Methylglyoxal Alone or Combined with Light-Emitting Diodes/Complex Electromagnetic Fields Represent an Effective Response to Microbial Chronic Wound Infections
Abstract
1. Introduction
- (i)
- To investigate the inhibitory effect of LED and CMFs therapy, alone and combined with MGO against clinical pathogenic isolates of S. aureus, P. aeruginosa, and C. albicans;
- (ii)
- To evaluate the effect of LED, CMFs, and their synergistic combination with MGO on P. aeruginosa swimming, swarming, and twitching motility;
- (iii)
- To assess the potential changes in cellular membrane permeability and fluidity induced by LED, CMFs, and their synergistic combination with MGO on S. aureus, P. aeruginosa and C. albicans strains;
- (iv)
- To determine the potential interaction between MGO and a target enzyme of P. aeruginosa by docking analysis.
2. Results
2.1. Antimicrobial Activity of MGO
2.2. Planktonic Optical Density
2.3. Synergism
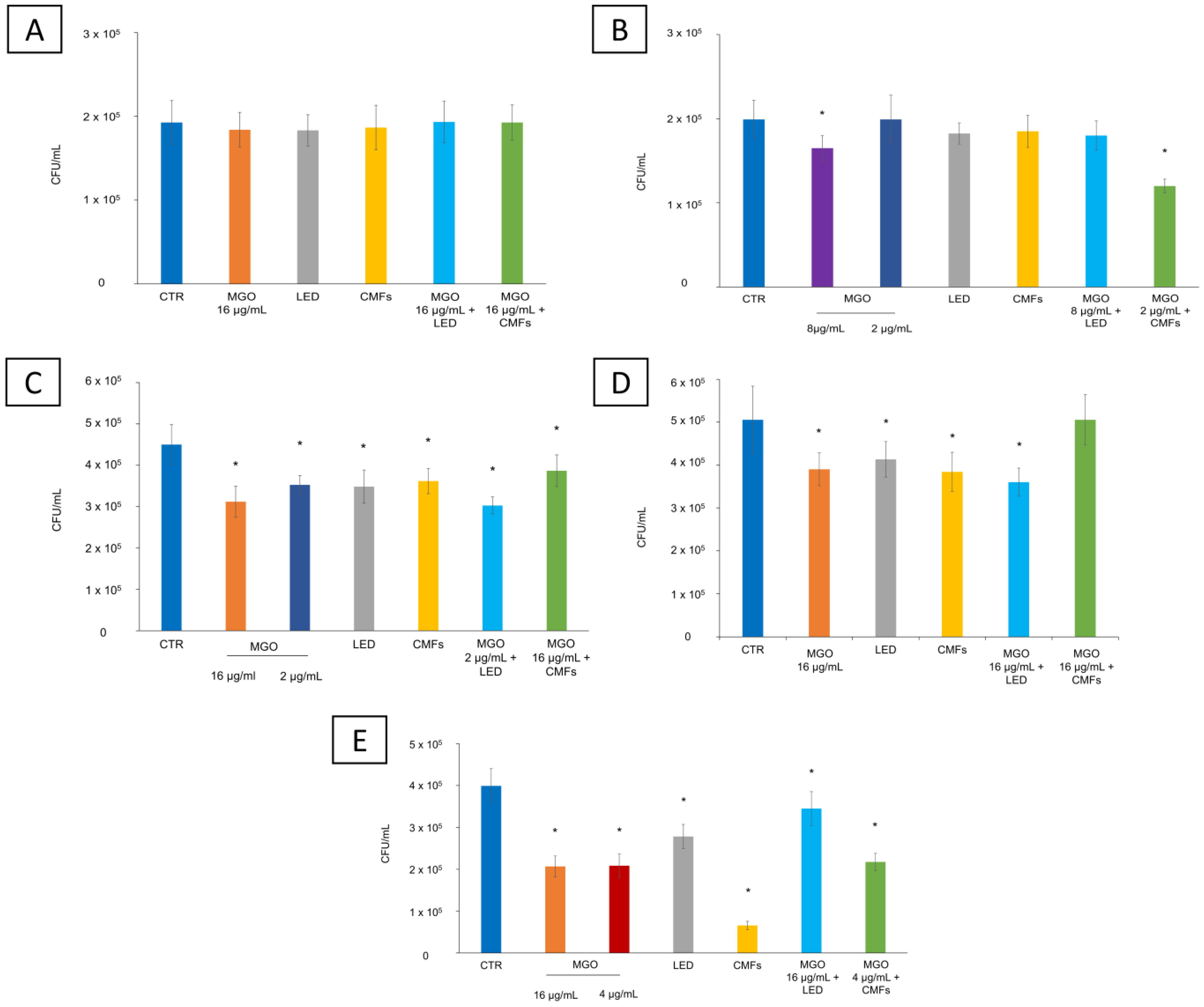
2.4. CFU/mL Reduction
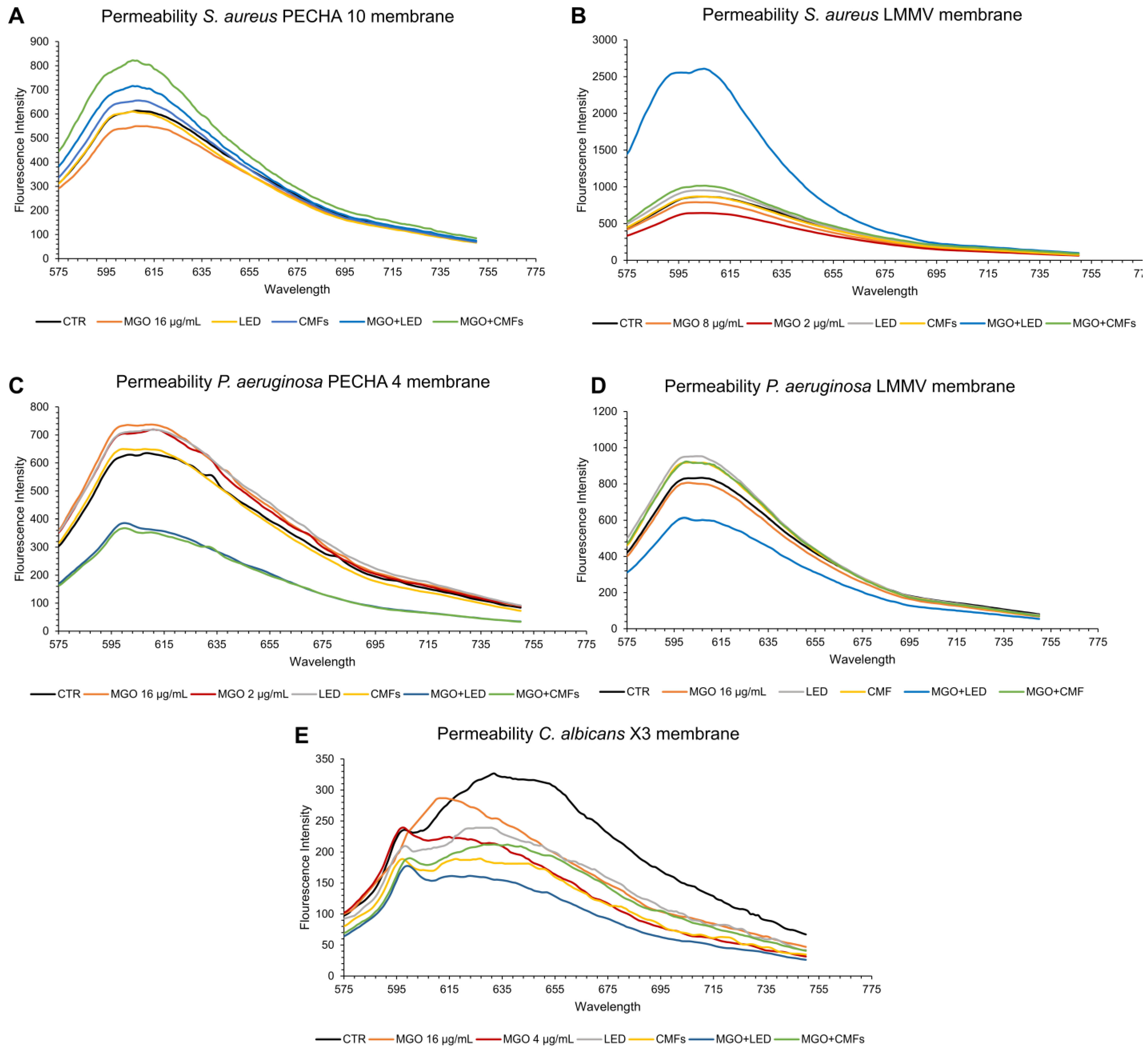
2.5. Membrane Permeability
2.6. Membrane Fluidity
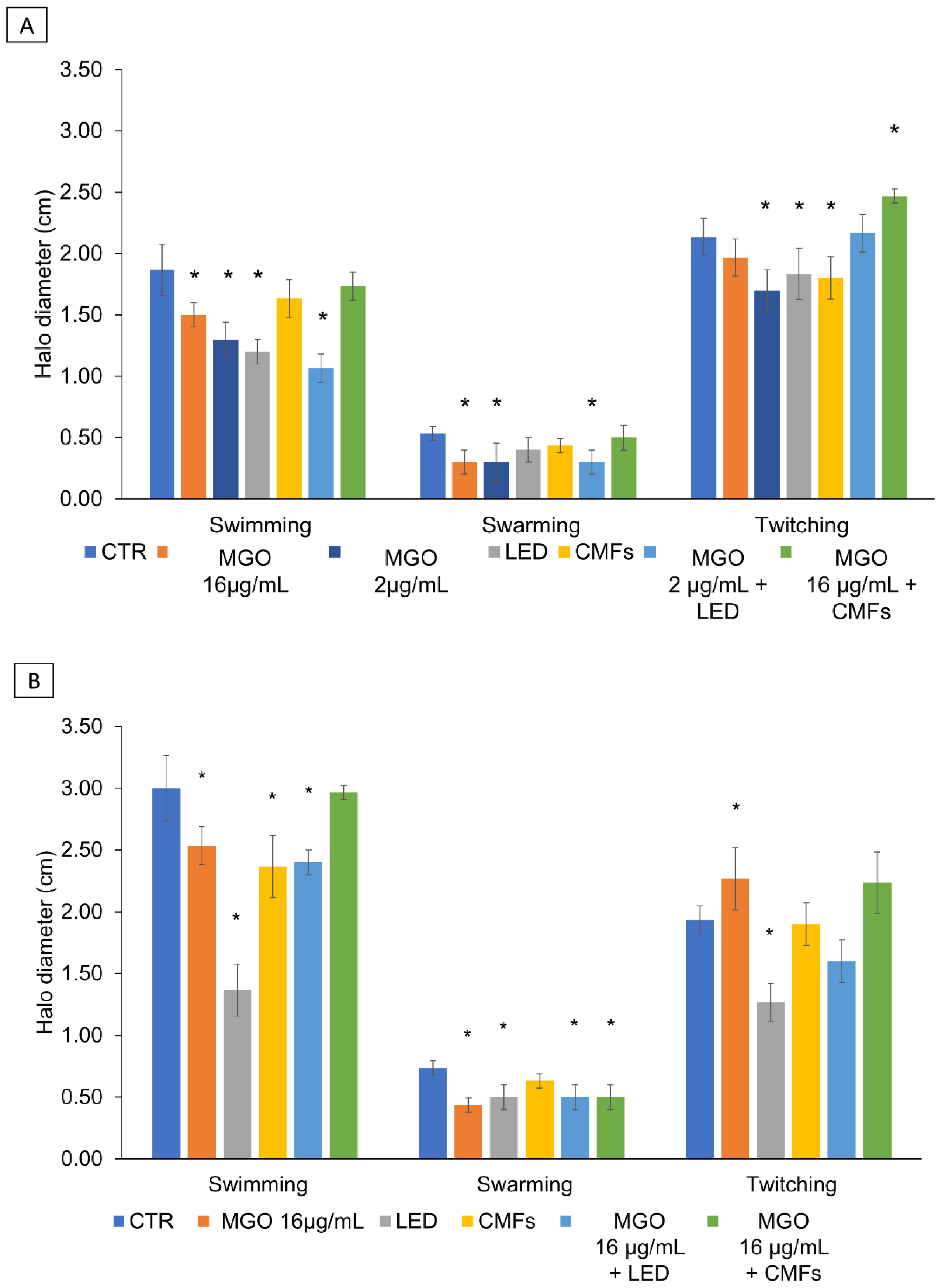
2.7. Swimming, Swarming, and Twitching Motility
2.8. Docking Studies
3. Discussion
4. Materials and Methods
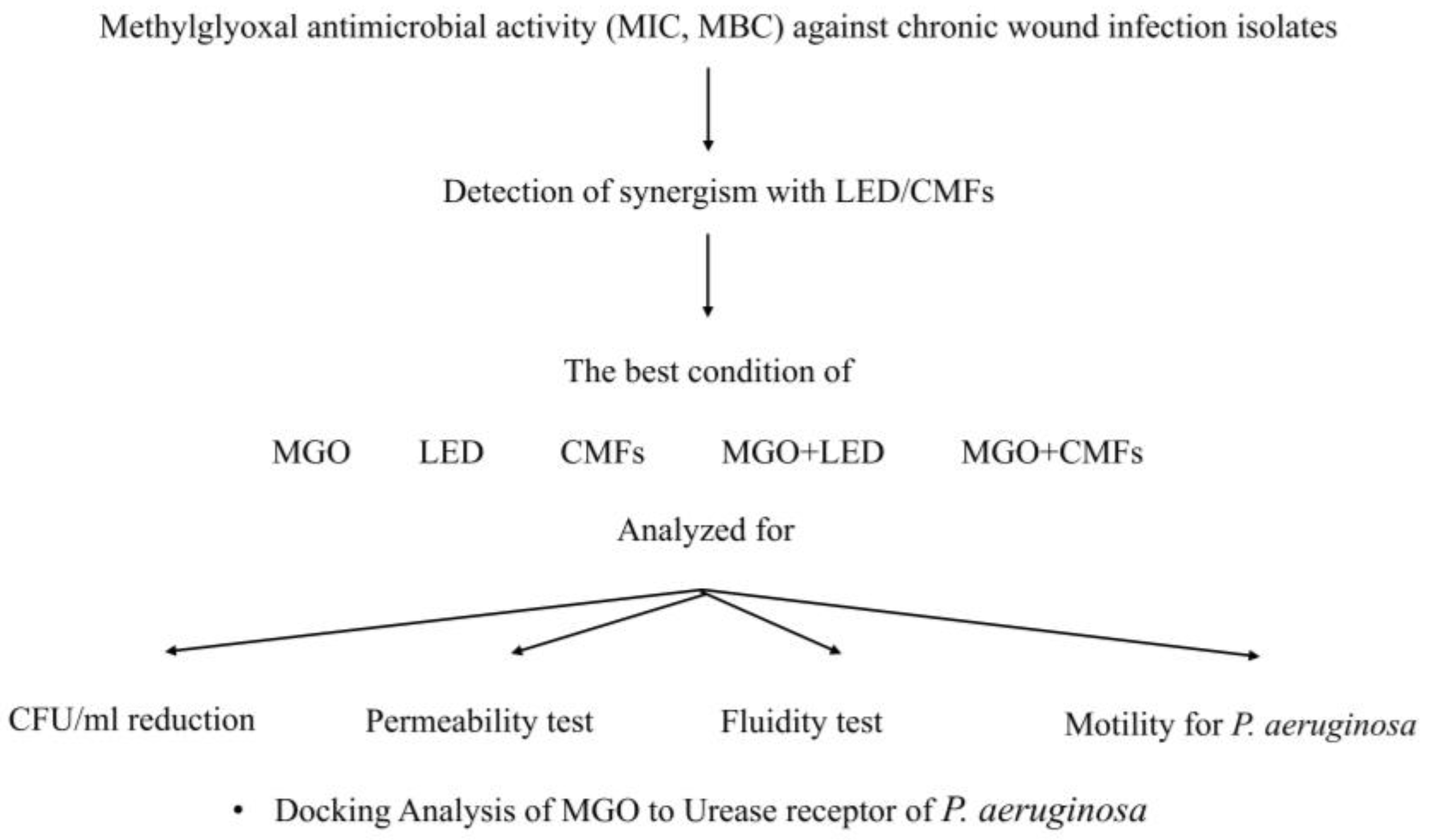
4.1. Experimental Plan
4.2. Microbial Strains
4.3. Material and Devices
4.4. Antimicrobial Susceptibility Assay
4.5. Planktonic Optical Density
4.6. Synergism
4.7. CFU/mL Reduction
4.8. Membrane Permeability
4.9. Membrane Fluidity
4.10. Swimming, Swarming, and Twitching Motility Assay
4.11. Building of the P. aeruginosa Model
4.12. Docking Studies
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| CFU/mL | Colony forming units per milliliter |
| CMFs | Complex Electromagnetic Fields |
| FIC I | Fractional Inhibitory Concentration Index |
| GPexc | Laurdan generalized polarization |
| LED | Light-Emitting Diodes |
| MGO | Methylglyoxal |
| MBC | Minimum Bactericidal Concentration |
| MFC | Minimum Fungicidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| PDT | Photodynamic therapy |
References
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef] [PubMed]
- Chiș, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Țincu, A.L.; Gligor, F.G.; Mureșan, M.L.; Dobrea, C.M. Microbial Resistance to Antibiotics and Effective Antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic Wound Infections: The Role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert. Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Di Giulio, M.; Di Lodovico, S.; Fontana, A.; Traini, T.; Di Campli, E.; Pilato, S.; D’Ercole, S.; Cellini, L. Graphene Oxide Affects Staphylococcus aureus and Pseudomonas aeruginosa Dual Species Biofilm in Lubbock Chronic Wound Biofilm Model. Sci. Rep. 2020, 10, 18525. [Google Scholar] [CrossRef]
- Alves, P.M.; Al-Badi, E.; Withycombe, C.; Jones, P.M.; Purdy, K.J.; Maddocks, S.E. Inter-action between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathog. Dis. 2018, 76, fty003. [Google Scholar] [CrossRef] [PubMed]
- Short, B.; Bakri, A.; Baz, A.; Williams, C.; Brown, J.L.; Ramage, G. There Is More to Wounds than Bacteria: Fungal Biofilms in Chronic Wounds. Curr. Clin. Microbiol. Rep. 2023, 10, 9–16. [Google Scholar] [CrossRef]
- Bandara, H.M.H.N.; Wood, D.L.A.; Vanwonterghem, I.; Hugenholtz, P.; Cheung, B.P.K.; Samaranayake, L.P. Fluconazole Resistance in Candida albicans Is Induced by Pseudomonas aeruginosa Quorum Sensing. Sci. Rep. 2020, 10, 7769. [Google Scholar] [CrossRef]
- Kim, W.S.; Calderhead, R.G. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Ther. 2011, 20, 205–215. [Google Scholar] [CrossRef]
- Kajagar, B.M.; Godhi, A.S.; Pandit, A.; Khatri, S. Efficacy of Low Level Laser Therapy on Wound Healing in Patients with Chronic Diabetic Foot Ulcers—A Randomised Control Trial. Indian J. Surg. 2012, 74, 359–363. [Google Scholar] [CrossRef]
- Kerppers, I.I.; de Lima, C.J.; Fernandes, A.B.; Villaverde, A.B. Effect of Light-Emitting Diode (ʎ 627 Nm and 945 Nm ʎ) Treatment on First Intention Healing: Immunohistochemical Analysis. Lasers Med. Sci. 2014, 30, 397–401. [Google Scholar] [CrossRef]
- Dai, T.; Hamblin, M.R. Visible Blue Light Is Capable of Inactivating Candida albicans and Other Fungal Species. Photomed. Laser Surg. 2017, 35, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Trentini, P.; Tripodi, D.; Spoto, G.; D’Ercole, S. In Vitro Antimicrobial Activity of LED Irradiation on Pseudomonas aeruginosa. J. Photochem. Photobiol. B 2017, 168, 25–29. [Google Scholar] [CrossRef]
- Petrini, M.; Spoto, G.; Scarano, A.; D’Arcangelo, C.; Tripodi, D.; Di Fermo, P.; D’Ercole, S. Near-Infrared LEDS Provide Persistent and Increasing Protection against E. faecalis. J. Photochem. Photobiol. B 2019, 197, 111527. [Google Scholar] [CrossRef]
- Jagdeo, J.; Nguyen, J.K.; Ho, D.; Wang, E.T.; Austin, E.; Mamalis, A.; Kaur, R.; Kraeva, E.; Schulman, J.M.; Li, C.-S.; et al. Safety of Light Emitting Diode-Red Light on Human Skin: Two Randomized Controlled Trials. J. Biophotonics 2020, 13, e201960014. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Aboulwafa, M.; Elnakib, M.; Saleh, S. Extremely Low Frequency Electromagnetic Field (ELF-EMF) as a Promising Tool for Treatment against Multi-Drug Resistant Bacteria. Arch. Pharm. Sci. Ain Shams Univ. 2024, 8, 146–162. [Google Scholar] [CrossRef]
- D’Ercole, S.; Di Lodovico, S.; Iezzi, G.; Pierfelice, T.V.; D’Amico, E.; Cipollina, A.; Piattelli, A.; Cellini, L.; Petrini, M. Complex Electromagnetic Fields Reduce Candida albicans Planktonic Growth and Its Adhesion to Titanium Surfaces. Biomedicines 2021, 9, 1261. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Petrini, M.; D’Amico, E.; Di Fermo, P.; Diban, F.; D’Arcangelo, S.; Piattelli, A.; Cellini, L.; Iezzi, G.; Di Giulio, M.; et al. Complex Magnetic Fields Represent an Eco-Sustainable Technology to Counteract the Resistant Candida albicans Growth without Affecting the Human Gingival Fibroblasts. Sci. Rep. 2023, 13, 22067. [Google Scholar] [CrossRef]
- Benya, P.D.; Kavanaugh, A.; Zakarian, M.; Söderlind, P.; Jashashvili, T.; Zhang, N.; Waldorff, E.I.; Ryaby, J.T.; Billi, F. Pulsed Electromagnetic Field (PEMF) Transiently Stimulates the Rate of Mineralization in a 3-Dimensional Ring Culture Model of Osteogenesis. PLoS ONE 2021, 16, e0244223. [Google Scholar] [CrossRef] [PubMed]
- Patiño, O.; Grana, D.; Bolgiani, A.; Prezzavento, G.; Miño, J.; Merlo, A.; Benaim, F. Pulsed Electromagnetic Fields in Experimental Cutaneous Wound Healing in Rats. J. Burn. Care Res. 1996, 17, 528–531. [Google Scholar] [CrossRef]
- Akdag, M.Z.; Dasdag, S.; Uzunlar, A.K.; Ulukaya, E.; Oral, A.Y.; Çelik, N.; Akşen, F. Can Safe and Long-Term Exposure to Extremely Low Frequency (50 Hz) Magnetic Fields Affect Apoptosis, Reproduction, and Oxidative Stress? Int. J. Radiat. Biol. 2013, 89, 1053–1060. [Google Scholar] [CrossRef]
- White, R. Manuka Honey in Wound Management: Greater than the Sum of Its Parts? J. Wound Care 2016, 25, 539–543. [Google Scholar] [CrossRef][Green Version]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-Type Honeys Can Eradicate Biofilms Produced by Staphylococcus aureus strains with Different Biofilm-Forming Abilities. PeerJ 2014, 2, e326. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The Origin of Methylglyoxal in New Zealand Manuka (Leptospermum scoparium) Honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Brighina, S.; Restuccia, C.; Arena, E.; Palmeri, R.; Fallico, B. Antibacterial Activity of 1,2-Dicarbonyl Compounds and the Influence of the in Vitro Assay System. Food Chem. 2019, 311, 125905. [Google Scholar] [CrossRef] [PubMed]
- Rückriemen, J.; Klemm, O.; Henle, T. Manuka Honey (Leptospermum scoparium) Inhibits Jack Bean Urease Activity due to Methylglyoxal and Dihydroxyacetone. Food Chem. 2017, 230, 540–546. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and Quantification of Methylglyoxal as the Dominant Antibacterial Constituent of Manuka (Leptospermum scoparium) Honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kilty, S.J.; Duval, M.; Chan, F.T.; Ferris, W.; Slinger, R. Methylglyoxal: (Active Agent of Manuka Honey) in Vitro Activity against Bacterial Biofilms. Int. Forum Allergy Rhinol. 2011, 1, 348–350. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies according to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The Unusual Antibacterial Activity of Medical-Grade Leptospermum Honey: Antibacterial Spectrum, Resistance and Transcriptome Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-Bacterial, Anti-Biofilm and Anti-Quorum Sensing Activities of Honey: A Review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef]
- Campbell, A.K.; Naseem, R.; Holland, I.B.; Matthews, S.B.; Wann, K.T. Methylglyoxal and Other Carbohydrate Metabolites Induce Lanthanum-Sensitive Ca2+ Transients and Inhibit Growth in E. coli. Arch. Biochem. Biophys. 2007, 468, 107–113. [Google Scholar] [CrossRef]
- Konieczna, I.; Zarnowiec, P.; Kwinkowski, M.; Kolesinska, B.; Fraczyk, J.; Kaminski, Z.; Kaca, W. Bacterial Urease and Its Role in Long-Lasting Human Diseases. Curr. Protein Pept. Sci. 2012, 13, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.; Wright, N.; Gardner, S.L.; Telzrow, C.L.; Wommack, A.J.; Vigueira, P.A. Manuka honey and methylglyoxal increase the sensitivity of Staphylococcus aureus to linezolid. Lett. Appl. Microbiol. 2018, 66, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Magrini, G. Methylglyoxal, the Major Antibacterial Factor in Manuka Honey: An Alternative to Preserve Natural Cosmetics? Cosmetics 2018, 6, 1. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and Chronic Wound Infections: Microbiological, Immuno-logical, Clinical and Therapeutic Distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef]
- Qi, X.; Shi, Y.; Zhang, C.; Cai, E.; Ge, X.; Xiang, Y.; Li, Y.; Zeng, B.; Shen, J. A Hybrid Hydrogel with Intrinsic Immunomodulatory Functionality for Treating Multidrug-Resistant Pseudomonas aeruginosa Infected Diabetic Foot Ulcers. ACS Mater. Lett. 2024, 6, 2533–2547. [Google Scholar] [CrossRef]
- Hayashi, K.; Fukushima, A.; Hayashi-Nishino, M.; Nishino, K. Effect of Methylglyoxal on Multidrug-Resistant Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 180. [Google Scholar] [CrossRef]
- Paramasivan, S.; Drilling, A.J.; Jardeleza, C.; Jervis-Bardy, J.; Vreugde, S.; Wormald, P.J. Methylglyoxal-Augmented Manuka Honey as a Topical Anti-Staphylococcus aureus biofilm Agent: Safety and Efficacy in an in Vivo Model. Int. Forum Allergy Rhinol. 2014, 4, 187–195. [Google Scholar] [CrossRef]
- Yang, M.; Ward, J.; Choy, K. Nature-Inspired Bacterial Cellulose/Methylglyoxal (BC/MGO) Nanocomposite for Broad-Spectrum Antimicrobial Wound Dressing. Macromol. Biosci. 2020, 20, 2000070. [Google Scholar] [CrossRef]
- Hampden-Martin, A.; Fothergill, J.; El Mohtadi, M.; Chambers, L.; Slate, A.J.; White-head, K.A.; Shokrollahi, K. Photodynamic Antimicrobial Chemotherapy Coupled with the Use of the Photosensitizers Methylene Blue and Temoporfin as a Potential Novel Treatment for Staphylococcus aureus in Burn Infections. Access Microbiol. 2021, 3, 000273. [Google Scholar] [CrossRef]
- Ishiwata, N.; Tsunoi, Y.; Sarker, R.R.; Haruyama, Y.; Kawauchi, S.; Sekine, Y.; Onuma, C.; Tsuda, H.; Saitoh, D.; Nishidate, I.; et al. Control of Burn Wound Infection by Methylene Blue Mediated Photodynamic Treatment with Light Emitting Diode Array Illumination in Rats. Lasers Surg. Med. 2021, 53, 1238–1246. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, J.; Wang, Y.; Xu, Z.; Zhao, L.; Zhao, J.; Hong, G.; Liu, T. Antimicrobial Photodynamic Therapy Combined with Antibiotic in the Treatment of Rats with Third-Degree Burns. Front. Microbiol. 2021, 12, 622410. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.; Bolognese, F.; Barbieri, P. Blue Light Enhances the Antimicrobial Activity of Honey against Pseudomonas aeruginosa. In Proceedings of the Light-Based Diagnosis and Treatment of Infectious Diseases, San Francisco, CA, USA, 27 January–1 February 2018; Volume 10479, pp. 34–44. [Google Scholar] [CrossRef]
- Ahmed, I.; Istivan, T.; Cosic, I.; Pirogova, E. Evaluation of the Effects of Extremely Low Frequency (ELF) Pulsed Electromagnetic Fields (PEMF) on Survival of the Bacterium Staphylococcus aureus. EPJ Nonlinear Biomed. Phys. 2013, 1, 5. [Google Scholar] [CrossRef]
- Ahmed, I.; Istivan, T.; Pirogova, E. Irradiation of Escherichia coli by Extremely-Low Frequency (ELF) Pulsed Electromagnetic Fields (PEMF): Evaluation of Bacterial Survival. J. Electromagn. Waves Appl. 2014, 29, 26–37. [Google Scholar] [CrossRef]
- Inhan-Garip, A.; Aksu, B.; Akan, Z.; Akakin, D.; Ozaydin, A.N.; San, T. Effect of Extremely Low Frequency Electromagnetic Fields on Growth Rate and Morphology of Bacteria. Int. J. Radiat. Biol. 2011, 87, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Novák, J.; Strašák, L.; Fojt, L.; Slaninová, I.; Vetterl, V. Effects of Low-Frequency Magnetic Fields on the Viability of Yeast Saccharomyces cerevisiae. Bioelectrochemistry 2007, 70, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fojt, L.; Klapetek, P.; Strašák, L.; Vetterl, V. 50Hz Magnetic Field Effect on the Morphology of Bacteria. Micron 2009, 40, 918–922. [Google Scholar] [CrossRef]
- Nomura, W.; Aoki, M.; Inoue, Y. Methylglyoxal Inhibits Nuclear Division through Alterations in Vacuolar Morphology and Accumulation of Atg18 on the Vacuolar Membrane in Saccharomyces cerevisiae. Sci. Rep. 2020, 10, 13887. [Google Scholar] [CrossRef]
- Zanotti, F.; Trentini, M.; Zanolla, I.; Tiengo, E.; Mantarro, C.; Dalla Paola, L.; Tremoli, E.; Sambataro, M.; Sambado, L.; Picari, M.; et al. Playing with Biophysics: How a Symphony of Different Electromagnetic Fields Acts to Reduce the Inflammation in Diabetic Derived Cells. Int. J. Mol. Sci. 2023, 24, 1754. [Google Scholar] [CrossRef]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How Methylglyoxal Kills Bacteria: An Ultrastructural Study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sztafrowski, D.; Muraszko, J.; Jasiura, A.; Bryk, P.; Urbanek, A.K.; Krasowska, A. The Alternating 50 Hz Magnetic Field Depending on the Hydrophobicity of the Strain Affects the Viability, Filamentation and Sensitivity to Drugs of Candida albicans. PLoS ONE 2023, 18, e0291438. [Google Scholar] [CrossRef] [PubMed]
- Oncul, S.; Cuce, E.M.; Aksu, B.; Inhan Garip, A. Effect of Extremely Low Frequency Electromagnetic Fields on Bacterial Membrane. Int. J. Radiat. Biol. 2016, 92, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, A.; Meddings, J.B.; Ratnam, S.; Sherman, P.M. Modulation of Host Cell Membrane Fluidity: A Novel Mechanism for Preventing Bacterial Adhesion. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G201–G208. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Influence of Growth and Treatment Temperature on Staphylococcus aureus Resistance to Pulsed Electric Fields: Relationship with Membrane Fluidity. Innov. Food Sci. Emerg. Technol. 2016, 37, 161–169. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Heat Resistance, Membrane Fluidity and Sublethal Damage in Staphylococcus aureus Cells Grown at Different Temperatures. Int. J. Food Microbiol. 2019, 289, 49–56. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Kim, Y.-M. Regulation and Controlling the Motility Properties of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 104, 33–49. [Google Scholar] [CrossRef]
- Tuttobene, M.R.; Pérez, J.F.; Pavesi, E.S.; Perez Mora, B.; Biancotti, D.; Cribb, P.; Altilio, M.; Müller, G.L.; Gramajo, H.; Tamagno, G.; et al. Light Modulates Important Pathogenic Determinants and Virulence in ESKAPE Pathogens Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus. J. Bacteriol. 2021, 203, e00566-20. [Google Scholar] [CrossRef]
- Burrows, L.L. Pseudomonas aeruginosa Twitching Motility: Type IV Pili in Action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef]
- Raouia, H.; Hamida, B.; Khadidja, A.; Ahmed, L.; Abdelwaheb, C. Effect of Static Magnetic Field (200 MT) on Biofilm Formation in Pseudomonas aeruginosa. Arch. Microbiol. 2019, 202, 77–83. [Google Scholar] [CrossRef]
- Kiselar, J.G.; Wang, X.; Dubyak, G.R.; El Sanadi, C.; Ghosh, S.K.; Lundberg, K.; Wil-liams, W.M. Modification of β-Defensin-2 by Dicarbonyls Methylglyoxal and Glyoxal Inhibits Antibacterial and Chemotactic Function in Vitro. PLoS ONE 2015, 10, e0130533. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, L.; Cianci, M.; Musiani, F.; Lente, G.; Palombo, M.; Ciurli, S. Inactivation of Urease by Catechol: Kinetics and Structure. J. Inorg. Biochem. 2017, 166, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, L.; Contaldo, U.; Musiani, F.; Cianci, M.; Bagnolini, G.; Roberti, M.; Ciurli, S. Inhibition of Urease, a Ni Enzyme: The Reactivity of a Key Thiol with Mono and Di Substituted Catechols Elucidated by Kinetic, Structural, and Theoretical Studies. Angew. Chem. Int. Ed. Engl. 2021, 60, 6029–6035. [Google Scholar] [CrossRef]
- Mazzei, L.; Cianci, M.; Benini, S.; Ciurli, S. The Structure of the Elusive Urease–Urea Complex Unveils the Mechanism of a Paradigmatic Nickel Dependent Enzyme. Angew. Chem. Int. Ed. Engl. 2019, 58, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Cotham, W.E.; Metz, T.O.; Ferguson, P.L.; Brock, J.W.; Hinton, D.J.; Thorpe, S.R.; Baynes, J.W.; Ames, J.M. Proteomic Analysis of Arginine Adducts on Glyoxal-Modified Ribonuclease. Mol. Cell. Proteom. 2004, 3, 1145–1153. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with Alphafold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Bacchetti, T.; D’Ercole, S.; Covone, S.; Petrini, M.; Di Giulio, M.; Di Fermo, P.; Diban, F.; Ferretti, G.; Cellini, L. Complex Chronic Wound Biofilms Are Inhibited in Vitro by the Natural Extract of Capparis spinose. Front. Microbiol. 2022, 13, 832919. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Lanotte, P. Genetic Features of Pseudomonas aeruginosa Isolates from Cystic Fibrosis Patients Compared with Those of Isolates from Other Origins. J. Med. Microbiol. 2004, 53, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; D’Aurizio, E.; Trubiani, O.; Grande, R.; Di Campli, E.; Di Giulio, M.; Di Bartolomeo, S.; Sozio, P.; Iannitelli, A.; Nostro, A.; et al. Viscoelastic Properties Of Staphylococcus aureus and Staphylococcus epidermidis mono-Microbial Biofilms. Microb. Biotechnol. 2009, 2, 634–641. [Google Scholar] [CrossRef]
- Nhan, T.X.; Leclercq, R.; Cattoir, V. Prevalence of Toxin Genes in Consecutive Clinical Isolates of Staphylococcus aureus and Clinical Impact. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 719–725. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine Leukocidin--Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Diban, F.; Di Fermo, P.; Petrini, M.; Fontana, A.; Di Giulio, M.; Piattelli, A.; D’Ercole, S.; Cellini, L. Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management. Int. J. Mol. Sci. 2022, 23, 6942. [Google Scholar] [CrossRef] [PubMed]
- Wikler, M.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard; CLSI (NCCLS): Wayne, PA, USA, 2006; Volume 26. [Google Scholar]
- Di Lodovico, S.; Dotta, T.C.; Cellini, L.; Iezzi, G.; D’Ercole, S.; Petrini, M. The Antibacterial and Antifungal Capacity of Eight Commercially Available Types of Mouthwash against Oral Microorganisms: An in Vitro Study. Antibiotics 2023, 12, 675. [Google Scholar] [CrossRef]
- Di Fermo, P.; Di Lodovico, S.; Amoroso, R.; De Filippis, B.; D’Ercole, S.; Di Campli, E.; Cellini, L.; Di Giulio, M. Searching for New Tools to Counteract the Helicobacter pylori Resistance: The Positive Action of Resveratrol Derivatives. Antibiotics 2020, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Evaluation of Membrane Fluidity of Multidrug-Resistant Isolates of Escherichia coli and Staphylococcus aureus in Presence and Absence of Antibiotics. J. Photochem. Photobiol. B 2018, 181, 150–156. [Google Scholar] [CrossRef]
- Issac Abraham, S.V.P.; Palani, A.; Ramaswamy, B.R.; Shunmugiah, K.P.; Arumugam, V.R. Antiquorum Sensing and Antibiofilm Potential of Capparis spinosa. Arch. Med. Res. 2011, 42, 658–668. [Google Scholar] [CrossRef]
- Turnbull, L.; Whitchurch, C.B. Motility Assay: Twitching Motility. Methods Mol. Biol. 2014, 1149, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Uranyl ex-traction by N, N-dialkylamide ligands studied by static and dynamic DFT simulations. In Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- DeLano, W.L. PyMOL Molecular Viewer; DeLano Scientific LLC: Palo Alto, CA, USA, 2008; Available online: http://www.pymol.org/ (accessed on 27 January 2025).


| MGO | S. aureus PECHA 10 | S. aureus LMMV | P. aeruginosa PECHA 4 | P. aeruginosa LMMV | C. albicans X3 |
|---|---|---|---|---|---|
| MIC (µg/mL) | 64 | 128 | 256 | 256 | 4096 |
| MBC/MFC (µg/mL) | 64 | 128 | 256 | 256 | 4096 |
| Synergistic Combinations (MGO+LED) (µg/mL MGO) | FIC Index | Synergistic Combinations (MGO+CMFs) (µg/mL MGO) | FIC Index | |
|---|---|---|---|---|
| S. aureus PECHA 10 | 16 * 32 | 0.250 0.500 | 16 * 32 | 0.250 0.500 |
| S. aureus LMMV | 8 * 16 32 64 | 0.062 0.125 0.250 0.500 | 2 * 4 8 16 32 64 | 0.015 0.031 0.062 0.125 0.250 0.500 |
| P. aeruginosa PECHA 4 | 2 * 4 8 1 32 64 128 | 0.008 0.015 0.031 0.062 0.125 0.250 0.500 | 16 * 32 64 128 | 0.062 0.125 0.250 0.500 |
| P. aeruginosa LMMV | 16 * 32 64 128 | 0.062 0.125 0.250 0.500 | 16 * 32 64 128 | 0.062 0.125 0.250 0.500 |
| C. albicans X3 | 16 * 32 64 128 256 512 1024 2048 | 0.004 0.008 0.015 0.031 0.062 0.125 0.250 0.500 | 4 * 8 16 32 64 128 256 512 1024 | 0.001 0.002 0.004 0.008 0.015 0.031 0.062 0.125 0.250 |
| GPexc Values | |||||
|---|---|---|---|---|---|
| S. aureus PECHA 10 | S. aureus LMMV | P. aeruginosa PECHA 4 | P. aeruginosa LMMV | C. albicans X3 | |
| CTR | 0.12 ± 0.01 | 0.02 ± 0.003 | 0.03 ± 0.01 | 0.19 ± 0.07 | −0.10 ± 0.005 |
| MGO 16 | 0.06 ± 0.005 | ND | 0.12 ± 0.006 | 0.22 ± 0.001 | 0.11 ± 0.002 |
| MGO 8 | ND | 0.17 ± 0.006 | ND | ND | ND |
| MGO 4 | ND | ND | ND | ND | −0.11 ± 0.004 |
| MGO 2 | ND | 0.17 ± 0.0002 | 0.11 ± 0.01 | ND | ND |
| LED | 0.006 ± 0.006 | 0.15 ± 0.0004 | 0.11 ± 0.003 | 0.25 ± 0.003 | −0.13 ± 0.004 |
| CMFs | 0.04 ± 0.0003 | 0.17 ± 0.0003 | 0.04 ± 0.005 | 0.25 ± 0.01 | 0.01 ± 0.01 |
| MGO + LED | 0.14 ± 0.006 | 0.02 ± 0.01 | 0.01 ± 0.001 | 0.04 ± 0.01 | −0.14 ± 0.01 |
| MGO + CMFs | 0.11 ± 0.003 | 0.03 ± 0.01 | 0.05 ± 0.001 | 0.06 ± 0.002 | 0.06 ± 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diban, F.; Di Fermo, P.; Di Lodovico, S.; Petrini, M.; Pilato, S.; Fontana, A.; Pinti, M.; Di Giulio, M.; Lence, E.; González-Bello, C.; et al. Methylglyoxal Alone or Combined with Light-Emitting Diodes/Complex Electromagnetic Fields Represent an Effective Response to Microbial Chronic Wound Infections. Antibiotics 2025, 14, 396. https://doi.org/10.3390/antibiotics14040396
Diban F, Di Fermo P, Di Lodovico S, Petrini M, Pilato S, Fontana A, Pinti M, Di Giulio M, Lence E, González-Bello C, et al. Methylglyoxal Alone or Combined with Light-Emitting Diodes/Complex Electromagnetic Fields Represent an Effective Response to Microbial Chronic Wound Infections. Antibiotics. 2025; 14(4):396. https://doi.org/10.3390/antibiotics14040396
Chicago/Turabian StyleDiban, Firas, Paola Di Fermo, Silvia Di Lodovico, Morena Petrini, Serena Pilato, Antonella Fontana, Morena Pinti, Mara Di Giulio, Emilio Lence, Concepción González-Bello, and et al. 2025. "Methylglyoxal Alone or Combined with Light-Emitting Diodes/Complex Electromagnetic Fields Represent an Effective Response to Microbial Chronic Wound Infections" Antibiotics 14, no. 4: 396. https://doi.org/10.3390/antibiotics14040396
APA StyleDiban, F., Di Fermo, P., Di Lodovico, S., Petrini, M., Pilato, S., Fontana, A., Pinti, M., Di Giulio, M., Lence, E., González-Bello, C., Cellini, L., & D’Ercole, S. (2025). Methylglyoxal Alone or Combined with Light-Emitting Diodes/Complex Electromagnetic Fields Represent an Effective Response to Microbial Chronic Wound Infections. Antibiotics, 14(4), 396. https://doi.org/10.3390/antibiotics14040396













