Impact of Cooking Procedures on Coccidiostats in Poultry Muscle
Abstract
1. Introduction
2. Results
2.1. Method Validation
2.2. Comparison Between Coccidiostats and Cooking Processes
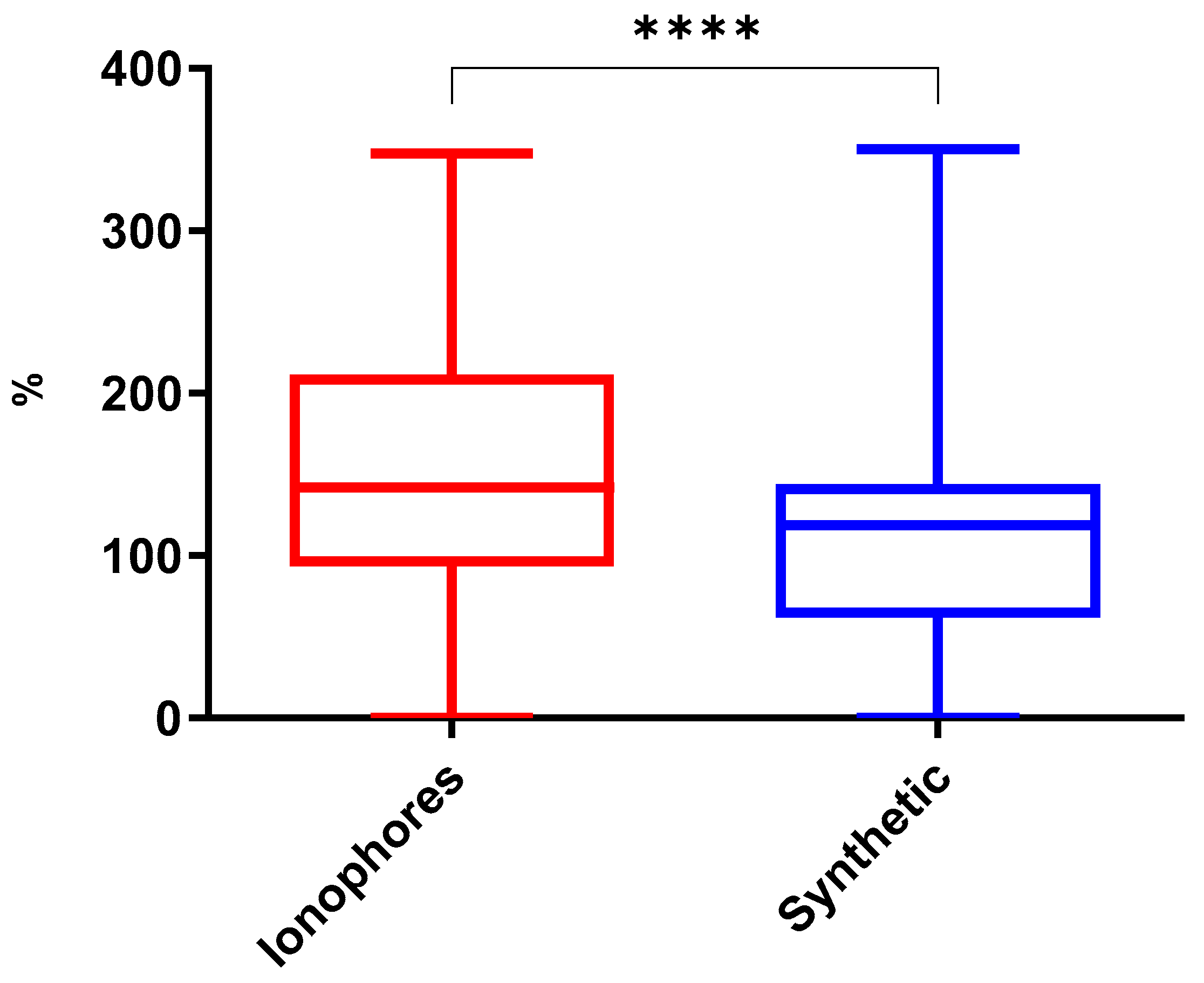
2.2.1. Comparison Between Groups of Coccidiostats
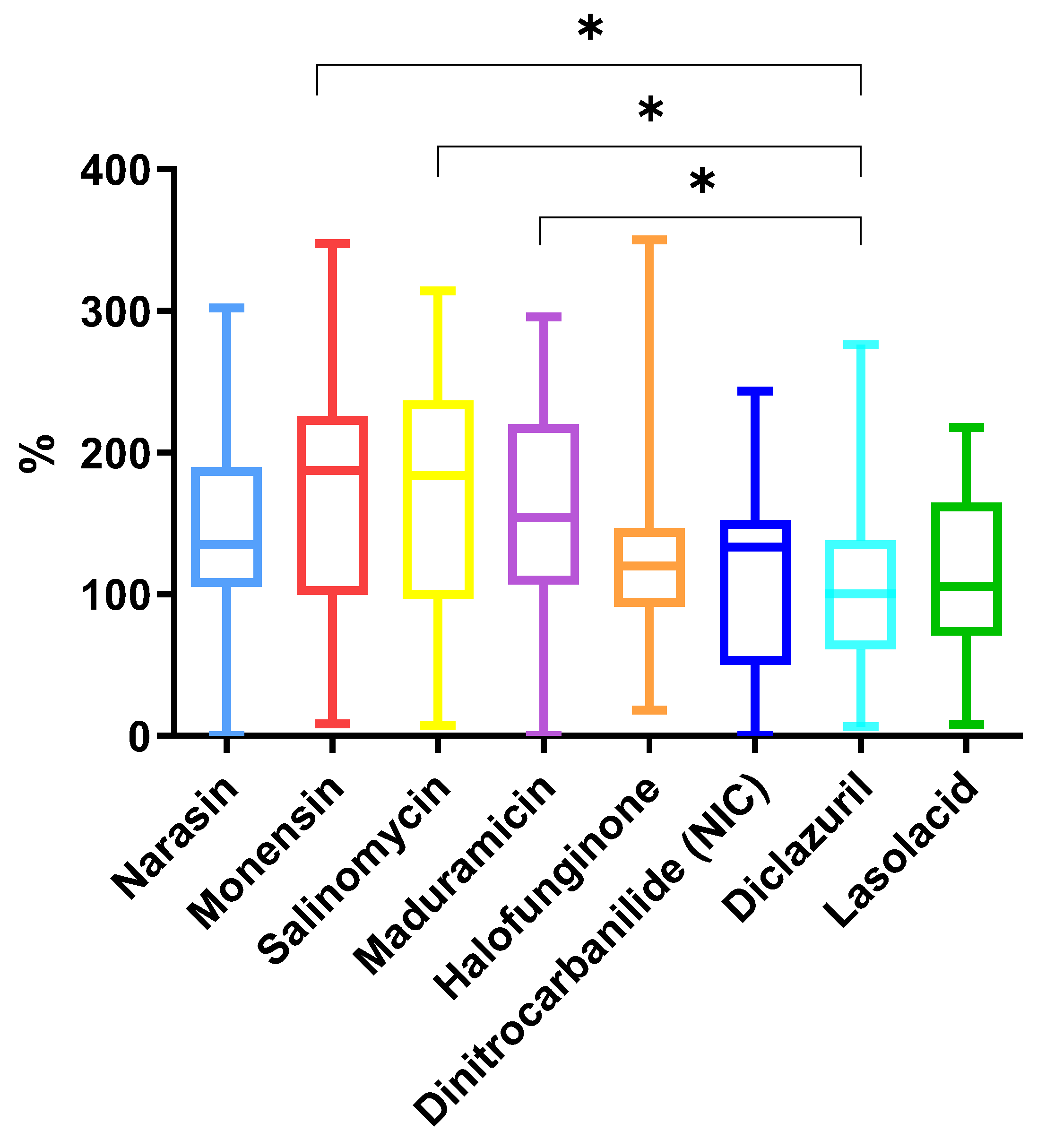
2.2.2. Comparison Between Coccidiostats
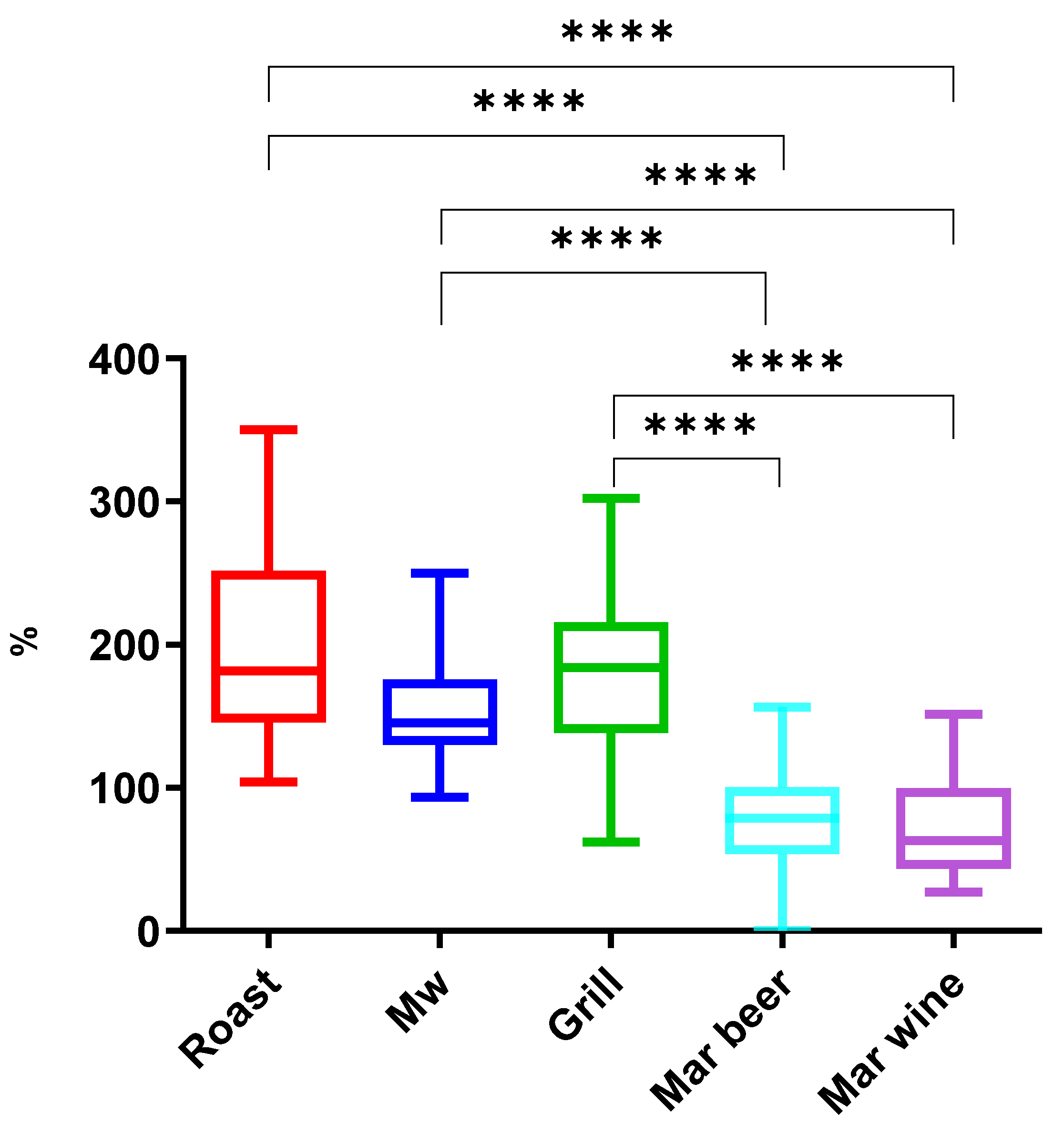
2.2.3. Comparison Between Cooking Methods
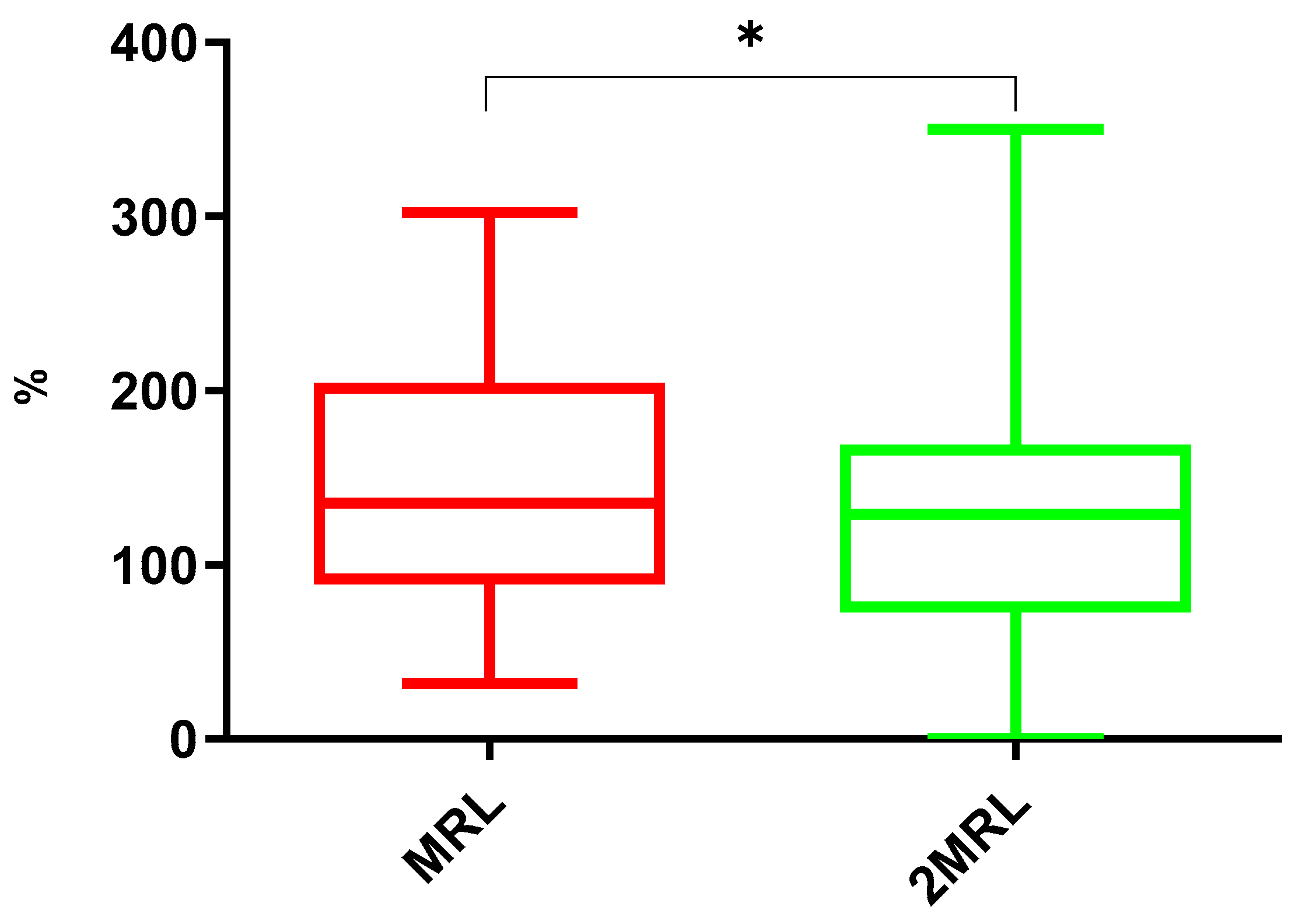
2.2.4. Comparison Between Maximum Residue Level and Twice-Maximum-Residue Level Concentrations
3. Materials and Methods
3.1. Sampling and Cooking Methods
3.2. Chemicals, Reagents, and Standard Solutions
3.3. Sample Extraction
3.4. UHPLC-MS/MS Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziam, H.; Abbas, A.; Abbas, R.Z.; Raza, M.A.; Hussain, K.; Younis, E.Z.; Radwan, I.T.; Selim, A. Avian Coccidiosis: Recent Advances in Alternative Control Strategies and Vaccine Development. Agrobiol. Rec. 2020, 1, 11–25. [Google Scholar] [CrossRef]
- FAO. Gateway to Poultry Production and Products. Available online: https://www.fao.org/poultry-production-products/en (accessed on 4 June 2025).
- Hamid, P.H.; Kristianingrum, Y.P.; Wardhana, A.H.; Prastowo, S.; Da Silva, L.M.H.R. Chicken Coccidiosis in Central Java, Indonesia: A Recent Update. Vet. Med. Int. 2018, 2018, 8515812. [Google Scholar] [CrossRef]
- Martins, R.R.; Silva, L.J.G.; Pereira, A.M.P.T.; Esteves, A.; Duarte, S.C.; Pena, A. Coccidiostats and poultry: A comprehensive review and current legislation. Foods 2022, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Liu, X.; Komabayashi, T.; Jeong, S.-I.; Selli, S. Natural Products for Infectious Diseases. Evid.-Based Complement. Altern. Med. 2016, 2016, 9459047. [Google Scholar] [CrossRef]
- Kadykalo, S.; Roberts, T.; Thompson, M.; Wilson, J.; Lang, M.; Espeisse, O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 2018, 51, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Bacila, D.M.; Lazzarotto, M.; Hansel, F.A.; Scheuermann, G.N.; Feddern, V.; Cunha Junior, A.; Igarashi-Mafra, L. Thermal profile of 4,4′-dinitrocarbanilide determined by thermogravimetry–differential scanning calorimetry–mass spectrometry (TG–DSC–MS) and pyrolysis–gas chromatography–mass spectrometry (Py–GC–MS). J. Therm. Anal. Calorim. 2019, 138, 697–701. [Google Scholar] [CrossRef]
- Monteiro, A.; Gonçalves, R.; Fradinho, M. Manual de Substâncias Indesejáveis; Associação Portuguesa dos Industriais de Alimentos Compostos para Animais: Lisboa, Portugal, 2022; ISBN 978-989-53558-2-2. [Google Scholar]
- Bello, A.; Henri, J.; Viel, A.; Mochel, J.P.; Poźniak, B. Ionophore coccidiostats—Disposition kinetics in laying hens and residues transfer to eggs. Poult. Sci. 2023, 102, 102280. [Google Scholar] [CrossRef]
- Augustine, P.C.; Smith, C.K.; Danforth, H.D.; Ruff, M.D. Effect of ionophorous anticoccidials on invasion and development of Eimeria: Comparison of sensitive and resistant isolates and correlation with drug uptake. Poult. Sci. 1987, 66, 960–965. [Google Scholar] [CrossRef]
- Abbas, F.; Thomas, P.; Cully-Duse, B.; Andronicos, N.M.; Winter, G. Cattle–compost–soil: The transfer of antibiotic resistance in livestock agriculture. Microbiologyopen 2023, 12, e1375. [Google Scholar] [CrossRef]
- Russell, J.B.; Houlihan, A.J. Ionophore resistance of ruminal bacteria and its potential impact on human health. FEMS Microbiol. Rev. 2003, 27, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Scientific Committee for Food Safety. The Risk of Development of Antimicrobial Resistance with the Use of Coccidiostats in Poultry Diets Opinion of the Panel on Animal Feed of the Norwegian Scientific Committee for Food Safety; Norwegian Scientific Committee for Food Safety: Oslo, Norway, 2015; ISBN 9788282591850. [Google Scholar]
- Zhao, D.; Suo, J.; Liang, L.; Liang, R.; Zhou, R.; Ding, J.; Liu, X.; Suo, X.; Zhang, S.; Tang, X. Innovative prevention and control of coccidiosis: Targeting sporogony for new control agent development. Poult. Sci. 2024, 103, 104246. [Google Scholar] [CrossRef] [PubMed]
- Nesse, L.L.; Bakke, A.M.; Eggen, T.; Hoel, K.; Kaldhusdal, M.; Ringø, E.; Yazdankhah, S.P.; Lock, E.-J.; Olsen, R.E.; Ørnsrud, R.; et al. The Risk of Development of Antimicrobial Resistance with the Use of Coccidiostats in Poultry Diets. Eur. J. Nutr. Food Saf. 2019, 11, 40–43. [Google Scholar] [CrossRef]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing veterinary drug residues in animal products: A review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef]
- EU. Regulation (EC) No 1831/2003. Off. J. Eur. Union 2003, 4, 29–43. [Google Scholar]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland. Chemical Residues and Contaminants in Foods of Animal Origin; Food Safety Authority of Ireland: Dublin, Ireland, 2015. [Google Scholar]
- Chicoine, A.; Erdely, H.; Fattori, V.; Finnah, A.; Fletcher, S.; Lipp, M.; Sanders, P.; Scheid, S. Assessment of veterinary drug residues in food: Considerations when dealing with sub-optimal data. Regul. Toxicol. Pharmacol. 2020, 118, 104806. [Google Scholar] [CrossRef]
- European Union Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009. Off. J. Eur. Union 2009, L 152, 11–22.
- Martins, R.R.; Pereira, A.M.P.T.; Silva, L.J.G.; Esteves, A.; Duarte, S.C.; Freitas, A.; Pena, A. Risk Assessment of Nine Coccidiostats in Commercial and Home-Raised Eggs. Foods 2023, 12, 1225. [Google Scholar] [CrossRef]
- Rose, M.D.; Rowley, L.; Shearer, G.; Farrington, W.H.H. Effect of Cooking on Veterinary Drug Residues in Food. 6. Lasalocid. J. Agric. Food Chem. 1997, 45, 927–930. [Google Scholar] [CrossRef]
- Huet, A.C.; Mortier, L.; Daeseleire, E.; Fodey, T.; Elliott, C.; Delahaut, P. Screening for the coccidiostats halofuginone and nicarbazin in egg and chicken muscle: Development of an ELISA. Food Addit. Contam. 2005, 22, 128–134. [Google Scholar] [CrossRef]
- Tarbin, J.A.; Bygrave, J.; Bigwood, T.; Hardy, D.; Rose, M.; Sharman, M. The effect of cooking on veterinary drug residues in food: Nicarbazin (dinitrocarbanilide component). Food Addit. Contam. 2005, 22, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, B.; Xie, K.; Liu, J.; Zhang, Y.; Wang, Y.; Guo, Y.; Zhang, G.; Dai, G.; Wang, J. Development and comparison of HPLC-MS/MS and UPLC-MS/MS methods for determining eight coccidiostats in beef. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1087–1088, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Cantón, L.; Lanusse, C.; Moreno, L. Veterinary drug residues in meat-related edible tissues. In New Aspects of Meat Quality; Elsevier: Amsterdam, The Netherlands, 2022; pp. 755–783. ISBN 9780323858793. [Google Scholar]
- Wu, M.; Cheng, X.; Wu, X.; Qian, H.; Wang, W. Effect of Cooking Methods on Amphenicols and Metabolites Residues in Livestock and Poultry Meat Spiked Tissues. Foods 2022, 11, 3497. [Google Scholar] [CrossRef]
- Efriem, S.; Britzi, M.; Soback, S.; Sabastian, C.; Mabjeesh, S.J. A Multi-Residue Analytical Method for Assessing the Effects of Stacking Treatment on Antimicrobial and Coccidiostat Degradation in Broiler Litter. Pharmaceuticals 2024, 17, 203. [Google Scholar] [CrossRef]
- Bacila, D.M.; Cunha, A.; Weber, I.F.; Scheuermann, G.N.; Coldebella, A.; Caron, L.; Molognoni, L.; Daguer, H.; Igarashi Mafra, L.; Feddern, V. Degradation of 4,4′-Dinitrocarbanilide in Chicken Breast by Thermal Processing. J. Agric. Food Chem. 2018, 66, 8391–8397. [Google Scholar] [CrossRef]
- Bacila, D.M.; Cunha, A.; Gressler, V.; Scheuermann, G.N.; Coldebella, A.; Caron, L.; Igarashi-Mafra, L.; Feddern, V. Detection of p-Nitroaniline Released from Degradation of 4,4′-Dinitrocarbanilide in Chicken Breast during Thermal Processing. J. Agric. Food Chem. 2019, 67, 9002–9008. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-D.; Yu, Z.-L.; He, Q.; Zeng, W.-C. Migration of phenolic compounds in meat during marinating process: Action rule, mass transfer and mechanism. LWT 2023, 185, 115192. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Romero-Gonzalez, R.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O.; Garrido-Frenich, A. Stability of antibacterial and coccidiostat drugs on chicken meat burgers upon cooking and in vitro digestion. Food Chem. 2020, 316, 126367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.R.; Pereira, A.M.P.T.; Silva, L.J.G.; Duarte, S.C.; Freitas, A.; Pena, A. Impact of Cooking Procedures on Coccidiostats in Poultry Muscle. Antibiotics 2025, 14, 586. https://doi.org/10.3390/antibiotics14060586
Martins RR, Pereira AMPT, Silva LJG, Duarte SC, Freitas A, Pena A. Impact of Cooking Procedures on Coccidiostats in Poultry Muscle. Antibiotics. 2025; 14(6):586. https://doi.org/10.3390/antibiotics14060586
Chicago/Turabian StyleMartins, Rui R., André M. P. T. Pereira, Liliana J. G. Silva, Sofia C. Duarte, Andreia Freitas, and Angelina Pena. 2025. "Impact of Cooking Procedures on Coccidiostats in Poultry Muscle" Antibiotics 14, no. 6: 586. https://doi.org/10.3390/antibiotics14060586
APA StyleMartins, R. R., Pereira, A. M. P. T., Silva, L. J. G., Duarte, S. C., Freitas, A., & Pena, A. (2025). Impact of Cooking Procedures on Coccidiostats in Poultry Muscle. Antibiotics, 14(6), 586. https://doi.org/10.3390/antibiotics14060586










