Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties
Abstract
1. Introduction
2. Results
2.1. Antibiofilm Activity of Honey
2.2. Swimming Motility
2.3. The Prebiotic Activity of Rosaceae Honey
2.3.1. Microbial Adhesion to Solvent
2.3.2. Antibiofilm Activity Exhibited by the Probiotics’ Culture Supernatants
Crystal Violet Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Test
3. Discussion
3.1. Antibiofilm Activity
3.2. Swimming Motility
3.3. Prebiotic Activity of Honey
4. Materials and Methods
4.1. Minimal Inhibitory Concentration (MIC)
4.2. Antibiofilm Activity Exhibited by the Honey
4.3. Evaluation of Honey’s Effect on the Metabolic Activity of Sessile Cells
4.4. Swimming Motility of Pathogens
4.5. Prebiotic Activity of the Honey
4.5.1. Growth of Lactic Acid Bacteria in the Presence of Honey
4.5.2. Microbial Adhesion to Solvent
4.5.3. Antibiofilm Activity of the Supernatants of the LAB Grown in the Presence of the Honey
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Infection Prevention and Control; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. WHO Bacterial Priority Pathogens List; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Medina, E.; Pieper, D.H. How to Overcome the Antibiotic Crisis; Stadler, M., Dersch, P., Eds.; Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2016; Volume 398. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, A.; Mitra, R.; Kundu, S.; Banerjee, P.; Chowdhury, A.A.; Ghosh, S. Escaping the ESKAPE pathogens: A review on antibiofilm potential of nanoparticles. Microb. Pathog. 2024, 194, 106842. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Zafer, M.M.; Mohamed, G.A.; Ibrahim, S.R.; Ghosh, S.; Bornman, C.; Elfaky, M.A. Biofilm-mediated infections by multidrug-resistant microbes: A comprehensive exploration and forward perspectives. Arch. Microbiol. 2024, 206, 101. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7. [Google Scholar] [CrossRef]
- MaChado, A.; Zamora-Mendoza, L.; Alexis, F.; Álvarez-Suarez, J.M. Use of plant extracts, bee-derived products, and probiotic-related applications to fight multi-drug-resistant pathogens in the post-antibiotic era. Future Pharmacol. 2023, 3, 535–567. [Google Scholar] [CrossRef]
- Onyango, L.A.; Liang, J. Manuka honey as a non-antibiotic alternative against Staphylococcus spp. and their small colony variant (SCVs) phenotypes. Front. Cell. Inf. Microbiol. 2024, 14, 1380289. [Google Scholar] [CrossRef]
- Fratianni, F.; Amato, G.; d’Acierno, A.; Ombra, M.N.; De Feo, V.; Coppola, R.; Nazzaro, F. In vitro prospective healthy and nutritional benefits of different Citrus monofloral honeys. Sci. Rep. 2023, 13, 1088. [Google Scholar] [CrossRef]
- Mustar, S.; Nurliayana, I. A sweeter pill to swallow: A review of honeybees and honey as a source of probiotic and prebiotic products. Foods 2022, 11, 2102. [Google Scholar] [CrossRef]
- Mohan, A.; Quek, S.Y.; Gutierrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of honey in improving the gut microbial balance. Food Qual. Saf. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Report: USA Honey Market. 2024. Available online: https://www.ams.usda.gov/mnreports/fvmhoney.pdf (accessed on 9 March 2025).
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-bacterial, anti-biofilm and anti-quorum sensing activities of honey: A review. J. Ethnopharmacol. 2023, 317, 11683. [Google Scholar] [CrossRef]
- Roby, M.; Fathy, Y.; Esmail, A.H.; Mohdaly, A.; Hassanien, M. Evaluation of Egyptian honeys and their floral origins: Phenolic compounds, antioxidant activities, and antimicrobial characteristics. Environ. Sci. Pollut. Res. 2020, 27, 20748–20756. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A. Comparative Antibacterial and Antibiofilm Activities of Manuka Honey and Egyptian Clover Honey. Asian J. Appl. Sci. 2014, 2, 984. [Google Scholar]
- Ibáñez, E.; Bicchi, C.; Capozzi, F.; Chen, Y.; Coppola, F.; Fanali, S.; Ferreira, S.R.S.; Fischer, M.; Gavahian, M.; Gavara, R.; et al. Future trends in Food Science and Foodomics: A perspective view by the Editorial Team of Exploration of Foods and Foodomics. Explor. Foods Foodomics 2024, 2, 707–766. [Google Scholar] [CrossRef]
- Molan, P.C. The Antibacterial Activity of Honey. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef]
- Santonocito, D.; Messina, L.; Greco, V.; Giuffrida, A.; Puglia, C.; Di Giulio, M.; Inturri, R.; Vaccaro, S. Almond hull extract valorization: From waste to food recovery to counteract Staphylococcus aureus and Escherichia coli in formation and mature biofilm. Foods 2023, 13, 3834. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Qamash, R.A.; Bulkhi, R.A.; Bifari, J.A.; Bakhsh, O.S.; Hawsawi, K.O.; Matuure, E.Y.; Sulaimani, K.A.; Hakim, A.T.; Mujahid, M.S. Biological studies of the activity of Manuka honey against Carbapenem-resistant Enterobacterales (CRE) bacteria. Saudi Med. J. 2024, 45, 876. [Google Scholar] [CrossRef]
- Qin, C.; Yang, G.; Wu, S.; Zhang, H.; Zhu, C. Synthesis, physicochemical characterization, antibacterial activity, and biocompatibility of quaternized hawthorn pectin. Int. J. Biol. Macromol. 2022, 213, 1047–1056. [Google Scholar] [CrossRef]
- Orhan, I.; Özçelik, B.; Kartal, M.; Özdeveci, B.; Duman, H. HPLC quantification of vitexine-2 ″-O-rhamnoside and hyperoside in three Crataegus species and their antimicrobial and antiviral activities. Chromatographia 2007, 66, 153–157. [Google Scholar] [CrossRef]
- Das, H.; Samanta, A.K.; Kumar, S.; Roychoudhury, P.; Sarma, K.; Akter, F.; Subudhi, P.K.; Dutta, T.K. Exploration of antimicrobial, antibiofilm and antiquorum sensing activity of the Himalayan yellow raspberry (Rubus ellipticus) against clinical isolates of Escherichia coli and Staphylococcus aureus. Indian J. Anim. Res 2024, 58, 1005–1010. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Colautti, A.; Camprini, L.; Ginaldi, F.; Comi, G.; Reale, A.; Coppola, F.; Iacumin, L. Safety traits, genetic and technological characterization of Lacticaseibacillus rhamnosus strains. LWT 2024, 207, 116578. [Google Scholar] [CrossRef]
- Fratianni, F.; De Giulio, B.; Amato, G.; De Feo, V.; Coppola, R.; Nazzaro, F. In vitro prebiotic effects and antibacterial activity of five leguminous honeys. Foods 2022, 12, 3338. [Google Scholar] [CrossRef]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: A review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, E.; Mortman, N.J.; Vargas-Cuebas, G.; Tai, A.K.; Isberg, R.R. A global regulatory system links virulence and antibiotic resistance to envelope homeostasis in Acinetobacter baumannii. PLoS Pathog. 2018, 14, e1007030. [Google Scholar] [CrossRef]
- Allen, J.L.; Tomlinson, B.R.; Casella, L.G.; Shaw, L.N. Regulatory networks important for survival of Acinetobacter baumannii within the host. Curr.Opin. Microbiol. 2020, 55, 74–80. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Da Silva, E.P.; De Martinis, E.C.P. Current knowledge and perspectives on biofilm formation: The case of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2013, 97, 957–968. [Google Scholar] [CrossRef]
- Wortel, I.M.; Kim, S.; Liu, A.Y.; Ibarra, E.C.; Miller, M.J. Listeria motility increases the efficiency of epithelial invasion during intestinal infection. PLoS Pathog. 2022, 18, e1011028. [Google Scholar] [CrossRef]
- Khalil, M.A.; Sonbol, F.I.A.L.; Aboshady, T.A.; Alqurashi, A.S.; Ali, S.S. Exploring the Therapeutic Potentials of Exopolysaccharides Derived from Lactic Acid Bacteria and Bifidobacteria: Antioxidant, Antitumor, and Periodontal Regeneration. Front. Microbiol. 2022, 13, 803688. [Google Scholar] [CrossRef]
- Jacobsen, C.; Rosenfeldt, V.; Hayford, A.; Møller, P.; Michaelsen, K.; Paerregaard, A.; Sandström, B.; Tvede, M.; Jakobsen, M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Phùng, T.T.T.; Dupont, S.; Beney, L.; Chanut, J.; Karbowiak, T. Unlocking Probiotic Potential: Physicochemical Approaches to Evaluate Probiotic Bacterial Adhesion Potential to the Intestinal Tract. Molec. Nutr. Food Res. 2025, e202400705. [Google Scholar] [CrossRef]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2021, 27, 4179. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Churey, J.J.; Worobo, R.W. Antimicrobial activity of bacterial isolates from different floral sources of honey. Int. J. Food Microbiol. 2008, 126, 240–244. [Google Scholar] [CrossRef]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Saftić Martinović, L.; Birkic, N.; Pavlešić, T.; Planinić, A.; Gobin, I.; Mišetić Ostojić, D.; Pedisić, S. Chemical Characterization of Rare Unifloral Honeys of Ailanthus (Ailanthus altissima), Fennel (Foenicum vulgare), and Raspberry (Rubus idaeus) and their Antimicrobial and Antioxidant Activity. Agric. Res. 2025, 14, 130–142. [Google Scholar] [CrossRef]
- Russell, N.J.; Stöhr, W.; Plakkal, N.; Cook, A.; Berkley, J.A.; Adhisivam, B.; Agarwal, R.; Ahmed, N.U.; Balasegaram, M.; Ballot, D.; et al. Patterns of antibiotic use, pathogens, and prediction of mortality in hospitalized neonates and young infants with sepsis: A global neonatal sepsis observational cohort study (NeoOBS). PLoS Med. 2023, 20, e1004179. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhargava, A. Biofilms and human health. Biotechnol. Lett. 2016, 38, 1–22. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Maggio, F.; Rossi, C.; Serio, A.; Chaves-Lopez, C.; Casaccia, M.; Paparella, A. Anti-biofilm mechanisms of action of essential oils by targeting genes involved in quorum sensing, motility, adhesion, and virulence: A review. Int. J. Food Microbiol. 2024, 426, 110874. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, M. Strategies to Block Bacterial Pathogenesis by Interference with Motility and Chemotaxis. Curr. Top. Microbiol. Immunol. 2016, 398, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.; Maddocks, S.E.; Cooper, R.A. Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J. Antimicrob. Chemother. 2015, 70, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Sierra, J.; Martínez-Guevara, J.L.; Pool-Yam, L.; Magaña-Ortiz, D.; Yam-Puc, A.; Ortiz-Vázquez, E. Effects of phenolic and protein extracts from Melipona beecheii honey on pathogenic strains of Escherichia coli and Staphylococcus aureus. Food Sci. Biotechnol. 2020, 29, 1013–1021. [Google Scholar] [CrossRef]
- Yadav, P.; Shrestha, S.; Basyal, D.; Tiwari, A.; Sah, R.; Sah, A.K.; Yadav, B.; Willcox, M.; Mishra, S.K. Characterization and Biofilm Inhibition of Multidrug-Resistant Acinetobacter baumannii Isolates. Int. J. Microbiol. 2023, 2024, 5749982. [Google Scholar] [CrossRef]
- Eijkelkamp, B.A.; Stroeher, U.H.; Hassan, K.A.; Papadimitrious, M.S.; Paulsen, I.T.; Brown, M.H. Adherence and motility characteristics of clinical Acinetobacter baumannii isolates. FEMS Microbiol. Lett. 2011, 323, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Khan, F.; Tabassum, N.; Kim, Y.-M. Motility of Acinetobacter baumannii: Regulatory systems and controlling strategies. Appl. Microbiol. Biotechnol. 2024, 108, 1–13. [Google Scholar] [CrossRef]
- Martínez, A.C.M.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of essential oils on growth inhibition, biofilm formation and membrane integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Carabarin-Lima, A.; León-Izurieta, L.; Rocha-Gracia, R.d.C.; Castañeda-Lucio, M.; Torres, C.; Gutiérrez-Cazarez, Z.; González-Posos, S.; de la Peña, C.F.M.; Martinez-Laguna, Y.; Lozano-Zarain, P. First evidence of polar flagella in Klebsiella pneumoniae isolated from a patient with neonatal sepsis. J. Med. Microbiol. 2016, 65, 729–737. [Google Scholar] [CrossRef]
- Halstead, F.D.; Webber, M.A.; Rauf, M.; Burt, R.; Dryden, M.; Oppenheim, B.A. In vitro activity of an engineered honey, medical-grade honeys, and antimicrobial wound dressings against biofilm-producing clinical bacterial isolates. J. Wound Care 2016, 25, 93–94. [Google Scholar] [CrossRef]
- Pannella, G.; Lombardi, S.J.; Coppola, F.; Vergalito, F.; Iorizzo, M.; Succi, M.; Tremonte, P.; Iannini, C.; Sorrentino, E.; Coppola, R. Effect of biofilm formation by Lactobacillus plantarum on the malolactic fermentation in model wine. Foods 2020, 9, 797. [Google Scholar] [CrossRef]
- Rosendale, D.I.; Maddox, I.S.; Miles, M.C.; Rodier, M.; Skinner, M.; Sutherland, J. High-throughput microbial bioassays to screen potential New Zealand functional food ingredients intended to manage the growth of probiotic and pathogenic gut bacteria. Int. J. Food Sci. Technol. 2008, 43, 2257–2267. [Google Scholar] [CrossRef]
- Kajiwara, S.; Gandhi, H.; Ustunol, Z. Effect of honey on the growth of and acid production by human intestinal Bifidobacterium spp.: An in vitro comparison with commercial oligosaccharides and inulin. J. Food Prot. 2002, 65, 214–218. [Google Scholar] [CrossRef]
- Chick, H.; Shin, H.S.; Ustunol, Z. Growth and acid production by lactic acid bacteria and bifidobacteria grown in skim milk containing honey. J. Food Sci. 2001, 66, 478–481. [Google Scholar] [CrossRef]
- Popa, D.; Ustunol, Z. Influence of sucrose, high fructose corn syrup and honey from different floral sources on growth and acid production by lactic acid bacteria and bifidobacteria. Int. J. Dairy Technol. 2011, 64, 247–253. [Google Scholar] [CrossRef]
- Conway, P.L.; Stern, R.; Tran, L. The Valueadding Potential of Prebiotic Components of Australian Honey; RIRDC Project No. PRJ-000041 RIRDC Publication No. 09/0179; RIRDC Publication: Barton, Australia, 2010; 30p, ISBN 1 74151 975 6. ISSN 1440-6845. [Google Scholar]
- Celebioglu, H.U.; Erden, Y.; Ozel, H.B. In vitro cytotoxic effects of lactobacilli grown with lime honey on human breast and colon cancer cells. Food Biosci. 2021, 41, 101020. [Google Scholar] [CrossRef]
- Shamala, T.; Shri-Jyothi, Y.; Saibaba, P. Stimulatory effect of honey on multiplication of lactic acid bacteria under in vitro and in vivo conditions. Lett. Appl. Microbiol. 2000, 30, 453–455. [Google Scholar] [CrossRef]
- Kgozeimeh, F.; Golestannejad, Z.; Tofighi, M.; Ayen, A.; Doostmohamadi, M.; Gavanji, S.; Bakhtari, A. Antibacterial Activity of Milk Vetch Flower Honey against Four Bacteria of Human Oral Flora: Streptococcus mutans, Lactobacillus casei, Lactobasillus rhamnosus and Lactobasillus plantarum. Annu. Res. Rev. Biol. 2014, 4, 3335. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Caputo, L.; Amato, G.; De Feo, V.; Coppola, R.; Nazzaro, F. Polyphenols Content and In Vitro α-Glycosidase Activity of Different Italian Monofloral Honeys, and Their Effect on Selected Pathogenic and Probiotic Bacteria. Microorganisms 2021, 9, 1694. [Google Scholar] [CrossRef]
- Pompilio, A.; Kaya, E.; Lupetti, V.; Catelli, E.; Bianchi, M.; Maisetta, G.; Esin, S.; Di Bonaventura, G.; Batoni, G. Cell-free supernatants from Lactobacillus strains exert antibacterial, antibiofilm, and antivirulence activity against Pseudomonas aeruginosa from cystic fibrosis patients. Microbes Infect. 2024, 26, 105301. [Google Scholar] [CrossRef]
- Saini, P.; Ayyanna, R.; Kumar, R.; Bhowmick, S.K.; Bhaskar, V.; Dey, B. Restriction of growth and biofilm formation of ESKAPE pathogens by caprine gut-derived probiotic bacteria. Front. Microbiol. 2024, 15, 1428808. [Google Scholar] [CrossRef]
- Lavrentiev, M.A.; Kaliuzhnaia, O.S.; Khokhlenkova, N.V.; Dvinskykh, N.V. Biotechnological research in the development of a functional product with a probiotic component. News Pharm. 2022, 104. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef]
- Rodica, M.; Mihaiela, C. Neuroprotection induced by honey compounds. In Natural Molecules in Neuroprotection and Neurotoxicity; Academic Press: Cambridge, MA, USA, 2023; pp. 1563–1586. [Google Scholar] [CrossRef]
- Fratianni, F.; Amato, G.; Ombra, M.N.; De Feo, V.; Nazzaro, F.; De Giulio, B. Chemical Characterization and Biological Properties of Leguminous Honey. Antioxidants 2024, 13, 482. [Google Scholar] [CrossRef]
- Karataş, Ş.; Aktümsek, A.; Duru, M.E. An investigation of the biological activity of monofloral honey produced in south-western Anatolia. Int. J. Second. Metab. 2021, 8, 300–311. [Google Scholar] [CrossRef]
- Küçükaydın, S.; Tel-Çayan, G.; Çayan, F.; Taş-Küçükaydın, M.; Eroğlu, B.; Duru, M.E.; Öztürk, M. Characterization of Turkish Astragalus honeys according to their phenolic profiles and biological activities with a chemometric approach. Food Biosci. 2023, 53, 102507. [Google Scholar] [CrossRef]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey-loaded alginate/PVA nanofibrous membrane as a potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- De la Mora-López, D.S.; Madera-Santana, T.J.; Olivera-Castillo, L.; Castillo-Ortega, M.M.; López-Cervantes, J.; Sánchez-Machado, D.I.; Ayala-Zavala, J.F.; Soto-Valdez, H. Production and performance evaluation of chitosan/collagen/honey nanofibrous membranes for wound dressing applications. Int. J. Biol. Macromol. 2024, 275, 133809. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cellgrowth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Francolino, R.; Martino, M.; Nazzaro, F.; Sirignano, C.; Fratianni, F.; Coppola, F.; De Martino, L.; Formisano, C.; De Feo, V. Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy. Plants 2025, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Uba, A.I.; Abul, N.; Gulcin, I.; Koyuncu, I.; Yuksekdag, O.; Kumar, M.; Ponnaiya, S.; Tessappan, S.; Nazzaro, F.; et al. A multifunctional natural treasure based on a “one stone, many birds” strategy for designing health-promoting applications: Tordylium apulum. Food Biosci. 2024, 62, 105088. [Google Scholar] [CrossRef]
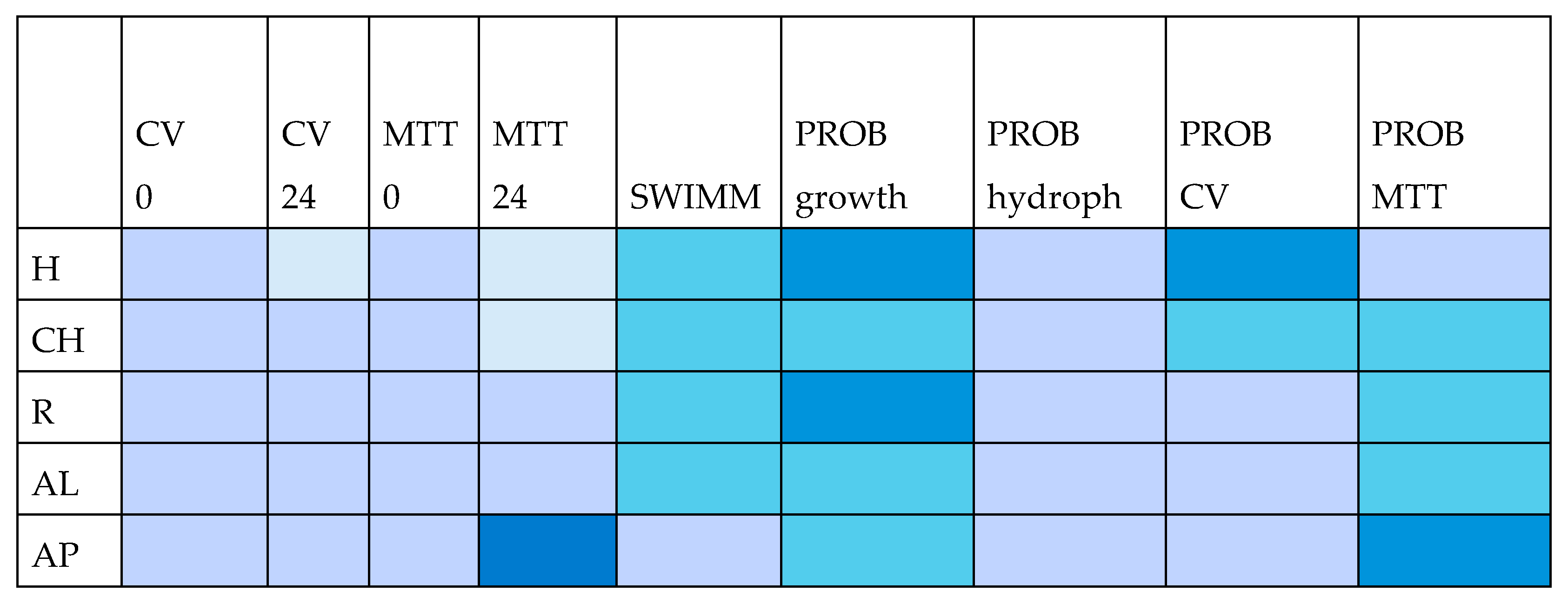
| MIC | AB | EC | KP | LM | PA | SA |
|---|---|---|---|---|---|---|
| H | 34.0 ± 2.0 a | 34.0 ± 1.0 a | >50 b | 33.0 ± 1.0 a | 34.0 ± 1.0 a | 32.0 ± 1.0 a |
| CH | 32.0 ± 1.0 a | 34.0± 1.0 a | 40.0 ± 2.0 a | 32.0 ± 1.0 a | >50 b | 32.0 ± 1.0 a |
| R | 32.0 ± 1.0 a | 32.0± 1.0 a | >50 b | 32.0± 1.0 a | 40.0± 2.0 a | 40.0 ± 2.0 a |
| AL | 32.0 ± 1.0 a | 32.0± 1.0 a | >50 b | 33.0± 1.0 a | >50 b | 40.0 ± 2.0 a |
| AP | 33.0 ± 2.0 a | 30.0± 1.0 a | >50 b | 34.0± 1.0 a | 40.0 ± 2.0 a | 40.0 ± 1.0 a |
| C | 28.0 ± 1.0 | 28.0 ± 2.0 | 32.0± 1.0 a | 30.0 ± 2.0 | 28.0 ± 2.0 | 34.0 ± 1.0 |
| CV 0 (%) | AB | EC | KP | LM | PA | SA |
| H | 57.94 ± 0.37 c | 47.82 ± 0.27 c | 6.89 ± 0.91 a | 55.87 ± 0.18 c | 4.24 ± 0.99 a | 46.25 ± 0.26 c |
| CH | 43.49 ± 0.11 b | 55.39 ± 0.67 c | 29.66 ± 0.87 b | 54.89 ± 0.35 c | 0.00 ± 0.00 | 51.25 ± 0.35 c |
| R | 54.60 ± 0.71 c | 52.99 ± 0.54 c | 0.00 ± 0.00 | 54.40 ± 0.55 c | 31.88 ± 0.8 b | 34.30 ± 0.38 b |
| AL | 52.24 ± 0.28 c | 57.59 ± 0.27 c | 0.00 ± 0.00 | 48.08 ± 0.30 c | 7.86 ± 0.14 a | 28.76 ± 0.45 b |
| AP | 59.43 ± 0.21 c | 54.16 ± 0.57 c | 0.00 ± 0.00 | 44.53 ± 0.45 b | 22.73 ± 0.12 b | 39.86 ± 0.67 b |
| CV24 (%) | AB | EC | KP | LM | PA | SA |
| H | 5.05 ± 0.08 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 26.26 ± 0.15 b | 0.00 ± 0.00 |
| CH | 2.29 ± 0.20 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 20.23 ± 0.16 a | 39.95 ± 0. 05 b |
| R | 14.33 ± 0.35 a | 0.00 ± 0.00 | 1.17 ± 0.04 | 0.00 ± 0.00 | 12.48 ± 0.41 a | 30.13 ± 0.15 b |
| AL | 12.87 ± 0.13 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 33.43 ± 0.26 b | 33.08 ± 0.19 b | 10.95 ± 0.11 a |
| AP | 9.71 ± 0.54 a | 0.77 ± 0.06 | 21.58 ± 0.94 a | 18.82 ± 0.13 a | 38.01 ± 0.94 b | 20.11 ± 0.15 a |
| MTT 0 (%) | AB | EC | KP | LM | PA | SA |
| H | 0.00 ± 0.00 | 27.38 ± 0.84 b | 52.66 ± 0.32 c | 50.16 ± 2.01 c | 19.83 ± 1.13 a | 0.00 ± 0.00 |
| CH | 27.16 ± 0.04 b | 32.96 ± 0.40 b | 56.47 ± 0.56 c | 51.32 ± 1.45 c | 25.66 ± 0.32 b | 0.00 ± 0.00 |
| R | 0.00 ± 0.00 | 35.98 ± 0.62 b | 43.78 ± 1.67 b | 53.39 ± 0.43 c | 12.86 ± 0.21 a | 0.00 ± 0.00 |
| AL | 2.57 ± 0.09 a | 30.18 ± 0.51 b | 33.08 ± 0.57 b | 46.90 ± 0.56 c | 1.78 ± 0.87 a | 0.00 ± 0.00 |
| AP | 0.00 ± 0.00 | 25.37 ± 0.32 b | 24.24 ± 0.26 b | 40.27 ± 0.38 b | 0.23 ± 0.21 | 0.00 ± 0.00 |
| MTT 24 (%) | AB | EC | KP | LM | PA | SA |
| H | 37.57 ± 2.45 b | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| CH | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.36 ± 0.31 a | 0.00 ± 0.00 | 0.00 ± 0.00 |
| R | 32.20 ± 2.64 b | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 15.91 ± 0.62 a | 0.00 ± 0.00 |
| AL | 46.47 ± 3.76 c | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.70 ± 0.16 a | 0.00 ± 0.00 | 5.17 ± 0.33 a |
| AP | 54.36 ± 3.67 c | 41.54 ± 2.23 b | 0.00 ± 0.00 | 5.49 ± 0.11 a | 0.00 ± 0.00 | 19.52 ± 1.57 a |
| AB | EC | KP | LM | PA | SA | |
|---|---|---|---|---|---|---|
| H | 10 ±1.0 c | 10 ± 1.5 a | 12 ± 0.5 a | 10 ±1.0 a | 8 ± 0.5 a | 15 ± 0.5 |
| CH | 15 ±1.0 b | 7 ± 0.5 b | 10 ±1.0 a | 7 ±1.0 a | 7 ±1.0 a | 15 ± 1.5 |
| R | 15 ±1.5 b | 15± 1.5 | 12 ± 1.5 a | 7 ±1.0 a | 13 ±2.0 a | 10 ±1.5 a |
| AL | 18 ± 1.5 b | 15± 1.5 | 15 ± 1.0 a | 10 ±2.0 a | 9 ±1.0 a | 10 ± 0.6 a |
| AP | 20 ± 1.0 a | 10 ±1.0 a | 13 ± 0.5 a | 10 ± 1.5 a | 13 ±1.0 a | 10 ± 0.5 a |
| CO | 25 ± 1.0 | 15 ± 1.5 | 15 ± 1.5 | 12 ± 1.0 | 12 ± 1.5 | 15 ± 1.0 |
| CO | H | CH | R | AL | AP | |
|---|---|---|---|---|---|---|
| LB | 0.603 ± 0.17 | 1.348 ± 0.11 b | 1.222 ± 0.11 b | 1.302 ± 0.12 b | 1.332 ± 0.14 b | 1.392 ± 0.15 b |
| LC | 0.504 ± 0.11 | 1.512 ± 0.12 b | 1.323 ± 0.14 b | 1.622 ± 0.14 b | 1.470 ± 0.15 b | 1.483 ± 0.14 b |
| LG | 0.449 ± 0.08 | 1.377 ± 0.13 b | 1.296 ± 0.11 b | 1.431 ± 0.14 b | 1.387 ± 0.14 b | 1.385 ± 0.11 b |
| LP | 0.125 ± 0.07 | 1.431 ± 0.13 d | 1.331 ± 0.12 d | 1.351 ± 0.11 d | 1.403 ± 0.13 d | 1.442 ± 0.13 d |
| LR | 0.182 ± 0.06 | 1.412 ± 0.13 d | 1.376 ± 0.14 d | 1.435 ± 0.13 d | 1.441 ± 0.14 d | 1.445 ± 0.14 d |
| CO | H | CH | R | AL | AP | |
|---|---|---|---|---|---|---|
| LB | 3.71 ± 0.25 | 10.5 ± 1.13 a | 4.66 ± 0.54 | 8.95 ± 0.77 a | 12.7 ± 1.15 a | 7.97 ± 0.67 a |
| LC | 0.50 ± 0.00 | 4.23 ± 0.31 a | 0.47 ± 0.00 | 10.13 ± 1.21 a | 2.26 ± 0.14 a | 0.84 ± 0.71 |
| LG | 3.12 ± 0.28 | 0.12 + 0.02 | 0.10 + 0.02 | 6.57 ± 0.53 a | 0.09 ± 0.01 | 4.07 ± 0.35 a |
| LP | 0.78 ± 0.00 | 0.30 + 0.03 | 5.87 ± 0.60 a | 0.36 + 0.02 | 0.32 ± 0.02 | 0.34 + 0.02 |
| LR | 0.89 ± 0.09 | 0.72 + 0.06 | 0.85 + 0.08 | 0.74 + 0.09 | 0.76 ± 0.09 | 0.80 + 0.06 |
| CV | H | CH | R | AL | AP | CO | |
|---|---|---|---|---|---|---|---|
| L. bulgaricus | AB | 47.68 ± 3.33 b | 49.33 ± 4.04 | 42.94 ± 4.04 b | 26.71 ± 1.57 a | 38.14 ± 2.22 b | 45.76 ± 3.57 b |
| EC | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.43 ± 0.57 a | 0.00 ± 0.00 | 0.91 ± 0.57 | |
| KP | 72.15 ± 4.44 c | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 33.06 ± 2.34 b | |
| LM | 44.35 ± 4.01 b | 0.00 ± 0.00 | 8.90 ± 1.02 a | 11.92 ± 0.78 a | 15.20 ± 0.67 a | 17.69 ± 1.33 a | |
| PA | 29.28 ± 2.03 b | 0.00 ± 0.00 | 29.62 ± 3.01 b | 4.03 ± 0.57 a | 13.47 ± 1.02 a | 0.00 ± 0.00 | |
| SA | 10.05 0.56 a | 18.07 ± 0.98 a | 19.80 ± 1.45 a | 5.87 ± 1.02 a | 26.58 ± 2.04 b | 5.07 ± 0.13 a | |
| L. casei | AB | 44.97 ± 3.67 b | 44.77 ± 4.03 b | 38.24 ± 3.01 b | 57.99 ± 2.25 c | 53.71 ± 4.01 c | 43.01 ± 3.98 b |
| EC | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.59 ± 1.02 a | 0.00 ± 0.00 | 26.35 ± 2.04 b | 18.96 ± 1.03 a | |
| KP | 72.15 ± 2.34 c | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 30.62 ± 2.2 b | 34.22 ± 2.87 b | |
| LM | 44.35 ± 3.34 b | 0.00 ± 0.00 | 19.46 a ± 1.03 | 30.31 ± 2.92 b | 21.35 ± 1.44 a | 63.95 ± 1.25 c | |
| PA | 29.28 ± 1.55 b | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 20.82 ± 2.03 a | |
| SA | 10.05 ± 0.67 a | 18.07 ± 1.13 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 25.80 ± 2.09 b | |
| L. gasseri | AB | 14.63 ± 1.02 a | 23.83 ± 1.44 a | 25.75 ± 2.01 b | 12.31 ± 0.2 a | 14.23 ± 1.02 a | 29.45 ± 2.09 b |
| EC | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 21.13 ± 1.17 | |
| KP | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| LM | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PA | 0.00 ± 0.00 | 34.43 ± 1.57 b | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| SA | 7.02 ± 1.90 a | 60.62 ± 2.44 c | 28.02 ± 2.12 b | 0.00 ± 0.00 | 27.72 ± 2.09 b | 0.00 ± 0.00 | |
| L. plantarum | AB | 50.71 ± 4.03 c | 13.27 ± 0.57 a | 42.02 ± 2.32 b | 39.68 ± 3.01 b | 24.05 ± 2.12 a | 0.00 ± 0.00 |
| EC | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 14.51 a ± 1.02 | 7.53 a ± 0.57 | 22.23 a ± 0.67 | |
| KP | 0.00 ± 0.00 | 1.44 ± 0.05 | 0.00 ± 0.00 | 1.92 ± 0.06 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| LM | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PA | 0.00 ± 0.00 | 22.97 ± 0.23 a | 0.00 ± 0.00 | 24.69 ± 1.34 a | 26.52 ± 1.55 b | 37.63 ± 1.67 b | |
| SA | 24.93 ± 2.01 a | 21.90 ± 0.57 a | 0.00 ± 0.00 | 15.48 ± 0.13 a | 27.01 ± 1.71 b | 38.59 ± 2.04 b | |
| L. rhamnosus | AB | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| EC | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.95 ± 0.07 a | |
| KP | 3.12 ± 0.05 a | 13.06 ± 0.56 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.81 ± 0.11 a | |
| LM | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PA | 44.16 ± 1.98 c | 14.91 ± 1.02 a | 0.47 ± 0.03 | 0.00 ± 0.00 | 9.28 ± 0.77 a | 0.00 ± 0.00 | |
| SA | 16.99 ± 0.32 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| MTT | H | CH | R | AL | AP | MRS | |
|---|---|---|---|---|---|---|---|
| L. bulgaricus | AB | 11.83 ± 0.57 a | 41.14 ± 1.75 b | 55.13 ± 5.01 c | 67.64 ± 4.43 c | 62.81 ± 4.07 c | 57.93 ± 4.41 c |
| EC | 94.43 ± 1.66 d | 47.70 ± 2.22 b | 35.94 ± 4.75 b | 44.39 ± 4.98 b | 43.54 ± 2.02 b | 34.04 ± 2.57 b | |
| KP | 0.00 ± 0.00 | 18.51 ± 1.76 a | 36.21 ± 1.87 b | 48.08 ± 1.34 b | 46.70 ± 1.13 b | 21.08 ± 0.67 a | |
| LM | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.60 ± 0.00 a | 0.00 ± 0.00 | 14.09 ± 0.98 a | 10.95 ± 0.57 a | |
| PA | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.81 ± 0.03 a | 0.00 ± 0.00 | 4.28 ± 0.10 a | 3.73 ± 0.11 a | |
| SA | 24.60 ± 1.21 a | 36.13 ± 2.31 b | 47.39 ± 3.81 b | 45.98 ± 3.07 b | 47.74 ± 4.44 b | 31.76 ± 3.11 b | |
| L. casei | AB | 67.91 ± 4.57 c | 66.67 ± 3.76 c | 70.76 ± 2.12 c | 59.02 ± 3.87 c | 69.35 ± 3.76 c | 55.89 ± 4.21 c |
| EC | 44.18 ± 1.57 b | 53.99 ± 3.02 c | 46.54 ± 2.98 b | 51.06 ± 3.02 b | 41.26 ± 3.15 b | 34.86 ± 2.78 b | |
| KP | 65.50 ± 2.43 b | 48.50 ± 3.26 b | 45.27 ± 3.21 b | 45.30 ± 3.88 b | 52.80 ± 3.01 b | 24.32 ± 0.57 a | |
| LM | 7.76 ± 1.11 a | 18.67 ± 2.44 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 12.70 ± 0.76 a | |
| PA | 26.30 ± 2.09 b | 19.46 ± 2.09 a | 2.62 ± 0.43 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| SA | 45.57 ± 3.54 b | 51.68 ± 2.98 b | 31.04 ± 1.67 b | 29.80 ± 1.12 b | 37.70 ± 1.45 b | 41.99 ± 1.24 b | |
| L. gasseri | AB | 51.69 ± 4.01 b | 57.34 ± 4.87 c | 66.33 ± 3.01 c | 66.87 ± 2.44 c | 66.69 ± 2.57 c | 62.97 ± 3.01 c |
| EC | 67.81 ± 3.91 c | 60.79 ± 3.65 c | 66.26 ± 2.87 c | 63.36 ± 2.09 c | 68.74 ± 2.76 c | 57.89 ± 1.67 c | |
| KP | 81.77 ± 1.45 d | 88.03 ± 1.98 d | 84.06 ± 1.57 d | 82.59 ± 1.13 d | 87.61 ± 1.13 d | 89.73 ± 1.43 d | |
| LM | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PA | 0.00 ± 0.00 | 0.00 ± 0.00 | 16.01 ± 0.14 a | 22.22 ± 0.12 a | 0.00 ± 0.00 | 1.90 ± 0.05 a | |
| SA | 0.00 ± 0.00 | 2.03 ± 0.06 a | 0.00 ± 0.00 | 19.31 ± 1.12 a | 22.42 ± 1.44 a | 26.11 ± 0.43 b | |
| L. plantarum | AB | 36.79 ± 3.37 b | 32.14 ± 2.42 b | 58.47 ± 4.91 c | 34.52 ± 2.32 b | 40.52 ± 3.01 b | 0.00 ± 0.00 |
| EC | 42.54 ± 1.11 b | 40.23 ± 2.02 b | 46.46 ± 3.98 b | 50.06 ± 3.34 b | 49.30 ± 3.01 b | 13.79 ± 2.67 a | |
| KP | 50.28 ± 1.57 b | 60.04 ± 2.67 c | 46.61 ± 2.25 b | 59.32 ± 3.91 c | 58.44 ± 4.67 c | 29.40 ± 1.57 b | |
| LM | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.31 ± 0.34 a | 23.12 ± 0.57 a | 6.06 ± 0.43 a | 3.15 ± 0.05 a | |
| PA | 0.00 ± 0.00 | 16.30 ± 0.57 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 13.45 ± 1.12 a | 0.00 ± 0.00 | |
| SA | 29.80 ± 2.91 b | 39.86 ± 3.05 b | 28.13 ± 2.03 b | 22.00 ± 1.13 a | 26.99 ± 1.67 b | 0.00 ± 0.00 | |
| L. rhamnosus | AB | 16.08 ± 0.67 a | 36.52 ± 1.25 b | 41.27 ± 2.71 b | 46.06 ± 1.61 b | 35.21 ± 1.23 b | 14.22 ± 1.21 a |
| EC | 53.09 ± 3.11 c | 33.41 ± 2.54 b | 52.95 ± 3.65 c | 52.55 ± 3.97 c | 56.43 ± 4.09 c | 59.68 ± 3.92 c | |
| KP | 53.81 ± 3.67 | 37.01 ± 0.89 b | 69.21 ± 2.13 c | 59.54 ± 3.03 c | 59.53 ± 4.12 c | 57.72 ± 4.24 c | |
| LM | 56.66 ± 2.09 c | 41.29 ± 4.65 b | 48.94 ± 3.74 b | 50.90 ± 4.45 b | 49.70 ± 4.21 b | 60.75 ± 3.12 c | |
| PA | 56.85 ± 1.14 c | 44.29 ± 3.85 b | 47.16 ± 3.05 b | 39.54 ± 4.01 b | 40.29 ± 1.43 b | 43.04 ± 3.41 b | |
| SA | 50.25 ± 5.21 c | 40.54 ± 3.67 b | 43.39 ± 3.13 b | 44.79 ± 4.35 b | 36.95 ± 3.02 b | 51.44 ± 4.15 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, F.; Abdalrazeq, M.; Fratianni, F.; Ombra, M.N.; Testa, B.; Zengin, G.; Ayala Zavala, J.F.; Nazzaro, F. Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties. Antibiotics 2025, 14, 298. https://doi.org/10.3390/antibiotics14030298
Coppola F, Abdalrazeq M, Fratianni F, Ombra MN, Testa B, Zengin G, Ayala Zavala JF, Nazzaro F. Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties. Antibiotics. 2025; 14(3):298. https://doi.org/10.3390/antibiotics14030298
Chicago/Turabian StyleCoppola, Francesca, Manar Abdalrazeq, Florinda Fratianni, Maria Neve Ombra, Bruno Testa, Gokhan Zengin, Jesus Fernando Ayala Zavala, and Filomena Nazzaro. 2025. "Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties" Antibiotics 14, no. 3: 298. https://doi.org/10.3390/antibiotics14030298
APA StyleCoppola, F., Abdalrazeq, M., Fratianni, F., Ombra, M. N., Testa, B., Zengin, G., Ayala Zavala, J. F., & Nazzaro, F. (2025). Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties. Antibiotics, 14(3), 298. https://doi.org/10.3390/antibiotics14030298










