Abstract
The human gut microbiota—an intricate and dynamic ecosystem—plays a pivotal role in metabolic regulation, immune modulation, and the maintenance of intestinal barrier integrity. Although antibiotic therapy is indispensable for managing bacterial infections, it profoundly disrupts gut microbial communities. Such dysbiosis is typified by diminished diversity and shifts in community structure, especially among beneficial bacterial genera (e.g., Bifidobacterium and Eubacterium), and fosters antibiotic-resistant strains and the horizontal transfer of resistance genes. These alterations compromise colonization resistance, increase intestinal permeability, and amplify susceptibility to opportunistic pathogens like Clostridioides difficile. Beyond gastrointestinal disorders, emerging evidence associates dysbiosis with systemic conditions, including chronic inflammation, metabolic syndrome, and neurodegenerative diseases, underscoring the relevance of the microbiota–gut–brain axis. The recovery of pre-existing gut communities post-antibiotic therapy is highly variable, influenced by drug spectrum, dosage, and treatment duration. Innovative interventions—such as fecal microbiota transplantation (FMT), probiotics, synbiotics, and precision microbiome therapeutics—have shown promise in counteracting dysbiosis and mitigating its adverse effects. These therapies align closely with antibiotic stewardship programs aimed at minimizing unnecessary antibiotic use to preserve microbial diversity and curtail the spread of multidrug-resistant organisms. This review emphasizes the pressing need for microbiota-centered strategies to optimize antibiotic administration, promote long-term health resilience, and alleviate the disease burden associated with antibiotic-induced dysbiosis.
1. Introduction
The human gut microbiota—a complex consortium of trillions of microorganisms—exerts fundamental roles in metabolism, immunomodulation, and pathogen defense. In addition, it influences the enteric nervous system (ENS), a dense network of neurons and glial cells governing gut motility, secretion, absorption, immune responses, and intestinal permeability [1]. Thus, perturbations in microbial communities (dysbiosis) correlate not only with gastrointestinal but also systemic pathologies [2]. Dysbiosis arises from environmental perturbations, immune imbalances, or antibiotics [3]. While antibiotics play a vital role in treating bacterial infections, they can drastically diminish gut microbial diversity, undermining metabolic functions [3,4,5]. This breakdown in microbial homeostasis compromises colonization resistance and facilitates the horizontal gene transfer of antibiotic resistance genes (ARGs), complicating infection control [6,7,8]. Broad-spectrum antibiotics (e.g., β-lactams, fluoroquinolones) are especially disruptive, eradicating key beneficial taxa (e.g., Bifidobacterium, Eubacterium) and promoting the expansion of multidrug-resistant organisms, such as vancomycin-resistant enterococci [2,8,9]. Dysbiosis-related changes extend to extra-intestinal conditions, including metabolic syndrome, chronic inflammation, and neurodegenerative disorders, underlining the microbiota–gut–brain axis’ significance [10]. This axis mediates neuroimmune and neuroendocrine signaling, affecting both the central nervous system (CNS) and ENS [1,11,12]. Alterations in critical genera (e.g., Akkermansia, Clostridium) can impair gut motility and reduce the expression of neuronal markers crucial to ENS stability [2,13]. Moreover, antibiotic-induced dysbiosis intensifies gut disorders by increasing intestinal permeability, delaying transit time, and compromising ENS neuronal integrity [1,2,14]. Clinically, these dysregulations heighten infection risks (e.g., C. difficile) and bloodstream infections, particularly in critically ill individuals [3,15]. The intestinal barrier—maintained by epithelial and immune cells—becomes more permeable under dysbiosis [16], wherein specialized epithelial cells (Paneth, goblet, enteroendocrine) secrete antimicrobial peptides and sustain mucosal integrity [17].
Multiple therapeutic strategies have emerged to counter dysbiosis. Fecal microbiota transplantation (FMT), probiotics, and synbiotics have proven effective in restoring gut microbial diversity and resilience [3]. Concurrently, antibiotic stewardship programs strive to restrain unnecessary use, maintaining beneficial taxa and diminishing selective pressures driving ARGs [8]. Recent evidence highlights the essential interplay between intestinal epithelial and immune cells in sustaining microbial homeostasis [9,17,18,19]. Ultimately, the gut microbiota underpins an array of physiologic and pathophysiologic processes. This review elucidates the mechanisms behind antibiotic-induced dysbiosis—including microbial imbalances, the emergence of resistance, and compromised host–microbiome interactions—and advocates integrating microbiota-focused strategies into clinical practice to optimize antibiotic usage, encourage robust health, and lessen disease risk.
2. Material and Methods
This review systematically examines the effects of antibiotics on the composition and function of the gut microbiota by conducting a comprehensive analysis of the existing literature. Studies published between 2012 and 2025 were identified through searches in PubMed, Scopus, and Web of Science using keywords such as “gut microbiota”, “dysbiosis”, “antibiotic resistance”, and “microbial diversity”. Inclusion criteria prioritized studies reporting both quantitative and qualitative changes in microbial communities following antibiotic exposure. Particular attention was given to broad-spectrum antibiotics—especially β-lactams and fluoroquinolones—due to their well-documented impact on beneficial taxa such as Bifidobacterium and Faecalibacterium. Data were extracted on changes in major bacterial phyla, including Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, to evaluate shifts in community structure. This review also focuses on mechanisms of antibiotic resistance, including horizontal gene transfer and selective pressures favoring multidrug-resistant strains. Clinical implications such as increased risk of Clostridioides difficile infection and decreased short-chain fatty acid production were also analyzed. Furthermore, therapeutic strategies aimed at restoring microbial balance—such as fecal microbiota transplantation (FMT) and synbiotics—were evaluated for their effectiveness in enhancing resilience and colonization resistance. This review is based exclusively on secondary data; no new experimental research was conducted. All ethical standards for systematic literature reviews were followed. Based on the findings, we propose recommendations for antibiotic stewardship that emphasize the importance of targeted therapies to minimize dysbiosis and curb the spread of antibiotic resistance. The review adheres to the PRISMA Extension for Scoping Reviews (PRISMA-ScR) checklist [20], which was employed to ensure methodological rigor and transparent reporting. A visual summary of the study selection process is presented in Figure 1. Key reporting items addressed in this study are summarized in the PRISMA-ScR checklist (Table 1).
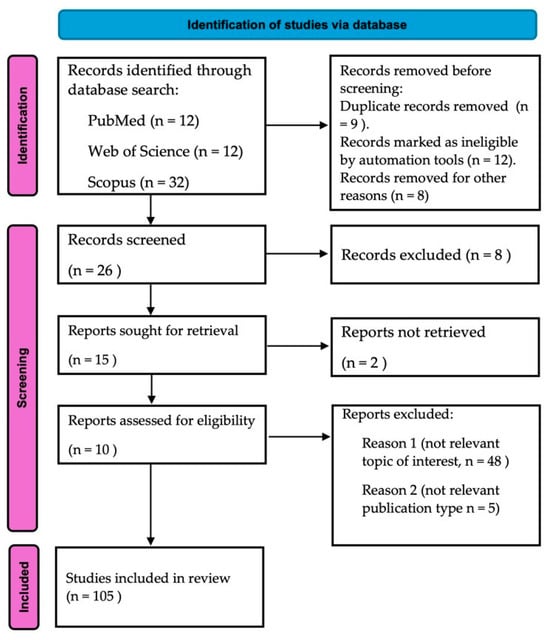
Figure 1.
Study flowchart following the PRISMA Extension for Scoping Reviews (PRISMA-ScR) guidelines [20]. The diagram illustrates the sequential phases of identification, screening, eligibility assessment, and the final inclusion of studies. It provides a clear overview of the number of records retrieved, excluded, and ultimately included in the review.

Table 1.
PRISMA Extension for Scoping Reviews (PRISMA-ScR) checklist [20]. This table summarizes the key reporting items, their corresponding descriptions, and the extent to which each was addressed in the present study. The checklist supports methodological rigor and ensures comprehensive reporting throughout the review process.
3. The Human Intestinal Microbiota: Composition and Function
3.1. Taxonomic and Functional Diversity
The human gastrointestinal microbiota is a vast, dynamic milieu of bacteria, archaea, fungi, viruses, and protozoa that collectively orchestrate nutrient processing, immunological modulation, and broader health outcomes [21]. Microbial density varies along the gastrointestinal tract: the small intestine has fewer microbes due to higher oxygen levels and faster transit, while the colon supports a richer and more stable community [22]. Predominant bacterial phyla include Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia, each contributing to essential physiological functions. For instance, Firmicutes (Clostridium, Faecalibacterium) drive short-chain fatty acid (SCFA) production, while Bacteroidetes (Bacteroides, Prevotella) specialize in polysaccharide breakdown [23]. Additional minor constituents like Akkermansia muciniphila help sustain mucosal integrity [22,23]. Microbial diversity and composition shift over the lifespan and under external influences (e.g., diet, environment) [21].
These gut-resident microorganisms are pivotal in metabolic homeostasis, nutrient assimilation, and immune regulation. They ferment complex carbohydrates to produce SCFAs like acetate, propionate, and butyrate, fueling colonocytes and reducing inflammation [21]. In addition, they synthesize vitamins (e.g., vitamin B complex, vitamin K) and participate in amino acid metabolism and gut–brain axis signaling via microbial-derived neurotransmitter precursors [24]. Immune function is likewise modulated by the microbiota, as bacterial metabolites foster regulatory T-cell development, modulate antimicrobial peptide release, and interact with innate immune sensors to maintain immune balance [19]. A well-balanced and diverse gut microbiota thus not only deters pathogenic overgrowth (colonization resistance), but also preserves intestinal barrier function [25]. Conversely, dysbiosis—characterized by altered diversity and elevated pro-inflammatory taxa—underlies inflammatory bowel disease, obesity, type 2 diabetes, colorectal cancer, and certain neurological disorders [26]. Understanding the symbiotic relationships among microbes, dietary patterns, and host pathways is integral to developing targeted interventions (e.g., probiotics, prebiotics, FMT) aimed at re-establishing microbial homeostasis and preventing disease [27].
3.2. Role in Host Physiology and Immune System Modulation
Within the gastrointestinal tract, a highly tuned interplay between the host and its resident microbial communities underpins metabolic, immune, and overall physiologic stability [28,29]. SCFAs (notably butyrate, acetate, and propionate)—key microbial byproducts—modulate immune function by mitigating inflammation, shaping immune cell phenotypes, and reinforcing epithelial integrity [30,31]. Interactions between gut microbes and the immune system are primarily mediated through pattern recognition receptors (PRRs) on intestinal epithelial and antigen-presenting cells [24,32]. The innate immune system relies on epithelial barriers, antimicrobial peptides, and tissue-resident immune cells (e.g., macrophages, dendritic cells, innate lymphoid cells) to regulate microbial loads [33,34]. Dysbiosis, however, can breach these protective mechanisms, raising intestinal permeability and triggering chronic inflammation that propels inflammatory bowel disease, metabolic syndromes, and neurological dysfunctions via gut–brain axis pathways [35,36]. Adaptive immunity refines these responses; mucosa-associated lymphoid structures orchestrate immunoglobulin A (IgA) production and T-cell differentiation, fostering tolerance toward commensals or mounting immune responses to pathogens [37,38]. Meanwhile, specific SCFAs are particularly influential in encouraging regulatory T-cell (Treg) populations, diminishing inflammation, and supporting epithelial homeostasis [39]. Hence, the gut acts as an “immunological command center” moderated by microbial signals that drive context-dependent immune outcomes [40]. Advances in microbiome-based therapies—including probiotics, prebiotics, and FMT—offer new avenues for restoring microbial balance and bolstering immune resilience [41,42]. As ongoing research sheds light on these interactions, microbiome-targeted interventions promise potential for preventing and managing numerous immunologically mediated conditions.
3.3. Microbiota Stability and Resilience
Gut microbiota stability (i.e., sustained composition/function over time) and resilience (i.e., recovery after external stress) are critical for warding off dysbiosis-associated pathologies [43]. Microbial diversity, functional redundancy, and robust host–microbe signaling foster this equilibrium [44,45]. While broad-spectrum antibiotics strongly disrupt beneficial taxa, the microbiota retains an inherent capacity to rebound through functional redundancy, β-lactamase activity, and recolonization [46]. Diet also significantly modulates stability: high-fiber diets encourage SCFA-producing bacteria and fortify gut barrier integrity, whereas Western-style diets elevate disease risk by suppressing beneficial taxa [45,47]. Emerging interventions (e.g., probiotics, FMT, microbiome-driven dietary adjustments) underscore the growing impetus for “microbiome-first medicine” that prioritizes gut microbial health [47,48].
4. Impact of Antibiotics on the Intestinal Microbiota
Although antibiotics are vital for treating bacterial infections, they can dramatically disrupt the gut microbiota, a delicate ecosystem essential for immune maturation, metabolism, and overall health (Figure 2). Broad-spectrum agents often drive dysbiosis—resulting in a loss of diversity and the distortion of community composition—by depleting beneficial genera (Bifidobacterium, Faecalibacterium) and altering key phyla (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria) [49,50]. Weakened colonization resistance paves the way for opportunistic pathogens (e.g., C. difficile, Salmonella typhimurium) [51,52], while reduced SCFA production further undercuts gut homeostasis [6,49]. Antibiotics can also accelerate antibiotic resistance via horizontal gene transfer [6]. Recovery from these perturbations hinges on antibiotic spectrum, dose, and duration, potentially taking months or years in some cases [49]. Early-life disruptions may incur lasting immunological, metabolic, and cognitive repercussions. Probiotic or synbiotic supplementation and selective antibiotic regimens can mitigate such adverse outcomes, highlighting antibiotic stewardship’s criticality [50,53]. See Table 2 for an overview of microbiome-based therapeutic approaches.
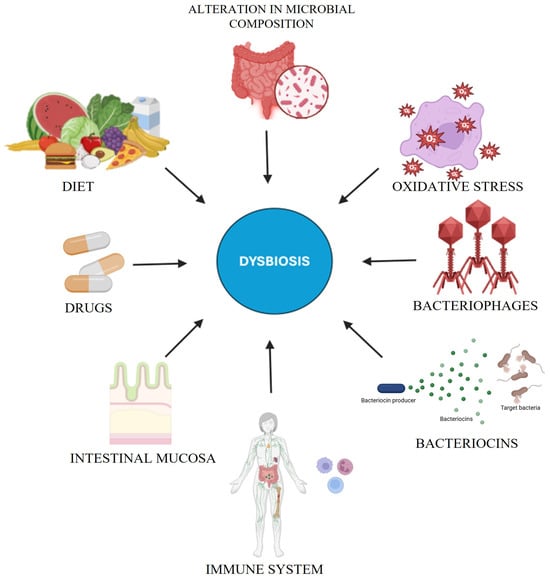
Figure 2.
This diagram depicts the multifactorial contributors to dysbiosis, a disruption of microbial homeostasis. Key factors include diet, drugs, the intestinal mucosa, and the immune system, which regulate microbial stability. Oxidative stress, bacteriophages, and bacteriocins further modulate microbial composition, while microbial alterations can sustain dysbiosis, compromising gut and systemic homeostasis.

Table 2.
Advantages, disadvantages, and future directions of microbiome-based therapeutics. A concise overview of microbiome-based therapies, emphasizing their benefits, limitations, and future prospects for enhanced precision and clinical effectiveness.
5. Mechanistic and Technological Insights into Dysbiosis and Antimicrobial Resistance
Dysbiosis—defined by the loss of beneficial microbes, the overgrowth of pathogens, and reduced microbial diversity—results from multifactorial disturbances, such as antibiotic exposure, immune dysregulation, metabolic imbalance, and environmental stressors [54,55]. These changes not only disrupt gut homeostasis, but also promote the emergence and spread of antimicrobial resistance.
5.1. Disruption of Microbial Homeostasis and Pathophysiological Consequences
A stable gut ecosystem is primarily sustained by dominant phyla such as Firmicutes and Bacteroidetes, which modulate immune function, produce short-chain fatty acids (SCFAs), and maintain mucosal integrity [56]. Disruptive factors—including antibiotic use, low-fiber diets, and inflammation—can destabilize this balance, favoring the expansion of opportunistic pathogens like Enterobacteriaceae [57]. Dysbiosis increases intestinal permeability, facilitating the translocation of microbial components (e.g., lipopolysaccharides) into systemic circulation and triggering inflammation [55,58]. Concurrently, the loss of SCFA-producing bacteria impairs the differentiation of regulatory T cells (Tregs), intensifying inflammatory responses [56].
5.2. Epithelial Crosstalk and Systemic Implications
The interaction between gut epithelial cells and the microbiota plays a critical role in immune education and barrier integrity. Specialized epithelial cells—such as tuft and Paneth cells—engage in bidirectional communication with commensals to uphold intestinal architecture and immune surveillance [57,58]. The disruption of this signaling axis not only contributes to gastrointestinal disorders, but also exerts systemic effects, including immune dysfunction and neurological disturbances via the gut–immune and gut–brain axes [55,58,59].
5.3. Dysbiosis and Antibiotic Resistance
Antibiotic-induced dysbiosis accelerates the horizontal transfer of antimicrobial resistance genes (ARGs) within the gut microbiota, compromising colonization resistance and enabling the proliferation of multidrug-resistant organisms [6,50,60]. The depletion of key taxa—such as Faecalibacteriu prausnitzii and Bifidobacterium spp.—further increases susceptibility to resistant strains, including extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae [61,62]. Emerging interventions like fecal microbiota transplantation (FMT), targeted probiotics, and postbiotics aim to counter these risks while preserving the integrity of the broader microbial ecosystem [4,50].
5.4. Integrative Multi-Omics and Computational Approaches
To unravel the complexity of antibiotic-driven dysbiosis, cutting-edge multi-omics platforms—such as metagenomics, metatranscriptomics, metabolomics, and metaproteomics—are increasingly employed [63,64]. These technologies allow the high-resolution profiling of microbiota composition, functional pathways, and resilience dynamics [65,66]. For instance, Zhernakova et al. [67] used multi-omics to identify the antibiotic-associated depletion of Faecalibacterium and reduced butyrate synthesis—key indicators of microbiota stability. Similarly, Manor et al. [68] applied genome-scale metabolic models to predict SCFA production under various antibiotic regimens, informing the design of microbiota-preserving therapies.
Machine learning further supports these efforts by detecting critical “tipping points” within microbial networks that predict dysbiosis onset. Shi et al. [69], for example, demonstrated how Lactobacillus-based interventions can modulate immune responses and restore microbial balance following β-lactam-induced dysbiosis.
5.5. Biomarker Discovery and Immune Network Mapping
Targeted metabolomics has identified bile acid derivatives and aromatic amino acid metabolites as early biomarkers of dysbiosis and ARG propagation [70]. These indicators are now incorporated into experimental diagnostic platforms to guide therapeutic decisions [71]. Simultaneously, systems biology approaches have enabled the detailed mapping of immune–microbial interactions, revealing key regulatory nodes such as IL-22-mediated epithelial repair [72,73].
5.6. Translational Therapeutics and Clinical Applications
Innovative microbiota-based therapies—shaped by these mechanistic insights—include synthetic microbial consortia like RePOOPulate, designed to restore fermentation capacity and mucosal immunity in Clostridioides difficile infections [74], and bacteriophage cocktails that selectively target resistant pathogens while sparing commensals [75]. As computational tools evolve, these precision-guided approaches are transforming microbiome therapeutics, enabling individualized antibiotic stewardship and limiting resistance development [68,76].
5.7. Clinical Restoration Strategies and Regulatory Challenges
Microbiome-targeted interventions—including probiotics, synbiotics, and FMT—have shown promise in treating C. difficile infection, inflammatory bowel disease, and metabolic syndromes [69,70,71,72,73,74,75,76,77,78,79,80]. Nevertheless, concerns about donor variability, safety, and regulatory consistency—especially for FMT and live biotherapeutic products—persist [81,82]. Standardization in manufacturing, the development of defined microbial formulations, and clear regulatory pathways will be crucial for ensuring safe and scalable implementation.
6. Cutting-Edge Mechanistic Insights and Innovative Therapeutic Frontiers
Recent advances in microbiome science have catalyzed a shift from empirical treatments to precision interventions, grounded in the mechanistic understanding of host–microbiota dynamics in the context of antibiotic perturbation [83,84]. This section highlights breakthrough strategies that leverage systems-level insights for targeted microbiota restoration.
6.1. Systems Biology and Network Analysis of Host–Microbiota Interactions
Contemporary systems biology enables the modeling of complex microbial ecosystems with unprecedented resolution. For example, studies by Zmora et al. [85] have integrated single-cell transcriptomics with spatial omics to reveal how antibiotics disrupt mucosal-layer colonization by key commensals, leading to impaired IL-10 signaling and increased susceptibility to inflammation. These network analyses have elucidated central regulatory nodes, such as Akkermansia muciniphila-driven mucin degradation pathways, which can be therapeutically modulated to restore epithelial integrity [86].
6.2. Precision Microbiome Therapeutics: Next-Generation Strategies
Novel therapeutic strategies now focus on restoring specific microbiome functions rather than broad recolonization. For instance, Petrof et al. [87] demonstrated the efficacy of a defined microbial ecosystem (RePOOPulate) in resolving recurrent C. difficile infection, offering a safer alternative to fecal microbiota transplantation. Similarly, bacteriophage therapy has shown promise in selectively depleting pathogenic Escherichia coli while preserving beneficial taxa, as reported in mouse models by Li et al. [88]. Postbiotics—non-viable microbial products such as butyrate-rich vesicles or purified microbial enzymes—are being developed to restore metabolic signaling and epithelial function without introducing live microbes, reducing the risk of unintended colonization or immune reactions [89].
6.3. Translational Implications and Future Directions
The convergence of high-resolution omics, predictive analytics, and user-centered bioinformatics tools paves the way for real-time, personalized microbiome monitoring. Platforms like MICROSCOPE and the Human Microbiome Cloud (HMC) now offer clinicians dashboards to track microbial shifts and receive evidence-based recommendations for intervention [90]. Pilot studies integrating these tools into antimicrobial stewardship protocols have reported a reduced incidence of microbiota-associated adverse effects and lower rates of multidrug-resistant organism colonization [91]. Looking ahead, clinical protocols may incorporate routine microbiome profiling to inform dynamic treatment adjustments—such as the pre-emptive administration of narrow-spectrum antibiotics, adjunctive synbiotics, or phage cocktails—to harmonize infection control with the preservation of host–microbial homeostasis.
7. Knowledge Gaps and Future Directions
Despite significant advances in microbiome science, critical knowledge gaps remain that limit the precision, scalability, and safe clinical deployment of microbiota-targeted interventions. A key challenge is the substantial inter-individual variability in microbiota recovery following antibiotic exposure, influenced by host genetics, immune status, lifestyle, and antibiotic pharmacodynamics [92]. While some individuals achieve microbial restoration within weeks, others exhibit prolonged dysbiosis, increasing susceptibility to chronic inflammation and disease [46,54]. This variability is compounded by the absence of validated biomarkers of microbiome resilience, which hinders risk stratification and the development of personalized therapies [47,70]. Importantly, host-related factors such as age, diet, and immune competence have a significant impact on both the extent of dysbiosis and the pace of microbiota recovery. For instance, elderly individuals and immunocompromised patients often experience more profound and prolonged microbiota disruptions [93,94].
Diet, particularly fiber intake and dietary diversity, also plays a key role in shaping the trajectory of microbial reconstitution post-antibiotics [95,96]. These dimensions remain underexplored in current clinical frameworks and deserve greater emphasis in future study designs.
Another critical issue is the methodological heterogeneity in studies investigating antibiotic-induced microbiome alterations. While shotgun metagenomics enables the high-resolution taxonomic and functional profiling of microbial communities, it lacks the ability to capture viable and metabolically active strains. Conversely, culture-based methods, although limited in scope, offer functional insights and strain-level isolation for downstream applications [97,98]. A systematic comparison of these approaches—and potentially integrative methodologies—is needed to standardize outcome measures and facilitate cross-study comparison.
The lack of standardization in fecal microbiota transplantation (FMT) also presents a major translational barrier. Although FMT remains highly effective for recurrent Clostridioides difficile infections [52], it carries risks related to safety, reproducibility, and the transmission of antimicrobial resistance genes or undesirable metabolic traits [67,88]. Consequently, attention is shifting toward defined microbial consortia, engineered live biotherapeutics, and postbiotics, which offer improved control, reproducibility, and regulatory compliance [79,90].
Moreover, although the gut–immune and gut–brain axes are increasingly recognized as critical mediators of systemic responses to dysbiosis [10,11], the mechanistic links between microbial disruption and downstream host dysfunction remain poorly defined. For example, the antibiotic-induced depletion of short-chain fatty acid (SCFA)-producing taxa such as Faecalibacterium prausnitzii may impair immune homeostasis and contribute to chronic inflammatory states [76].
To address these gaps and enhance the translational potential of microbiome-based interventions, future research should prioritize the following:
- Longitudinal, multi-omics studies to define microbial and host signatures of resilience and vulnerability [70,74];
- Standardized FMT protocols, including donor screening, microbial quality control, and long-term safety monitoring [52];
- The clinical validation of next-generation microbiome-based therapeutics, with clearly defined safety and efficacy profiles [79,88];
- Mechanistic studies integrating host transcriptomics, immunophenotyping, and metabolomics to elucidate causal pathways linking dysbiosis to disease [74,76].
8. Future Perspectives
Looking ahead, precision microbiome medicine is expected to transform clinical approaches to managing antibiotic-induced dysbiosis and related disorders. Advances in metagenomics, metabolomics, and machine learning are enabling the development of real-time diagnostics that can guide individualized treatment decisions and minimize collateral damage to the gut microbiota [93,94,95]. Artificial intelligence-driven analytics can now predict microbiome vulnerability and recovery potential, thereby supporting dynamic antibiotic stewardship strategies [91,96]. For example, narrow-spectrum antibiotics and β-lactamase inhibitors are being evaluated as microbiota-sparing alternatives to broad-spectrum regimens [97]. In parallel, personalized microbiota restoration therapies, such as targeted probiotics, synthetic microbial consortia, and postbiotic compounds, are under active development [90,98].
Furthermore, personalized dietary interventions—such as prebiotic-enriched or fiber-rich diets—may be employed to promote microbial resilience and maintain homeostasis during and after antibiotic therapy [49,99]. These strategies align with a broader shift toward “microbiome-first medicine”, where therapeutic decisions account for host–microbiota dynamics [48]. To ensure successful clinical translation, healthcare systems should undertake the following:
- Invest in infrastructure for point-of-care microbiome analysis [100];
- Develop regulatory frameworks for the approval and monitoring of microbiota-based therapeutics [98];
- Promote interdisciplinary collaboration among clinicians, microbiologists, computational biologists, and regulatory bodies [81,92].
This paradigm shift has the potential to reduce antibiotic-associated complications, preserve microbial diversity, and enhance patient outcomes through sustainable, individualized microbiome management.
9. Conclusions
Antibiotic therapy remains essential for the management of bacterial infections, yet its unintended disruption of the gut microbiota presents significant clinical challenges [101]. Broad-spectrum agents often compromise beneficial taxa, facilitate pathogen overgrowth, and impair host–microbiome homeostasis [102]. The heterogeneity of microbiome recovery highlights the need for precision approaches in mitigating antibiotic-induced dysbiosis, as outcomes depend on antibiotic class, dosage, duration, and host-specific factors [103]. Emerging strategies—including targeted probiotics, microbiome-based therapeutics, and integrative multi-omics tools—offer new opportunities to preserve or restore microbial balance [104]. Moving forward, personalized antibiotic regimens supported by real-time microbiome monitoring and rational microbial restoration will be critical to improving patient outcomes and promoting sustainable antibiotic stewardship [105].
Author Contributions
Conceptualization, P.A. and G.C.; methodology, R.V.; validation, G.A.F., R.V. and P.A.; formal analysis, G.C.; investigation, G.A.F.; resources, P.A.; writing—original draft preparation, P.A. and G.C.; writing—review and editing, R.V., G.A.F., P.A. and G.C.; visualization, G.C., G.A.F., R.V. and P.A.; supervision, P.A.; funding acquisition, R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AAD | Antibiotic-Associated Diarrhea |
| ARGs | Antibiotic Resistance Genes |
| CDI | Clostridioides Difficile Infection |
| CNS | Central Nervous System |
| ENS | Enteric Nervous System |
| ESBL | Extended-Spectrum β-Lactamase |
| FDA | Food and Drug Administration |
| FMT | Fecal Microbiota Transplantation |
| FOS | Fructooligosaccharides |
| GOS | Galactooligosaccarides |
| GRAS | Generally Recognized As Safe |
| HMC | Human Microbiome Cloud |
| IBD | Inflammatory Bowel disease |
| IBS | Irritable Bowel Syndrome |
| NEC | Necrotizing Enterocolitis |
| PRRS | Pattern Recognition Receptors |
| RCTs | Randomized Controlled Trials |
| SCFAs | Short-Chain Fatty Acids |
References
- Bernabè, G.; Shalata, M.E.M.; Zatta, V.; Bellato, M.; Porzionato, A.; Castagliuolo, I.; Brun, P. Antibiotic Treatment Induces Long-Lasting Effects on Gut Microbiota and the Enteric Nervous System in Mice. Antibiotics 2023, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.P.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Shah, T.; Baloch, Z.; Shah, Z.; Cui, X.; Xia, X. The Intestinal Microbiota: Impacts of Antibiotics Therapy, Colonization Resistance, and Diseases. Int. J. Mol. Sci. 2021, 22, 6597. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Antibiotic-Therapy-Induced Gut Dysbiosis Affecting Gut Microbiota—Brain Axis and Cognition: Restoration by Intake of Probiotics and Synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef]
- Leo, S.; Lazarevic, V.; von Dach, E.; Kaiserc, L.; Prendki, V.; Schrenzel, J.; Huttner, B.D.; Huttner, A. Effects of antibiotic duration on the intestinal microbiota and resistome: The PIRATE RESISTANCE project, a cohort study nested within a randomized trial. EBioMedicine 2021, 71, 103566. [Google Scholar] [CrossRef]
- Theophilus, R.J.; Taft, D.H. Antimicrobial Resistance Genes (ARGs), the Gut Microbiome, and Infant Nutrition. Nutrients 2023, 15, 3177. [Google Scholar] [CrossRef]
- Bhalodi, A.A.; van Engelen, T.S.R.; Virk, H.S.; Wiersinga, W.J. Impact of antimicrobial therapy on the gut microbiome. J. Antimicrob. Chemother. 2019, 74 (Suppl. S1), i6–i15. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan LK, S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Sig. Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Geng, Z.H.; Zhu, Y.; Li, Q.L.; Zhao, C.; Zhou, P.H. Enteric Nervous System: The Bridge Between the Gut Microbiota and Neurological Disorders. Front. Aging Neurosci. 2022, 14, 810483. [Google Scholar] [CrossRef] [PubMed]
- Aljeradat, B.; Kumar, D.; Abdulmuizz, S.; Kundu, M.; Almealawy, Y.F.; Batarseh, D.R.; Atallah, O.; Ennabe, M.; Alsarafandi, M.; Alan, A.; et al. Neuromodulation and the Gut-Brain Axis: Therapeutic Mechanisms and Implications for Gastrointestinal and Neurological Disorders. Pathophysiol. 2024, 31, 244–268. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef] [PubMed]
- Thabet, E.; Dief, A.E.; Arafa, S.A.; Yakout, D.; Ali, M.A. Antibiotic-induced gut microbe dysbiosis alters neurobehavior in mice through modulation of BDNF and gut integrity. Physiol. Behav. 2024, 283, 114621. [Google Scholar] [CrossRef]
- Gonzales-Luna, A.J.; Carlson, T.J.; Garey, K.W. Gut microbiota changes associated with Clostridioides difficile infection and its various treatment strategies. Gut Microbes 2023, 15, 2223345. [Google Scholar] [CrossRef]
- Prame Kumar, K.; Ooi, J.D.; Goldberg, R. The interplay between the microbiota, diet and T regulatory cells in the preservation of the gut barrier in inflammatory bowel disease. Front. Microbiol. 2023, 14, 1291724. [Google Scholar] [CrossRef]
- Yao, Y.; Shang, W.; Bao, L.; Peng, Z.; Wu, C. Epithelial-immune cell crosstalk for intestinal barrier Homeostasis. Eur. J. Immunol. 2024, 54, e2350631. [Google Scholar] [CrossRef]
- Dhingra, G.G.; Kumar, R.; Sood, U.; Hira, P.; Kaur, J.; Lal, R. Microbiome and Human Health: From Dysbiosis to Therapeutic Interventions. In Role of Microbes in Sustainable Development; Sobti, R., Kuhad, R.C., Lal, R., Rishi, P., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, W.; Liang, Z.; Wang, J.; Zeng, Z.; Kołat, D.; Zhao, L. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 33. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Straus, S.E. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Hollister, E.B.; Gao, C.; Versalovic, J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 2014, 146, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional Roles of B Vitamins in the Gut and Gut Microbiome. Mol. Nutr. Food Res. 2020, 64, e2000426. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Fakharian, F.; Thirugnanam, S.; Welsh, D.A.; Kim, W.-K.; Rappaport, J.; Bittinger, K.; Rout, N. The Role of Gut Dysbiosis in the Loss of Intestinal Immune Cell Functions and Viral Pathogenesis. Microorganisms 2023, 11, 1849. [Google Scholar] [CrossRef]
- Shukla, V.; Singh, S.; Verma, S.; Verma, S.; Abbas Rizvi, A.; Abbas, M. Targeting the microbiome to improve human health with the approach of personalized medicine: Latest aspects and current updates. Clin. Nutr. ESPEN 2024, 63, 813–820. [Google Scholar] [CrossRef]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Bessa, L.A. Microbial Diversity: The Gap between the Estimated and the Known. Diversity 2018, 10, 46. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Chatterjee, S. Chapter 1—Innate and Adaptive Immunity: Barriers and Receptor-Based Recognition. In Immunity and Inflammation in Health and Disease; Shampa Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–13. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. J. Nat. 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, J.; Cai, P.; Xia, Y.; Yi, C.; Shang, A.; Akanyibah, F.A.; Mao, F. The emerging role of the gut microbiota and its application in inflammatory bowel disease. Biomed. Pharmacother. 2024, 179, 117302. [Google Scholar] [CrossRef] [PubMed]
- Mousa, R.S.; Invernizzi, P.; Mousa, H.S. Innate immune cells in the pathogenesis of inflammatory bowel disease—From microbial metabolites to immune modulation. Front. Gastroenterol. 2024, 3, 1452430. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Sai, A.; Shetty, G.B.; Shetty, P.; Nanjeshgowda, H.L. Influence of gut microbiota on autoimmunity: A narrative review. BBI-Integrative 2024, 5, 100046. [Google Scholar] [CrossRef]
- Feng, C.; Jin, C.; Liu, K.; Yang, Z. Microbiota-derived short chain fatty acids: Their role and mechanisms in viral infections. Biomed. Pharmacother. 2023, 160, 114414. [Google Scholar] [CrossRef]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef]
- Tan, J.; Ni, D.; Ribeiro, R.V.; Pinget, G.V.; Macia, L. How Changes in the Nutritional Landscape Shape Gut Immunometabolism. Nutrients 2021, 13, 823. [Google Scholar] [CrossRef]
- Crouch, L.I.; Rodrigues, C.S.; Bakshani, C.R.; Tavares-Gomes, L.; Gaifem, J.; Pinho, S.S. The role of glycans in health and disease: Regulators of the interaction between gut microbiota and host immune system. Semin. Immunol. 2024, 73, 101891. [Google Scholar] [CrossRef]
- Baldi, S.; Mundula, T.; Nannini, G.; Amedei, A. Microbiota shaping—The effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: A systematic review. World. J. Gastroenterol. 2021, 27, 6715–6732. [Google Scholar] [CrossRef] [PubMed]
- Ramond, P.; Galand, P.E.; Logares, R. Microbial functional diversity and redundancy: Moving forward. FEMS Microbiol. Rev. 2025, 49, fuae031. [Google Scholar] [CrossRef] [PubMed]
- Pihelgas, S.; Ehala-Aleksejev, K.; Adamberg, S.; Kazantseva, J.; Adamberg, K. The gut microbiota of healthy individuals remains resilient in response to the consumption of various dietary fibers. Sci. Rep. 2024, 14, 22208. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- D’humières, C.; Delavy, M.; Alla, L.; Ichou, F.; Gauliard, E.; Ghozlane, A.; PrediRes Study Group. Perturbation and resilience of the gut microbiome up to 3 months after β-lactams exposure in healthy volunteers suggest an important role of microbial β-lactamases. Microbiome 2024, 12, 50. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano GA, D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Ducarmon, Q.R.; Kuijper, E.J.; Olle, B. Opportunities and Challenges in Development of Live Biotherapeutic Products to Fight Infections. J. Infect. Dis. 2021, 223, S283–S289. [Google Scholar] [CrossRef]
- Rogers, A.W.L.; Tsolis, R.M.; Bäumler, A.J. Salmonella versus the Microbiome. Microbiol. Mol. Biol. Rev. 2020, 85, e00027-19. [Google Scholar] [CrossRef]
- Zikou, E.; Koliaki, C.; Makrilakis, K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines 2024, 12, 1871. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicro. Prot. 2023, 15, 1626–1643. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N. Mitigation of antibiotic resistance using probiotics, prebiotics and synbiotics. A review. Environ. Chem. Lett. 2022, 20, 1295–1308. [Google Scholar] [CrossRef]
- Ferrer, M.; Méndez-García, C.; Rojo, D.; Barbas, C.; Moy, A. Antibiotic use and microbiome function. Biochem. Pharmacol. 2017, 134, 114–126. [Google Scholar] [CrossRef]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Moreira de Gouveia, M.I.; Bernalier-Donadille, A.; Jubelin, G. Enterobacteriaceae in the Human Gut: Dynamics and Ecological Roles in Health and Disease. Biology 2024, 13, 142. [Google Scholar] [CrossRef]
- Gierynska, M.; Szulc-Dabrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota—A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef]
- Zeng, M.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal. Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Coutry, N.; Gasmi, I.; Herbert, F.; Jay, P. Mechanisms of intestinal dysbiosis: New insights into tuft cell functions. Gut Microbes 2024, 16, 2379624. [Google Scholar] [CrossRef]
- Pezzino, S.; Sofia, M.; Greco, L.P.; Litrico, G.; Filippello, G.; Sarvà, I.; La Greca, G.; Latteri, S. Microbiome Dysbiosis: A Pathological Mechanism at the Intersection of Obesity and Glaucoma. Int. J. Mol. Sci. 2023, 24, 1166. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Morgan, E.W.; Perdew, G.H.; Patterson, A.D. Multi-Omics Strategies for Investigating the Microbiome in Toxicology Research. Toxicol Sci. 2022, 187, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Shayista, H.; Nagendra Prasada, M.N.; Niranjan Raj, S.; Prasad, A.; Lakshmi, S.; Ranjini, H.K.; Manju, K.; Ravikumara; Chouhan, R.S.; Khohlova, O.Y.; et al. Complexity of antibiotic resistance and its impact on gut microbiota dynamics. Eng. Microbiol. 2025, 5, 100187. [Google Scholar] [CrossRef]
- Liwinski, T.; Elinav, E. Harnessing the microbiota for therapeutic purposes. Am. J. Transplant. 2020, 20, 1469–1760. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S. Microbiota restoration for recurrent Clostridioides difficile: Getting one step closer every day! J. Intern. Med. 2021, 290, 294–309. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, J.; Narbad, A.; Chen, Q. Advances in Lactobacillus Restoration for β-Lactam Antibiotic-Induced Dysbiosis: A System Review in Intestinal Microbiota and Immune Homeostasis. Microorganisms 2023, 11, 179. [Google Scholar] [CrossRef]
- Guo, C.; Yong, W.; Yao, B.; Song, L.; Liang, L. Diagnostic and clinical relevance of targeted metabolomic analysis of serum bile acid profiles in acute pancreatitis. BMC Gastroenterol. 2025, 25, 181. [Google Scholar] [CrossRef]
- Jacob, M.; Malkawi, A.; Albast, N.; Bougha, S.A.; Lopata, A.; Dasouki, M.; Rahman, A.M.A. A targeted metabolomics approach for clinical diagnosis of inborn errors of metabolism. Anal. Chim. Acta 2018, 1025, 141–153. [Google Scholar] [CrossRef]
- Bystron, J.M.; Dziekiewicz, A.M. Modulation of gut microbiota in the therapy of mental disorders—New therapeutic strategies. Med. Srod. 2024, 27, 66–71. [Google Scholar] [CrossRef]
- Crabtree, D.; Seidler, K.; Barrow, M. Pathophysiological mechanisms of gut dysbiosis and food allergy and an investigation of probiotics as an intervention for atopic disease. Clin. Nutr. ESPEN 2025, 65, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; Aleya, S.; Alsubih, M.; Aleya, L. Microbiome Dynamics: A Paradigm Shift in Combatting Infectious Diseases. J. Pers. Med. 2024, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, T.; Soffer, N.; Sulakvelidze, A.; Nielsen, D.S. A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions, while preserving a non-targeted representative commensal normal microbiota. Gut Microbes. 2018, 9, 391–399. [Google Scholar] [CrossRef]
- Colarusso, A.V.; Goodchild-Michelman, I.; Rayle, M.; Zomorrodi, A.R. Computational modeling of metabolism in microbial communities on a genome-scale. Curr. Opin. Syst. Biol. 2021, 26, 46–57. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Taylor, J.W. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef]
- Mohr, A.E.; Ortega-Santos, C.P.; Whisner, C.M.; Klein-Seetharaman, J.; Jasbi, P. Navigating Challenges and Opportunities in Multi-Omics Integration for Personalized Healthcare. Biomedicines 2024, 12, 1496. [Google Scholar] [CrossRef]
- Stein, C.M.; Weiskirchen, R.; Damm, F.; Strzelecka, P.M. Single-cell omics: Overview, analysis, and application in biomedical science. J. Cell. Biochem. 2021, 122, 1571–1578. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–cell communication: New insights and clinical implications. Sig. Transduct. Target Ther. 2024, 9, 196. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. BioDrugs 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 2019, 25, 803–814.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, A.; Jiang, A.; Qi, C.; Liu, Z.; Cheng, Q.; Luo, P. Computational frameworks transform antagonism to synergy in optimizing combination therapies. Npj Digit. Med. 2025, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Elinav, E. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef]
- Khalili, L.; Park, G.; Nagpal, R.; Salazar, G. The Role of Akkermansia muciniphila on Improving Gut and Metabolic Health Modulation: A Meta-Analysis of Preclinical Mouse Model Studies. Microorganisms 2024, 12, 1627. [Google Scholar] [CrossRef]
- Petrof, E.O.; Gloor, G.B.; Vanner, S.J.; Weese, S.J.; Carter, D.; Daigneault, M.C.; Allen-Vercoe, E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013, 1, 3. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, F.; Li, Y.; Pan, S.; Wang, H.; Yang, Z.; Wang, Z.; Hu, Z.; Yu, J.; Barritt, J.D.; et al. Bacteriophages allow selective depletion of gut bacteria to produce a targeted-bacterium-depleted mouse model. Cell Rep Methods 2022, 2, 100324. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 5, 3094. [Google Scholar] [CrossRef]
- Bai, D.; Ma, C.; Xun, J.; Luo, H.; Yang, H.; Lyu, H.; Zhu, Z.; Gai, A.; Yousuf, S.; Peng, K.; et al. MicrobiomeStatPlots: Microbiome statistics plotting gallery for meta-omics and bioinformatics. Imeta 2025, 4, e70002. [Google Scholar] [CrossRef]
- Khadse, S.N.; Ugemuge, S.; Singh, C. Impact of Antimicrobial Stewardship on Reducing Antimicrobial Resistance. Cureus 2023, 15, e49935. [Google Scholar] [CrossRef]
- Casotti, M.C.; Meira, D.D.; Alves, L.N.R.; Bessa, B.G.d.O.; Campanharo, C.V.; Vicente, C.R.; Aguiar, C.C.; Duque, D.D.A.; Barbosa, D.G.; Santos, E.d.V.W.d.; et al. Translational Bioinformatics Applied to the Study of Complex Diseases. Genes 2023, 14, 419. [Google Scholar] [CrossRef]
- Pennisi, F.; Pinto, A.; Ricciardi, G.E.; Signorelli, C.; Gianfredi, V. The Role of Artificial Intelligence and Machine Learning Models in Antimicrobial Stewardship in Public Health: A Narrative Review. Antibiotics 2025, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, M.O.; Jain, A.; Joseph, C.E.; Edison, L.K. Microbiome-Driven Therapeutics: From Gut Health to Precision Medicine. Gastrointest. Disord. 2025, 7, 7. [Google Scholar] [CrossRef]
- Calder, P.C.; Ortega, E.F.; Meydani, S.N.; Adkins, Y.; Stephensen, C.B.; Thompson, B.; Zwickey, H. Nutrition, Immunosenescence, and Infectious Disease: An Overview of the Scientific Evidence on Micronutrients and on Modulation of the Gut Microbiota. Adv. Nutr. 2022, 13, S1–S26. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Kollia, Z.; Verykios, V.S.; Nikolaou, M. Immunosenescence: How Aging Increases Susceptibility to Bacterial Infections and Virulence Factors. Microorganisms 2024, 12, 2052. [Google Scholar] [CrossRef]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Patel, P.G.; Patel, A.C.; Chakraborty, P.; Gosai, H.B. Impact of Dietary Habits, Ethnicity, and Geographical Provenance in Shaping Human Gut Microbiome Diversity. In Probiotics, Prebiotics, Synbiotics, and Postbiotics; Kothari, V., Kumar, P., Ray, S., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Du, P.; Fan, R.; Zhang, N.; Wu, C.; Zhang, Y. Advances in Integrated Multi-omics Analysis for Drug-Target Identification. Biomolecules 2024, 14, 692. [Google Scholar] [CrossRef]
- Ongaro, A.E.; Ndlovu, Z.; Sollier, E.; Otieno, C.; Ondoa, P.; Street, A.; Kersaudy-Kerhoas, M. Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices. Lab Chip 2022, 22, 3122–3137. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Ma, Z.; Zuo, T.; Frey, N.; Rangrez, A.Y. A systematic framework for understanding the microbiome in human health and disease: From basic principles to clinical translation. Sig. Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef]
- Sahle, Z.; Engidaye, G.; Shenkute Gebreyes, D.; Adenew, B.; Abebe, T.A. Fecal microbiota transplantation and next-generation therapies: A review on targeting dysbiosis in metabolic disorders and beyond. SAGE Open Med. 2024, 12, 20503121241257486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, X.; Sheng, H.; Shen, X.; Sun, X.; Yan, Y.; Yuan, Q. Engineering probiotics as living diagnostics and therapeutics for improving human health. Microb. Cell Fact. 2020, 19, 56. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).