Efficient Removal of Tetracyclines and Their Metabolites from Wastewater Using Purified Stevensite: Adsorption Capacity, Reusability, and Antibiotic Decontamination
Abstract
1. Introduction
2. Results and Discussion
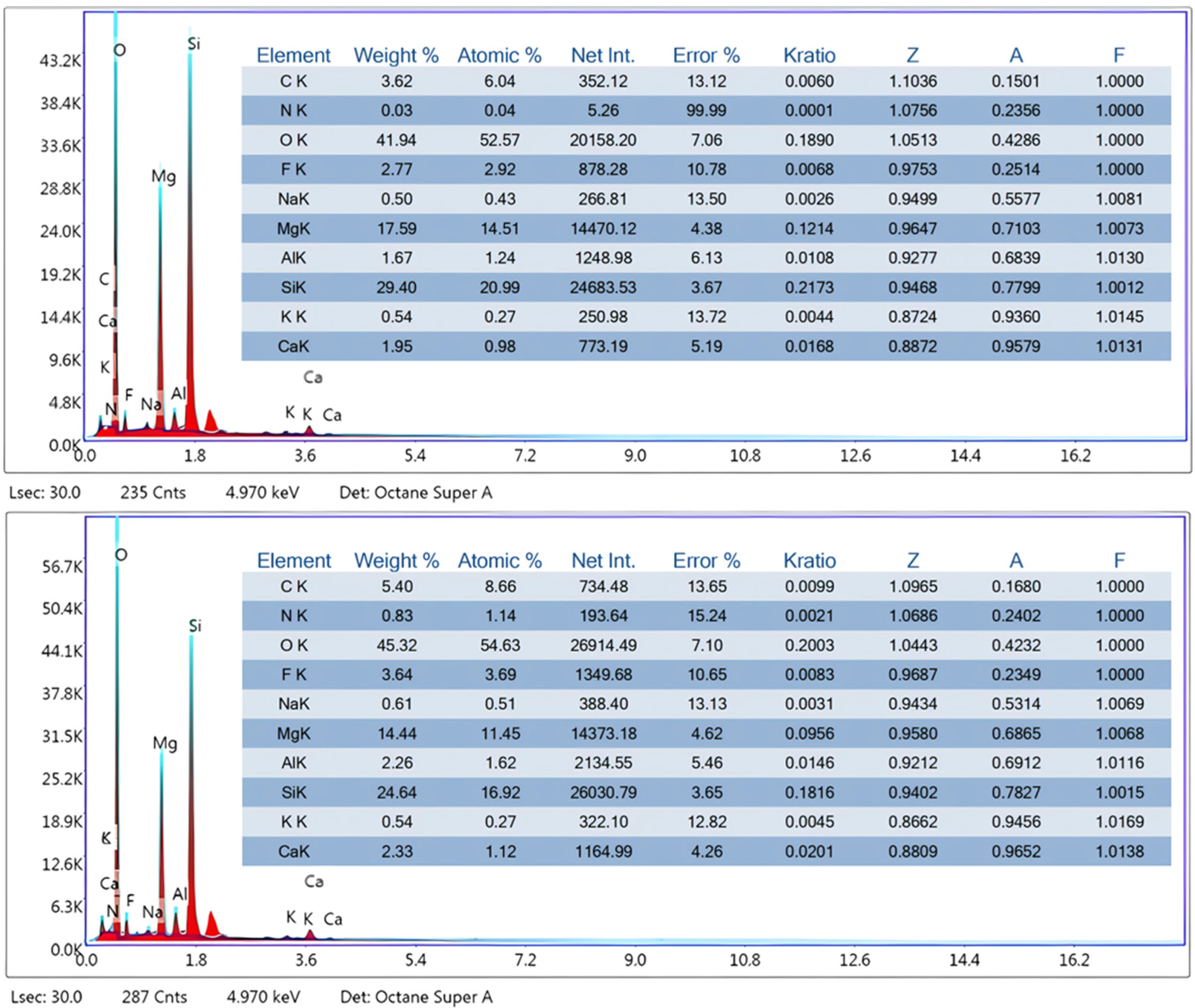
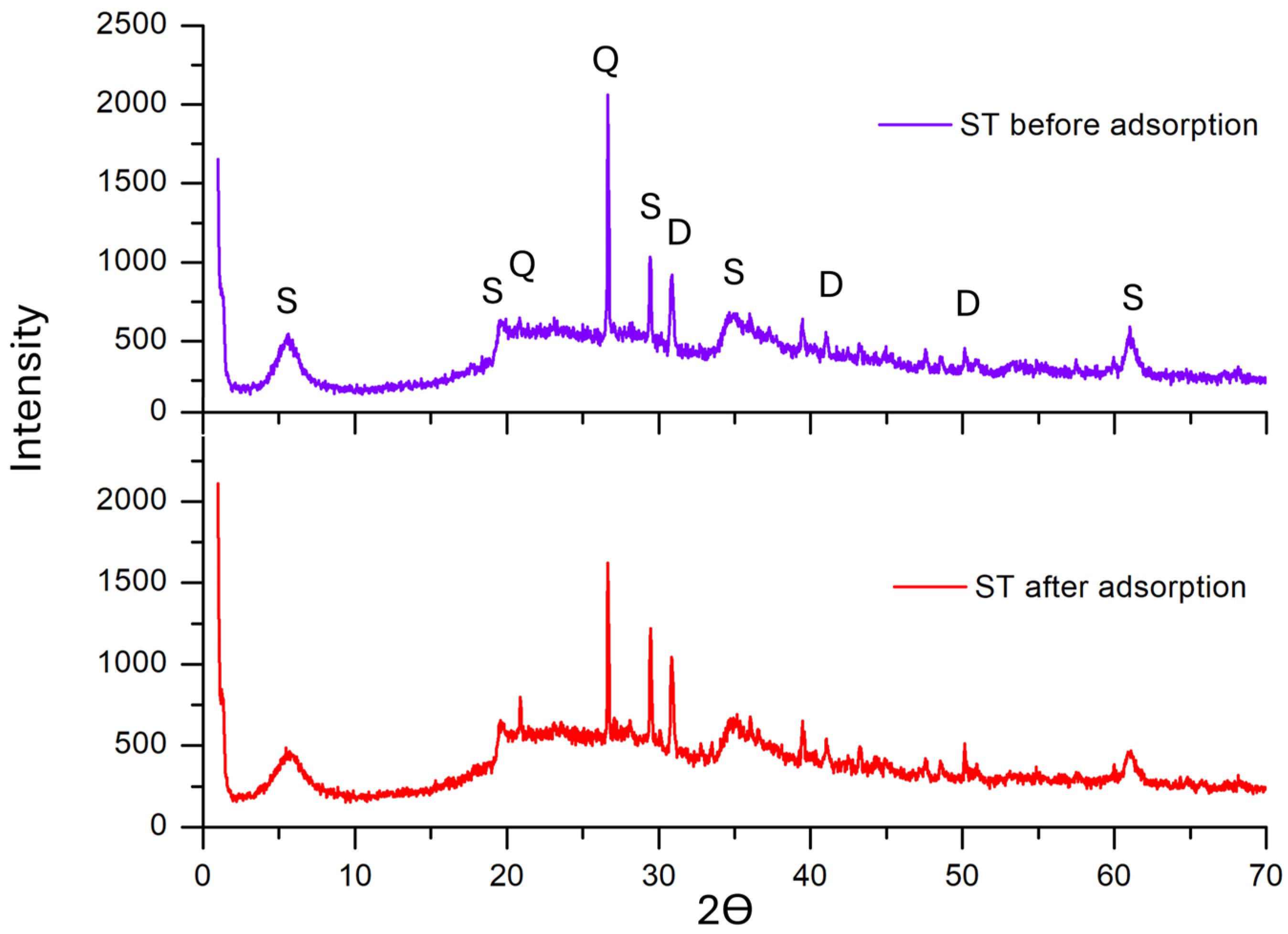
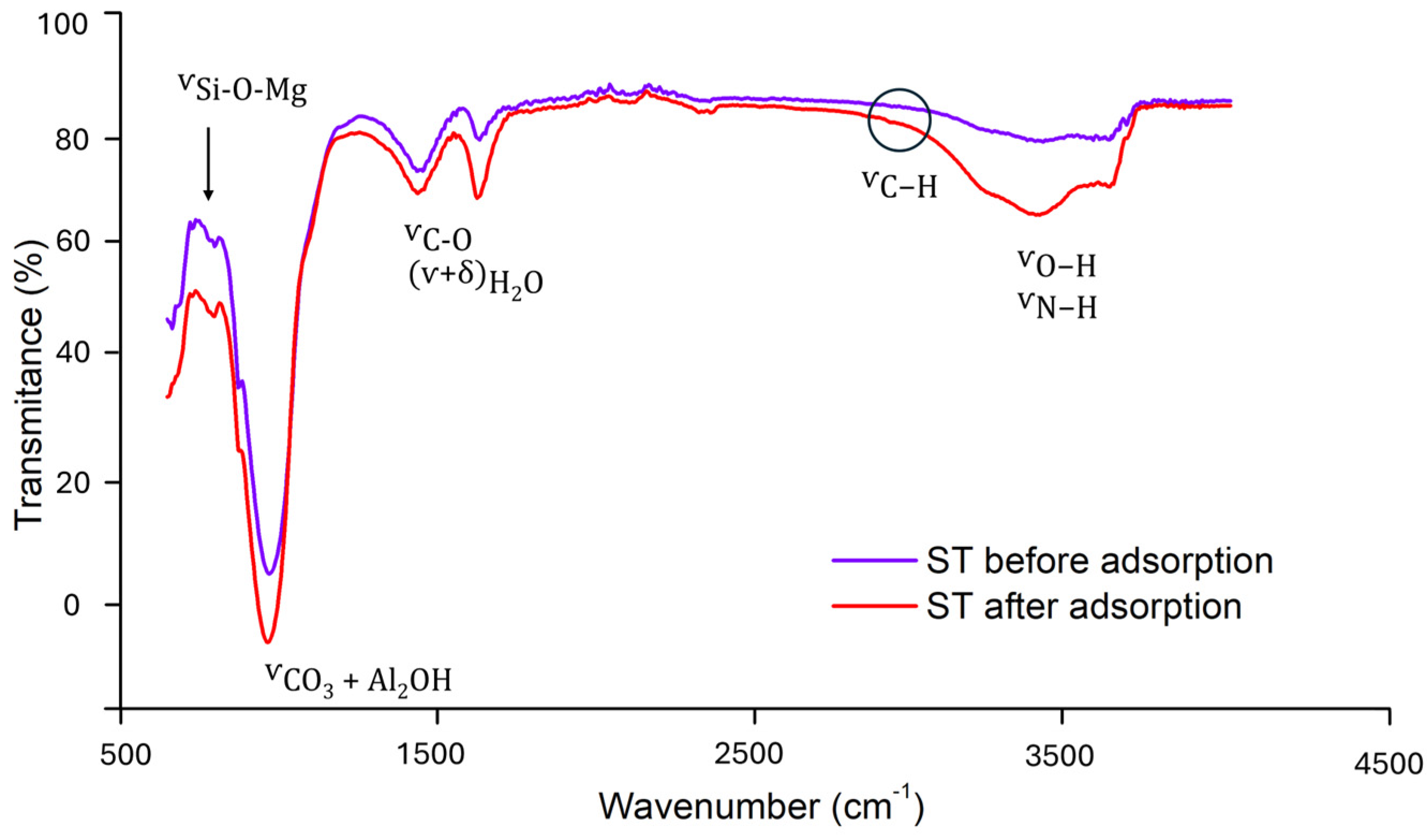
2.1. Adsorbent Characterization
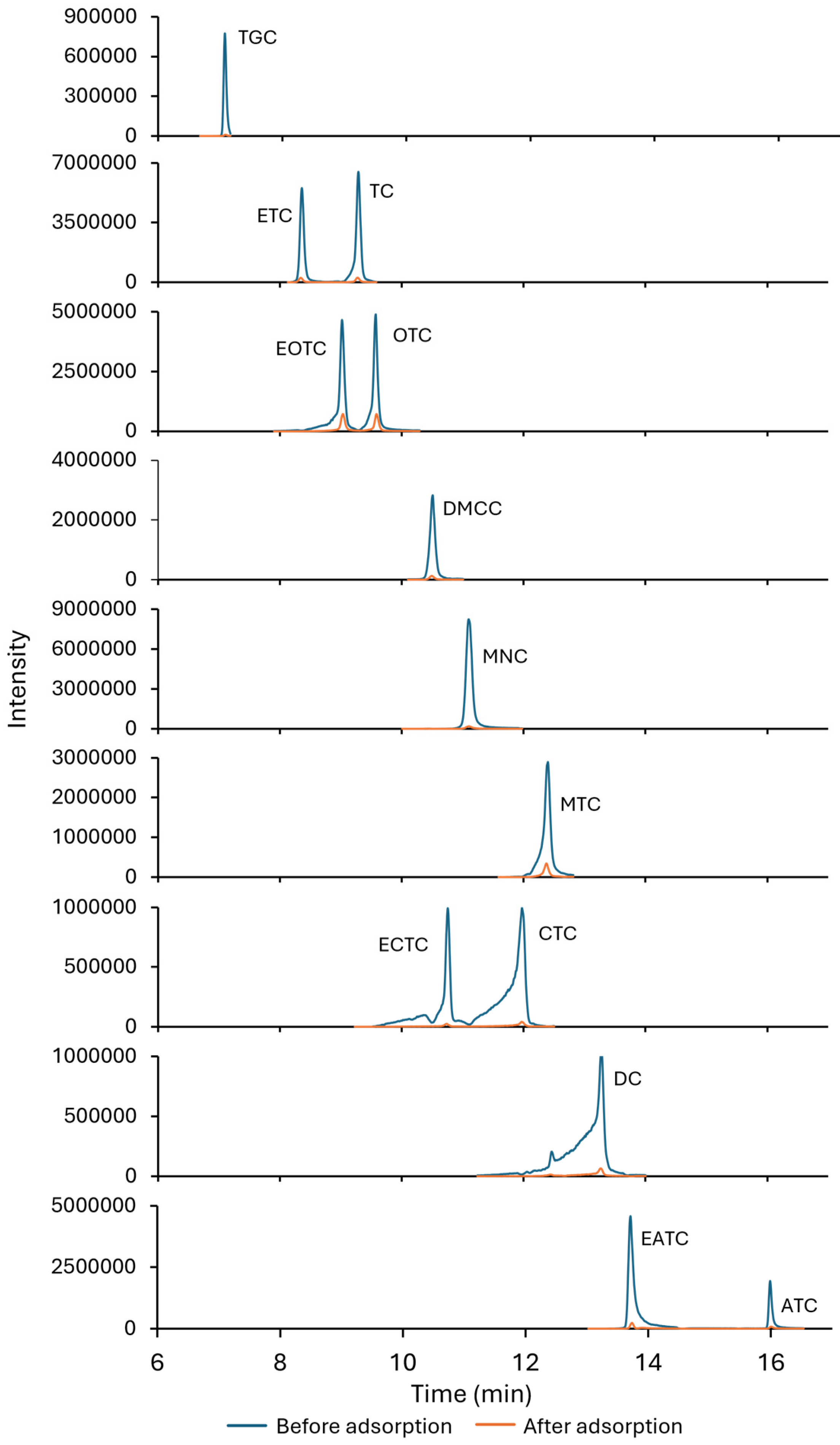
2.2. Adsorption Studies
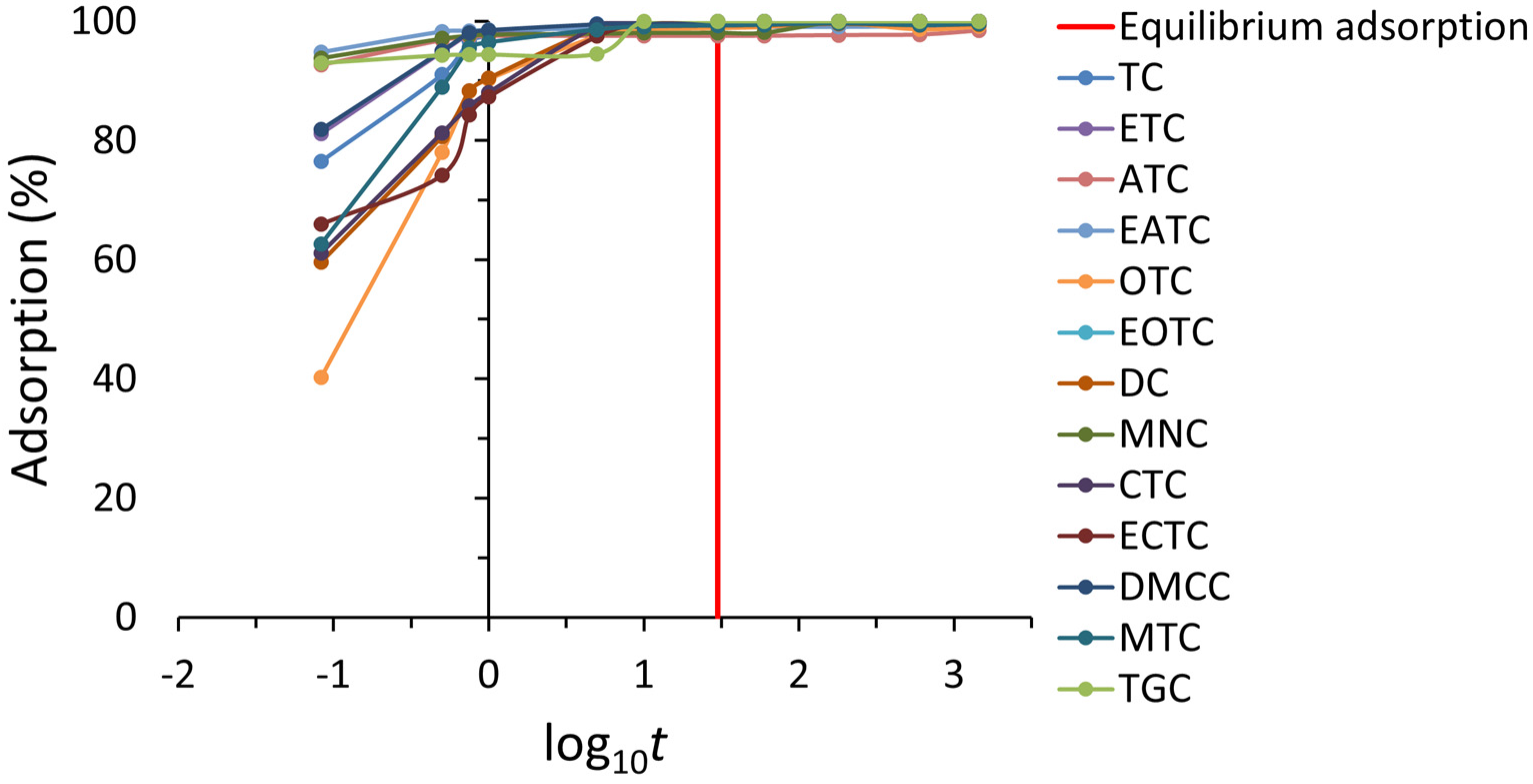
2.2.1. Kinetic Studies
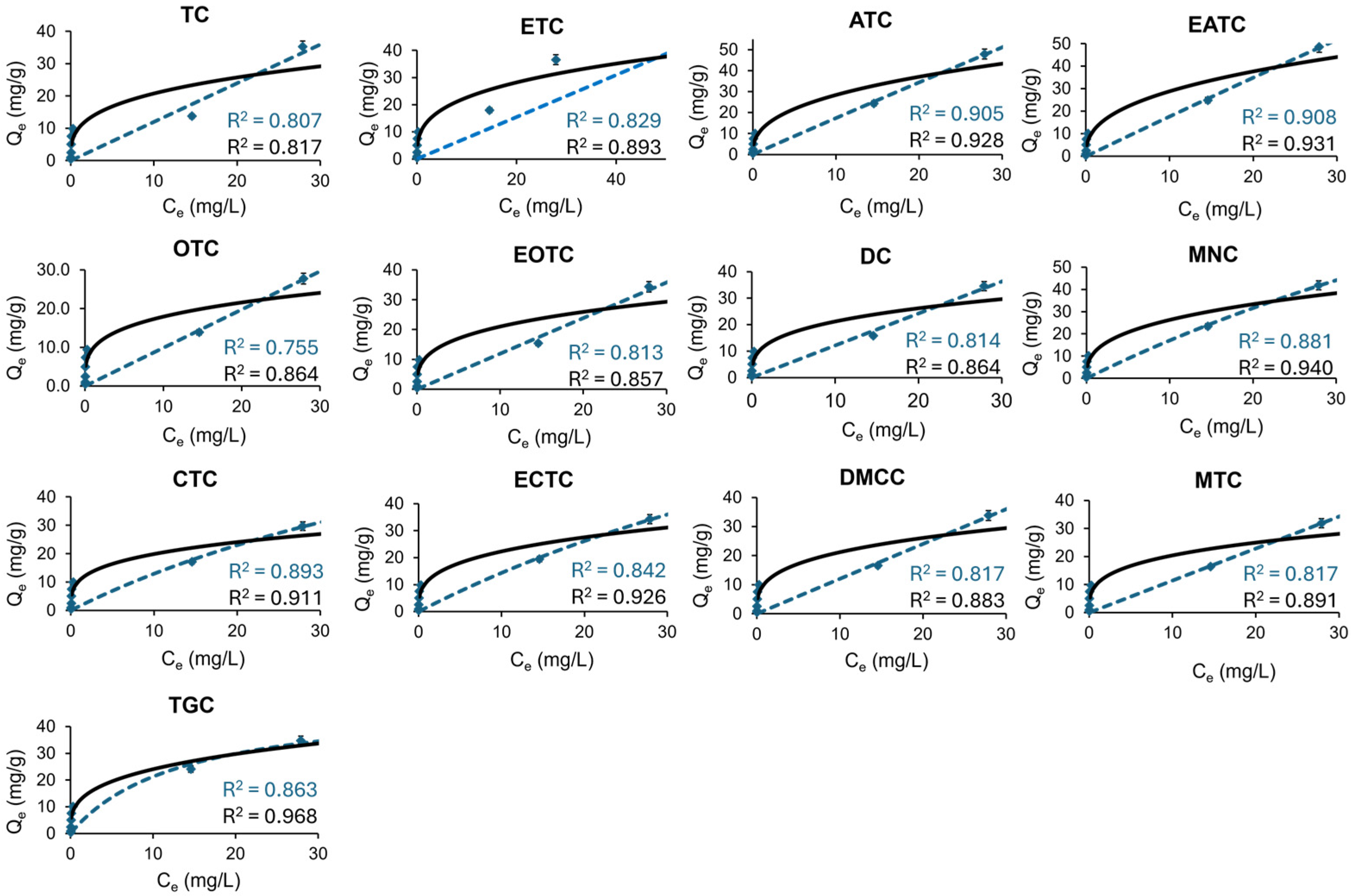
2.2.2. Isotherm Studies
2.2.3. Influence of Environmental Conditions: pH, Salt, and Organic Content
2.3. Mechanistic Study
2.4. Desorption and Reuse
2.5. Adsorption in Real Environmental Matrices
| Clay Material | Tetracycline(s) | Adsorbent Dose (g L−1) | Tetracycline Concentration (mg L−1) | qmax (mg g−1) | % Adsorption | Equilibrium Time | References |
|---|---|---|---|---|---|---|---|
| Kaolinite group | |||||||
| Kaolinite | TC | 4 1 | 50 | 47 | 90%; ~100% | 4 h; 24 h | [42] |
| Kaolinite | TC | 1 | 0.113 mM | - | 90% | 24 h | [43] |
| Illite group | |||||||
| Illite | TC | 10 | 1000 | 32 | - | 8 h | [44] |
| Vermiculite group | |||||||
| Vermiculite | OTC | 2 | 160 | 37 | 77% | 24 h | [45] |
| Palygorskite group | |||||||
| palygorskite | TC | 0.5 | 200 | 99 | 93% | 2 h | [46] |
| Smectite group | |||||||
| Montmorillonite | TC | 0.2 | 0.113 mM | - | 90% | 24 h | [43] |
| Montmorillonite (SWy-2) | TC | 0.5 | 250 | 227 | 70.76%; 78.64% | 0.25 h; 6 h | [47] |
| Montmorillonite | TC | 0.2 | 100 | 250 | ~100% | 24 h | [39] |
| Montmorillonite | TC | 25 | 450 | 287 | >85% | 24 h | [35] |
| Ca-bentonite | TC | 0.2 | 250 | 284 | - | 7 days | [40] |
| Bentonite | TC | 1.25 | 60 | 537 | - | 8 days | [48] |
| Bentonite | TC | 0.4 | 200 | 157 | <60% | 4.2 h | [31] |
| Maknessy–Mazzouna | TC | 50 | 1000 µM | 368 | ~100% | 24 h | [49] |
| Stevensite | OTC, TC, and CTC | 5 | 1000 | 126–140 | ~100% | 24 h | [16] |
| Stevensite | TC, OTC, CTC, DC, MTC, MNC, DMCC, TGC, ETC, ATC, EATC, EOTC, and ECTC | >86 (for all compounds) | >99% | 0.5 h | present study |
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Purification and Pretreatment of Stevensite (ST)
3.3. Characterization Analysis
3.4. Batch Adsorption Experiments
3.5. LC-MS/MS Analysis
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATC | anhydrotetracycline |
| CTC | chlortetracycline |
| Ce | equilibrium concentration |
| DC | doxycycline |
| DMCC | demeclocycline |
| dMRM | dynamic multiple reaction monitoring |
| DOM | dissolved organic matter |
| EDS | energy-dispersive X-ray spectroscopy |
| EATC | 4-epianhydrotetracycline |
| ECTC | 4-epichlortetracycline |
| EOTC | 4-epioxytetracycline |
| ESI | electrospray ionization |
| ETC | 4-epitetracycline |
| FTIR | Fourier transform infrared spectroscopy |
| k1 | PFO kinetic constants |
| k2 | PSO kinetic constants |
| KF | Freundlich constant (L mg−1) |
| KL | Langmuir dissociation constant (L mg−1) |
| LC-MS/MS | liquid chromatography coupled to tandem mass spectrometry |
| MNC | minocycline |
| MTC | metacycline |
| n | Freundlich constant related to the adsorption strength of the adsorbent |
| NRMSE | normalized root mean square error |
| OTC | oxytetracycline |
| PFO | pseudo-first order |
| PSO | pseudo-second order |
| q | equilibrium adsorption capacity (mg g−1) |
| qe | amount of tetracyclines adsorbed at equilibrium (mg g−1) |
| qmax | maximum amount adsorbed within a monolayer (mg g−1) |
| qt | amount of tetracyclines adsorbed at determined time (mg g−1) |
| QQQ | triple quadrupole mass spectrometer |
| SEM | scanning electron microscopy |
| ST | stevensite |
| TC | tetracycline |
| TGC | tigecycline |
| XRD | X-ray diffraction |
References
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazard. Mater. Lett. 2024, 5, 100105. [Google Scholar]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar]
- Antos, J.; Piosik, M.; Ginter-Kramarczyk, D.; Zembrzuska, J.; Kruszelnicka, I. Tetracyclines contamination in European aquatic environments: A comprehensive review of occurrence, fate, and removal techniques. Chemosphere 2024, 353, 141519. [Google Scholar]
- Mahamallik, P.; Saha, S.; Pal, A. Tetracycline degradation in aquatic environment by highly porous MnO2 nanosheet assembly. Chem. Eng. J. 2015, 276, 155–165. [Google Scholar]
- Hou, J.; Wang, C.; Mao, D.; Luo, Y. The occurrence and fate of tetracyclines in two pharmaceutical wastewater treatment plants of Northern China. Environ. Sci. Pollut. Res. 2016, 23, 1722–1731. [Google Scholar]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic effects of tetracycline and its degradation products on freshwater green algae. Ecotoxicol. Environ. Saf. 2019, 174, 43–47. [Google Scholar] [CrossRef]
- Alacahan, Ö.F.; Özyonar, F. Removal of tetracycline using different treatment processes; electrocoagulation, ultrasound and ultrasound assisted electrocoagulation. Environ. Process. 2024, 11, 58. [Google Scholar]
- Liu, Z.; Wen, H.; Jiang, S.; Xu, J.; Chen, P. A comparative study of antibiotic treatment by different charged nanofiltration membranas. Desalination 2025, 597, 118316. [Google Scholar] [CrossRef]
- Nikzad, M.; Mousavi, S.Y.; Heydarian, M.; Rahmani, S.; Shabanian, S.R.; Hejazi, F. A review on recent advances in photodegradation of tetracycline in aqueous media. J. Iran. Chem. Soc. 2024, 21, 887–902. [Google Scholar]
- Mustapha, N.; Gouda, M.H.; Rafea, M.A.; Salerno, M.; Ahmed, A.M.; Elessawy, N.A. Utilizing a novel green ternary polymeric nanocomposite material to remove tetracycline antibiotic effectively from aqueous solutions. Absorption 2024, 30, 1881–1891. [Google Scholar] [CrossRef]
- Gharous, M.; Martín, J.; Mejías, C.; Bounab, L.; Ghoukairi, M.; Santos, J.L.; Aparicio, I.; Alonso, E. Methionine-stevensite derived bionanocomposite: A green and efficient adsorbent for the removal of antibiotics. Environ. Technol. Innov. 2024, 34, 103591. [Google Scholar]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar]
- Bouna, L.; Rhouta, B.; Maury, F.; Jada, A.; Senocq, F.; Lafont, M.-C. Photocatalytic activity of TiO2/stevensite nanocomposites for the removal of Orange G from aqueous solutions. Clay Miner. 2014, 49, 417–428. [Google Scholar]
- Hacıosmanoğlu, G.G.; Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review. J. Environ. Manag. 2022, 317, 115397. [Google Scholar]
- Bounori, Y.; Berkani, M.; Haddak, N.; Idirene, M.; Zatout, S.; Tensaout, S.; Foura, G.; Meziti, C.; Haroune, S. Removal of malachite green from aqueous solution by kerolite/stevensite mixed-layer clay: Full factorial design analysis. Int. J. Environ. Anal. Chem. 2024, 1–24. [Google Scholar] [CrossRef]
- Antón-Herrero, R.; García-Delgado, C.; Alonso-Izquierdo, M.; García-Rodríguez, G.; Cuevas, J.; Eymar, E. Comparative adsorption of tetracyclines on biochars and stevensite: Looking for the most effective adsorbent. Appl. Clay Sci. 2018, 160, 162–172. [Google Scholar]
- Ben Seddik, N.; Raissouni, I.; Draoui, K.; Ait Aghzzaf, A.; Chraka, A.; Aznag, B.; Chaouket, F.; Bouchta, D. Calcite, the main corrosion inhibitor contained in the raw clay (Rhassoul) of brass in 3% NaCl medium. Mediterr. J. Chem. 2019, 9, 382–391. [Google Scholar]
- Fernández, R.; Ruiz, A.I.; García-Delgado, C.; González-Santamaría, D.E.; Antón-Herrero, R.; Yunta, F.; Poyo, C.; Hernández, A.; Eymar, E.; Cuevas, J. Stevensite-based geofilter for the retention of tetracycline from water. Sci. Total Environ. 2018, 645, 146–155. [Google Scholar]
- Carvalho, T.; Neves, R.; Hildebrando, E.; Betega de Paiva, L.; Valenzuela-Diaz, F.R. Organophilic synthetic stevensite-Zn: Synthesis and characterization, an alternative simple method. Minerals 2022, 12, 1568. [Google Scholar] [CrossRef]
- Brião, G.V.; da Silva, M.G.C.; Vieira, M.G.A.; Chu, K.H. Correlation of type II adsorption isotherms of water contaminants using modified BET equations. Colloid Interface Sci. Commun. 2022, 46, 100557. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Chfaira, R.; Mounir, C. Interfacial electrochemical properties of natural Moroccan Ghassoul (stevensite) clay in aqueous suspension. Heliyon 2020, 6, e03634. [Google Scholar] [PubMed]
- Ahrouch, M.; Gatica, J.M.; Draoui, K.; Bellido, D.; Vidal, H. Lead removal from aqueous solution by means of integral natural clays honeycomb monoliths. J. Hazard. Mater. 2019, 365, 519–530. [Google Scholar] [PubMed]
- Bentahar, Y.; Draoui, K.; Hurel, C.; Khairoun, S.; Ajouyed, O.; Marmier, N. Physico-chemical characterization and valorization of swelling and nonswelling Moroccan clays in basic dye removal from aqueous solutions. J. Afr. Earth Sci. 2019, 154, 80–88. [Google Scholar] [CrossRef]
- Azaryouh, L.; Abara, H.; Kassab, Z.; Ablouh, E.; Aboulkas, A.; El Achaby, M.; Draoui, K. Hybrid carbonaceous adsorbents based on clay and cellulose for cadmium recovery from aqueous solution. RSC Adv. 2023, 13, 6954–6965. [Google Scholar]
- Allaoui, S.; Bennani, M.N.; Ziyat, H.; Qabaqous, O.; Tijani, N.; Ittobane, N. Kinetic study of the adsorption of polyphenols from olive mill wastewater onto natural clay: Ghassoul. J. Chem. 2020, 2020, 7293189. [Google Scholar]
- El Mahbouby, A.; Ezaier, Y.; Zyade, S.; Mechnou, I. Elaboration and characterization of organo-ghassoul (Moroccan clay) as an adsorbent using cationic surfactant for anionic dye adsorption. Phys. Chem. Res. 2023, 11, 913–928. [Google Scholar]
- de Santiago-Buey, C.; Suárez-Barrios, M.; García-Romero, E.; Doval-Montoya, M. Mg-Rich Smectite “precursor” Phase in the Tagus Basin, Spain. Clays Clay Miner. 2000, 48, 366–373. [Google Scholar]
- Madejová, J.; Komadel, P. Baseline studies of FTIR the Clay Minerals Society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Turan, B.; Sarıgöl, G.; Demircivi, P. Adsorption of tetracycline antibiotics using metal and clay embedded cross-linked chitosan. Mater. Chem. Phys. 2022, 279, 125781. [Google Scholar]
- Maged, A.; Iqbal, J.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Tuning tetracycline removal from aqueous solution onto activated 2:1 layered clay mineral: Characterization, sorption and mechanistic studies. J. Hazard. Mater. 2019, 387, 121320. [Google Scholar]
- Li, Y.; Wang, J.; Huang, Z.; Qian, C.; Tian, Y.; Duan, Y. An Eu-doped Zr-metal-organic framework for simultaneous detection and removal of antibiotic tetracycline. J. Environ. Chem. Eng. 2021, 9, 106012. [Google Scholar]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef]
- Mustapha, S.; Shuaib, D.T.; Ndamitso, M.M.; Etsuyankpa, M.B.; Sumaila, A.; Mohammed, U.M.; Nasirudeen, M.B. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods. Appl. Water Sci. 2019, 9, 142. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Vallés, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Nunes Filho, F.G.; Silva Filho, E.C.; Osajima, J.A.; de Melo Alves, A.P.; Fonseca, M.G. Adsorption of tetracycline using chitosan–alginate–bentonite composites. Appl. Clay Sci. 2023, 239, 106952. [Google Scholar]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef]
- Lenart-Boroń, A.; Prajsnar, J.; Guzik, M.; Boroń, P.; Grad, B.; Żelazny, M. Antibiotics in Groundwater and River Water of Białka—A Pristine Mountain River. Appl. Sci. 2022, 12, 12743. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Gao, S.; Geng, J.; Wang, X. Adsorption of tetracycline (TC) onto montmorillonite: Cations and humic acid effects. Geoderma 2012, 183–184, 12–18. [Google Scholar]
- Ortiz-Ramos, U.; Leyva-Ramos, R.; Mendoza-Mendoza, E.; Aragón-Piña, A. Removal of tetracycline from aqueous solutions by adsorption on raw Ca-bentonite: Effect of operating conditions and adsorption mechanism. Chem. Eng. J. 2022, 432, 134428. [Google Scholar]
- Wang, Z.; Muhammad, Y.; Tang, R.; Lu, C.; Yu, S.; Song, R.; Tong, Z.; Han, B.; Zhang, H. Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacine. Sep. Purif. Technol. 2021, 274, 119059. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Li, S.; Han, R.; Wang, G. Insights into tetracycline adsorption onto kaolinite and montmorillonite: Experiments and modeling. Environ. Sci. Pollut. Res. 2015, 22, 17031–17040. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Jean, J.-S.; Jiang, W.-T.; Wang, C.-J.; Lin, K.-H. Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl. Clay Sci. 2012, 67–68, 158–163. [Google Scholar] [CrossRef]
- Liu, S.; Wu, P.; Yu, L.; Li, L.; Gong, B.; Zhu, N.; Dang, Z.; Yang, C. Preparation and characterization of organo-vermiculite based on phosphatidylcholine and adsorption of two typical antibiotics. Appl. Clay Sci. 2017, 137, 160–167. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Yu, T.-L.; Munkhbayer, S.; Kuo, T.-H.; Hung, Y.-C.; Jean, J.-S.; Lin, K.-H. Sorptive removal of tetracycline from water by palygorskite. J. Hazard. Mater. 2009, 165, 148–155. [Google Scholar] [CrossRef]
- Shang, J.; Huang, M.; Zhao, L.; He, P.; Liu, Y.; Pan, H.; Cao, S.; Liu, X. Adsorption performance and mechanisms of tetracycline on clay minerals in estuaries and nearby coastal areas. ACS Omega 2024, 9, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ramos, U.; Leyva-Ramos, R.; Mendoza-Mendoza, E.; Carrasco-Marín, F.; Bailón-García, E.; Villela-Martínez, D.E.; Valdez-García, G.D. Modeling adsorption rate of Trimethoprim, tetracycline and chlorphenamine from aqueous solutions onto natural bentonite clay. Elucidating mass transfer mechanisms. Chem. Eng. J. 2024, 493, 152666. [Google Scholar] [CrossRef]
- Hamdi, S.; Gharbi-Khelifi, H.; Barreiro, A.; Mosbahi, M.; Cela-Dablanca, R.; Brahmi, J.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Issaoui, M.; Álvarez-Rodríguez, E. Tetracycline adsorption/desorption by raw and activated Tunisian clays. Environ. Res. 2024, 242, 117536. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- DrugBank Database. Available online: https://go.drugbank.com/ (accessed on 4 December 2024).
- ChemicalBook Database. Available online: https://www.chemicalbook.com/ (accessed on 4 December 2024).
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef]



| Kinetic Parameters | TGC | ETC | EOTC | TC | OTC | DMCC | ECTC | MNC | CTC | MTC | DC | EATC | ATC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo-first order: | |||||||||||||
| k1 (min−1) | 0.026 | 0.380 | 0.463 | 0.532 | 0.423 | 0.274 | 0.467 | 0.321 | 0.452 | 0.390 | 0.492 | 0.165 | 3.946 |
| ±0.015 | ±0.002 | ±0.003 | ±0.005 | ±0.008 | ±0 | ±0.002 | ±0 | ±0.019 | ±0.006 | ±0.007 | ±0.002 | ±0.004 | |
| qecal (mg·g−1) | 0.063 | 0.046 | 0.140 | 0.221 | 0.290 | 0.056 | 0.26 | 0.013 | 0.285 | 0.155 | 0.229 | 0.012 | 0.048 |
| ±0.004 | ±0.002 | ±0.007 | ±0.004 | ±0.04 | ±0 | ±0.18 | ±0 | ±0.06 | ±0.011 | ±0.008 | ±0.002 | ±0.003 | |
| qeexp (mg·g−1) | 1.03 | 1.05 | 1.05 | 1.07 | 1.29 | 1.28 | 0.983 | 1.14 | 1.32 | 1.58 | 1.17 | 1.09 | 0.991 |
| R2 | 0.259 | 0.738 | 0.871 | 0.868 | 0.879 | 0.600 | 0.986 | 0.717 | 0.493 | 0.774 | 0.962 | 0.436 | 0.596 |
| Pseudo-second order: | |||||||||||||
| k2 (g·mg−1·min−1) | 19.9 | 21.4 | 11.1 | 56.0 | 6.2 | 25.8 | 11.5 | 3.84 | 13.0 | 6.88 | 8.65 | 9.76 | 2.11 |
| ±0.13 | ±0.63 | ±1.8 | ±12 | ±1.9 | ±0 | ±2 | ±0.02 | ±2 | ±1.2 | ±1.3 | ±1.0 | ±1.1 | |
| qecal (mg·g−1) | 1.034 | 1.0500 | 1.051 | 1.066 | 1.280 | 1.280 | 0.983 | 1.138 | 1.317 | 1.5750 | 1.167 | 1.085 | 0.990 |
| ±0 | ±0.0002 | ±0 | ±0 | ±0.0013 | ±0 | ±0 | ±0 | ±0 | ±0.001 | ±0.001 | ±0.0004 | ±0.005 | |
| qeexp (mg·g−1) | 1.034 | 1.051 | 1.052 | 1.067 | 1.288 | 1.280 | 0.983 | 1.138 | 1.317 | 1.575 | 1.168 | 1.086 | 0.991 |
| R2 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Model Parameters | TGC | ETC | EOTC | TC | OTC | DMCC | ECTC | MNC | CTC | MTC | DC | EATC | ATC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir: | |||||||||||||
| qmax (mg·g−1) | 86.1 | 856.3 | 1000 | 1000 | 1000 | 1000 | 221.6 | 262.7 | 625.6 | 609.2 | 777.2 | 851.1 | 883.6 |
| KL (mg·g−1) | 0.029 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.007 | 0.007 | 0.003 | 0.002 | 0.002 | 0.002 | 0.002 |
| R2 | 0.863 | 0.829 | 0.813 | 0.807 | 0.755 | 0.817 | 0.842 | 0.881 | 0.893 | 0.817 | 0.814 | 0.908 | 0.905 |
| Freundlich: | |||||||||||||
| KF (mg1−n·Ln·g−1) | 12.0 | 11.0 | 10.6 | 10.4 | 9.8 | 10.9 | 11.3 | 12.0 | 11.8 | 10.8 | 10.7 | 11.8 | 11.7 |
| n | 0.325 | 0.320 | 0.315 | 0.323 | 0.288 | 0.313 | 0.321 | 0.349 | 0.369 | 0.311 | 0.314 | 0.389 | 0.385 |
| R2 | 0.968 | 0.893 | 0.857 | 0.817 | 0.864 | 0.883 | 0.926 | 0.940 | 0.911 | 0.891 | 0.864 | 0.931 | 0.928 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Criado, N.; Martín-Pozo, L.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Efficient Removal of Tetracyclines and Their Metabolites from Wastewater Using Purified Stevensite: Adsorption Capacity, Reusability, and Antibiotic Decontamination. Antibiotics 2025, 14, 395. https://doi.org/10.3390/antibiotics14040395
García-Criado N, Martín-Pozo L, Martín J, Santos JL, Aparicio I, Alonso E. Efficient Removal of Tetracyclines and Their Metabolites from Wastewater Using Purified Stevensite: Adsorption Capacity, Reusability, and Antibiotic Decontamination. Antibiotics. 2025; 14(4):395. https://doi.org/10.3390/antibiotics14040395
Chicago/Turabian StyleGarcía-Criado, Noelia, Laura Martín-Pozo, Julia Martín, Juan Luis Santos, Irene Aparicio, and Esteban Alonso. 2025. "Efficient Removal of Tetracyclines and Their Metabolites from Wastewater Using Purified Stevensite: Adsorption Capacity, Reusability, and Antibiotic Decontamination" Antibiotics 14, no. 4: 395. https://doi.org/10.3390/antibiotics14040395
APA StyleGarcía-Criado, N., Martín-Pozo, L., Martín, J., Santos, J. L., Aparicio, I., & Alonso, E. (2025). Efficient Removal of Tetracyclines and Their Metabolites from Wastewater Using Purified Stevensite: Adsorption Capacity, Reusability, and Antibiotic Decontamination. Antibiotics, 14(4), 395. https://doi.org/10.3390/antibiotics14040395









