Abstract
Background/Objectives: Wine pomace is a rich source of bioactive phenolic compounds with potential health benefits. This study aimed to evaluate the antipathogenic and antioxidant properties of ethanol and ethyl acetate extracts from wine pomace of three grape varietals (Tannat, Bonarda, and Malbec) to explore their potential as natural alternatives for mitigating bacterial virulence in Pseudomonas aeruginosa. Methods: Successive exhaustion extractions were performed using solvents of increasing polarity (ethyl acetate and ethanol). The phenolic content was quantified, and the antioxidant activity was evaluated using standard assays. The antipathogenic activity against P. aeruginosa was assessed by measuring biofilm formation, elastase and protease activity, pyocyanin production, and swarming motility. Quorum sensing (QS) inhibition was tested using a violacein production assay in Chromobacterium violaceum. Results: Ethanol was more effective at extracting phenolic compounds, with Tannat exhibiting the highest total phenolic content (162.5 µg GAE/mg). HPLC-DAD analysis identified 16 phenolic acids, 18 flavonoids, and 3 stilbenes across the extracts. The ethanol extracts showed strong antioxidant activity (phosphomolybdenum reducing capacity 67–128 μg AAE/mg, ABTS•+ scavenging 37–71 µg/mL, Fe3+ reducing power 31–68 µg/mL) and inhibited biofilm formation (up to 61%), elastase (up to 41%), and protease (up to 46%) activities in P. aeruginosa. The extracts also reduced pyocyanin production (up to 78%) and swarming motility (up to 68%), suggesting interference with QS. Moreover, the extracts inhibited violacein production in C. violaceum, confirming QS inhibition (up to 26%). Conclusions: Among the extracts, ethanol-extracted Tannat pomace showed the most substantial antipathogenic and antioxidant activities. The results add value to wine pomace by suggesting its use as natural extracts rich in phenolic compounds, capable of controlling the bacterial virulence of Pseudomonas aeruginosa without promoting the development of resistance.
1. Introduction
Microbial contamination of foods is a major public health concern, affecting consumers, regulatory agencies, and food industries worldwide. Foodborne pathogens, including bacteria, viruses, fungi, and parasites, are responsible for millions of illnesses worldwide, with an estimated 600 million people affected annually [1]. These pathogens pose a particular risk to vulnerable populations, such as children under five, pregnant women, the elderly, and individuals with compromised immune systems. In severe cases, foodborne infections can lead to debilitating diseases, such as meningitis, and even death [1,2].
Food contamination by pathogenic microorganisms during processing and storage represents a great health and hygiene risk and produces significant economic losses for the food industry [3]. Among these microorganisms, Pseudomonas aeruginosa is particularly noteworthy. Commonly found in natural environments such as soil, freshwater, and marine habitats, as well as on abiotic surfaces like clinical instruments and food processing equipment [4,5], this bacterium readily attaches to food surfaces and forms biofilms. Its presence has been documented in a variety of products including dairy, meat, water, and plant-based foods [6]. Biofilm formation not only facilitates cross-contamination after processing but also complicates cleaning processes, thereby threatening human health [7].
Beyond its role in clinical infections, P. aeruginosa has emerged as a significant foodborne pathogen. Its rapid growth on food surfaces, coupled with the production of oxidized compounds and slimy substances, accelerates spoilage and raises concerns among consumers and food safety regulators [6,8]. Moreover, its ability to thrive in diverse environments and develop resistance to multiple antibiotics underscores the need for innovative control strategies.
P. aeruginosa organizes population behaviors, such as biofilm formation, swarming motility, and virulence factor production (pyocyanin, elastase, protease) by a cell-to-cell communication mechanism called Quorum sensing (QS) [9]. This mechanism allows bacteria to sense their microbial population through signal molecules named autoinducers. The most common signal molecules used by Gram-negative bacteria are N-acyl-homoserine lactones (AHLs) [10,11]. In many cases, the presence of these molecules would be a health risk, since they can remain in a wide variety of foods with no possibility of inactivation at 100 °C [12,13,14].
The search for QS inhibitors (QSI) is an alternative and safer approach to interrupting bacterial communication and controlling bacterial virulence factors [15,16]. Accordingly, the bioactivities of natural products like potential QSI as antipathogenic substances could be an important strategy to prevent food spoilage and foodborne diseases [17,18,19].
In this context, the food industry generates large quantities of waste and byproducts. These byproducts constitute a rich bioactive compound source, which may be applied in the food, feed, cosmetic, and pharmaceutical industries [20]. In the northwest region of Argentina, specifically Cafayate (Salta), wine production is one of the most developed activities. During wine production, tons of waste made up essentially of skins, seeds, and stems called grape pomace is generated [21,22,23]. This is characterized by a high content of polyphenol compounds that are partially extracted during the winemaking process. Generally, grape pomace contains 25–35% of the total weight of grape processed [24,25,26]. These large amounts of byproducts generated by wineries constitute a serious environmental problem [27,28].
Previous studies reported the antimicrobial activity of grape extracts against planktonic cultures of Gram-positive and Gram-negative bacteria, and the potential of grape pomace phenolic compounds to be used as preservatives [29,30,31]. However, this study seeks to interrupt bacterial communication (or QS mechanism) as an attractive strategy to current bacterial control practices employed in industrial settings [32,33,34].
This work aims to add value to red grape wine pomace as a natural alternative for preventing bacterial infections by interfering with the different QS-dependent virulence factors of P. aeruginosa.
2. Results and Discussion
Vitis vinifera L. is a valuable source of bioactive molecules, including lipids, proteins, carbohydrates, and polyphenols, which account for approximately 5–8% of its composition, depending on the grape cultivar [35]. Grape pomace is known for its high polyphenol content and exhibits superior antioxidant activity compared to other plant extracts [36]. Recovering V. vinifera pomace aligns with the principles of the circular economy, a model that promotes the reuse of products and natural resources to extend their life cycle while minimizing waste and byproducts [35].
In the present study, successive exhaustion extractions were performed on wine pomace from the three varietals using sequential extraction with ethyl acetate followed by ethanol. These solvents were used to obtain phenolic compounds of medium to high polarity, respectively. These secondary metabolites are known for their antioxidant, antimicrobial, and anti-inflammatory properties [37,38]. Therefore, they are considered ideal candidates for inhibiting pathogenic microorganisms. The composition of grape pomace extracts can vary depending on the extraction technique used [39], and factors such as geographical origin, climate, grape variety, and winemaking process further influence their phenolic composition and biological activity [40].
Several techniques have been employed to recover polyphenols from wine byproducts. The most commonly used industrial method is conventional solvent extraction, often solid–liquid or maceration, to obtain bioactive compounds from plant matrices. Water and ethanol are the solvents most employed for food, pharmaceutical, and cosmetic applications due to their safety and effectiveness [41]. The choice of solvent significantly influences the quantity and distribution of extracted phenolic compounds, affecting both the antimicrobial and antioxidant activities of the extracts [42].
Not all phenolic compounds can be efficiently isolated using a single solvent due to their varied polarities and solubilities. Employing a sequential extraction approach overcomes these limitations by gradually increasing the polarity of the solvent. This strategy enables the recovery of a broader range of polyphenols, resulting in a more comprehensive profile of bioactive compounds. Furthermore, it simplifies compound identification by reducing the number of compounds extracted per solvent, which improves the efficiency of analytical techniques [43,44,45].
In this work, the total phenolic content of the ethanolic extracts was 3 to 4 times greater than that of the ethyl acetate extracts (Table 1), emphasizing the superior effectiveness of ethanol in extracting phenolic compounds. Among the varietals, Tannat showed the highest total phenolic content, followed by Bonarda, which may be attributed to the distinct composition of the Tannat varietal (higher content of tannins, flavones/flavonols, and non-flavonoid phenolics). In contrast, the anthocyanin content in Bonarda ethanolic extracts was 1.5 to 2.6 times higher than in the other varietals. The enhanced extraction efficiency of ethanol, particularly for flavonoids and tannins, highlights its potential for producing bioactive-rich extracts with health-promoting properties. Previous studies have shown that for grape pomace, ethanol is particularly effective at recovering phenolic compounds [46,47,48], while in other plant matrices, the ethyl acetate fraction has demonstrated higher phenolic recovery [49,50].

Table 1.
The polyphenol composition of the extracts.
The HPLC-DAD analysis (Table 2) allowed the identification of 16 phenolic acids, 18 flavonoids, and 3 stilbenoids in the extracts, with most of these compounds present in the three wine varietals. The ethanolic extracts generally exhibited higher compound concentrations than the ethyl acetate extracts. In general, phenolic acids were more concentrated in ethanolic extracts due to their solubility properties, with 4-O-caffeoylquinic acid, vanillic acid, and chlorogenic acid among the most abundant. Similar trends were observed for flavonoids, with ethanolic extracts containing higher concentrations of (+)-catechin, epicatechin, rutin, myricetin, quercetin, and phloridzin. Notably, ethyl acetate extracts had higher levels of quercetin-3-O-glucopyranoside than their corresponding ethanolic extracts, while kaempferol and naringin were found in comparable concentrations across both solvent systems. Between the varietals, notable quantitative differences were observed; for instance, the ethanolic extract of Tannat had higher levels of key phenolic acids, as well as catechin and rutin. On the other hand, Bonarda extracts exhibited higher concentrations of 4,5-di-O-caffeoylquinic acid than the other extracts. The vanillic acid content in Bonarda’s ethyl acetate extract was 2 to 2.4 times higher than that of the other ethyl acetate extracts and comparable to levels in the ethanolic extracts. Stilbenoids, such as trans-polydatin, resveratrol, and trans-epsilon viniferin, were detected in almost all varieties except for the ethyl acetate extract of the Malbec variety.

Table 2.
Phenolic compounds quantified (mg/100 g DW) in wine pomace extracts through HPLC-DAD.
The antioxidant activity of the extracts was evaluated through different method-ologies (Table 3). All extracts have antioxidant capacity, mainly the ethanolic extracts, with the ethanolic extract of Tannat being the most active (lowest IC50 value). These results correlate with the concentration of phenolic compounds. Although the extracts are slightly less active than pure compounds used as standards, they have potential ap-plications in food preservation, nutraceuticals, or cosmetics, where oxidative stability is key.

Table 3.
The antioxidant capacity of the wine pomace extracts.
The rise in antimicrobial resistance globally has emerged as a significant public health concern, necessitating the search for new antimicrobial agents. The food industry generates substantial byproducts rich in bioactive compounds, mainly phenolic compounds, which have garnered significant attention from researchers and industry due to their antioxidant and antimicrobial properties [51]. However, research on grape byproducts has largely focused on their antibiotic effects rather than their ability to counteract pathogenic mechanisms [52,53]. Only a limited number of studies have explored the antipathogenic properties of wine pomace, and in grape varieties different from those in the present study. For instance, Viola et al. [48] demonstrated that extracts from Torrontés grape pomace possess both antioxidant and antibiofilm activities against P. aeruginosa and Staphylococcus aureus. Interestingly, while Cabernet sauvignon grape pomace extracts obtained via accelerated solvent extraction exhibited P. aeruginosa and Staphylococcus epidermidis biofilm inhibition, those derived from conventional ethanolic and methanolic methods did not show significant antibiofilm effects [39]. Additionally, Sateriale et al. [54] (2024) reported that polyphenolic extracts from Aglianico grape pomace effectively inhibited biofilm formation by S. aureus and Bacillus cereus.
Regarding biofilm formation by P. aeruginosa (Figure 1), ethanolic extracts from the pomace of the three varietals, at a concentration of 100 µg/mL, inhibited biofilm formation in both strains: PAO1, clinical isolate, and LVP 60, contaminated water isolate. The inhibition rates were 40% and 54% for Bonarda, 26% and 37% for Malbec, and 34% and 61% for Tannat, respectively. Notably, only the ethyl acetate extract of Bonarda inhibited biofilm formation in both strains, with 45% and 57% inhibition rates. Unlike ciprofloxacin, these extracts did not inhibit bacterial growth but significantly reduced biofilm production, suggesting an antipathogenic effect that does not involve a traditional antibiotic action. This inhibition of biofilms is particularly relevant, since biofilms contribute to bacterial resistance and persistence. The ability of these extracts to reduce biofilm formation without killing bacteria could prevent the development of bacterial resistance, offering an alternative to controlling pathogenic infections.
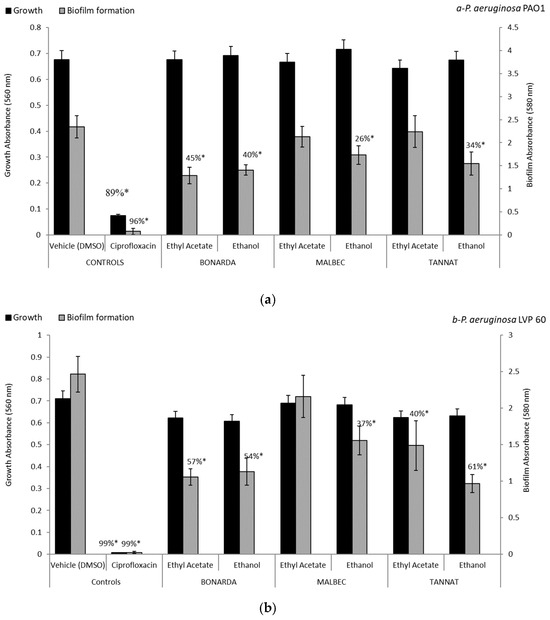
Figure 1.
Biofilm formation and growth of Pseudomonas aeruginosa PAO1 (a) and LVP 60 (b) in the presence and absence of 100 μg/mL of pomace extracts and ciprofloxacin (1 μg/mL). The values represent the means ± SD. * The values are significantly different at p ≤ 0.05 compared to the respective DMSO control.
The biological activity of many phenolic compounds has been studied individually, providing partial support for the results obtained in this study. For instance, proanthocyanidins (condensed tannins) from grape seed extract have been shown to suppress biofilm formation by Escherichia coli and Salmonella Typhimurium effectively [35]. Several natural compounds, including p-coumaric acid, gallic acid, ferulic acid, quercetin, caffeic acid, ursolic acid, and rutin, have been highlighted for their effectiveness in controlling P. aeruginosa biofilm formation [55,56]. Ivanov et al. [57] reported that rutin and ferulic acid significantly reduced biofilm formation, while catechin inhibited P. aeruginosa biofilm formation on surfaces [58]. Additionally, resveratrol at 500 µM partially disrupted the compact structure of P. aeruginosa PAO1 biofilm [59]. Kaempferol has also exhibited inhibitory effects against both Gram-positive and Gram-negative bacteria [60]. Chlorogenic acid has shown significant activity against P. aeruginosa, while vanillic acid displayed strong inhibitory effects against Enterobacter, with a minimum inhibitory concentration (MIC) of 800 µg/mL [61,62]. The highest biofilm attenuation was observed at twice the MIC of vanillic acid, highlighting its dose-dependent antibiofilm efficacy [63]. These compounds are believed to exert their effects through QS inhibition and the suppression of autoinducer production in P. aeruginosa [64].
The connection between virulence factor production and antioxidant activity is well established. The accumulation of reactive oxygen species (ROS) within cells induces oxidative stress, and biofilm development. ROS promotes microbial adhesion, enhancing biofilm formation [65]. Oxidative stress is also recognized as a critical mechanism in inflammatory processes, especially in Gram-negative infections of the intestinal mucosa [66,67]. Studies have reported the high antioxidant activity of grape pomace, indicating its potential as a source of natural antioxidants [23,36,41,48]. Moreover, the antioxidant activity correlates positively with phenolic compound concentration and antibiofilm activity. This aligns with previous findings, where methanol and ethyl acetate extracts from Torrontés wine pomace, which contained the highest total polyphenol levels, exhibited the strongest ABTS•+ and nitric oxide scavenging capacity, the highest Fe3⁺ reducing power, and the most significant biofilm inhibition. There is a direct relationship between polyphenol content, antioxidant potential, and antibiofilm properties in winemaking byproducts [48].
The extracts also showed significant inhibition regarding elastase activity in P. aeruginosa (Figure 2). Elastase is a key virulence factor in P. aeruginosa, contributing to tissue damage and immune evasion during infections. In the PAO1 and LVP 60 strains, the extracts inhibited elastase activity in a range of 16% to 51%, with the ethanolic extracts being the most effective. Inhibition rates were 41% and 30% for Bonarda, 34% and 11% for Malbec, and 41% and 23% for Tannat, for the PAO1 and LVP 60 strains, respectively. Notably, the ethyl acetate extract of Bonarda showed significant elastase inhibition in the PAO1 strain, with an inhibition rate of 50% at a concentration of 100 µg/mL. Inhibiting elastase production can weaken the pathogenic potential of bacteria without exerting selective pressure for resistance development, as seen with conventional antibiotics, highlighting the potential of these extracts as natural alternatives for mitigating bacterial virulence in clinical or industrial settings.
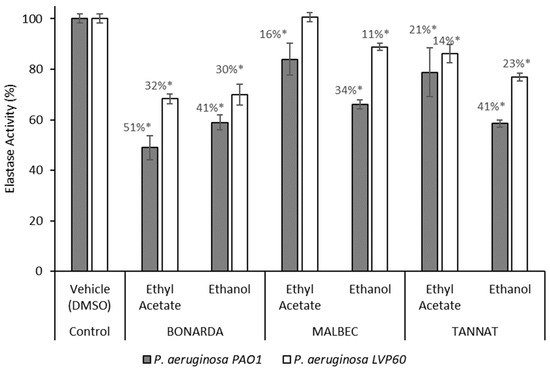
Figure 2.
Elastase activity of Pseudomonas aeruginosa PAO1 and LVP 60 in the presence and absence of 100 μg/mL of pomace extracts. The values represent the means ± SD. * The values are significantly different at p ≤ 0.05, compared to the respective DMSO control.
On the other hand, the protease activity assays demonstrated that the P. aeruginosa strains grew in the presence of the ethanolic extracts of the three pomace varietals, exhibiting inhibition of the protease activity in a range of 10% to 46%, with the LVP 60 strain being the most sensitive (Figure 3). Notably, Bonarda and Tannat extracts were more active than Malbec, showing stronger inhibition of protease activity. The ethyl acetate extract from Bonarda was the only one to inhibit protease activity in both strains, with inhibition rates of 22% for PAO1 and a remarkable 66% for LVP 60. The higher sensitivity of LVP 60 and the increased activity of Bonarda and Tannat suggest varietal differences in the bioactive compound profiles of the pomace extracts, which could be exploited for strain-specific therapeutic strategies. These findings highlight the potential of pomace extracts as natural virulence modulators, offering a complementary approach to traditional antimicrobial therapies by targeting bacterial virulence factors rather than promoting resistance.
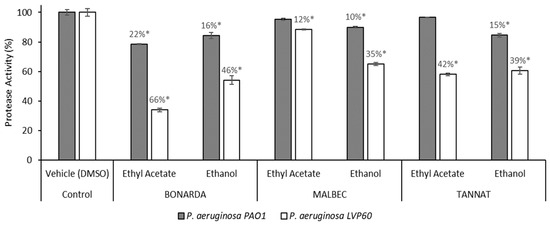
Figure 3.
Protease activity of Pseudomonas aeruginosa PAO1 and LVP 60 in the presence and absence of 100 μg/mL of pomace extracts. The values represent the means ± SD. * The values are significantly different at p ≤ 0.05, compared to the respective DMSO control.
The enzymes proteases and elastases, regulated by the LasR and RhlR QS systems, play a critical role in host tissue invasion and immune system evasion. Chlorogenic acid, detected in wine pomace, can inhibit elastase activity [61]. Additionally, flavonoids such as myricetin, quercetin, and kaempferol, also present in wine pomace, have demonstrated human neutrophil elastase inhibition [68,69]. An ethanolic extract from alperujo, an olive industry byproduct, at a concentration of 100 μg/mL, significantly reduced elastase activity in P. aeruginosa strains LVP 60 by 99% and PAO1 by 81% [70]. Resveratrol (250 µM) and quercetin (500 µM) inhibited proteolytic activity in P. aeruginosa by 35.9% and 34.0%, respectively [71]. Quercetin significantly inhibited protease production by P. aeruginosa PAO1 (43%) Ouyang et al. [72].
Pyocyanin is recognized as one of the most significant virulence factors in P. aeruginosa. It plays a crucial role in its pathogenicity and exhibits antimicrobial properties against various bacterial and fungal species [73]. Consequently, targeting the inhibition of this virulence factor serves as a valuable indicator of a compound’s efficacy as a QSI [59,71]. The extracts effectively inhibited the pigment pyocyanin, another virulence factor produced by P. aeruginosa (Figure 4). Ethanolic extracts outperformed ethyl acetate extracts in reducing pyocyanin production in both bacterial strains, with inhibition levels ranging from 18% to 78%. The Tannat ethanolic extract showed the most potent effect, decreasing pyocyanin production by 47% in PAO1 and 78% in LVP 60. This was followed by the Bonarda ethanolic extract, which achieved 41% inhibition in PAO1 and 48% in LVP 60. Among the ethyl acetate extracts, Bonarda pomace was the most effective, inhibiting pyocyanin production by 48% in PAO1 and 36% in LVP 60. Ugurlu et al. [74] demonstrated that cinnamic acid, vanillic acid, ferulic acid, and caffeic acid reduced pyocyanin production by 9–21% at sub-MIC, while Ouyang et al. [72] reported that quercetin inhibited pyocyanin production by 58% at 53 μM. Likewise, chlorogenic acid has been shown to reduce pyocyanin production [61].
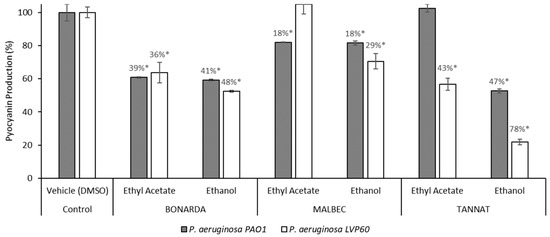
Figure 4.
Pyocyanin production by Pseudomonas aerguninosa PAO1 and LVP 60 in the presence and absence of 100 μg/mL of pomace extracts. The values represent the means ± SD. * The values are significantly different at p ≤ 0.05, compared to the respective DMSO control.
Swarming motility, closely linked to virulence and antibiotic resistance, is another adaptive mechanism contributing to biofilm formation and infection persistence in various microorganisms [75,76]. All extracts attenuated swarming motility in both P. aeruginosa strains, with inhibition ranging from 17% to 68% (Table 4). This reduction in swarming motility suggests that the extracts may interfere with QS mechanisms, which are crucial for regulating collective behaviors in P. aeruginosa. Cranberry proanthocyanidins and other tannins have been reported to completely inhibit P. aeruginosa swarming motility [77]. Gallic acid and naringenin inhibited swarming by more than 20%, and quercetin reached 50%, while resveratrol had no significant effect [71]. In contrast, Nunes Sagini et al. [39] informed that grape extract of Cabernet sauvignon increased P. aeruginosa P14 swarming.

Table 4.
Migration (swarming) percentage of P. aeruginosa.
Altering QS can reduce biofilm formation, virulence factor production, and antibiotic resistance. Two assays were conducted to evaluate anti-QS activity. The Chromobacterium violaceum CECT 494 assay detects inhibitors of AHL synthesis, while the C. violaceum CV026 assay identifies quenchers of AHL signals. In the C. violaceum CECT 494 biosensor assay, pomace extracts reduced violacein production 20–26% without affecting bacterial growth (Figure 5). Vanillic acid, a known QS inhibitor present at varying concentrations in the extracts, served as a positive control, showing inhibition rates of 33% and 47% at 10 and 100 µg/mL, respectively. Additionally, wine pomace extracts competitively inhibited the interaction between AHL and its receptor CviR, impeding violacein production. This effect was evidenced by cloudy, colorless areas observed around the wells containing extracts (Figure 6).
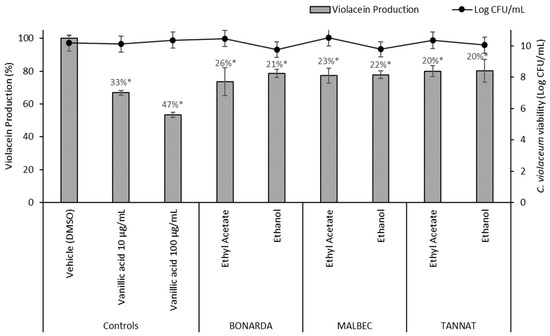
Figure 5.
Violacein production by Chromobacterium violaceum CECT 494 in the presence of 100 μg/mL of pomace extracts and the controls (DMSO and vanillic acid). The values represent the means ± SD. * The values are significantly different at p ≤ 0.05, compared to the DMSO.
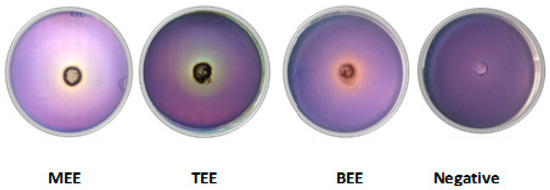
Figure 6.
Quorum sensing biosensor assay with Chromobacterium violaceum CV026 performed with wine pomace extracts at 5 mg/well (MEE: Malbec ethanolic extract, TEE: Tannat ethanolic extract, and BEE: Bonarda ethanolic extract) and DMSO (negative control).
Resveratrol has demonstrated the ability to inhibit QS, reducing violacein production in C. violaceum ATCC 12472 by 60% at 6.0 μM without affecting bacterial growth. In contrast, gallic acid and phloridizin did not impact violacein production [78]. Similarly, Duarte et al. [79] reported violacein inhibition by resveratrol, suggesting that its anti-QS activity is related to mimicking QS signals, thereby disrupting bacterial communication. Bali et al. [80] also found that gallic acid did not exhibit anti-QS activity in the biosensor strain. However, tests using only wild-type C. violaceum strains are insufficient to fully understand the QS inhibitory mechanism, as violacein inhibition may result from reduced autoinducer production (inhibition of AHL synthesis) or interference with the AHL-dependent transcriptional activator [66]. Thus, besides C. violaceum ATCC 12472, additional assays with strains like C. violaceum CV026, a mutant unable to produce AHLs but capable of responding to exogenous AHLs, may be necessary [81].
To evaluate the safety of the extracts, an acute toxicity test was performed using Artemia salina (a complex organism). The ethanolic extracts from Bonarda, Malbec, and Tannat wine pomace showed no signs of toxicity at concentrations up to 1000 μg/mL. However, the ethyl acetate extracts could not be tested due to solubility issues that hindered proper dilution. As reported by Nguta et al. [82], extracts with LD50 values (the concentration that kills 50% of A. salina larvae) above 1000 μg/mL are classified as non-toxic.
It is well established that the distribution and concentration of total polyphenolic compounds, as well as specific polyphenolic constituents, vary considerably across Vitis vinifera cultivars [83]. In this study, the ethanolic extract of Tannat showed superior antioxidant properties in all assays, along with greater inhibition of P. aeruginosa biofilm formation, pyocyanin production, and swarming motility compared to the other extracts. It also demonstrated elastase and protease inhibition levels comparable to the Bonarda extract and higher than those of Malbec. Furthermore, all extracts attenuated QS similarly. These findings may be correlated with the higher levels of total phenolics, flavones/flavonols, non-flavonoids, and tannins in the Tannat extract, supporting the link between phenolic content, antioxidant capacity, and the antipathogenic potential of wine pomace extracts.
Pure phenolic compounds are not approved as food preservatives; however, botanical extracts rich in polyphenols can be incorporated into perishable foods [84]. Given that polyphenols from wine pomace offer a natural and safe alternative for extending the shelf life of food products by preventing oxidation and spoilage caused by pathogens, the present findings highlight the potential of grape pomace as a food preservative. Grape pomace powder or extracts have been successfully used as preservatives in cheeses, yogurts, and gelatin, enhancing their stability [85,86,87,88,89]. Additionally, grape seed extract has been applied to fresh fish and fish products, effectively reducing lipid oxidation and inhibiting microbial growth [89,90,91,92].
3. Materials and Methods
3.1. Sampling and Extraction
The studies were performed with wine pomace, Vitis vinifera L., cv. Malbec, cv. Bonarda, and cv. Tannat obtained from wineries located in Cafayate, Salta, Argentina. Fresh wine pomace samples were collected and placed in ice-cooled boxes during transportation to the laboratory, where they were stored at –20 °C until processing.
The solid–liquid extraction method employed was maceration (1 kg dry pomace/L) by agitation for 24 h at 25 °C. To obtain extracts containing various classes of compounds, solvents with different polarities were used. The extraction process was carried out successively, beginning with ethyl acetate (moderate polarity), followed by ethanol 96° (high polarity), until exhaustion. This approach allowed for the selective extraction of different groups of bioactive compounds based on their solubility in each solvent.
After extraction, the resulting extracts were filtered using a Whatman Nº1 filter paper (Cytiva, Piscataway, NJ, USA) and concentrated through vacuum evaporation with a rotary evaporator (Rotavapor Buchi R-300, BUCHI, Flawil, Switzerland) at 30 °C. The dry extracts (DW) were then re-suspended in dimethyl sulfoxide (DMSO) for subsequent phytochemical and biological analyses.
3.2. Phytochemical Analysis
3.2.1. Determination of Total Phenolic and Nonflavonoid Compounds
The total phenolic content of grape pomace extracts was determined using the Folin–Ciocalteu reagent (Biopack, Buenos Aires, Argentina), following the method described by Viola et al. (2018) [48]. Gallic acid was used as the reference standard to create a calibration curve, and the results were expressed as µg gallic acid equivalents per mg of dry weight (µg GAE/mg). Nonflavonoid phenolics were quantified by measuring the remaining total phenolic content in the supernatant after the precipitation of flavonoids with acidic formaldehyde [48]. The results were expressed as µg GAE/mg.
3.2.2. Determination of Flavones/Flavonols
Flavones/Flavonols were determined following the procedure reported by Tapia et al. [23]. Samples were reacted with AlCl3, and the absorbance was measured at 420 nm. The amount of flavonoid was calculated using a linear regression equation obtained from a quercetin calibration curve. The flavonoid content was reported as µg quercetin equivalents per mg of dry weight (µg QE/mg).
3.2.3. Determination of Anthocyanins
The assessment of the total anthocyanin content was carried out using the pH differential method, and the results were expressed as µg of cyanidin-3-glucoside equivalents per mg of extract (µg C3GLE/mg) according to Tapia et al. [23].
3.2.4. Determination of Condensed Tannins
The total condensed tannins content was determined using 4-dimethylaminocinnamaldehyde (DMAC), as described by Viola et al. [48]. The absorbance was measured at 640 nm using a spectrophotometer. Proanthocyanidin B2 was used as the standard drug, and the results were expressed as µg of proanthocyanidin B2 equivalents per mg of dry weight (µg PB2E/mg).
3.2.5. Identification of Phenolic Compounds Using HPLC-DAD
Phenolic compounds were identified using a Shimadzu HPLC system (Kyoto, Japan) equipped with a Gemini C18 column (Phenomenex, Torrance, CA, USA) (250 × 4.6 mm, 5 μm) at 25 °C, following the method described by Moreira et al. [93]. The solvent system used was methanol (A) and water (B), both acidified with 0.1% formic acid. The gradient program ranged from 20% to 100% A over 100 min, with reconditioning phases before each injection. Detection was performed at 280, 320, and 360 nm, depending on the phenolic compound’s absorption maxima. Extracts were dissolved in methanol/water (20:80) and filtered before injection. Quantification was based on calibration curves of pure standards, expressed as mg per 100 g of dry extract.
3.3. Antioxidant Activity Assays
3.3.1. Total Antioxidant Activity
Pomace extracts were tested spectrophotometrically at 695 nm for their total antioxidant activity following the technique described by Tapia et al. [23]. Briefly, the reaction mixture, containing 1000 µL of a 4 nM ammonium molybdate solution and 200 µg DW/mL of pomace extract, was left to react in a water bath for 90 min at 95 °C. Ascorbic acid was used to plot the standard curve (R2 = 0.9903, p ≤ 0.05), and results were expressed as micrograms of ascorbic acid equivalents per mg of dry weight (µg AAE/mg DW).
3.3.2. ABTS•+ Free Radical Scavenging Activity
Pomace extracts were evaluated for their capacity to scavenge ABTS•+ radical [48]. Concentrations of 5–100 µg DW/mL of orujo extracts reacted with an ABTS•+ solution (absorbance of 0.7 at 734 nm); while Trolox (2.5–7.5 µg/mL) were used as positive controls. The IC50 (the concentration necessary to scavenge 50% of the ABTS•+ free radicals) was determined by linear regression analysis plotted with the percentage of scavenging obtained from the absorbance read at 734 nm after 6 min of incubation.
3.3.3. Nitric Oxide Scavenging Activity
Pomace extracts (100–1000 μg DW/mL) were put to react with sodium nitroprusside (100 mM) and Griess reagent (Sigma-Aldrich, St. Louis, MO, USA) to determine their capacity to depurate nitric oxide at 550 nm following the technique described by Viola et al. [48]. Ascorbic acid (25–400 µg/mL) was used as a positive control. To determine the concentration required to scavenge 50% of the nitric oxide radical (IC50), a regression curve was constructed by plotting the scavenging concentration against the sample concentration.
3.3.4. Iron III Reducing Power
The capacity of orujo extracts (100–500 µg DW/mL) to reduce Fe3+ to its ferrous form was determined spectrophotometrically at 700 nm according to Viola et al. [48]. BHT (3–13 µg/mL) was used as a positive control. The concentration that reduces 50% of the Fe3+ (RC50) was determined by linear regression analysis.
3.4. Antipathogenic Analysis
3.4.1. Bacterial Strains
Two strains of P. aeruginosa (PAO1 and LVP 60) were used as models for studying virulence of Gram-negative bacteria. The strain PAO1 isolated from infected wounds is widely used for research on opportunistic pathogens [94] and the strain LVP 60 was isolated from drinking water samples [95].
The strains used for the anti-quorum sensing (anti-QS) tests were Chromobacterium violaceum CECT 494 (wild type) and C. violaceum CV026 (mutant), both obtained from the Spanish Type Culture Collection (CECT) in Valencia, Spain.
The strains were activated from frozen stocks stored at −80 °C in Luria–Bertani (LB) broth with 20% glycerol for P. aeruginosa strains, and in LB Tryptein broth with 20% glycerol for C. violaceum strains. The revival process involves two successive passages of the frozen cultures in fresh broth at 37 °C to ensure optimal activation.
3.4.2. Bacterial Growth
In a 96-well microtiter polystyrene plate, 195 µL of an overnight culture of P. aeruginosa grown in Luria–Bertani (LB) medium diluted to reach the appropriate inoculum (OD 560 nm:0.1) was mixed with 5 µL of pomace extracts’ solutions (100 µg/mL final concentration), and incubated statically for 24 h. Bacterial growth was detected as turbidity (560 nm) using a microplate spectrophotometer (MultiskanGo, Thermo Fisher Scientific, Waltham, MA, USA).
Inhibition of bacterial growth mediated by the pomace was assessed by comparison with bacterial growth in the control wells containing 2.5% DMSO. Ciprofloxacin at 1 µg/mL was incorporated into the bioassay as a negative control.
3.4.3. Biofilm Formation Assay
The biofilm quantification was studied as described by Viola et al. [48]. Biofilms formed after 24 h incubation of bacterial cultures prepared as described in the previous paragraph were stained with 200 µL of an aqueous solution of crystal violet (0.05%, w/v) for 20 min, two washes with water removed unbound stains, and dried crystal violet bound to biofilm was solubilized with 200 µL acetic acid 30% during 30 min at 37 °C with shaking. Absorbance at 580 nm of crystal violet solution was determined using a microplate spectrophotometer (MultiskanGo, Thermo Fisher Scientific, Waltham, MA, USA). The following formula calculated biofilm inhibition (%):
Biofilm inhibition (%) = [(Control OD595 nm − Experimental OD595 nm)/Control OD595 nm] × 100.
3.4.4. Elastase Activity
Elastase activity was evaluated using an elastin-Congo red conjugate, a substrate specific for elastase, as described by Viola et al. [70]. In summary, 500 μL of a 5 mg/mL substrate solution dissolved in Tris-HCl buffer (pH 8.0) was combined with 500 μL of overnight culture supernatant from P. aeruginosa grown in Mueller-Hinton (MH) medium, with and without the extracts (final concentration 100 µg/mL). The mixture was incubated for 24 h at 37 °C and 150 rpm. Post-incubation, the samples were centrifuged at 10,000 rpm for 10 min, and the absorbance of the supernatant was measured at 495 nm using a microplate reader. A control assay was performed concurrently using a mixture of MH medium-Tris-HCl buffer (1:1). Elastase inhibition (%) was calculated by the following formula:
Enzyme inhibition (%) = [(Control OD495 nm -Experimental OD494 nm)/Control OD495 nm] × 100.
3.4.5. Protease Activity
To assess azocasein proteolytic activity, P. aeruginosa strains were incubated with and without pomace extracts at 37 °C for 48 h. Proteolytic activity in the cell-free supernatant was measured using the method of Gupta et al. [96]. In brief, 100 μL of supernatant from treated or untreated cultures was mixed with 400 μL of 0.3% azocasein in 0.05 M Tris-HCl (pH 7.5) and incubated at 37 °C for 1 h. The reaction was stopped with 10% trichloroacetic acid and centrifugation at 3500× g for 5 min. The absorbance of the clear supernatant was measured at 420 nm. Protease inhibition (%) was calculated by the following formula:
Enzyme inhibition (%) = [(Control OD420 nm − Experimental OD420 nm)/Control OD420 nm] × 100.
3.4.6. Pyocyanin Quantification
The quantification of pycyanin was carried out according to the method described by Díaz et al. [97]. Nine milliliters of cell-free supernatant from both treated and untreated cultures were extracted with 9 mL of chloroform (mixed for 15 s and left to stand to allow phase separation). The entire chloroform phase (light blue/turquoise in color) was collected, and 1 mL of 0.2 M HCl was added, followed by vortexing for approximately 15 s and then allowing it to rest. A 200 µL aliquot of the upper phase (turned pink/fuchsia), was taken and placed in a 96-well microplate to measure the absorbance at 520 nm.
Pyocyanin inhibition (%) = [(Control OD520 nm − Experimental OD520 nm)/Control OD520 nm] × 100.
3.4.7. Swarming Motility
The effect of pomace extracts on the motility of P. aeruginosa strains was assessed using the methodology described by Viola et al. [70]. Each pomace extract was mixed in two concentrations (250 µg/mL and 500 µg/mL), with swarm agar (0.5%) medium. Then, each P. aeruginosa strain was point inoculated and incubated at 37 °C for 24 h. DMSO was used as a negative control (1%, v/v). The effect of pomace extracts on swarming motility was determined by measuring circular turbid zones in comparison with those in the control. The motility measurements were made using Image J 1.47 V software.
3.4.8. Bioassay for the Detection of Anti-QS Activity
For the C. violaceum CECT 494 assay, violacein production, regulated by AHL autoinducers, was quantified in LB cultures treated with extracts (100 µg/mL), vanillic acid (10 and 100 µg/mL), or DMSO (control). After 24 h of incubation at 28 °C, violacein was extracted and its concentration was measured by absorbance at 585 nm. Bacterial viability was evaluated through serial dilution plating [70].
In the C. violaceum CV026 assay, violacein production is dependent on the exogenous addition of C6-HSL (short-chain AHLs). Agar plates supplemented with C6-HSL were inoculated with C. violaceum CV026, and wells loaded with extracts were examined for QS inhibition, indicated by a colorless zone surrounding the wells [70].
3.5. Toxicity Assay
The acute toxicity levels of pomace extracts, with concentrations from 250 to 1000 μg/mL, were evaluated using the Artemia salina test [23]. The negative control wells contained DMSO to a final concentration lower than 0.3%. Survival percentages were calculated by comparing the number of survivors in the test wells with respect to the negative control.
3.6. Statistical Analysis
All data are expressed as mean value ± SD of triplicate and octuplicate samples in phytochemical analysis and microbiological test, respectively. Statistical significance was analyzed using Tukey’s t-test at p < 0.05 (software InfoStat, Student Version, 2020), considering a confidence level of 95%.
4. Conclusions
Ethanolic extracts of wine pomace, particularly those derived from Tannat, exhibited strong antioxidant and antipathogenic activities by effectively inhibiting key virulence factors in P. aeruginosa. These results suggest that such extracts have potential as natural alternatives for combating bacterial contamination and mitigating the risk of resistance development.
However, while the in vitro antipathogenic effects are promising, further research is needed to assess their efficacy in real-world applications. In particular, their potential as biofilm inhibitors on various surfaces within the food industry and on food packaging where contamination is a major concern. Additionally, future studies should expand the range of pathogens tested to fully elucidate the antimicrobial potential of these natural extracts.
Author Contributions
Conceptualization, M.E.A., M.R.A. and E.C.; Methodology, C.M.V., M.E.D., R.T.-C. and M.M.M.; Software, C.M.V., M.E.D., R.T.-C. and M.M.M.; Formal Analysis, Writing—Review & Editing, M.E.A., M.R.A., E.C., M.A.B. and F.R.; Supervision, M.E.A., M.R.A., E.C. and F.R.; Project Administration, M.E.A. and M.R.A.; Funding Acquisition, M.E.A., M.R.A., E.C. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from the SCAIT-UNT (PIUNT D702, D708, and D715), the Agencia Nacional de Promoción Científica y Técnica ANPCyT (PICT 2018-02071 and 02514, PICT 2021-0060, PICT-2021-I-A-00439 I) and The Consejo Nacional de Investigaciones Científicas y Técnicas, CONICET (PUE 0021).This work received financial support from the PT national funds (FCT/MECI, Fundação para a Ciência e Tecnologia and Ministério da Educação, Ciência e Inovação) through the project UID/50006 -Laboratório Associado para a Química Verde—Tecnologias e Processos Limpos.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
María Rosa Alberto gratefully acknowledge the financial support of Fundación Williams (Fondos Complementarios para Proyectos de Investigación con Impacto en el Territorio Argentino 2024) that made this work possible. Manuela M. Moreira and Francisca Rodrigues are thankful for their contracts (2023.05993.CEECIND/CP2842/CT0009, DOI: 10.54499/2023.05993.CEECIND/CP2842/CT0009 and 2023.06819.CEECIND/CP2842/CT0008, DOI: 10.54499/2023.06819.CEECIND/CP2842/CT0008,, respectively) financed by FCT/MCTES—CEEC Individual Program Contract. The authors also are grateful to Cafayate wineries for providing the wine pomace.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Abu-Okail, A. An overview of the public health challenges in diagnosing and controlling human foodborne pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.C.; Bhowmik, S.; Ali, A. Conventional and advanced detection techniques of foodborne pathogens: A comprehensive review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef]
- Moi, I.M.; Ibrahim, Z.; Abubakar, B.M.; Katagum, Y.M.; Abdullahi, A.; Yiga, G.A.; Ayuba, I. Properties of Foodborne Pathogens and Their Diseases; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial biofilms in the food industry—A comprehensive review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.H.; Ho, C.T. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A typical biofilm forming pathogen and an emerging but underestimated pathogen in food processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef]
- Nahar, S.; Ha, A.J.W.; Byun, K.H.; Hossain, M.I.; Mizan, M.F.R.; Ha, S.D. Efficacy of flavourzyme against Salmonella typhimurium, Escherichia coli, and Pseudomonas aeruginosa biofilms on food-contact surfaces. Int. J. Food Microbiol. 2021, 336, 108897. [Google Scholar] [CrossRef]
- Urgancı, N.N.; Yılmaz, N.; Koçer Alaşalvar, G.; Yıldırım, Z. Pseudomonas aeruginosa and its pathogenicity. Turk. J. Agric. Food Sci. Technol. 2022, 10, 726–738. [Google Scholar] [CrossRef]
- Maiga, A.; Ampomah-Wireko, M.; Teng, L.H.; Min, F.Z.; Lin, Z.W.; Jie, Z.H.; Wu, C. Multidrug-resistant bacteria quorum-sensing inhibitors: A particular focus on Pseudomonas aeruginosa. Eur. J. Med. Chem. 2024, 281, 117008. [Google Scholar] [CrossRef]
- Xuan, G.; Lin, H.; Tan, L.; Zhao, G.; Wang, J. Quorum sensing promotes phage infection in Pseudomonas aeruginosa PAO1. mBio 2022, 13, e03174-21. [Google Scholar] [CrossRef]
- Díaz-Pérez, S.P.; Solis, C.S.; López-Bucio, J.S.; Valdez Alarcón, J.J.; Villegas, J.; Reyes-De la Cruz, H.; Campos-Garcia, J. Pathogenesis in Pseudomonas aeruginosa PAO1 biofilm-associated is dependent on the pyoverdine and pyocyanin siderophores by quorum sensing modulation. Microb. Ecol. 2023, 86, 727–741. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Grapefruit essential oils inhibit quorum sensing of Pseudomonas aeruginosa. Food Sci. Technol. Int. 2020, 26, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Acet, Ö.; Erdönmez, D.; Acet, B.Ö.; Odabaşı, M. N-acyl homoserine lactone molecules assisted quorum sensing: Effects, consequences, and monitoring of bacteria talking in real life. Arch. Microbiol. 2021, 203, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Caputo, L.; Brasca, M.; Fanelli, F. Recent Advances in the Mechanisms and Regulation of QS in Dairy Spoilage by Pseudomonas spp. Foods 2021, 10, 3088. [Google Scholar] [CrossRef]
- Elfaky, M.A. Unveiling the hidden language of bacteria: Anti-quorum sensing strategies for gram-negative bacteria infection control. Arch. Microbiol. 2024, 206, 124. [Google Scholar] [CrossRef]
- Naga, N.G.; Shaaban, M.I. Quorum Sensing and Quorum sensing inhibitors of natural origin. In Drug Discovery and Design Using Natural Products; Springer: Cham, Switzerland, 2023; pp. 395–416. [Google Scholar] [CrossRef]
- Luciardi, M.C.; Blázquez, M.A.; Cartagena, E.; Bardón, A.; Arena, M.E. Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT—Food Sci. Technol. 2016, 68, 373–380. [Google Scholar] [CrossRef]
- Nazareth, M.S.; Shreelakshmi, S.V.; Shetty, N.P. Identification and characterization of polyphenols from Carissa spinarum fruit and evaluation of their antioxidant and anti-quorum sensing activity. Curr. Microbiol. 2021, 78, 1277–1285. [Google Scholar] [CrossRef]
- Mangal, S.; Singh, V.; Chhibber, S.; Harjai, K. Natural bioactives versus synthetic antibiotics for the attenuation of quorum sensing-regulated virulence factors of Pseudomonas aeruginosa. Future Microbiol. 2022, 17, 773–787. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Salazar, P.B.; Fanzone, M.; Zabala, B.A.; Rodriguez Vaquero, M.J.; Cilli, E.; Barroso, P.A.; Acuña, L. A byproduct from the Valles Calchaquíes vineyards (Argentina) rich in phenolic compounds: A tool against endemic Leishmania dissemination. Environ. Sci. Pollut. Res. 2023, 30, 97377–97385. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising application of grape pomace and its agri-food valorization: Source of bioactive molecules with beneficial effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Tapia, P.E.; Silva, A.M.; Delerue-Matos, C.; Moreira, M.; Rodrigues, F.; Torres Carro, R.; Santi, M.D.; Ortega, M.G.; Blázquez, M.A.; Arena, M.E.; et al. Exploring the Phytochemical Composition and the Bioactive Properties of Malbec and Torrontés Wine Pomaces from the Calchaquíes Valleys (Argentina) for Their Sustainable Exploitation. Foods 2024, 13, 1795. [Google Scholar] [CrossRef] [PubMed]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.D.; Fierascu, R.C. Grapevine wastes: A rich source of antioxidants and other biologically active compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive components, applications, extractions, and health benefits of winery by-products from a circular bioeconomy perspective: A review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.D.C.; Madureira, J.; Margaça, F.M.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Taifouris, M.; El-Halwagi, M.; Martin, M. Evaluation of the economic, environmental, and social impact of the valorization of grape pomace from the wine industry. ACS Sustain. Chem. Eng. 2023, 11, 13718–13728. [Google Scholar] [CrossRef]
- Gabur, G.D.; Teodosiu, C.; Fighir, D.; Cotea, V.V.; Gabur, I. From Waste to Value in Circular Economy: Valorizing Grape Pomace Waste through Vermicomposting. Agriculture 2024, 14, 1529. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Poeta, P. Valorization of winemaking by-products as a novel source of antibacterial properties: New strategies to fight antibiotic resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of antibacterial and antioxidant properties of red (cv. Negramaro) and white (cv. Fiano) skin pomace extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial potential, antioxidant activity, and phenolic content of grape seed extracts from four grape varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Moreno-Chamba, B.; Salazar-Bermeo, J.; Navarro-Simarro, P.; Narváez-Asensio, M.; Martínez-Madrid, M.C.; Saura, D.; Valero, M. Autoinducers modulation as a potential anti-virulence target of bacteria by phenolic compounds. Int. J. Antimicrob. Agents 2023, 62, 106937. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Dereli, F.T.; Erzurumlu, Y.; Önem, E.; Arin, E.; Muhammed, M.T. Anti-quorum sensing activity of Vitis vinifera L. seed extract on some bacteria: A greener alternative against antimicrobial resistance. Erwerbs-Obstbau 2023, 65, 1931–1939. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in Bacterial Quorum Sensing: A Biopharmaceutical Perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Nicolosi, R.M.; Bonincontro, G.; Imperia, E.; Badiali, C.; De Vita, D.; Sciubba, F.; Dugo, L.; Guarino, M.P.L.; Altomare, A.; Simonetti, G.; et al. Protective Effect of Procyanidin-Rich Grape Seed Extract against Gram-Negative Virulence Factors. Antibiotics 2023, 12, 1615. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Valletta, A.; Pasqualetti, V.; Innocenti, M.; Giuliani, C.; Bellumori, M.; De Angelis, G.; Carnevale, A.; Locato, V.; Di Venanzio, C.; et al. Effects of Ionizing Radiation on Bio-Active Plant Extracts Useful for Preventing Oxidative Damages. Nat. Prod. Res. 2018, 33, 1106–1114. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Lima, M.D.C.; De Sousa, C.P.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; De Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Nunes Sagini, J.P.N.; Possamai Rossatto, F.C.P.; Souza, F.; Pilau, E.; Brandão Quines, C.B.; Ávila, D.S.; Ligabue-Braun, R.; Rigon Zimmer, A.R.; Inhoque Pereira, R.; Rigon Zimmer, K. Inhibition of Staphylococcus epidermidis and Pseudomonas aeruginosa biofilms by grape and rice agroindustrial residues. Microb. Pathog. 2024, 197, 107019. [Google Scholar] [CrossRef]
- Castangia, I.; Aroffu, M.; Fulgheri, F.; Abi Rached, R.; Corrias, F.; Sarais, G.; Nácher, A. From field to waste valorization: A preliminary study exploring the impact of the wine supply chain on the phenolic profile of three Sardinian pomace extracts. Foods 2024, 13, 1414. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Sailović, P.; Odžaković, B.; Bodroža, D.; Vulić, J.; Čanadanović-Brunet, J.; Zvezdanović, J.; Danilović, B. Polyphenolic Composition and Antimicrobial, Antioxidant, Anti-Inflammatory, and Antihyperglycemic Activity of Different Extracts of Teucrium montanum from Ozren Mountain. Antibiotics 2024, 13, 358. [Google Scholar] [CrossRef]
- Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves. Oxygen 2023, 3, 274–286. [Google Scholar] [CrossRef]
- Supriatno, S.; Lelifajri, L. Effect of sequential extraction on total phenolic content (TPC) and antioxidant activities (AA) of Luffa acutangula Linnaeus dried pulps. AIP Conf. Proc. 2018, 2002, 020062. [Google Scholar] [CrossRef]
- Nawaz, H.; Aslam, M.; Tul Muntaha, S.; Haq Nawaz, C. Effect of Solvent Polarity and Extraction Method on Phytochemical Composition and Antioxidant Potential of Corn Silk. Free. Radic. Antioxid. 2019, 9, 5–11. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Sousa, A.M.; Gando-Ferreira, L.M.; Quina, M.J. Grape Pomace as a Natural Source of Phenolic Compounds: Solvent Screening and Extraction Optimization. Molecules 2023, 28, 2715. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Viola, C.M.; Torres-Carro, R.; Cartagena, E.; Isla, M.I.; Alberto, M.R.; Arena, M.E. Effect of wine wastes extracts on the viability and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus strains. Evid.-Based Complement. Altern. Med. 2018, 2018, 9526878. [Google Scholar] [CrossRef]
- Sinan, K.I.; Chiavaroli, A.; Orlando, G.; Bene, K.; Zengin, G.; Cziáky, Z.; Jekő, J.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Menghini, L.; et al. Biopotential of Bersama abyssinica Fresen stem bark extracts: UHPLC profiles, antioxidant, enzyme inhibitory, and antiproliferative propensities. Antioxidants 2020, 9, 163. [Google Scholar] [CrossRef]
- Zekeya, N.; Ibrahim, M.; Mamiro, B.; Ndossi, H.; Kilonzo, M.; Mkangara, M.; Chacha, M.; Chilongola, J.; Kideghesho, J. Potential of natural phenolic antioxidant compounds from Bersama abyssinica (Meliathaceae) for treatment of chronic diseases. Saudi J. Biol. Sci. 2022, 29, 103273. [Google Scholar] [CrossRef]
- Silva, V.; Silva, A.; Ribeiro, J.; Aires, A.; Carvalho, R.; Amaral, J.S.; Barros, L.; Igrejas, G.; Poeta, P. Screening of Chemical Composition, Antimicrobial and Antioxidant Activities in Pomegranate, Quince, and Persimmon Leaf, Peel, and Seed: Valorization of Autumn Fruits By-Products for a One Health Perspective. Antibiotics 2023, 12, 1086. [Google Scholar] [CrossRef]
- Brown, C.; Jiang, X. Activities of muscadine grape skin and polyphenolic constituents against Helicobacter pylori. J. Appl. Microbiol. 2013, 114, 982–991. [Google Scholar] [CrossRef]
- Silva, A.; Martins, R.; Silva, V.; Fernandes, F.; Carvalho, R.; Aires, A.; Poeta, P. Red grape by-products from the demarcated Douro region: Chemical analysis, antioxidant potential and antimicrobial activity against food-borne pathogens. Molecules 2024, 29, 4708. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Pagliarulo, C. Vine-winery byproducts as a precious resource of natural antimicrobials: In vitro antibacterial and antibiofilm activity of grape pomace extracts against foodborne pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Lou, Z.; Tang, Y.; Song, X.; Wang, H. Metabolomics-based screening of biofilm-inhibitory compounds against Pseudomonas aeruginosa from burdock leaf. Molecules 2015, 20, 16266–16277. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as inhibitors of antibiotic resistant bacteria-mechanisms underlying rutin interference with bacterial virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, X.; Li, K.; Li, X.; Yu, K.; Liao, X.; Shi, B. Prevention of bacterial colonization based on self-assembled metal-phenolic nanocoating from rare-earth ions and catechin. ACS Appl. Mat. Interfaces 2020, 12, 22237–22245. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.F.; de Almeida, F.A.; Pinto, U.M. Exploring the antivirulence potential of phenolic compounds to inhibit quorum sensing in Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2025, 41, 32. [Google Scholar] [CrossRef]
- Fu, L.; Lu, W.; Zhou, X. Phenolic Compounds and in Vitro Antibacterial and Antioxidant Activities of Three Tropic Fruits: Persimmon, Guava, and Sweetsop. Biomed. Res. Int. 2016, 2016, 4287461. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Wang, L.; Zeng, W.; Sun, Y.; Zhou, C.; Zhou, T.; Shen, M. Effect of chlorogenic acid on the quorum-sensing system of clinically isolated multidrug-resistant Pseudomonas aeruginosa. J. Appl. Microbiol. 2022, 132, 1008–1017. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Hanganu, D.; Tiperciuc, B.; Nistor, A.; Vlase, A.M.; Laurian Vlase, L.; Puşcas, C.; Duma, M.; Login, C.C.; et al. Stachys Species: Comparative evaluation of phenolic profile and antimicrobial and antioxidant potential. Antibiotics 2023, 12, 1644. [Google Scholar] [CrossRef]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant Enterobacter hormaechei. J. Food Protec. 2020, 83, 576–583. [Google Scholar] [CrossRef]
- Shariati, A.; Noei, M.; Askarinia, M.; Khoshbayan, A.; Farahani, A.; Chegini, Z. Inhibitory effect of natural compounds on quorum sensing system in Pseudomonas aeruginosa: A helpful promise for managing biofilm community. Front. Pharmacol. 2024, 15, 1350391. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. Impact of quercitin against Salmonella typhimurium biofilm formation on food-contact surfaces and molecular mechanism pattern. Foods 2022, 11, 977. [Google Scholar] [CrossRef]
- Talavera, M.M.; Nuthakki, S.; Cui, H.; Jin, Y.; Liu, Y.; Nelin, L.D. Immunostimulated Arginase II Expression in Intestinal Epithelial Cells Reduces Nitric Oxide Production and Apoptosis. Front. Cell Dev. Biol. 2017, 5, 227133. [Google Scholar] [CrossRef][Green Version]
- Tang, X.; Liu, B.; Wang, X.; Yu, Q.; Fang, R. Epidermal Growth Factor, through Alleviating Oxidative Stress, Protect IPEC-J2 Cells from Lipopolysaccharides-Induced Apoptosis. Int. J. Mol. Sci. 2018, 19, 848. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, T.N.; Barros, A.O.; Nogueira, J.R.; Fruet, A.C.; Rodrigues, I.C.; Calcagno, D.Q.; Smith, M.A.C.; Souza, T.P.; Barros, S.B.M.; Vasconcellos, M.C.; et al. Anti-wrinkle and anti-whitening effects of jucá (Libidibia ferrea Mart.) extracts. Arch. Dermatol. Res. 2016, 308, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Casarini, T.P.A.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S. Dermatological applications of the flavonoid phloretin. Eur. J. Pharmacol. 2020, 889, 173593. [Google Scholar] [CrossRef]
- Viola, C.M.; Torres-Carro, R.; Verni, M.C.; del Valle Leal, E.; Dall’Acqua, S.; Rodrigues, F.; Arena, M.E. Interference in the production of bacterial virulence factors by olive oil processing waste. Food Biosc. 2022, 49, 101883. [Google Scholar] [CrossRef]
- Ahmed, S.A.K.S.; Rudden, M.; Smyth, T.J.; Dooley, J.S.G.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotech. 2019, 103, 3521–3535. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Abdul-Hussein, Z.; Atia, S. Antimicrobial effect of pyocyanin extracted from Pseudomonas aeruginosa. Eur. J. Exp. Biol. 2016, 6, 1–4. [Google Scholar]
- Ugurlu, A.; Karahasan Yagci, A.; Ulusoy, S.; Aksu, B.; Bosgelmez-Tinaz, G. Phenolic compounds affect production of pyocyanin, swarming motility and biofilm formation of Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2016, 6, 698–701. [Google Scholar] [CrossRef]
- Rütschlin, S.; Böttcher, T. Inhibitors of bacterial swarming behavior. Chemistry 2019, 26, 964–979. [Google Scholar] [CrossRef]
- Carette, J.; Nachtergael, A.; Duez, P.; Jaziri, M.; Rasamiravaka, T. Natural compounds inhibiting Pseudomonas aeruginosa biofilm formation by targeting quorum sensing circuitry. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef][Green Version]
- O’May, C.; Tufenkji, N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 2011, 77, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.; Lima, E.M.F.; Franco, B.D.G.M.; Pinto, U.M. Exploring Phenolic Compounds as Quorum Sensing Inhibitors in Foodborne Bacteria. Front. Microbiol. 2012, 12, 735931. [Google Scholar] [CrossRef]
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250. [Google Scholar] [CrossRef]
- Bali, E.B.; Türkmen, K.E.; Erdönmez, D.; Saglam, N. Comparative study of inhibitory potential of dietary phytochemicals against quorum sensing activity of and biofilm formation by Chromobacterium violaceum 12472, and swimming and swarming behavior of Pseudomonas aeruginosa PAO1. Food Technol. Biotechnol. 2019, 57, 212–221. [Google Scholar] [CrossRef]
- Rivera, M.L.C.; Hassimotto, N.M.A.; Bueris, V.; Sircili, M.P.; Almeida, F.A.; Pinto, U.M. Effect of Capsicum frutescens extract, capsaicin, and luteolin on quorum sensing regulated phenotypes. J. Food Sci. 2019, 84, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Biological screening of Kenyan medicinal plants using Artemia salina L. (Artemiidae). Pharmacologyonline 2011, 2, 458–478. [Google Scholar]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape Pomace as a Promising Antimicrobial Alternative in Feed: A Critical Review. J. Agric. Food Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.; Zeppa, G. Physicochemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J. Food Sci. Technol. 2016, 53, 1585–1596. [Google Scholar] [CrossRef]
- Demirkol, M.; Tarakci, Z. Effect of grape (Vitis labrusca L.) pomace dried by different methods on physicochemical, microbiological and bioactive properties of yoghurt. LWT 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT-Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Zhao, L.; Chen, L.; He, Y.; Yang, H. Vacuum impregnation of fish gelatin combined with grape seed extract inhibits protein oxidation and degradation of chilled tilapia fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. LWT 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Zhao, L.; He, Y.; Yang, H. Antimicrobial kinetics of nisin and grape seed extract against inoculated Listeria monocytogenes on cooked shrimps: Survival and residual effects. Food Control 2020, 115, 107278. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.L.; Hufnagle, W.O.; Kowallk, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.D.I.; Campos-Silva, R.; Macedo, A.J.; Blázquez, M.A.; Alberto, M.R.; Arena, M.E. Antibiofilm activity of coriander (Coriandrum sativum L.) grown in Argentina against food contaminants and human pathogenic bacteria. Ind. Crops Prod. 2020, 151, 112380. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, A.; Sandhu, P.; Daware, A.; Das, M.C.; Akhter, Y.; Bhattacharjee, S. Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: A study with plumbagin and gentamicin. J. Appl. Microbiol. 2017, 123, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.A.; González, S.N.; Alberto, M.R.; Arena, M.E. Human probiotic bacteria attenuate Pseudomonas aeruginosa biofilm and virulence by quorum-sensing inhibition. Biofouling 2020, 36, 597–609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).