Harnessing the Power of Antimicrobial Peptides: From Mechanisms to Delivery Optimization for Topical Infections
Abstract
1. Introduction
| Marketed Product | Type of Product | Company | Target Disease | Reference |
|---|---|---|---|---|
| Cubicin RF | Lipopeptide | Merck & Co., Inc. (Rahway, NJ, USA) | Skin infections | [19] |
| Daptomycin (cubicin) IV 4 mg/kg | Cyclic Lipopeptide | AuroMedics Pharma LLC (East Windsor, NJ, USA) | Skin infections | [20] |
| Polymyxin B vials | Polypeptide antibiotics | Xellia (Copenhagen, Denmark) | Acute urinary, meningeal or blood stream infections | [21,22] |
| Vancocin (vancomycin hydrochloride (1–2%) | Glycopeptides | Septicemia | [23] | |
| Dalvance/allergan (dalbavancin 500 mg/vial) | Second-generation lipoglycopeptide antibiotic | Melinta Therapeutics (Parsippany-Troy Hills, NJ, USA) FDA approval May 2014 | Acute skin structure infections | [24] |
| Telavancin | Semisynthetic peptide derivative | Theravance Biopharma (South San Francisco, CA, USA) | Serious bacterial skin infections | [25,26] |
| Orbactiv (oritavancin) | Semisynthetic lipoglycopeptide | Melinta Therapeutics | Acute skin structure infections | [27] |
| Omiganan pentahydrochloride | Synthetic analog of human defensin | Atopic dermatitis | [28,29] |
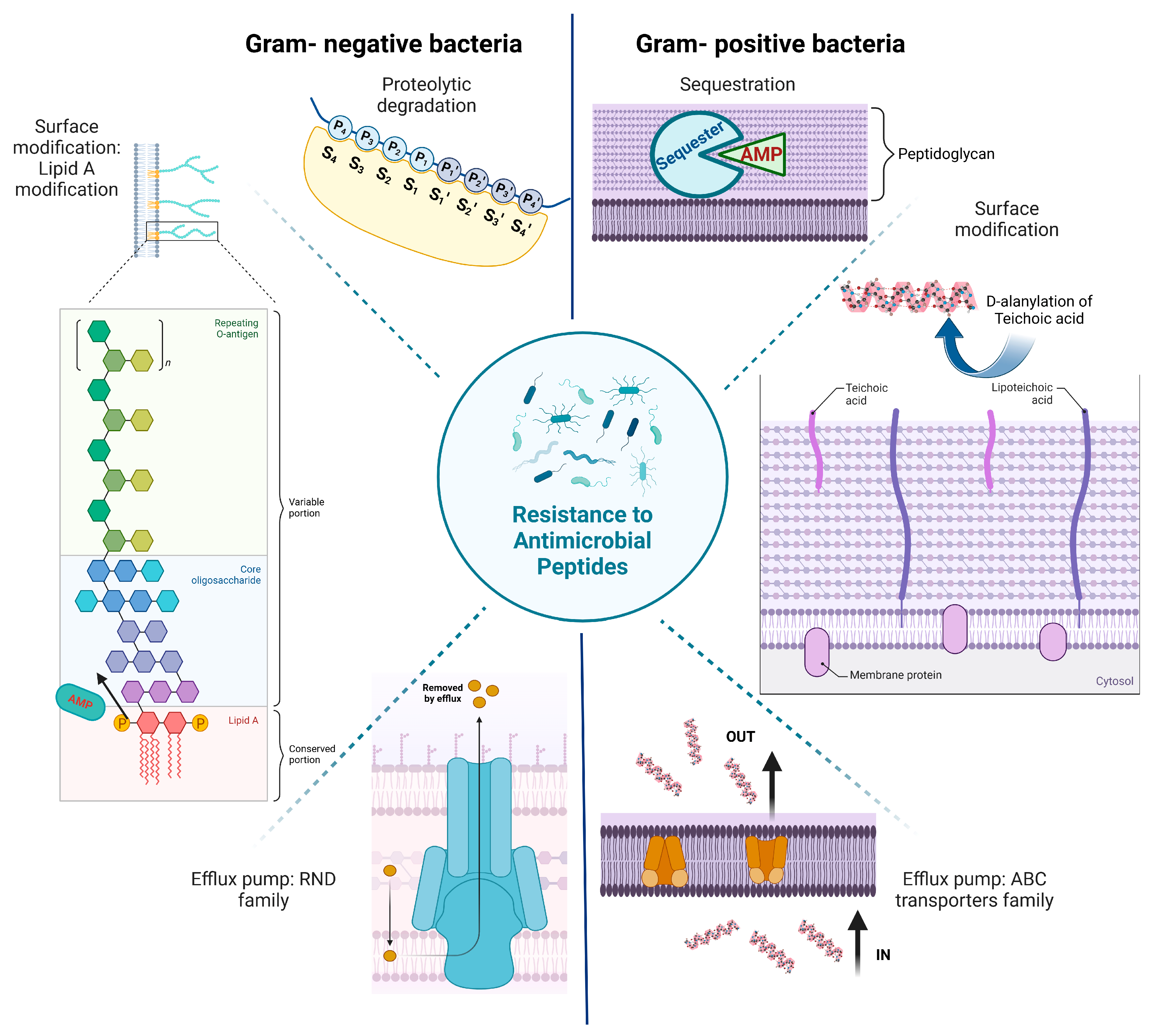
2. Resistance to Antimicrobial Peptides
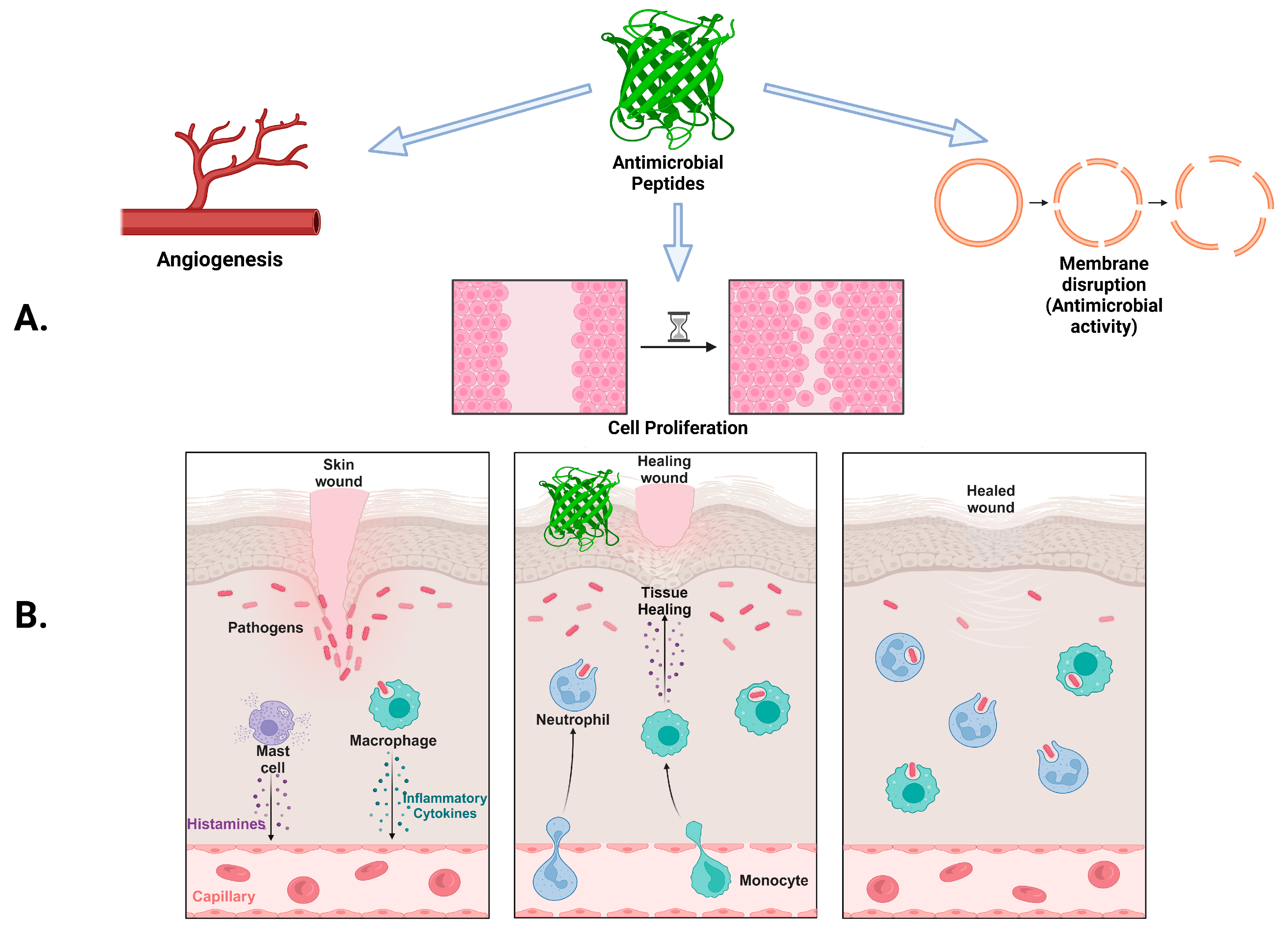
3. Skin Microbiome
4. Significance of pH in AMP Delivery for Topical Infections
5. Key Factors to Be Considered for Novel AMP Delivery
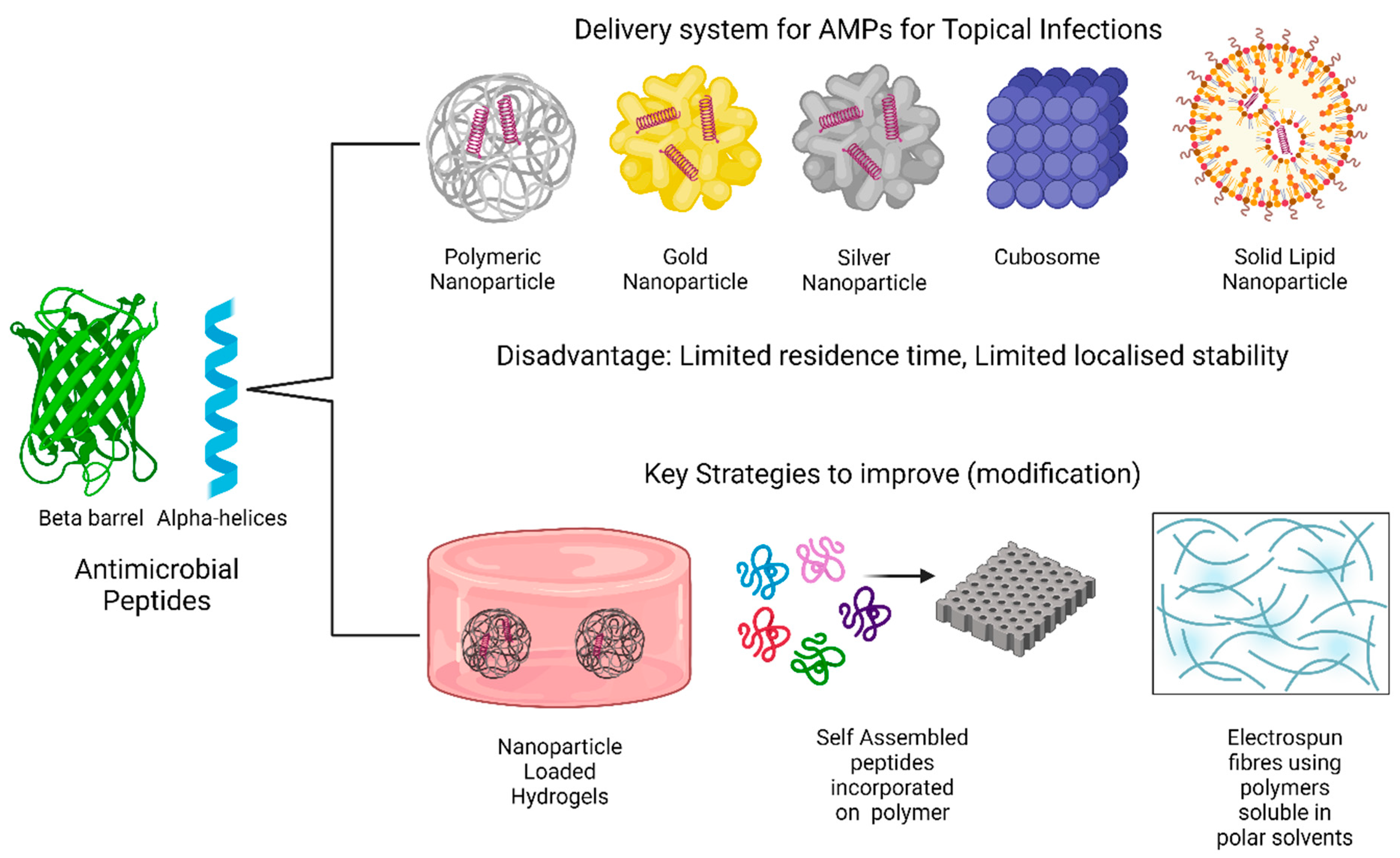
6. Emerging AMP Delivery Systems
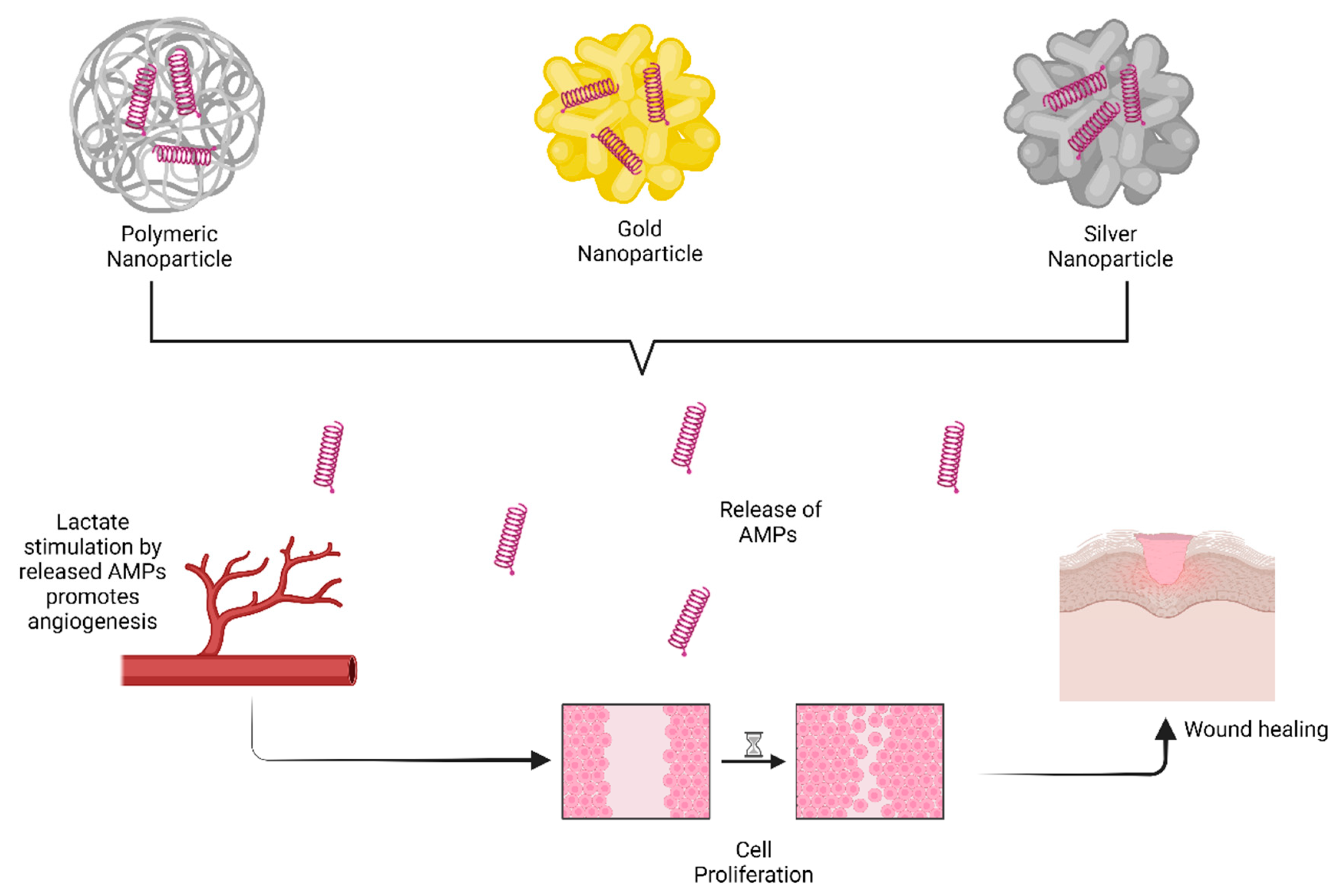
6.1. Nanoparticles
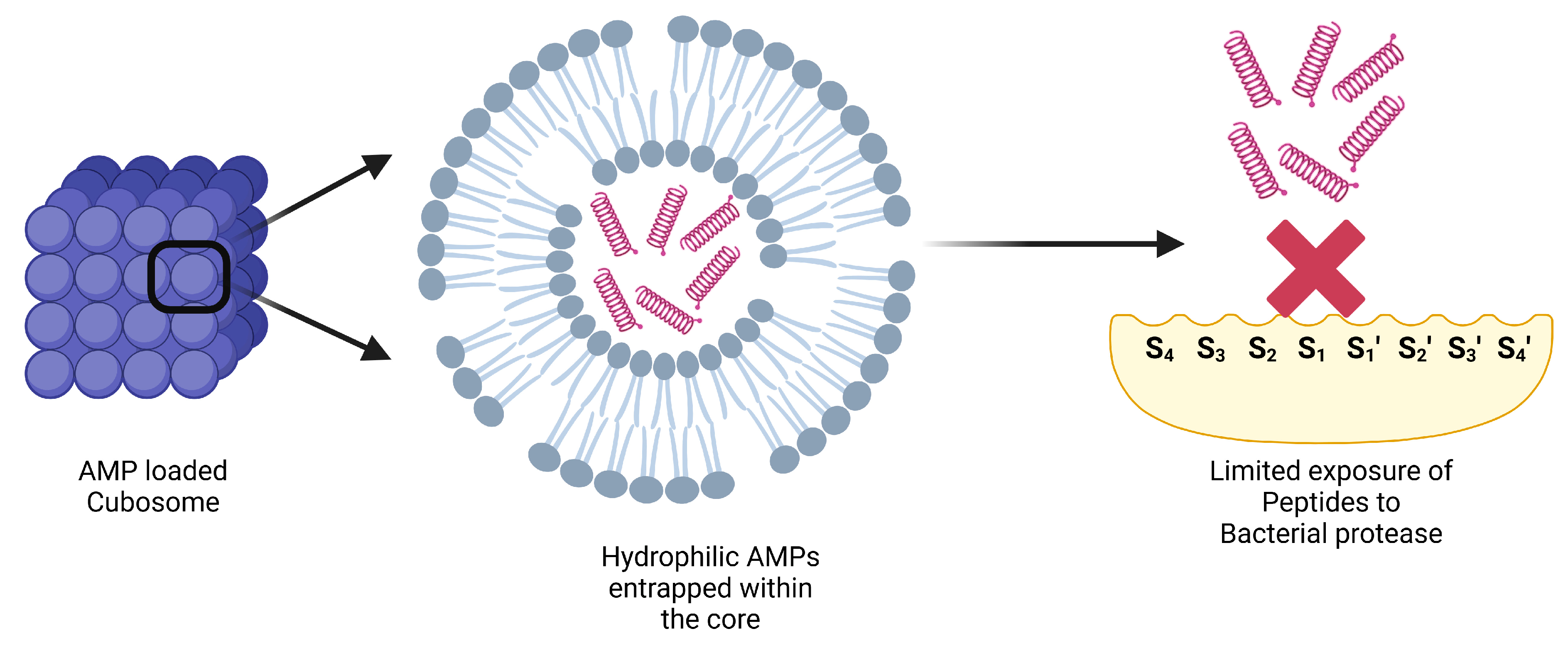
6.2. Cubosomes
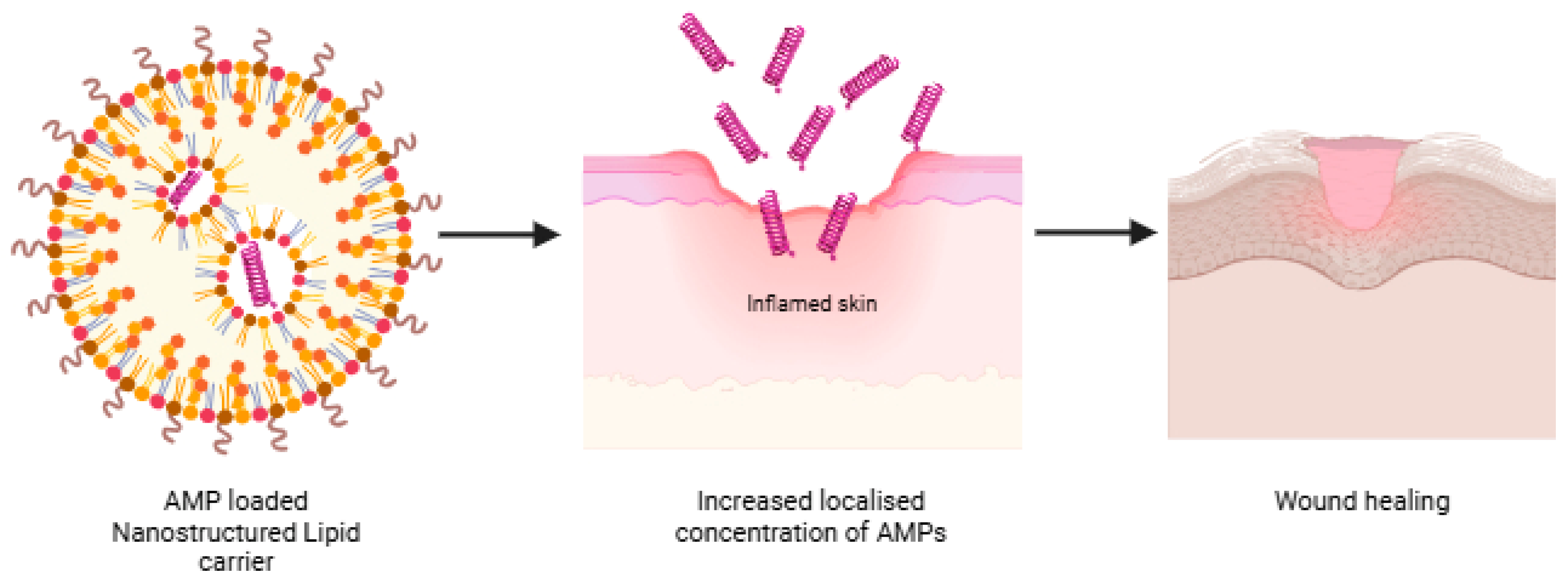
6.3. Nanostructured Lipid Carriers (NLCs)
7. Key Strategies to Improve AMP Delivery for Topical Infections
7.1. Hydrogels
7.2. Self-Assembling Peptides
7.3. Other Strategies to Improve AMP Delivery Systems
| AMP | Limitation | Strategies | Results | Reference |
|---|---|---|---|---|
| LL-37 | Proteolytic degradation |
|
| [64] |
| Amphiphilic peptides | Proteolytic degradation |
|
| [76] |
| Peptide (KIGAKI)3-NH2 | Conformational stability |
|
| [77] |
| AMP, SWLSKTAKKLFKKIPKKIPKKRFPRPR PWPRPNMI-NH 2, purity at >95%) | Less vascularization and prolonged inflammatory phase-Diabetic wound healing |
|
| [78] |
| Human antimicrobial peptide (AP-57) | Limited knowledge of its stability and efficacy |
|
| [79] |
| Octapeptide (IKFQFHFD) | Potential pH-switchable antimicrobial effect |
|
| [80] |
| Hydrophilic peptide (dalargin) | Lower encapsulation efficiency in PLGA nanoparticles |
|
| [81] |
| Nisin | Electrostatic repulsion with divalent cations associated with bacterial cell surface |
|
| [82] |
| Peptide Delivery System | AMP | Description | Result | Reference |
|---|---|---|---|---|
| Mesoporous silica nanoparticles (MSNs) | Nisin A (bacteriocin isolated from Lactococcus lactis subsp. Lactis) |
|
| [83] |
| Trichogin GA IV (short sequence), ampullosporin A (medium length sequence) |
|
| [84] | |
| Melittin |
|
| [85] | |
| Antimicrobial peptide conjugates | Aurein 2.2 (α-helical AMP) |
|
| [86] |
| Anoplin (decapeptide, short AMP) |
|
| [87] | |
| Nisin |
|
| [88] | |
| Bacteria-absorbing sponge | Host defense peptides (HDPs) (peptidomimetics) |
|
| [89] |
| Layered nanoclays | LL-37 |
|
| [90] |
| Carbon nanotubes | TP359 |
|
| [91] |
| Titanium nanoparticles | Lactoferrin-derived hLf1–11 |
|
| [92] |
8. Discussion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: An Overview of Our Work. Wellcome Trust. 2016. Available online: https://amr-review.org/sites/default/files/Tackling%20drug-resistant%20infections%20-%20An%20overview%20of%20our%20work_IncHealth_LR_NO%20CROPS.pdf (accessed on 31 March 2025).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Flohr, C.; Hay, R. Putting the Burden of Skin Diseases on the Global Map; Blackwell Publishing Ltd.: Oxford, UK, 2021; Volume 184, pp. 189–190. [Google Scholar]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [PubMed]
- Spann, C.T.; Taylor, S.C.; Weinberg, J.M. Topical antimicrobial agents in dermatology. Clin. Dermatol. 2003, 21, 70–77. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Bharath Prasad, A.; Mehta, C.H.; Nayak, U.Y. Antimicrobial peptide polymers: No escape to ESKAPE pathogens—A review. World J. Microbiol. Biotechnol. 2020, 36, 131. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical applications of antimicrobial peptides (AMPs): Where do we stand now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef]
- Grand View Research. Peptide Antibiotics Market Size, Share & Trends Analysis Report By Product Type, By Disease, By Route Of Administration (Oral, Injectable, Topical), By Distribution Channel, By Region, And Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/peptide-antibiotics-market-report (accessed on 24 August 2023).
- GlobeNewsWire. Antimicrobial Peptides Market Worth USD 539.32 Million by 2027|Growth, Size, Shares, Revenue, Types, Applications, Key Players, Top Countries, Growing Factors, Key Dynamics. Available online: https://www.globenewswire.com/news-release/2022/08/18/2500859/0/en/Antimicrobial-Peptides-Market-Worth-USD-539-32-million-by-2027-Growth-Size-Shares-Revenue-Types-Applications-Key-Players-Top-Countries-Growing-Factors-Key-Dynamics.html (accessed on 24 August 2023).
- Market, G.A.P. Global Antimicrobial Peptides Market—Industry Trends and Forecast to 2029. Available online: https://www.databridgemarketresearch.com/reports/global-antimicrobial-peptides-market (accessed on 24 August 2023).
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. Int. J. Mol. Sci. 2023, 24, 9031. [Google Scholar] [CrossRef]
- Porras-Luque, J. Topical antimicrobial agents in dermatology. Actas Dermo-Sifiliogr. 2007, 98, 29–39. [Google Scholar] [CrossRef]
- Caselli, L.; Malmsten, M. Skin and wound delivery systems for antimicrobial peptides. Curr. Opin. Colloid Interface Sci. 2023, 65, 101701. [Google Scholar] [CrossRef]
- Răileanu, M.; Borlan, R.; Campu, A.; Janosi, L.; Turcu, I.; Focsan, M.; Bacalum, M. No country for old antibiotics! Antimicrobial peptides (AMPs) as next-generation treatment for skin and soft tissue infection. Int. J. Pharm. 2023, 642, 123169. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Sibuyi, N.R.S.; Madiehe, A.M.; Meyer, M. Nanotechnology-based delivery systems for antimicrobial peptides. Pharmaceutics 2021, 13, 1795. [Google Scholar] [CrossRef]
- Patrulea, V.; Borchard, G.; Jordan, O. An update on antimicrobial peptides (AMPs) and their delivery strategies for wound infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, D.M.; Simou, J.; Roland, W.E. A review of daptomycin for injection (Cubicin) in the treatment of complicated skin and skin structure infections. Ther. Clin. Risk Manag. 2006, 2, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Arbeit, R.D.; Maki, D.; Tally, F.P.; Campanaro, E.; Eisenstein, B.I. Daptomycin 98-01 and 99-01 Investigators. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 2004, 38, 1673–1681. [Google Scholar] [CrossRef]
- Pharmaceuticals, X. Polymyxin Vials. Available online: https://www.xellia.com/products/Polymyxin%20B%20vials/ (accessed on 16 November 2023).
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an ‘old’class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef]
- Huvelle, S.; Godet, M.; Hecq, J.-D.; Gillet, P.; Jamart, J.; Galanti, L.M. Long-term Stability of Vancomycin Hydrochloride in Oral Solution: The Brand Name Versus a Generic Product. Int. J. Pharm. Compd. 2016, 20, 347–350. [Google Scholar] [PubMed]
- Ramdeen, S.; Boucher, H.W. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Expert Opin. Pharmacother. 2015, 16, 2073–2081. [Google Scholar] [CrossRef]
- Medscape. Vibativ (Telavancin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/vibativ-telavancin-345210 (accessed on 24 August 2023).
- Lampejo, T. Dalbavancin and telavancin in the treatment of infective endocarditis: A literature review. Int. J. Antimicrob. Agents 2020, 56, 106072. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Wang, J.; Cai, Y. Efficacy and safety of oritavancin for the treatment of acute bacterial skin and skin-structure infections: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2021, 25, 380–389. [Google Scholar] [CrossRef]
- Puar, N.; Chovatiya, R.; Paller, A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 21–31. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.; Hogendoorn, G.K.; Rijneveld, R.; Luijten, S.; van Alewijk, D.C.; van den Munckhof, E.H.; de Kam, M.L.; Feiss, G.L.; Prens, E.P. Pharmacodynamic effects of topical omiganan in patients with mild to moderate atopic dermatitis in a randomized, placebo-controlled, phase II trial. Clin. Transl. Sci. 2020, 13, 994–1003. [Google Scholar] [CrossRef]
- Joo, H.-S.; Fu, C.-I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150292. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updates 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Dowling, P.M. Peptide Antibiotics: Polymyxins, glycopeptides, bacitracin, and fosfomycin. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; John Wiley & Sons: New York, NY, USA, 2013; pp. 189–198. [Google Scholar]
- Vaara, M. New approaches in peptide antibiotics. Curr. Opin. Pharmacol. 2009, 9, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 2020, 66, 11–16. [Google Scholar] [CrossRef]
- Nørreslet, L.B.; Agner, T.; Clausen, M.-L. The skin microbiome in inflammatory skin diseases. Curr. Dermatol. Rep. 2020, 9, 141–151. [Google Scholar] [CrossRef]
- Guo, S.a.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Abdella, S.; Abid, F.; Youssef, S.H.; Kim, S.; Afinjuomo, F.; Malinga, C.; Song, Y.; Garg, S. pH and its applications in targeted drug delivery. Drug Discov. Today 2022, 28, 103414. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of acidic pH on wound healing in vivo: A novel perspective for wound treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [PubMed]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar]
- Sun, T.; Zhan, B.; Zhang, W.; Qin, D.; Xia, G.; Zhang, H.; Peng, M.; Li, S.-A.; Zhang, Y.; Gao, Y. Carboxymethyl chitosan nanoparticles loaded with bioactive peptide OH-CATH30 benefit nonscar wound healing. Int. J. Nanomed. 2018, 13, 5771–5786. [Google Scholar] [CrossRef]
- Dartora, V.F.; Passos, J.S.; Osorio, B.; Hung, R.-C.; Nguyen, M.; Wang, A.; Panitch, A. Chitosan hydrogels with MK2 inhibitor peptide-loaded nanoparticles to treat atopic dermatitis. J. Control. Release 2023, 362, 591–605. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Her, C.-H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef]
- Casciaro, B.; Moros, M.; Rivera-Fernandez, S.; Bellelli, A.; de la Fuente, J.M.; Mangoni, M.L. Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a (1-21) NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 2017, 47, 170–181. [Google Scholar] [CrossRef]
- Fahimirad, S.; Satei, P.; Ganji, A.; Abtahi, H. Wound healing capability of the double-layer Polycaprolactone/Polyvinyl alcohol-Chitosan lactate electrospun nanofiber incorporating Echinacea purpurea extract. J. Drug Deliv. Sci. Technol. 2023, 87, 104734. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bertossi, D.; Magistretti, P. Insights on the role of l-lactate as a signaling molecule in skin aging. Biogerontology 2023, 24, 709–726. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef] [PubMed]
- Comune, M.; Rai, A.; Chereddy, K.K.; Pinto, S.; Aday, S.; Ferreira, A.F.; Zonari, A.; Blersch, J.; Cunha, R.; Rodrigues, R. Antimicrobial peptide-gold nanoscale therapeutic formulation with high skin regenerative potential. J. Control. Release 2017, 262, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Marepally, S.; Vemula, P.; Xu, C. Inorganic nanoparticles for transdermal drug delivery and topical application. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–72. [Google Scholar]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [PubMed]
- Pivodová, V.; Franková, J.; Galandáková, A.; Ulrichová, J. In vitro AuNPs’ cytotoxicity and their effect on wound healing. Nanobiomedicine 2015, 2, 7. [Google Scholar]
- Mulet, X.; Boyd, B.J.; Drummond, C.J. Advances in drug delivery and medical imaging using colloidal lyotropic liquid crystalline dispersions. J. Colloid Interface Sci. 2013, 393, 1–20. [Google Scholar]
- Boge, L.; Bysell, H.; Ringstad, L.; Wennman, D.; Umerska, A.; Cassisa, V.; Eriksson, J.; Joly-Guillou, M.-L.; Edwards, K.; Andersson, M. Lipid-based liquid crystals as carriers for antimicrobial peptides: Phase behavior and antimicrobial effect. Langmuir 2016, 32, 4217–4228. [Google Scholar]
- Boge, L.; Umerska, A.; Matougui, N.; Bysell, H.; Ringstad, L.; Davoudi, M.; Eriksson, J.; Edwards, K.; Andersson, M. Cubosomes post-loaded with antimicrobial peptides: Characterization, bactericidal effect and proteolytic stability. Int. J. Pharm. 2017, 526, 400–412. [Google Scholar]
- Boge, L.; Västberg, A.; Umerska, A.; Bysell, H.; Eriksson, J.; Edwards, K.; Millqvist-Fureby, A.; Andersson, M. Freeze-dried and re-hydrated liquid crystalline nanoparticles stabilized with disaccharides for drug-delivery of the plectasin derivative AP114 antimicrobial peptide. J. Colloid Interface Sci. 2018, 522, 126–135. [Google Scholar]
- Meikle, T.G.; Zabara, A.; Waddington, L.J.; Separovic, F.; Drummond, C.J.; Conn, C.E. Incorporation of antimicrobial peptides in nanostructured lipid membrane mimetic bilayer cubosomes. Colloids Surf. B Biointerfaces 2017, 152, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gontsarik, M.; Buhmann, M.T.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. Antimicrobial peptide-driven colloidal transformations in liquid-crystalline nanocarriers. J. Phys. Chem. Lett. 2016, 7, 3482–3486. [Google Scholar] [CrossRef]
- Lozeau, L.D.; Rolle, M.W.; Camesano, T.A. A QCM-D study of the concentration-and time-dependent interactions of human LL37 with model mammalian lipid bilayers. Colloids Surf. B Biointerfaces 2018, 167, 229–238. [Google Scholar] [CrossRef]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Evaluation of strategies for improving proteolytic resistance of antimicrobial peptides by using variants of EFK17, an internal segment of LL-37. Antimicrob. Agents Chemother. 2009, 53, 593–602. [Google Scholar] [CrossRef]
- Grönberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Girbau, C.; Alonso, R.; Aguirre, J.J.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M. LL37 loaded nanostructured lipid carriers (NLC): A new strategy for the topical treatment of chronic wounds. Eur. J. Pharm. Biopharm. 2016, 108, 310–316. [Google Scholar] [CrossRef]
- Bae, C.S.; Ahn, T. Diacylglycerol in Cationic Nanoparticles Stimulates Oxidative Stress-Mediated Death of Cancer Cells. Lipids 2018, 53, 1059–1067. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Drechsler, M.; Ballauff, M. Thermosensitive core–shell particles as carriers for Ag nanoparticles: Modulating the catalytic activity by a phase transition in networks. Angew. Chem. Int. Ed. 2006, 45, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Vecchio, D.; Li, J.; Zhu, J.; Zhang, Q.; Fu, V.; Li, J.; Thamphiwatana, S.; Lu, D.; Zhang, L. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. Acs Nano 2014, 8, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhou, Y.; He, W.; Hua, D. A strategy for enhanced antibacterial activity against Staphylococcus aureus by the assembly of alamethicin with a thermo-sensitive polymeric carrier. Chem. Commun. 2016, 52, 896–899. [Google Scholar] [CrossRef]
- O’Brien, G.; Buckley, K.; Vanwalleghem, G.; Vanrenterghem, D.; Dharma, H.; Winter, R.L.; Douglass, J. A multi-centre, prospective, clinical in-market evaluation to assess the performance of Opsite™ Post-Op Visible dressings. Int. Wound J. 2010, 7, 329–337. [Google Scholar] [CrossRef]
- Dart, A.; Bhave, M.; Kingshott, P. Antimicrobial peptide-based electrospun fibers for wound healing applications. Macromol. Biosci. 2019, 19, 1800488. [Google Scholar] [CrossRef]
- Mi, G.; Shi, D.; Herchek, W.; Webster, T.J. Self-assembled arginine-rich peptides as effective antimicrobial agents. J. Biomed. Mater. Res. Part A 2017, 105, 1046–1054. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, C.; Zhao, X. Stimuli-responsive self-assembling peptides made from antibacterial peptides. Nanoscale 2013, 5, 6413–6421. [Google Scholar] [CrossRef]
- Wei, S.; Xu, P.; Yao, Z.; Cui, X.; Lei, X.; Li, L.; Dong, Y.; Zhu, W.; Guo, R.; Cheng, B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef]
- Li, X.; Fan, R.; Tong, A.; Yang, M.; Deng, J.; Zhou, L.; Zhang, X.; Guo, G. In situ gel-forming AP-57 peptide delivery system for cutaneous wound healing. Int. J. Pharm. 2015, 495, 560–571. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.-Y.; Zhao, Y.; Yang, Y.; Wang, W.; Wu, C.; Yang, B.; Zhang, Z.; Zhang, L.; Liu, Y. pH-switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano 2019, 13, 11686–11697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, F.; Benson, H. Effect of formulation factors on incorporation of the hydrophilic peptide dalargin into PLGA and mPEG-PLGA nanoparticles. Pept. Sci. 2008, 90, 644–650. [Google Scholar] [CrossRef]
- Taylor, T.M.; Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Characterization of antimicrobial-bearing liposomes by ζ-potential, vesicle size, and encapsulation efficiency. Food Biophys. 2007, 2, 1–9. [Google Scholar] [CrossRef]
- Flynn, J.; Mallen, S.; Durack, E.; O’Connor, P.M.; Hudson, S.P. Mesoporous matrices for the delivery of the broad spectrum bacteriocin, nisin A. J. Colloid Interface Sci. 2019, 537, 396–406. [Google Scholar] [CrossRef]
- Syryamina, V.N.; Samoilova, R.I.; Tsvetkov, Y.D.; Ischenko, A.V.; De Zotti, M.; Gobbo, M.; Toniolo, C.; Formaggio, F.; Dzuba, S.A. Peptides on the surface: Spin-label EPR and PELDOR study of adsorption of the antimicrobial peptides trichogin GA IV and ampullosporin a on the silica nanoparticles. Appl. Magn. Reson. 2016, 47, 309–320. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, T.; Lin, F.-C.; Zhang, B.; Zink, J.I. Supramolecular assemblies of heterogeneous mesoporous silica nanoparticles to co-deliver antimicrobial peptides and antibiotics for synergistic eradication of pathogenic biofilms. Acs Nano 2020, 14, 5926–5937. [Google Scholar] [CrossRef]
- Kumar, P.; Shenoi, R.A.; Lai, B.F.; Nguyen, M.; Kizhakkedathu, J.N.; Straus, S.K. Conjugation of aurein 2.2 to HPG yields an antimicrobial with better properties. Biomacromolecules 2015, 16, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Sørensen, K.K.; Hjálmarsdóttir, M.Á.; Sigurjónsson, Ó.E.; Jensen, K.J.; Másson, M.; Thygesen, M.B. Antimicrobial peptide shows enhanced activity and reduced toxicity upon grafting to chitosan polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef]
- Kanchanapally, R.; Nellore, B.P.V.; Sinha, S.S.; Pedraza, F.; Jones, S.J.; Pramanik, A.; Chavva, S.R.; Tchounwou, C.; Shi, Y.; Vangara, A. Antimicrobial peptide-conjugated graphene oxide membrane for efficient removal and effective killing of multiple drug resistant bacteria. RSC Adv. 2015, 5, 18881–18887. [Google Scholar] [CrossRef]
- Li, F.; Lin, L.; Chi, J.; Wang, H.; Du, M.; Feng, D.; Wang, L.; Luo, R.; Chen, H.; Quan, G. Guanidinium-rich lipopeptide functionalized bacteria-absorbing sponge as an effective trap-and-kill system for the elimination of focal bacterial infection. Acta Biomater. 2022, 148, 106–118. [Google Scholar] [CrossRef]
- Malekkhaiat Häffner, S.; Nyström, L.; Browning, K.L.; Mörck Nielsen, H.; Strömstedt, A.A.; Van Der Plas, M.J.; Schmidtchen, A.; Malmsten, M. Interaction of laponite with membrane components—Consequences for bacterial aggregation and infection confinement. ACS Appl. Mater. Interfaces 2019, 11, 15389–15400. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Ashmore, D.a.; Nath, S.d.; Kate, K.; Dennis, V.; Singh, S.R.; Owen, D.R.; Palazzo, C.; Arnold, R.D.; Miller, M.E. A novel covalent approach to bio-conjugate silver coated single walled carbon nanotubes with antimicrobial peptide. J. Nanobiotechnol. 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1–11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Pochert, A.; Gerber, M.; Raber, H.F.; Lindén, M. Influence of mesopore size and peptide aggregation on the adsorption and release of a model antimicrobial peptide onto/from mesoporous silica nanoparticles in vitro. Mol. Syst. Des. Eng. 2017, 2, 393–400. [Google Scholar] [CrossRef]
- Braun, K.; Pochert, A.; Lindén, M.; Davoudi, M.; Schmidtchen, A.; Nordström, R.; Malmsten, M. Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J. Colloid Interface Sci. 2016, 475, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A. Antimicrobial peptides: A new hope in biomedical and pharmaceutical fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Sommer, M.O.; Munck, C.; Toft-Kehler, R.V.; Andersson, D.I. Prediction of antibiotic resistance: Time for a new preclinical paradigm? Nat. Rev. Microbiol. 2017, 15, 689–696. [Google Scholar] [CrossRef]
- Wang, T.; Yin, L.; Ma, Z.; Zhang, Y. Chlorogenic Acid-Loaded Mesoporous Silica Nanoparticles Modified with Hexa-Histidine Peptides Reduce Skin Allergies by Capturing Nickel. Molecules 2022, 27, 1430. [Google Scholar] [CrossRef]
- Nystrom, L.; Nordstrom, R.; Bramhill, J.; Saunders, B.R.; Álvarez-Asencio, R.; Rutland, M.W.; Malmsten, M. Factors affecting peptide interactions with surface-bound microgels. Biomacromolecules 2016, 17, 669–678. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M.; Mohajerani, H. A novel pH-sensitive and magnetic starch-based nanocomposite hydrogel as a controlled drug delivery system for wound healing. Polym. Degrad. Stab. 2020, 179, 109255. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.-H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Gotz, F. Inactivation of the dlt Operon inStaphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhopadhyay, S.; Youssef, S.H.; Song, Y.; Nayak, U.Y.; Garg, S. Harnessing the Power of Antimicrobial Peptides: From Mechanisms to Delivery Optimization for Topical Infections. Antibiotics 2025, 14, 379. https://doi.org/10.3390/antibiotics14040379
Mukhopadhyay S, Youssef SH, Song Y, Nayak UY, Garg S. Harnessing the Power of Antimicrobial Peptides: From Mechanisms to Delivery Optimization for Topical Infections. Antibiotics. 2025; 14(4):379. https://doi.org/10.3390/antibiotics14040379
Chicago/Turabian StyleMukhopadhyay, Songhita, Souha H. Youssef, Yunmei Song, Usha Y. Nayak, and Sanjay Garg. 2025. "Harnessing the Power of Antimicrobial Peptides: From Mechanisms to Delivery Optimization for Topical Infections" Antibiotics 14, no. 4: 379. https://doi.org/10.3390/antibiotics14040379
APA StyleMukhopadhyay, S., Youssef, S. H., Song, Y., Nayak, U. Y., & Garg, S. (2025). Harnessing the Power of Antimicrobial Peptides: From Mechanisms to Delivery Optimization for Topical Infections. Antibiotics, 14(4), 379. https://doi.org/10.3390/antibiotics14040379








