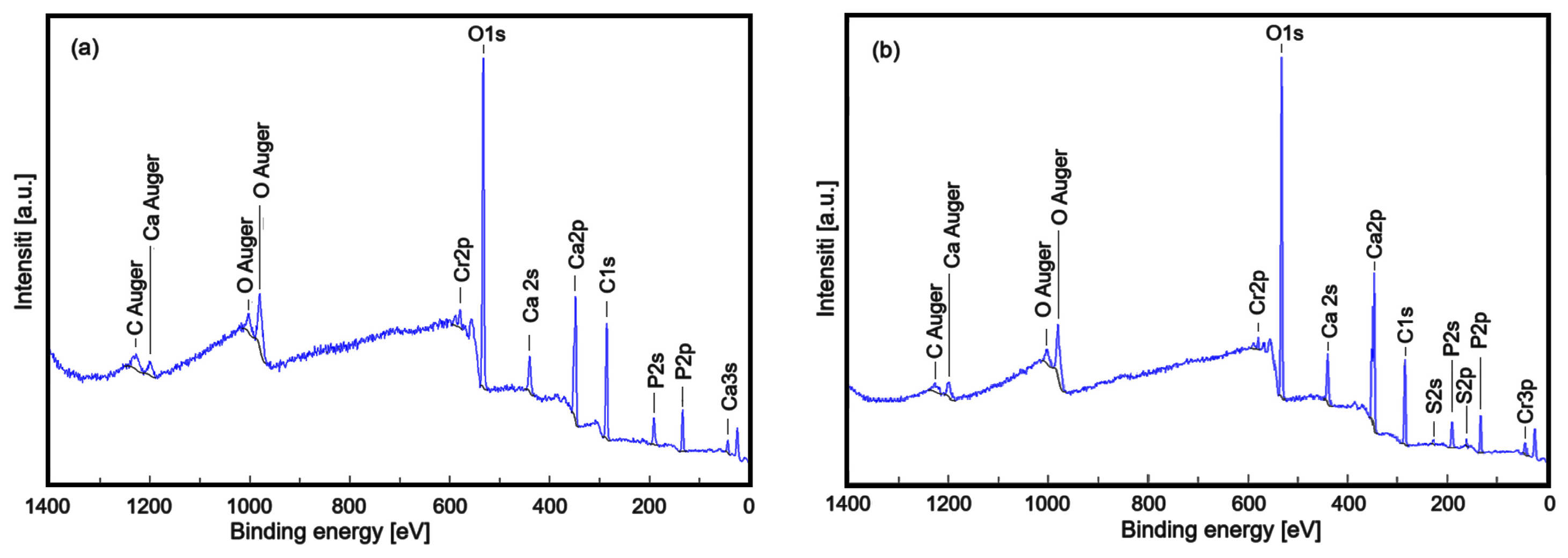

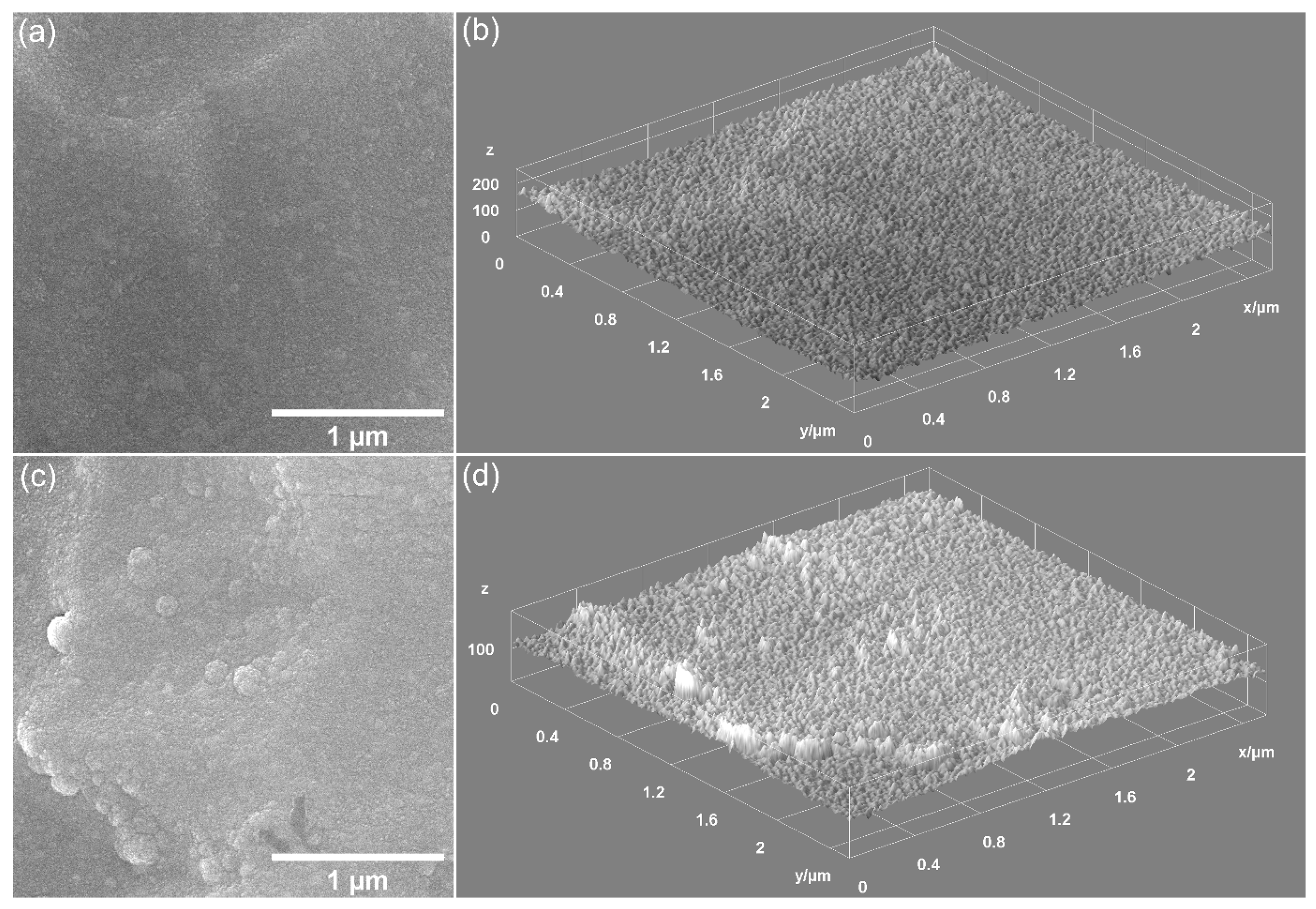
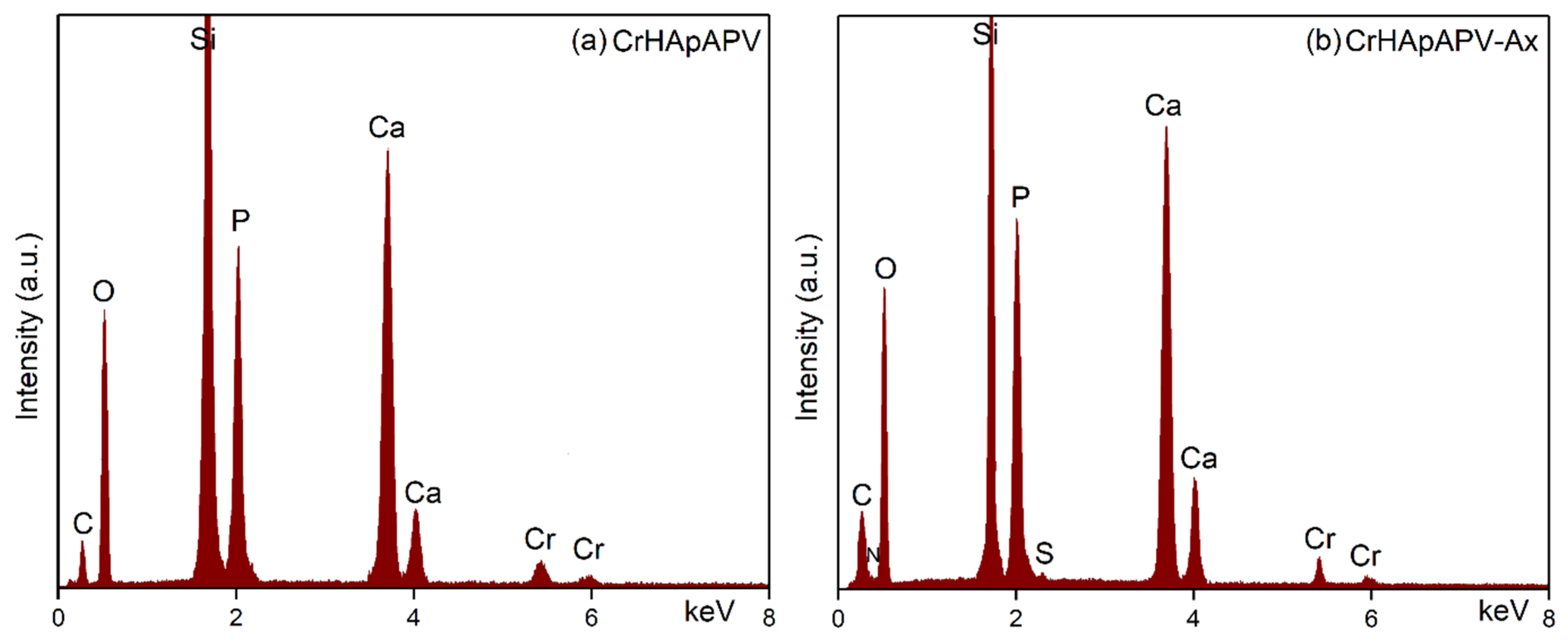

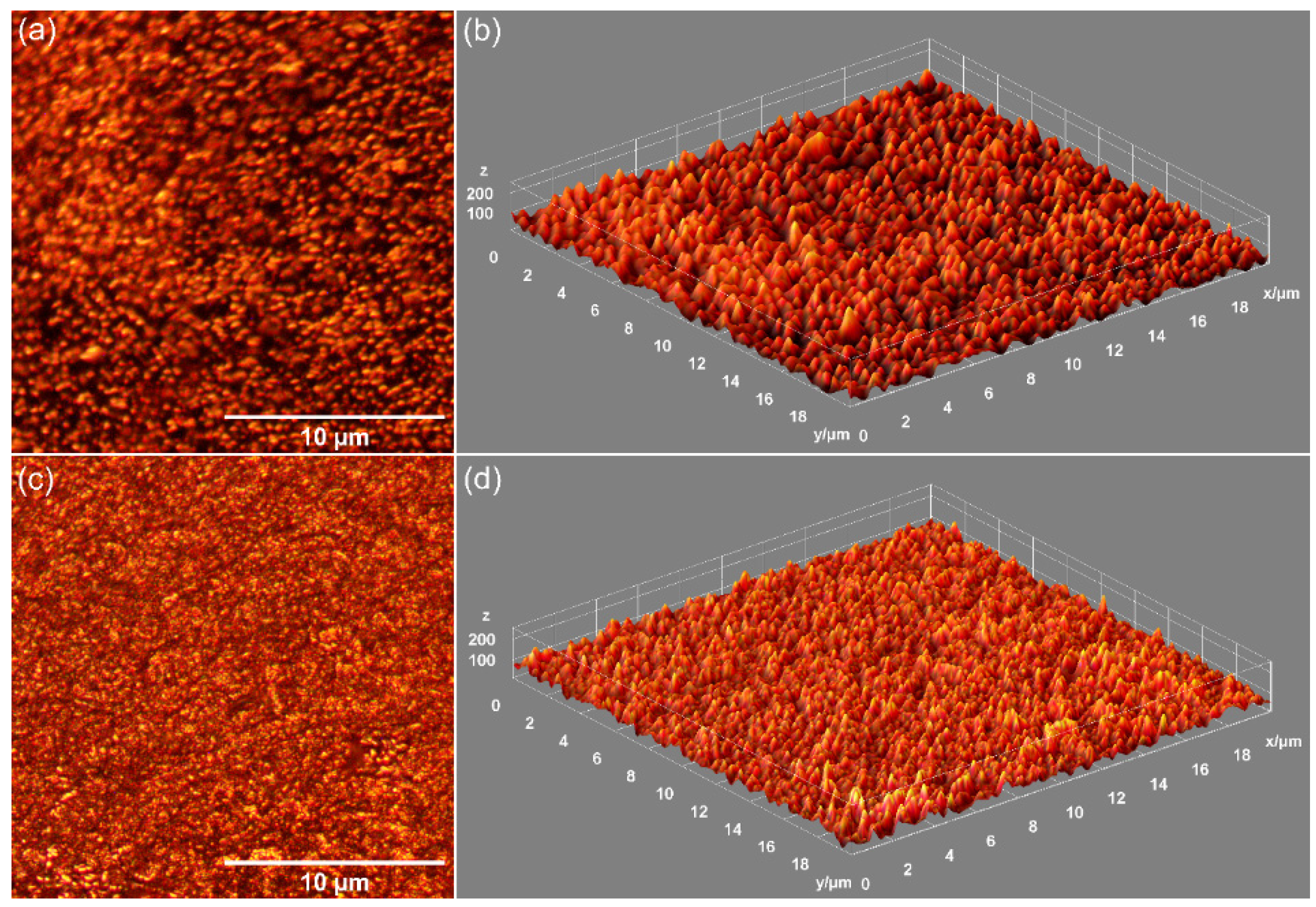
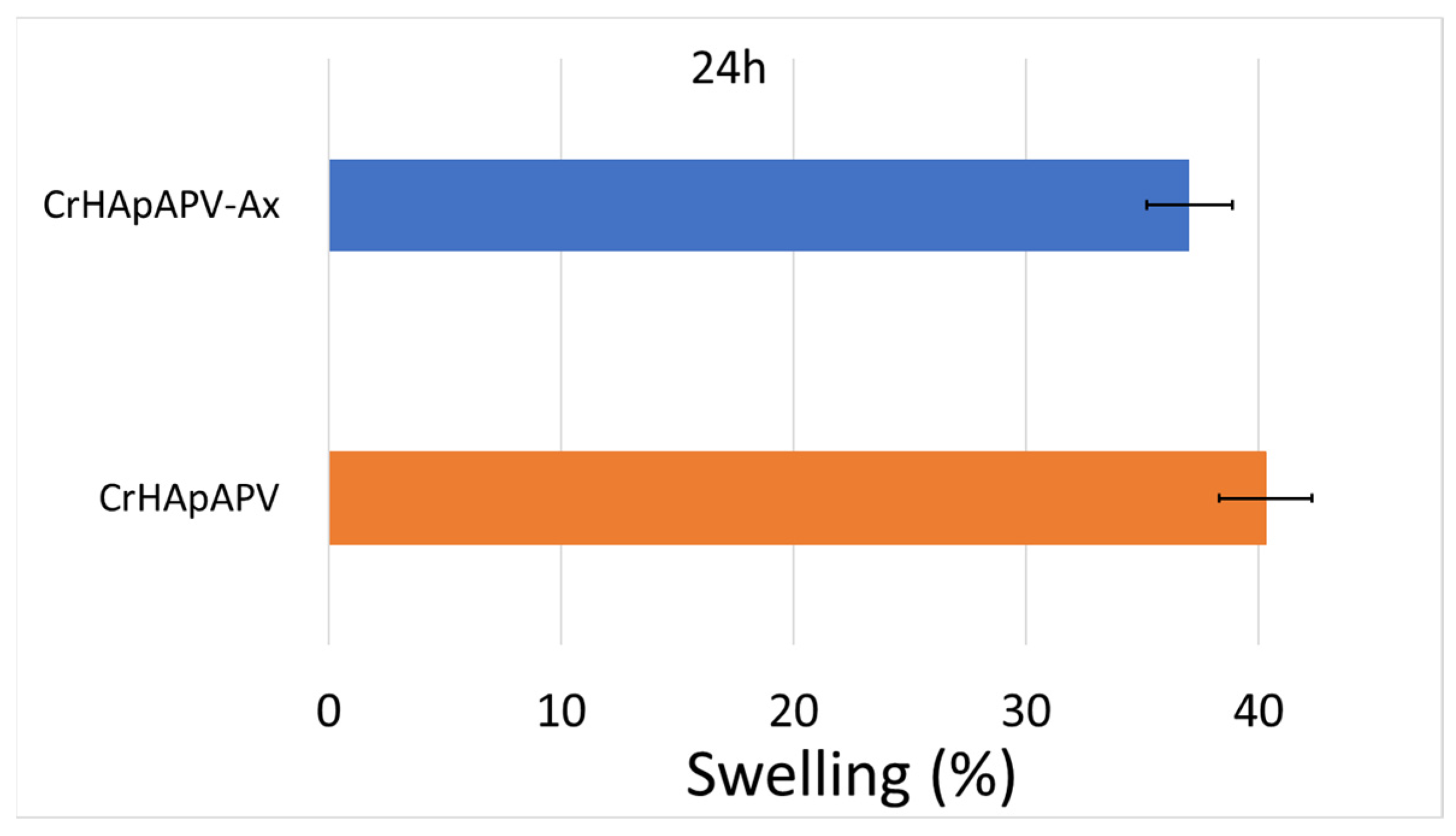
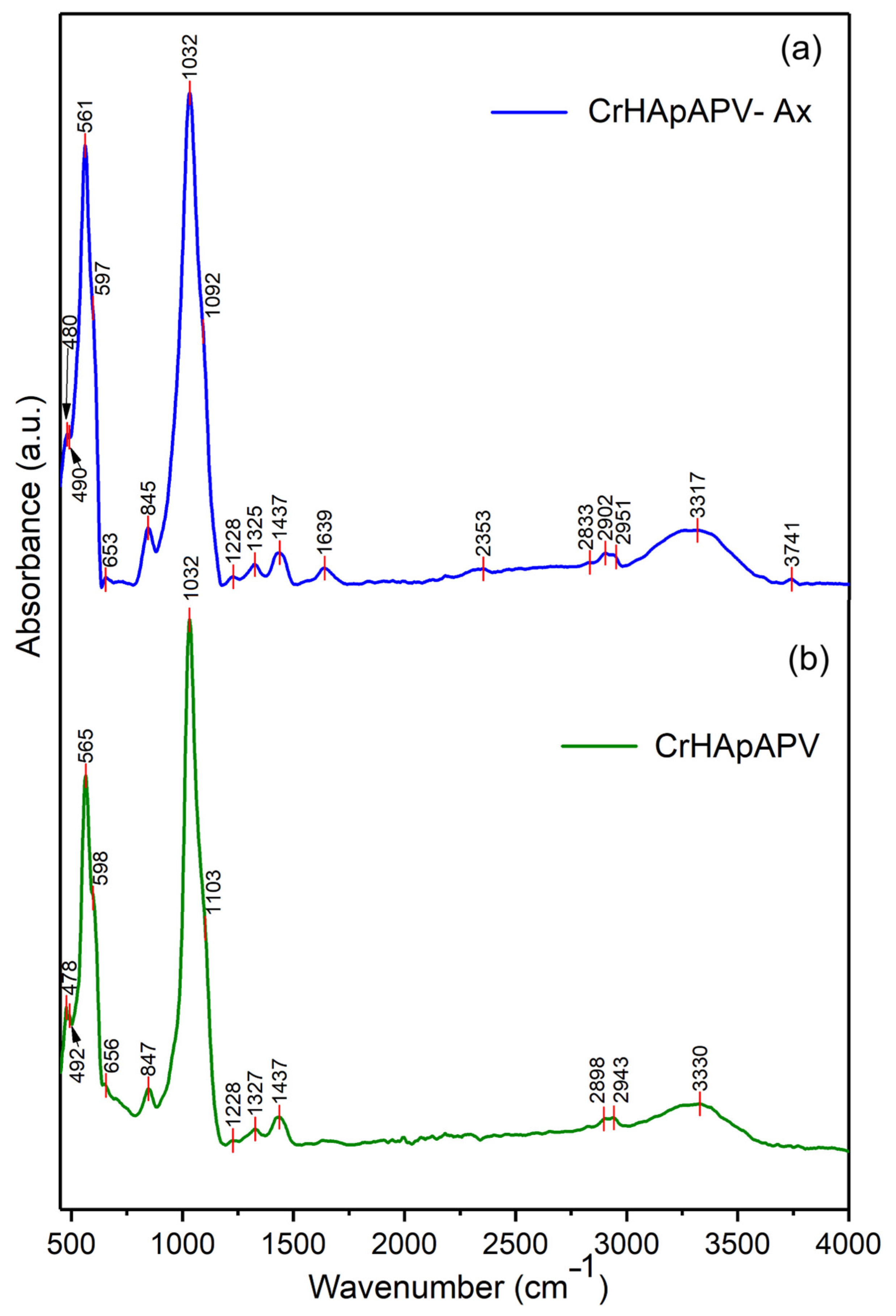
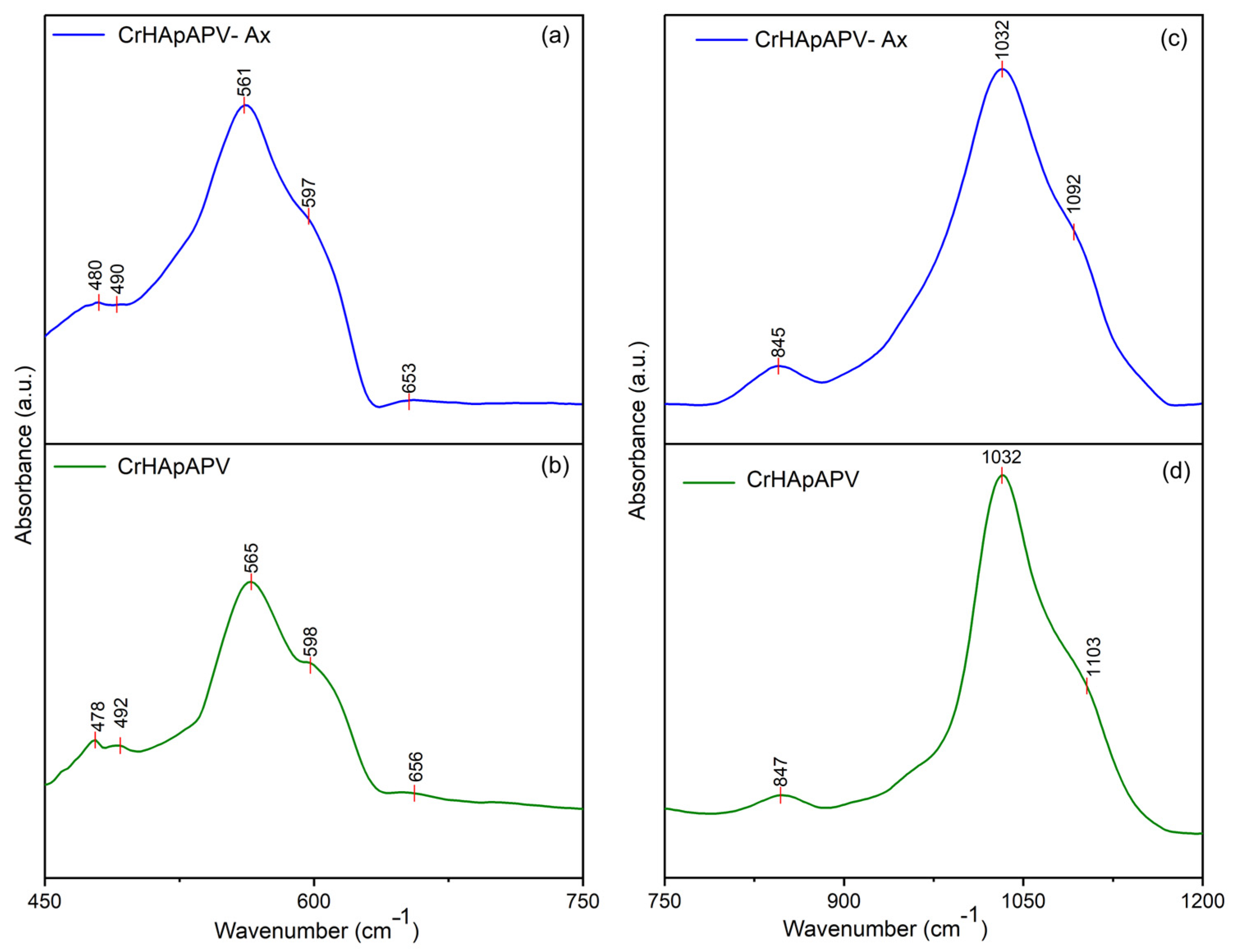


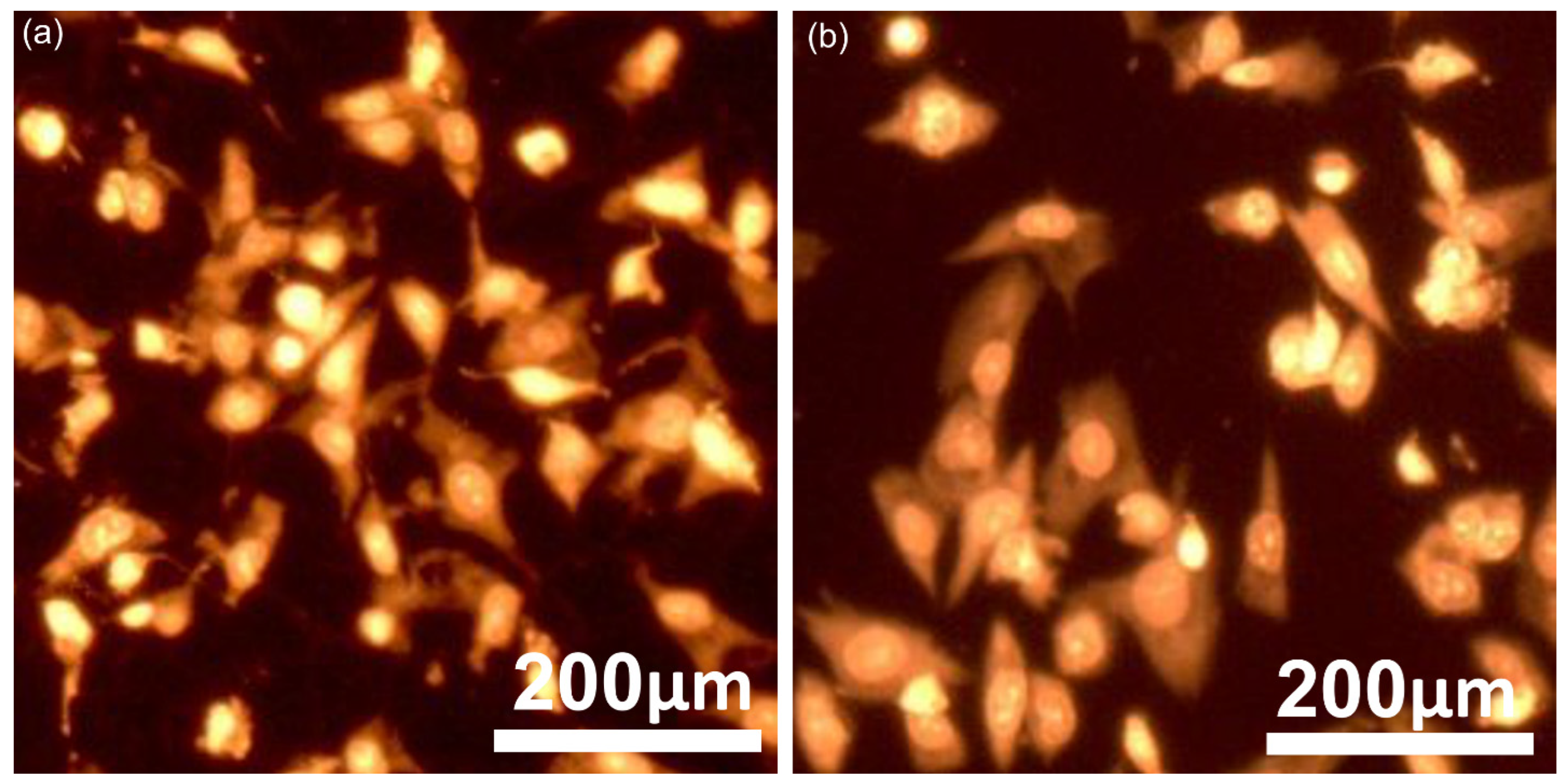
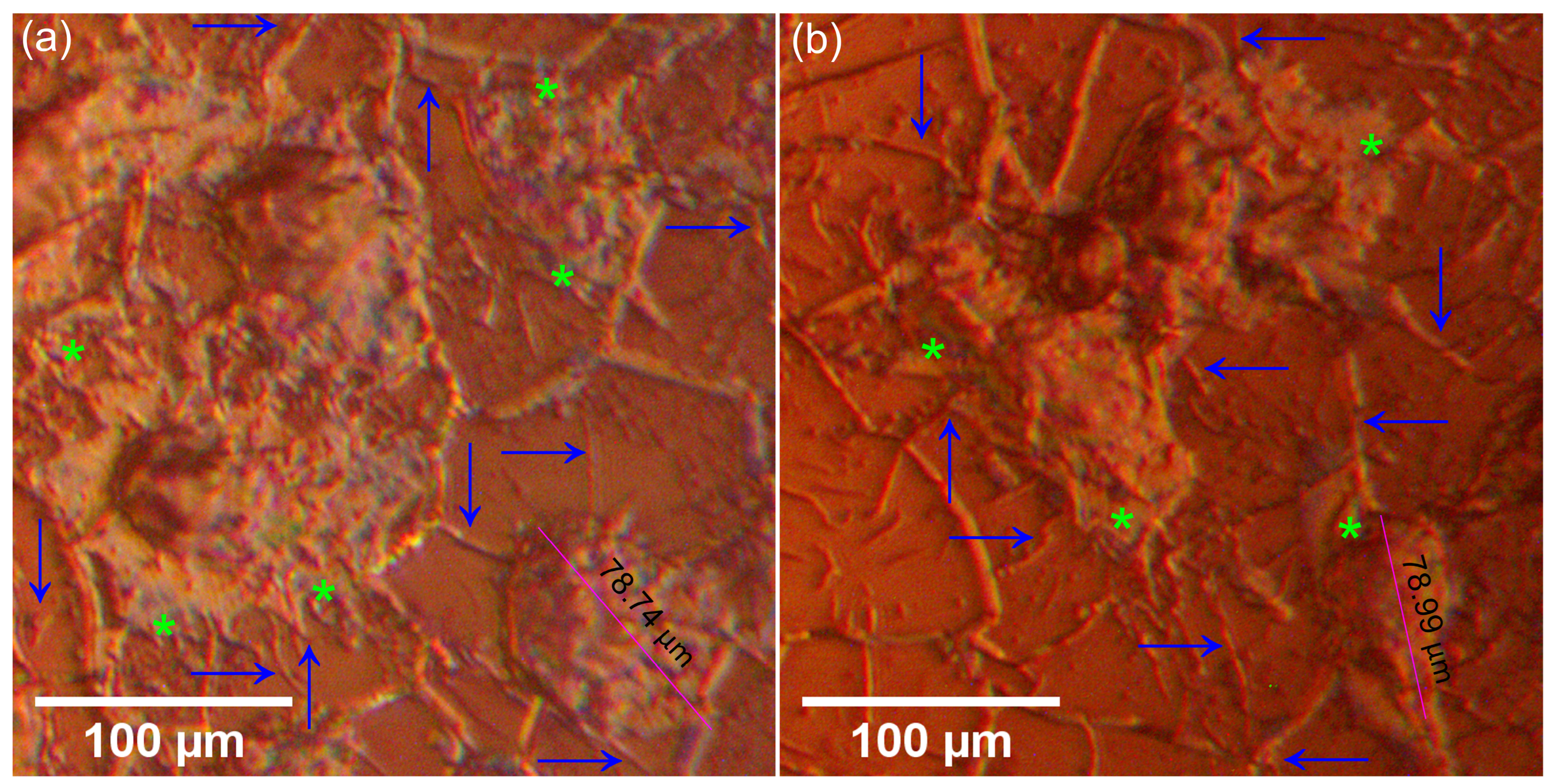
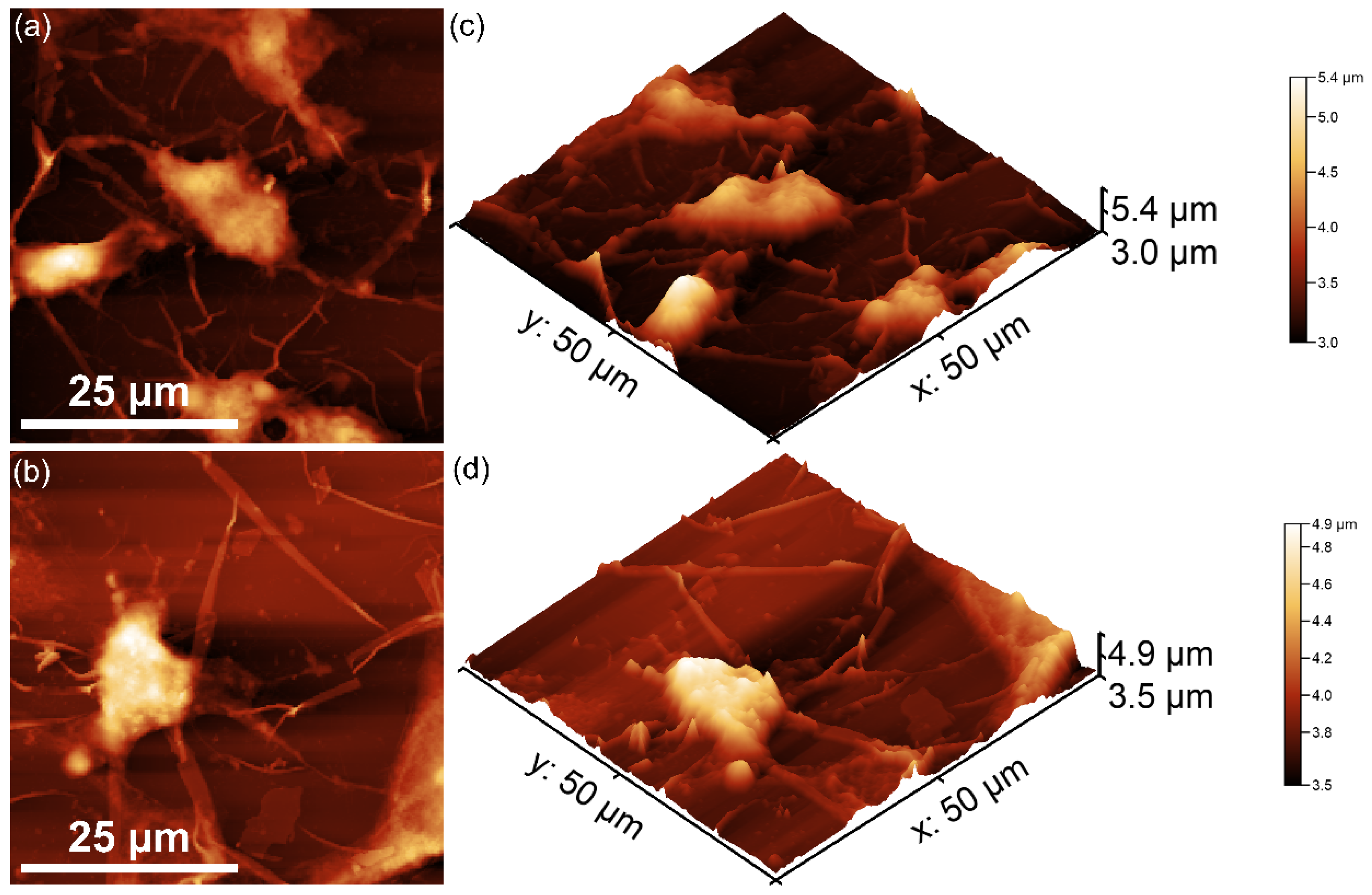
| Sample | Lattice Parameter (Å) | c/a Ratio | Volume of Unit Cell (Å)3 | Crystallite Size (nm) | |
|---|---|---|---|---|---|
| a | c | ||||
| CrHApAPV | 9.405 | 6.879 | 0.731 | 526.940 | 14.15 |
| CrHApAPV-Ax | 9.335 | 6.855 | 0.734 | 517.314 | 12.54 |
| Sample | Coating Thickness (nm) |
|---|---|
| CrHApAPV | 175 ± 10 nm |
| CrHApAPV-Ax | 188 ± 10 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, S.C.; Predoi, D.; Iconaru, S.L.; Rokosz, K.; Raaen, S.; Bleotu, C.; Predoi, M.V. Development of Chrome-Doped Hydroxyapatite in a PVA Matrix Enriched with Amoxicillin for Biomedical Applications. Antibiotics 2025, 14, 455. https://doi.org/10.3390/antibiotics14050455
Ciobanu SC, Predoi D, Iconaru SL, Rokosz K, Raaen S, Bleotu C, Predoi MV. Development of Chrome-Doped Hydroxyapatite in a PVA Matrix Enriched with Amoxicillin for Biomedical Applications. Antibiotics. 2025; 14(5):455. https://doi.org/10.3390/antibiotics14050455
Chicago/Turabian StyleCiobanu, Steluta Carmen, Daniela Predoi, Simona Liliana Iconaru, Krzysztof Rokosz, Steinar Raaen, Coralia Bleotu, and Mihai Valentin Predoi. 2025. "Development of Chrome-Doped Hydroxyapatite in a PVA Matrix Enriched with Amoxicillin for Biomedical Applications" Antibiotics 14, no. 5: 455. https://doi.org/10.3390/antibiotics14050455
APA StyleCiobanu, S. C., Predoi, D., Iconaru, S. L., Rokosz, K., Raaen, S., Bleotu, C., & Predoi, M. V. (2025). Development of Chrome-Doped Hydroxyapatite in a PVA Matrix Enriched with Amoxicillin for Biomedical Applications. Antibiotics, 14(5), 455. https://doi.org/10.3390/antibiotics14050455










