Mechanism Insight of Cell Death Signaling by Thymol Derivatives on Trypanosomatidae Protozoan Parasites
Abstract
1. Introduction
2. Results and Discussion
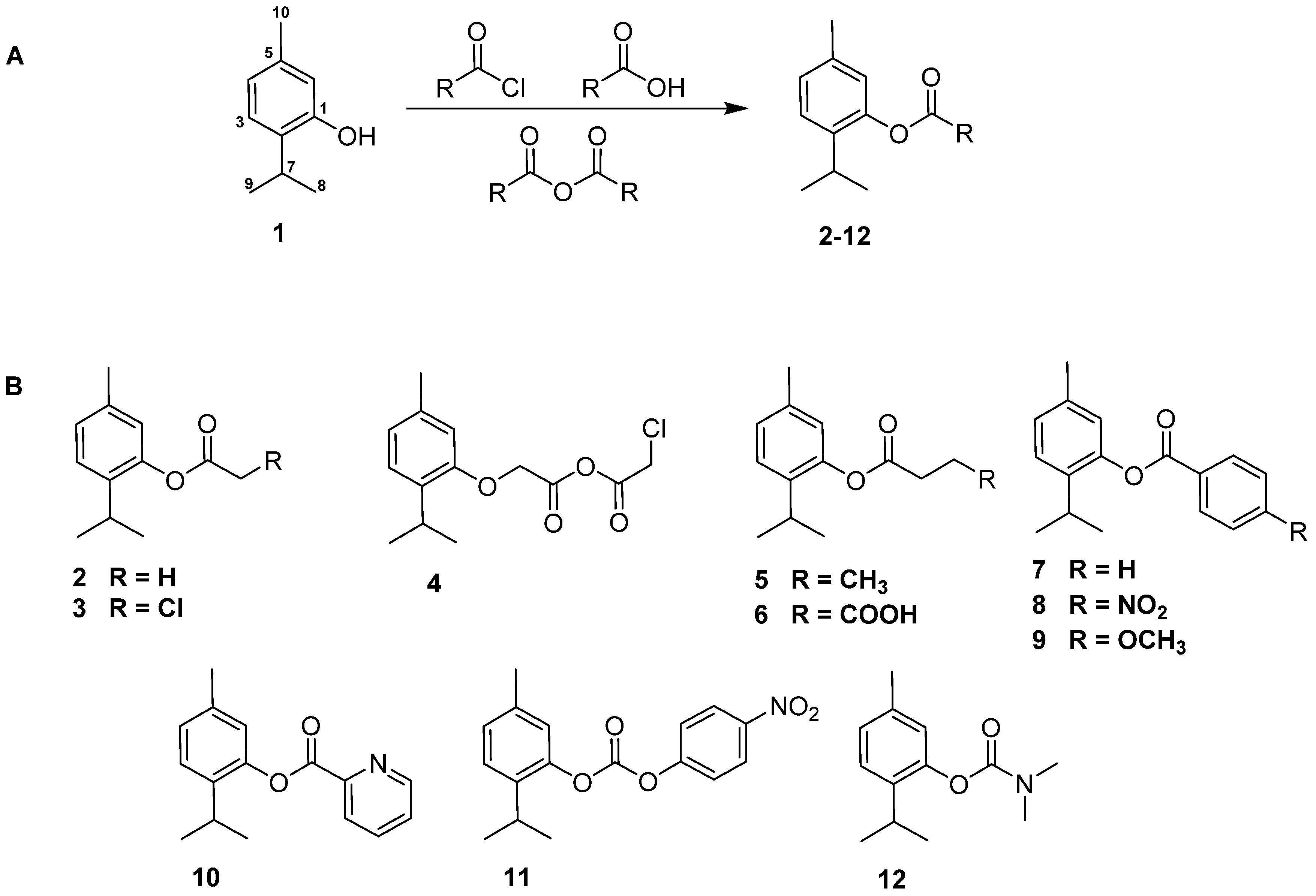
2.1. Chemistry
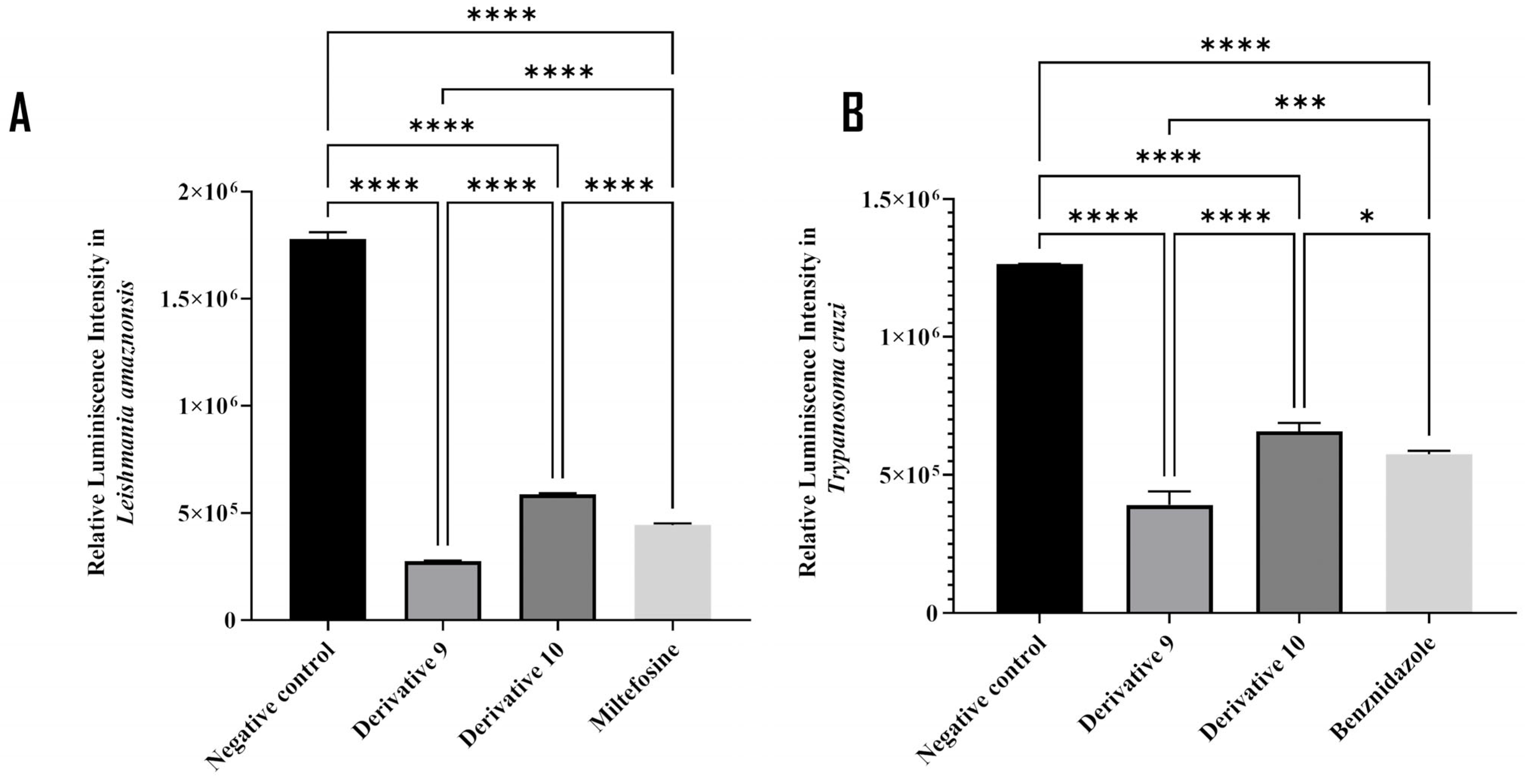
2.2. In Vitro Kinetoplastid Activity on L. amazonensis and T. cruzi
2.3. Preliminary Structure–Activity Relationship
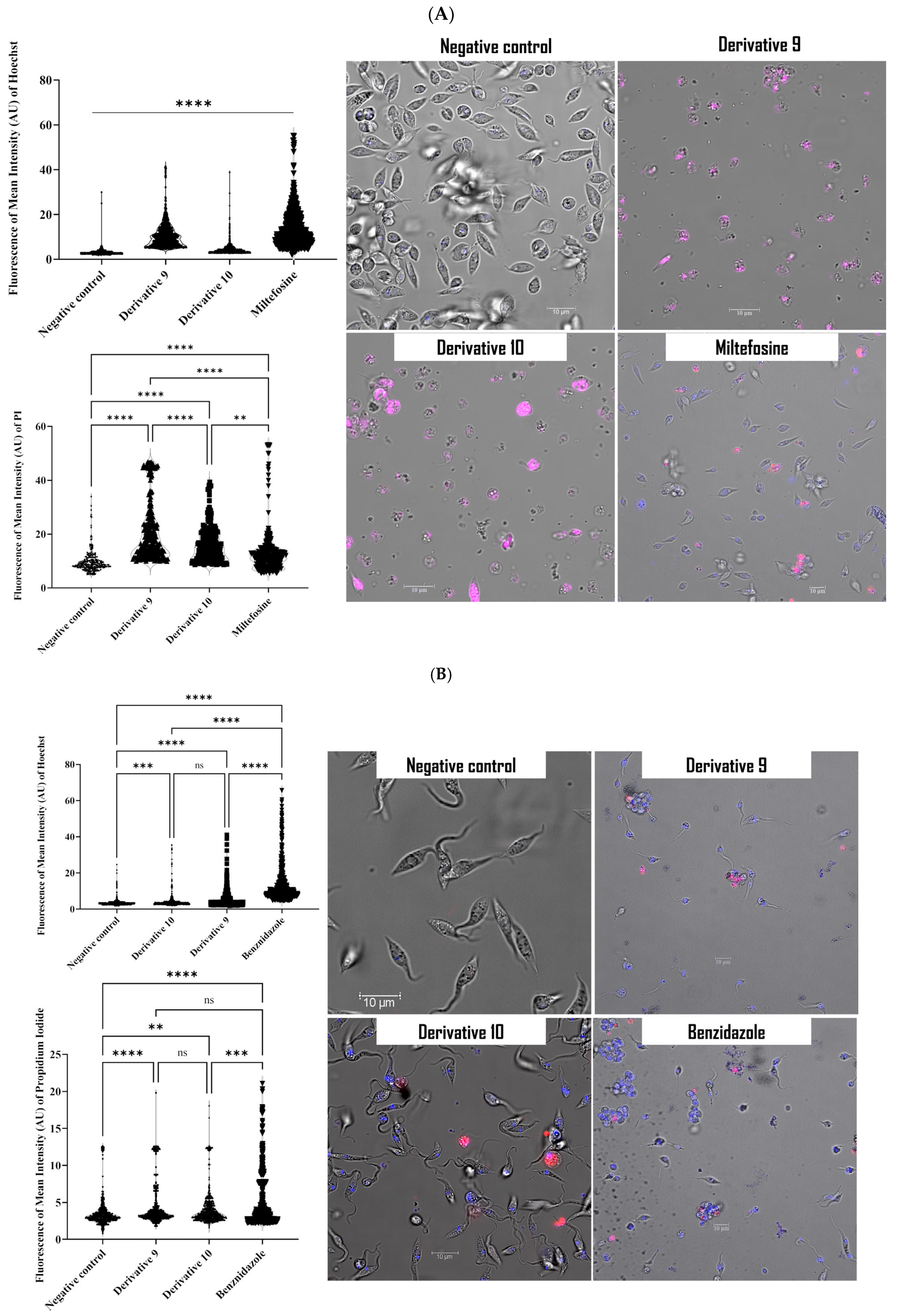
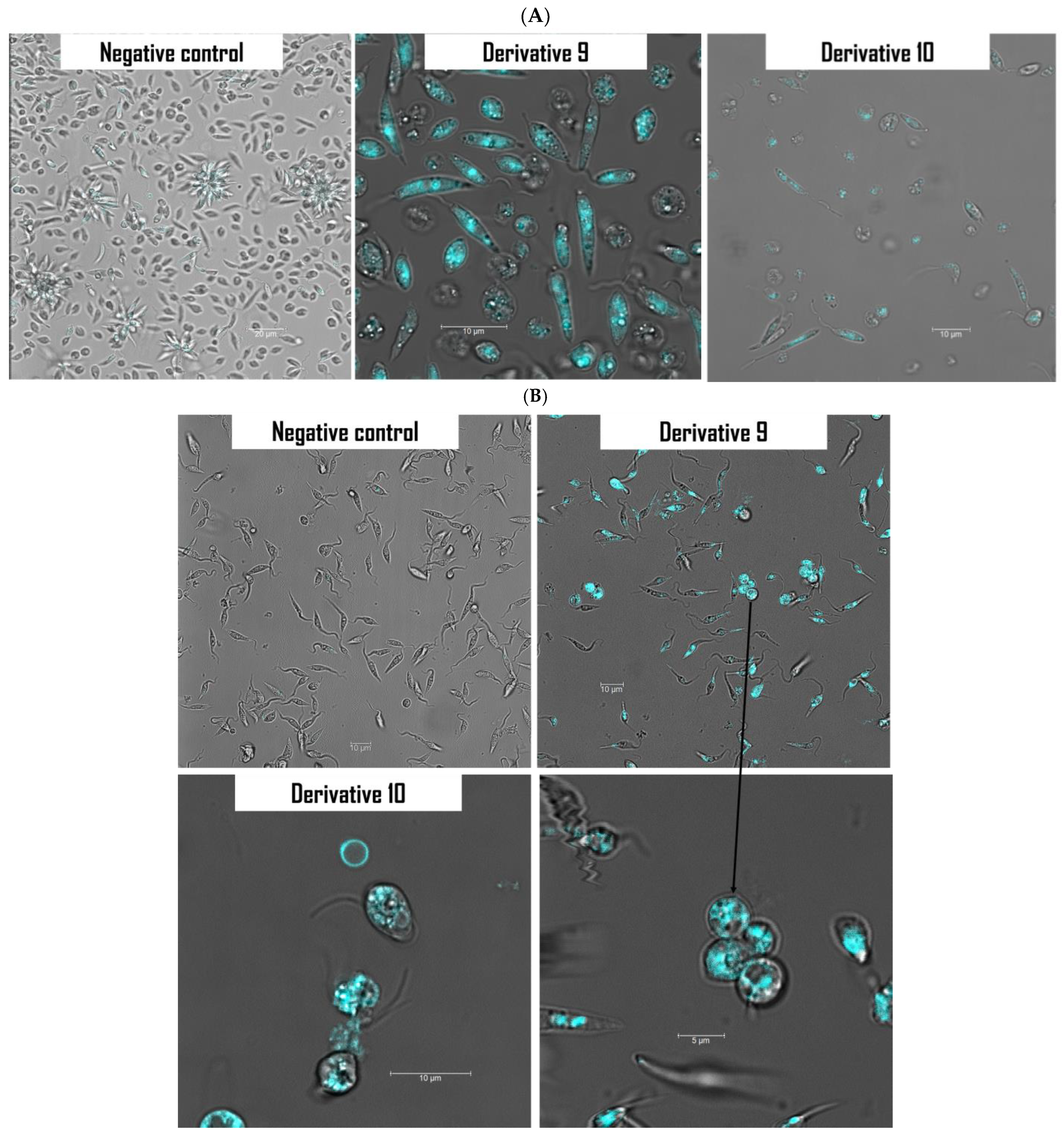
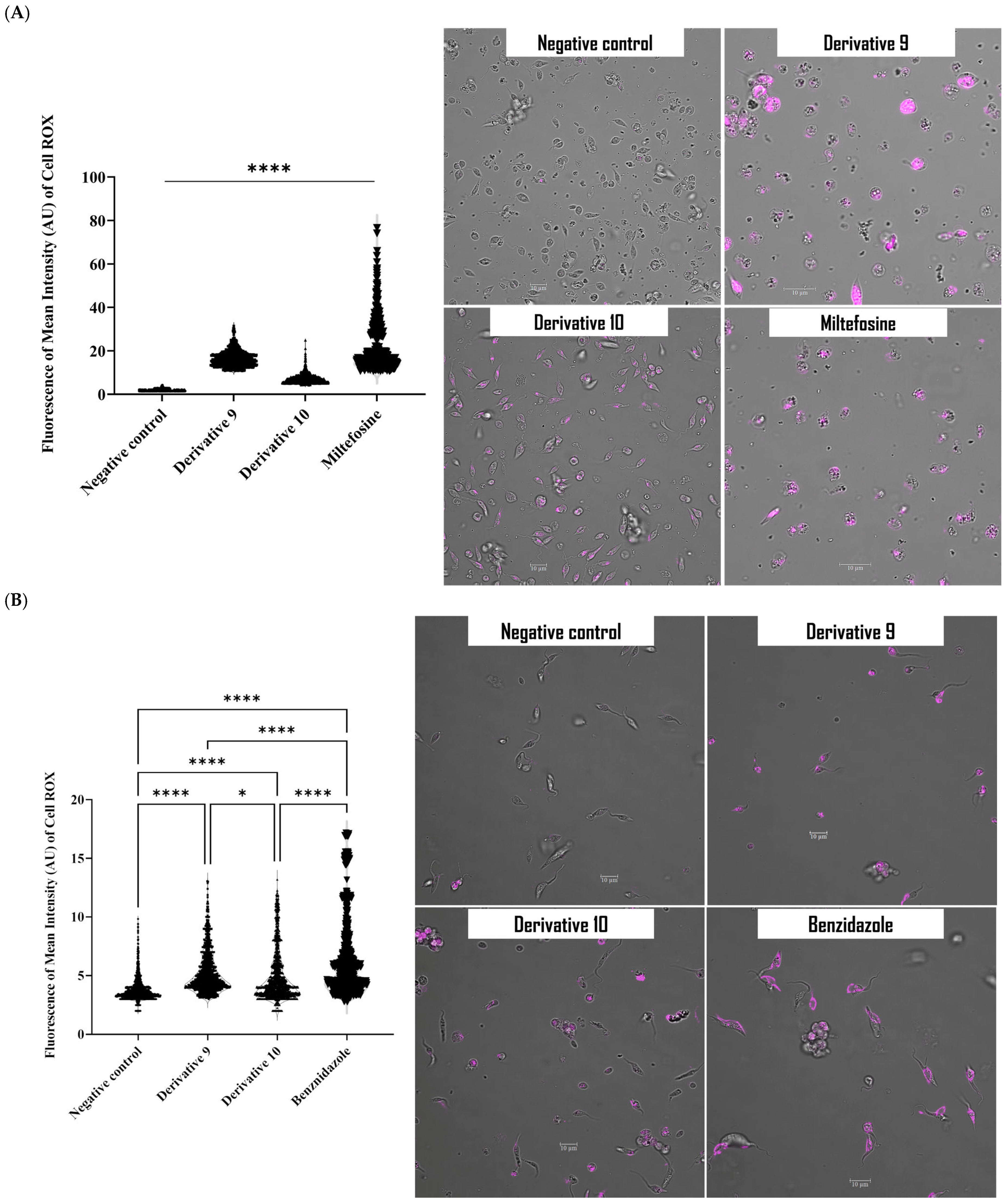
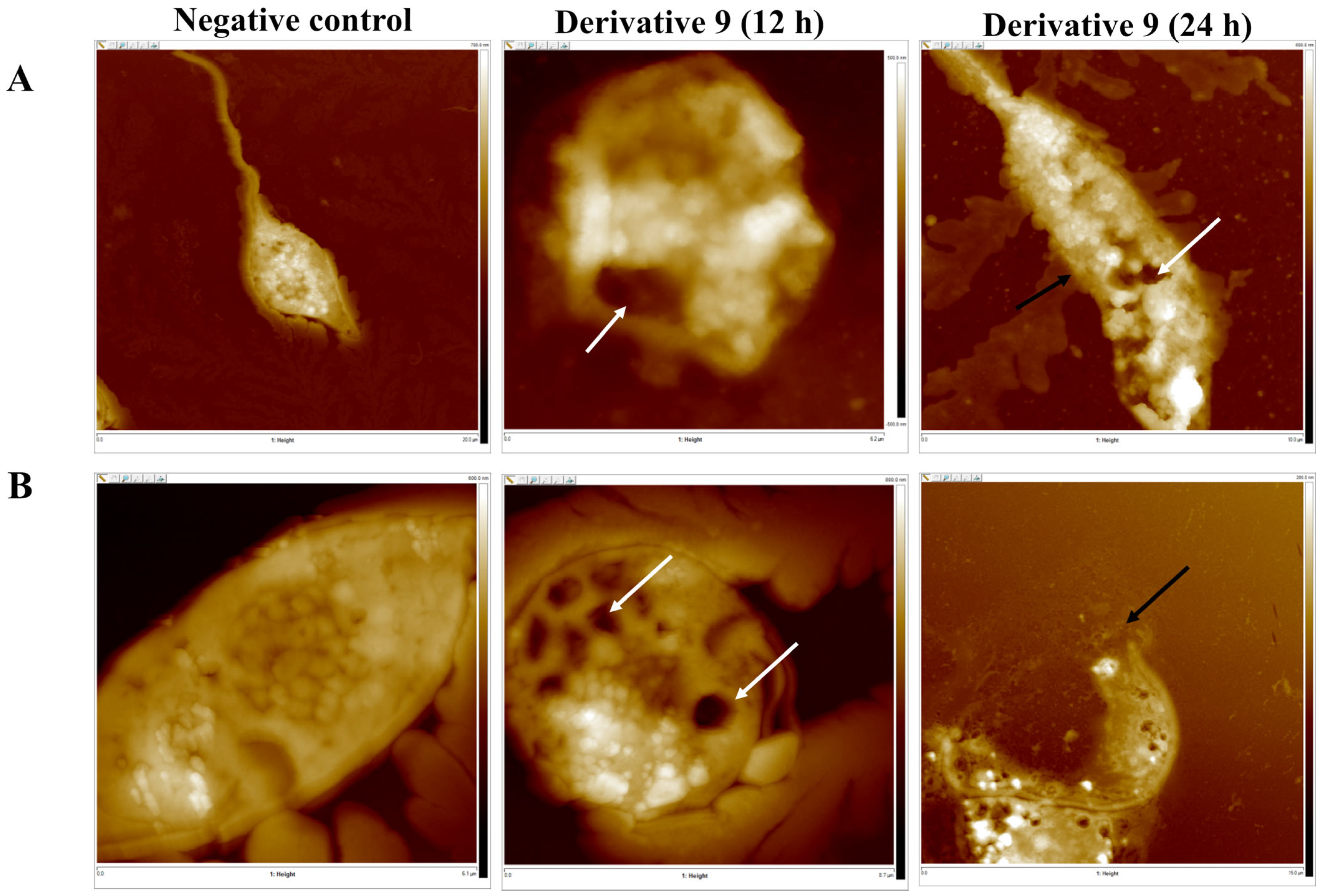
2.4. Mechanism of Action of Derivatives 9 and 10
2.5. Pharmacokinetic and Drug-Likeness Properties of Derivatives 9 and 10
3. Material and Methods
3.1. General Procedures
3.2. General Procedure of Derivatives Synthesis 2–12
3.2.1. Preparation of Derivatives 2–7 and 11–12
3.2.2. Preparation of Derivative 8
3.2.3. Preparation of Derivative 9
3.2.4. Preparation of Derivative 10
3.3. Biological Activity
3.3.1. In Vitro Effect on Promastigote Stage of Leishmania amazonensis
3.3.2. In Vitro Effect on Epimastigotes Stage of Trypanosoma cruzi
3.3.3. In Vitro Assay on the Intramacrophagic Stage
3.3.4. Cytotoxicity Assay on Murine Macrophages
3.4. Mechanism of Action Elucidation
3.4.1. Double-Stain Assay for Programmed Cell Death (PCD) Determination
3.4.2. Labeling of Autophagic Vacuoles Using Monodansylcadaverine (MDC) Staining
3.4.3. Plasma Membrane Permeability
3.4.4. Analysis of the Mitochondrial Function
3.4.5. Atomic Force Microscopy (AFM) Analysis
3.5. Statistical Analysis
3.6. Swiss ADME Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Veiga, C.R.P.; da Veiga, C.P.; Mamédio, D.F.; Su, Z. Innovative advances for neglected tropical disease (NTD): A global perspective from intellectual property. Technol. Soc. 2024, 78, 102682. [Google Scholar] [CrossRef]
- Filardy, A.A.; Guimarães-Pinto, K.; Nunes, M.P.; Zukeram, K.; Fliess, L.; Pereira, L.; Oliveira Nascimento, D.; Conde, L.; Morrot, A. Human Kinetoplastid Protozoan Infections: Where Are We Going Next? Front. Immunol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Jackson, A.P.; Otto, T.D.; Aslett, M.; Armstrong, S.D.; Bringaud, F.; Schlacht, A.; Hartley, C.; Sanders, M.; Wastling, J.M.; Dacks, J.B.; et al. Kinetoplastid phylogenomics reveals the evolutionary innovations associated with the origins of parasitism. Curr. Biol. 2016, 26, 161–172. [Google Scholar] [CrossRef]
- Landweber, L.F.; Horton, T.L.; Gott, J.M. Nucleic Acid Biodiversity: Rewriting DNA and RNA in Diverse Organisms. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013. [Google Scholar] [CrossRef]
- Gil-Antuñano, S.P.; Gold, S.; Abril, M.; Segovia-Hernández, M.; Cancelo-Hidalgo, M.J.; Flores-Chávez, M.; Pelayo-Delgado, I. Mother-to-child Chagas disease transmission: The challenge of detection and prevention in areas without the risk of vectorial transmission. Int. J. Gynaecol. Obstet. 2024, 164, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, V.I.; Martinez, E.G.; Leda, R.L.; Catunda, M.E.S.L.A.; Dias, A.d.S.; Padron-Antonio, Y.; Guerra, M.G.V.B. Resisting an invasion: A review of the triatomine vector (Kissing bug) defense strategies against a Trypanosoma sp infection. Acta Trop. 2023, 238, 106745. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Crespillo-Andújar, C.; Comeche, B.; Hamer, D.H.; Arevalo-Rodriguez, I.; Alvarez-Díaz, N.; Zamora, J.; Pérez-Molina, J.A. Use of benznidazole to treat chronic Chagas disease: An updated systematic review with a meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010386. [Google Scholar] [CrossRef]
- WHO, Leishmaniasis Fact Sheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 4 September 2024).
- Oryan, A.; Akbari, M. Worldwide risk factors in leishmaniasis. Asian Pac. J. Trop Med. 2016, 9, 925–932. [Google Scholar] [CrossRef]
- Younis, B.M.; Musa, A.M.; Monnerat, S.; Saeed, M.A.; Khalil, E.A.G.; Ahmed, A.E.; Ali, M.A.; Noureldin, A.; Ouattara, G.M.; Nyakaya, G.M.; et al. Safety and efficacy of paromomycin/miltefosine/liposomal amphotericin B combinations for the treatment of post-kala-azar dermal leishmaniasis in Sudan: A phase II, open label, randomized, parallel arm study. PLoS Negl. Trop. Dis. 2023, 17, e0011780. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, nwac206. [Google Scholar] [CrossRef]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects-A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 28, 1014. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Armson, A.; Lymbery, A.J.; Zahedi, A.; Ash, A. Medicinal plants as a source of antiparasitics: An overview of experimental studies. Pathog. Glob. Health 2023, 117, 535–553. [Google Scholar] [CrossRef]
- Games, E.; Guerreiro, M.; Santana, F.S.; Pinheiro, N.M.; de Oliveira, E.A.; Lopes, F.D.T.Q.S.; Olivo, C.R.; Tibério, I.F.L.C.; Martins, M.A.; Lago, J.H.G.; et al. Structurally related monoterpenes p-cymene, carvacrol and thymol isolated from essential oil from leaves of Lippia sidoides Cham. (Verbenaceae) protect mice against elastase-induced emphysema. Molecules 2016, 21, 1390. [Google Scholar] [CrossRef]
- Seraj, F.Q.M.; Heravi-Faz, N.; Soltani, A.; Ahmadi, S.S.; Shahbeiki, F.; Amir Talebpour, A.; Afshari, A.R.; Ferns, G.A.; Bahrami, A. Thymol has anticancer effects in U-87 human malignant glioblastoma cells. Mol. Biol. Rep. 2022, 49, 9623–9632. [Google Scholar] [CrossRef]
- Hikal, W.M.; Tkachenko, K.G.; Said-Al Ahl, H.A.; Sany, H.; Sabra, A.S.; Baeshen, R.S.; Bratovcic, A. Chemical composition and biological significance of thymol as antiparasitic. Open J. Ecol. 2021, 11, 240–266. [Google Scholar] [CrossRef]
- Bailén, M.; Illescas, C.; Quijada, M.; Martínez-Díaz, R.A.; Ochoa, E.; Gómez-Muñoz, M.T.; Navarro-Rocha, J.; González-Coloma, A. Anti-trypanosomatidae activity of essential oils and their main components from selected medicinal plants. Molecules 2023, 28, 1467. [Google Scholar] [CrossRef] [PubMed]
- Saatkamp, R.H.; Sanches, M.P.; Gambin, J.P.D.; Amaral, B.R.; de Farias, N.S.; Caon, T.; Müller, C.M.O.; Parize, A.L. Development of thymol nanoemulsions with potential application in oral infections. J. Drug Deliv. Sci. Technol. 2023, 87, 104855. [Google Scholar] [CrossRef]
- de Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.L.; Rondon, F.C.; Lobo, C.H.; de Alencar, A.N.M.; Arlindo Sales, A.D.; Rodrigues, A.P.R.; de Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef]
- Lazarevic, J.; Kolarevic, A.; Dordevic, A.; Stojanovic, G.; Smelcerovic, A.; Ciuffreda, P.; Santaniello, E. Synthesis, antimicrobial activity and in silico studies on thymol esters. Acta Chim. Slov. 2017, 64, 603–612. [Google Scholar] [CrossRef]
- Ben Youssef, M.; Omrani, A.; Sifaoui, I.; Hernández-Álvarez, E.; Bazzocchi, I.L.; Piñero, J.E.; Sebai, H.; Jimenez, I.A.; Lorenzo-Morales, J. Amoebicidal thymol analogues against brain-eating amoeba, Naegleria fowleri. Synthesis, biological evaluation, molecular mechanisms, and pharmacokinetic profile. Bioorg. Med. Chem. 2025, 159, 108346. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, B.M.; do Nascimento, P.G.G.; Souza, L.G.S.; de Farias, I.F.; da Silva, R.A.C.; de Lemos, T.L.G.; Monte, F.J.Q.; Oliveira, I.R.; Trevisan, M.T.S.; da Silva, H.C.; et al. Synthesis, larvicidal and acetylcholinesterase inhibitory activities of carvacrol/thymol and derivatives. Quim. Nova 2018, 41, 412–416. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Yang, Y.; Fan, L.; Su, F.; Ye, M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2019, 33, 1924–1930. [Google Scholar] [CrossRef]
- Sunyoto, T.; Potet, J.; Boelaert, M. Why miltefosine-a life-saving drug for leishmaniasis-is unavailable to people who need it the most. BMJ Glob. Health 2018, 3, e000709. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.S.; Concato, V.M.; Ganaza, A.F.M.; Quasne, A.C.; Ricci, B.; Dolce e Carvalho, P.V.; Della Colleta, G.H.; Lazarin-Bidóia, D.; Silva, T.F.; et al. The cytotoxic and anti-leishmanial activity of Oregano (Origanum vulgare) essential oil: An in vitro, in vivo, and in silico study. Ind. Crops. Prod. 2022, 187, 115367. [Google Scholar] [CrossRef]
- Mirahmad, A.; Ghoran, S.H.; Alipour, P.; Taktaz, F.; Hassan, S.; Naderian, M.; Moradalipour, A.; Faizi, M.; Kobarfard, F.; Ayatollahi, S.A. Oliveria decumbens Vent. (Apiaceae): Biological screening and chemical compositions. J. Ethnopharmacol. 2024, 318, 117053. [Google Scholar] [CrossRef]
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In vitro and in vivo effectiveness of carvacrol, thymol and linalool against Leishmania Infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef]
- Escobar, P.; Milena Leal, S.; Herrera, L.V.; Martínez, J.R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem. Inst. Oswaldo Cruz 2010, 105, 184–190. [Google Scholar] [CrossRef]
- Silva, A.R.S.T.; Scher, R.; Santos, F.V.; Ferreira, S.R.; Cavalcanti, S.C.H.; Correa, C.B.; Bueno, L.L.; Alves, R.J.; Souza, D.P.; Fujiwara, R.T.; et al. Leishmanicidal activity and Structure-activity relationships of essential oil constituents. Molecules 2017, 22, 815. [Google Scholar] [CrossRef]
- Mennai, I.; Lamera, E.; Slougui, N.; Benaicha, B.; Gasmi, S.; Samai, Z.; Rahmouni, N.; Bensouici, C.; Pinto, D.C.G.A. Chemical composition and antioxidant, antiparasitic, cytotoxicity and antimicrobial potential of the Algerian Limonium Oleifolium Mill. Essential oil and organic extracts. Chem. Biodivers. 2021, 18, e2100278. [Google Scholar] [CrossRef]
- Clemente, C.M.; Ravetti, S.; Allemandi, D.A.; Hergert, L.Y.; Pineda, T.; Robledo, S.M. Synthesis, in vitro antiprotozoal activity and cytotoxicity of new thymol carbonate derivatives. Chem. Select 2021, 6, 6597–6600. [Google Scholar] [CrossRef]
- Vaessen, E.M.J.; Timmermans, R.A.H.; Tempelaars, M.H.; Schutyser, M.A.I.; den Besten, H.M.W. Reversibility of membrane permeabilization upon pulsed electric field treatment in Lactobacillus plantarum WCFS. Sci. Rep. 2019, 9, 19990. [Google Scholar] [CrossRef]
- Fan, Y.; Ullman, E.; Zong, W.-X. The cellular decision between apoptosis and autophagy. Chin. J. Cancer 2013, 32, 121–129. [Google Scholar] [CrossRef]
- Dasari, S.K.; Bialik, S.; Levin-Zaidman, S.; Levin-Salomon, V.; Merrill, A.H.J.; Futerman, A.H.; Kimchi, A. Signalome-wide RNAi screen identifies GBA1 as a positive mediator of autophagic cell death. Cell Death Differ. 2017, 24, 1288–1302. [Google Scholar] [CrossRef]
- Bialik, S.; Dasari, S.K.; Kimchi, A. Autophagy-dependent cell death—where, how and why a cell eats itself to death. J. Cell Sci. 2018, 131, jcs215152. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. Swiss ADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Rao, S.P.S.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P.; et al. Drug discovery for kinetoplastid diseases: Future directions. ACS Infect. Dis. 2019, 5, 152–157. [Google Scholar] [CrossRef]
- Núñez, M.J.; Martínez, M.L.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Jiménez, I.A.; Lorenzo-Morales, J.; Piñero, J.E.; Bazzocchi, I.L. In vitro susceptibility of kinetoplastids to celastroloids from Maytenus chiapensis. Antimicrob. Agents. Chemother. 2021, 65, e02236-20. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Selective activity of oleanolic and maslinic acids on the amastigote form of Leishmania spp. Iran J. Pharm. Res. 2017, 16, 1190–1193. [Google Scholar]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. In vitro activity and mechanism of cell death induction of cyanomethyl vinyl ethers derivatives against Trypanosoma cruzi. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, B.; Orrenius, S. Current concepts in cell death. Curr. Protoc. Cell Biol. 2001, 11, 1–18. [Google Scholar] [CrossRef]
- Sifaoui, I.; Díaz-Rodríguez, P.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; López-Arencibia, A.; Salazar Villatoro, L.; Castelan-Ramírez, I.; Omaña-Molina, M.; Oliva, A.; Piñero, J.E.; et al. Pitavastatin loaded nanoparticles: A suitable ophthalmic treatment for Acanthamoeba Keratitis inducing cell death and autophagy in Acanthamoeba polyphaga. Eur. J. Pharm. Biopharm. 2022, 180, 11–22. [Google Scholar] [CrossRef]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Salazar-Villatoro, L.; Omaña-Molina, M.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. Acrylonitrile derivatives: In vitro activity and mechanism of cell death induction against Trypanosoma cruzi and Leishmania amazonensis. Int. J. Parasitol. Drugs Drug Resist. 2024, 24, 100531. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Marrero, A.R.; López-Arencibia, A.; Bethencout-Estrella, C.J.; Cen-Pacheco, F.; Sifaoui, I.; Hernández-Creus, A.; Duque-Ramírez, M.C.; Souto, M.L.; Hernández-Daranas, A.; Lorenzo-Morales, J.; et al. Antiprotozoal activities of marine polyether triterpenoids. Bioorg. Chem. 2019, 92, 103276. [Google Scholar] [CrossRef]




| Cmpds | L. amazonensis Promastigote | T. cruzi Epimastigote | Cytotoxicity | Selectivity Leishmania | Selectivity Trypanosoma |
|---|---|---|---|---|---|
| IC50 a | IC50 a | CC50 b | SI c | SI c | |
| 1 | 317.6 ± 21.4 | 206.0 ± 2.8 | >300 | >0.9 | >1.5 |
| 3 | 78.8 ± 15.9 | 49.5 ± 3.2 | >300 | >3.8 | >6.1 |
| 4 | 147.6 ± 10.0 | 123.7 ± 6.0 | 120.9 ± 0.4 | 0.8 | 0.9 |
| 5 | 216.5 ± 7.5 | 211.2 ± 7.3 | >300 | >1.4 | >1.4 |
| 7 | 56.0 ± 5.6 | 66.0 ± 1.7 | >300 | >5.4 | >4.5 |
| 8 | 76.4 ± 6.2 | 44.4 ± 2.5 | >300 | >3.9 | >6.8 |
| 9 | 29.1 ± 3.8 | 26.8 ± 2.2 | >300 | >10.3 | >11.2 |
| 10 | 34.4 ± 2.3 | 35.5 ± 2.5 | 254.5 ± 1.9 | 7.4 | 7.2 |
| 11 | 56.9 ± 7.2 | 66.9 ± 3.8 | >300 | >5.2 | >4.5 |
| 12 | 228.6 ± 21.4 | 157.8 ± 2.9 | >300 | >1.3 | >1.9 |
| M d | 6.5 ± 0.3 | 72.2 ± 8.9 | 11.1 | ||
| B e | 6.9 ± 0.8 | 399.9 ± 1.4 | 58.0 |
| Cmpds | L. amazonensis Intracellular | T. cruzi Intracellular | Cytotoxicity | Selectivity Leishmania | Selectivity Trypanosoma |
|---|---|---|---|---|---|
| IC50 a | IC50 a | CC50 b | SI c | SI d | |
| 9 | 15.2 ± 0.8 | 9.1 ± 0.5 | >300 | >19.7 | >33.0 |
| 10 | 25.3 ± 0.8 | 28.8 ± 5.6 | 254.5 ± 1.9 | 10.1 | 8.8 |
| M d | 3.1 ± 0.3 | 72.2 ± 8.9 | 23.3 | ||
| B e | 2.7 ± 0.4 | 399.9 ± 1.4 | 148.1 |
| Cmpds | Parameters of Lipinski’s Rule | Drug-Likeness | ||||||
|---|---|---|---|---|---|---|---|---|
| Log P | TPSA | MW | HBA | HBD | RB | Vs | ||
| 9 | 4.25 | 35.53 | 284.35 | 3 | 0 | 5 | 0 | Yes |
| 10 | 3.48 | 39.19 | 255.31 | 3 | 0 | 4 | 0 | Yes |
| Properties | Derivatives | Properties | Derivatives | ||
|---|---|---|---|---|---|
| 9 | 10 | 9 | 10 | ||
| BA | 0.55 | 0.55 | CYP1A2 | Yes | Yes |
| GI | High | High | CYP2C19 | Yes | Yes |
| BBB | Yes | Yes | CYP2C9 | No | Yes |
| P-gp | No | No | CYP2D6 | Yes | Yes |
| Log Kp | −4.63 | −5.51 | CYP3A4 | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omrani, A.; Ben Youssef, M.; Sifaoui, I.; Hernández-Álvarez, E.; Bethencourt-Estrella, C.J.; L. Bazzocchi, I.; Sebai, H.; Lorenzo-Morales, J.; Jiménez, I.A.; Piñero, J.E. Mechanism Insight of Cell Death Signaling by Thymol Derivatives on Trypanosomatidae Protozoan Parasites. Antibiotics 2025, 14, 383. https://doi.org/10.3390/antibiotics14040383
Omrani A, Ben Youssef M, Sifaoui I, Hernández-Álvarez E, Bethencourt-Estrella CJ, L. Bazzocchi I, Sebai H, Lorenzo-Morales J, Jiménez IA, Piñero JE. Mechanism Insight of Cell Death Signaling by Thymol Derivatives on Trypanosomatidae Protozoan Parasites. Antibiotics. 2025; 14(4):383. https://doi.org/10.3390/antibiotics14040383
Chicago/Turabian StyleOmrani, Amani, Meriam Ben Youssef, Ines Sifaoui, Eduardo Hernández-Álvarez, Carlos J. Bethencourt-Estrella, Isabel L. Bazzocchi, Hichem Sebai, Jacob Lorenzo-Morales, Ignacio A. Jiménez, and José E. Piñero. 2025. "Mechanism Insight of Cell Death Signaling by Thymol Derivatives on Trypanosomatidae Protozoan Parasites" Antibiotics 14, no. 4: 383. https://doi.org/10.3390/antibiotics14040383
APA StyleOmrani, A., Ben Youssef, M., Sifaoui, I., Hernández-Álvarez, E., Bethencourt-Estrella, C. J., L. Bazzocchi, I., Sebai, H., Lorenzo-Morales, J., Jiménez, I. A., & Piñero, J. E. (2025). Mechanism Insight of Cell Death Signaling by Thymol Derivatives on Trypanosomatidae Protozoan Parasites. Antibiotics, 14(4), 383. https://doi.org/10.3390/antibiotics14040383












