Molecular Characterization of Gram-Negative Bacilli Isolated from a Neonatal Intensive Care Unit and Phenotypic and Molecular Detection of ESBL and Carbapenemase
Abstract
1. Introduction
2. Results
2.1. Strains
2.2. Determination of Infection in Newborns
2.3. Biofilm Production
2.4. Production of Extended-Spectrum β-Lactamases
2.5. Production of Carbapenemases
2.6. Virulence Profile of Pseudomonas aeruginosa Isolates
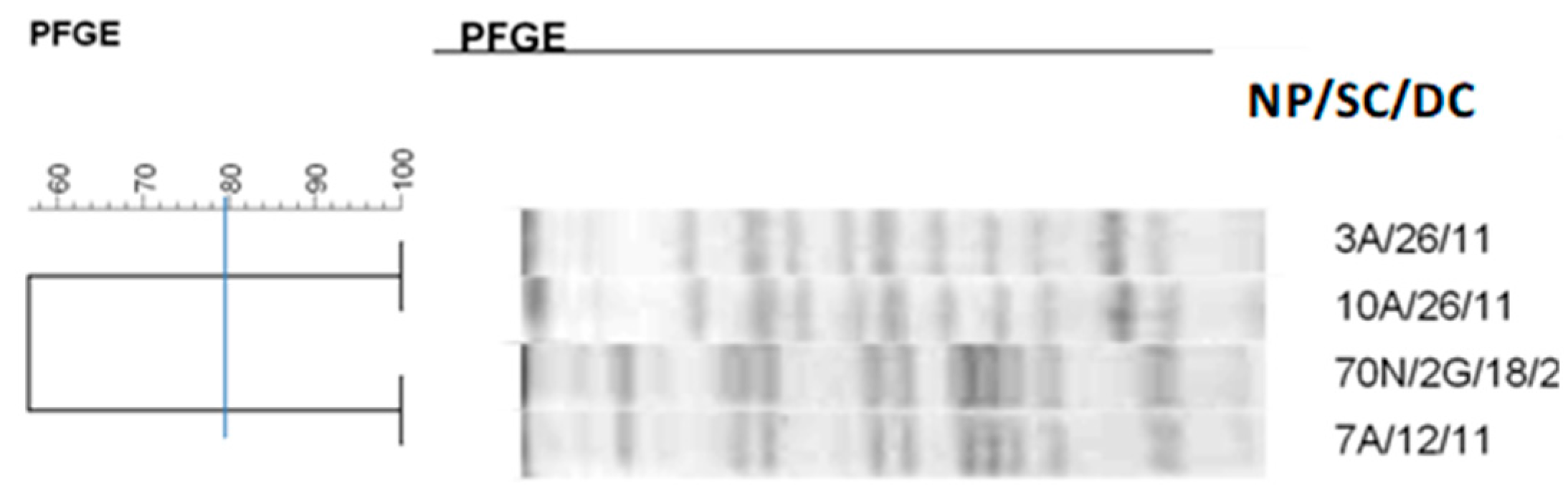
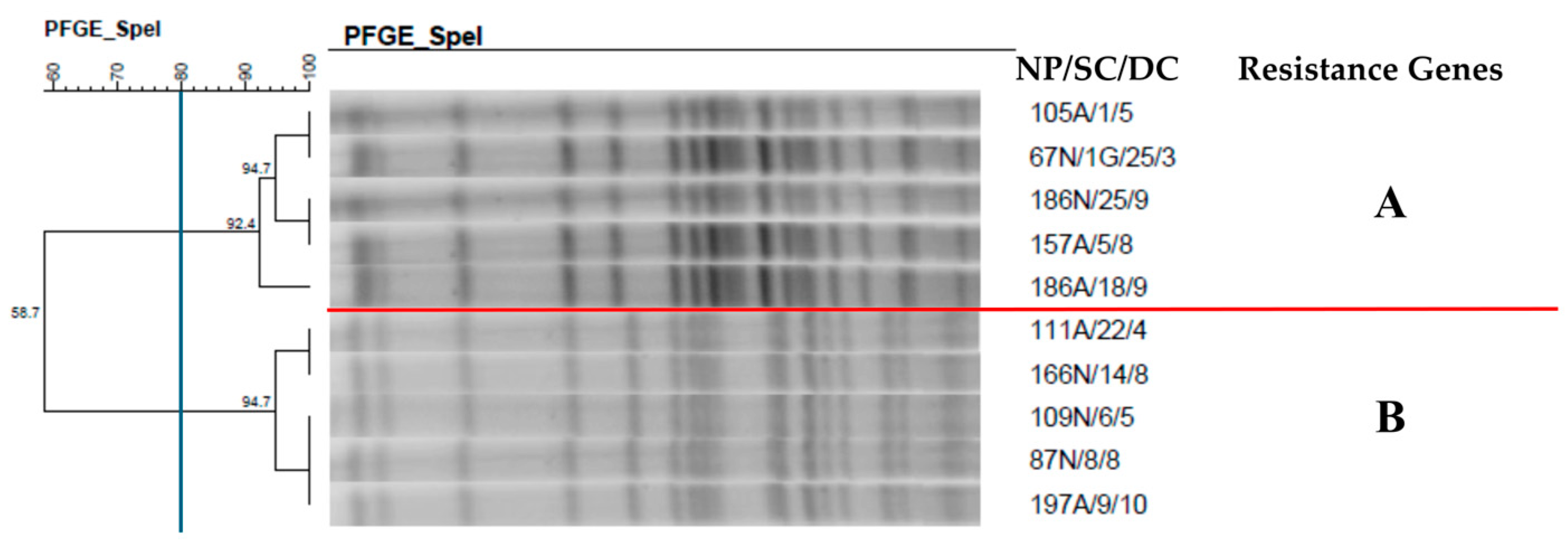
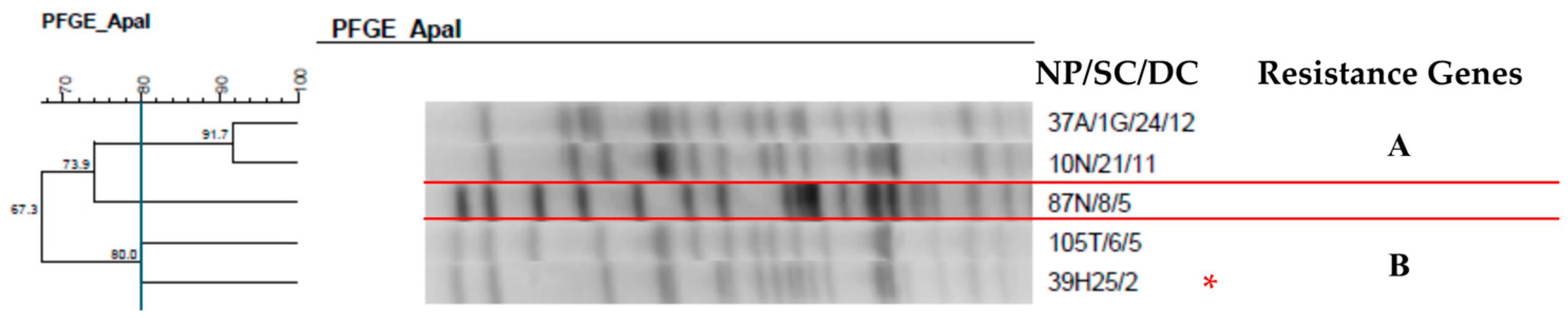
2.7. Clonal Profile of the Isolates–Pulsed-Field Gel Electrophoresis
2.8. Analysis of Risk Factors
3. Discussion
4. Materials and Methods
4.1. Study Location
- Common skin contaminating microorganisms: Corynebacterium spp. (excluding C. diphtheriae), Bacillus spp. (excluding B. anthracis), Propionibacterium spp., coagulase-negative Staphylococcus, Streptococcus of the viridans group, Aerococcus spp., and Micrococcus spp., isolated from at least two blood cultures collected at two different sites, with a maximum interval of 48 h between samplings.
- Coagulase-negative Staphylococcus isolated from at least one peripheral blood culture of a patient with a central vascular catheter.
4.2. Collection and Genotypic Identification of Microorganisms
4.3. Biofilm Production
4.4. Confirmation of the Production of Extended-Spectrum β-Lactamases
4.5. Molecular Characterization of Strains Producing Extended Spectrum β-Lactamases
4.6. Evaluation of the Virulence Profile of Pseudomonas aeruginosa Isolates
4.7. Pulsed-Field Gel Electrophoresis (PFGE)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zhang, H.; Yan, J.; Zhang, T. Literature review on the distribution characteristics and antimicrobial resistance of bacterial pathogens in neonatal sepsis. J. Matern. Fetal Neonatal Med. 2022, 35, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Medeiros, A.A. Evolution and dissemination of ß-lactamases accelerated by generations of ß-lactam antibiotics. Clin. Infect. Dis. 1997, 24, S19–S45. [Google Scholar] [CrossRef] [PubMed]
- Gold, H.S.; Moellering, R.C. Antimicrobial-drug resistance. N. Engl. J. Med. 1996, 335, 1445–1453. [Google Scholar] [CrossRef]
- Knothe, H.; Shah, P.; Krcmery, V.; Antal, M.; Mitsuhashi, S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 1983, 11, 315–317. [Google Scholar] [CrossRef]
- Miranda, G.; Castro, N.; Leaños, B. Clonal and horizontal dissemination of Klebsiella pneumoniae expressing SHV-5 extended-spectrum β-lactamase in a Mexican pediatric hospital. J. Clin. Microbiol. 2004, 42, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Boo, N.Y.; Ng, S.F.; Lim, V.K. A case-control study of risk factors associated with rectal colonization of extended-spectrum beta-lactamase producing Klebsiella spp. in newborn infants. J. Hosp. Infect. 2005, 61, 68–74. [Google Scholar] [CrossRef]
- Abdel-Hady, H.; Hawas, S.; El-Daker, M.; El-Kady, R. Extended-spectrum betalactamase producing Klebsiella pneumoniae in neonatal intensive care unit. J. Perinatol. 2008, 28, 685–690. [Google Scholar] [CrossRef]
- Stapleton, P.J.; Murphy, M.; McCallion, N.; Brennan, M.; Cunney, R.; Drew, R.J. Outbreaks of extended spectrum beta-lactamase-producing Enterobacteriaceae in neonatal intensive care units: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F72–F78. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-Specific Antibiotic Tolerance and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Top, E. Plasmid transfer in biofilms: A perspective on limitations and opportunities. npj Biofilms Microbiom. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Rocha, A.J.; Barsottini, M.R.O.; Rocha, R.R.; Laurindo, M.V.; Moraes, F.L.L.M.; Rocha, S.L. Pseudomonas aeruginosa: Virulence Factors and Antibiotic Resistance Genes. Review. Braz Arch Biol Techn. 2019, 62. [Google Scholar] [CrossRef]
- Sader, H.S.; Pignatari, A.C.; Leme, I.L.; Burattini, M.N.; Tancresi, R.; Hollis, R.J.; Jones, R.N. Epidemiologic typing of multiply drug resistant Pseudomonas aeruginosa isolated from an outbreak in an intensive care unit. Diagn. Microbiol. Infect. Dis. 1993, 17, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Pfaller, M.A.; Hollis, R.J. The use of molecular techniques in the epidemiology and control of hospital infections. Clin. Lab. Med. 1995, 15, 407–431. [Google Scholar] [CrossRef]
- Goldmann, D.; Leclair, J.; Macone, A. Bacterial colonization of neonates admitted to an intensive care environment. J. Pediatr. 1978, 93, 288–293. [Google Scholar] [CrossRef]
- Baier, C.; Pirr, S.; Ziesing, S.; Ebadi, E.; Hansen, G.; Bohnhorst, B.; Bange, F.C. Prospective surveillance of bacterial colonization and primary sepsis: Findings of a tertiary neonatal intensive and intermediate care unit. J. Hosp. Infect. 2019, 102, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Pestourie, N.; Garnier, F.; Barraud, O.; Bedu, A.; Ploy, M.C.; Mounier, M. Outbreak of AmpC β-lactamase-hyper-producing Enterobacter cloacae in a neonatal intensive care unit in a French teaching hospital. Am. J. Infect. Control 2014, 42, 456–458. [Google Scholar] [CrossRef]
- Ferry, A.; Plaisant, F.; Ginevra, C.; Dumont, Y.; Grando, J.; Claris, O.; Vandenesch, F.; Butin, M. Enterobacter cloacae colonisation and infection in a neonatal intensive care unit: Retrospective investigation of preventive measures implemented after a multiclonal outbreak. BMC Infect. Dis. 2020, 20, 682. [Google Scholar] [CrossRef]
- Artelt, T.; Kaase, M.; Bley, I.; Eiffert, H.; Mellmann, A.; Küster, H.; Lange, M.; Scheithauer, S. Transmission risk on a neonatal intensive care unit: Escherichia coli versus Klebsiella pneumoniae. Can. J. Infect. Dis. Med. Microbiol. 2018, 29, 1525072. [Google Scholar] [CrossRef]
- Berthelot, P.; Grattard, F.; Amerger, C.; Frery, M.C.; Lucht, F.; Pozzetto, B.; Fargier, P. Investigation of a nosocomial outbreak due to Serratia marcescens in a maternity hospital. Infect. Control Hosp. Epidemiol. 1999, 20, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; López, Y.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship between biofilm formation and antimicrobial resistance in Gram-negative bacteria. Microb. Drug Resist. 2019, 25, 72–79. [Google Scholar] [CrossRef]
- Bernardini, J.; Piraino, B.; Sorkin, M. Analysis of continuous ambulatory peritoneal dialysis-related Pseudomonas aeruginosa infections. Am. J. Med. 1987, 83, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Lanotte, P.; Watt, S.; Mereghetti, L.; Dartiguelongue, N.; Rastegar-Lari, A.; Goudeau, A.; Quentin, R. Genetic features of Pseudomonas aeruginosa isolates from cystic fibrosis patients compared with those of isolates from other origins. J. Med. Microbiol. 2004, 53, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Badamchi, A.; Masoumi, H.; Javadinia, S.; Asgarian, R.; Tabatabaee, A. Molecular detection of six virulence genes in Pseudomonas aeruginosa isolates detected in children with urinary tract infection. Microb. Pathog. 2017, 107, 44–47. [Google Scholar] [CrossRef]
- Heidary, Z.; Bandani, E.; Eftekhary, M.; Jafari, A.A. Virulence Genes Profile of Multidrug Resistant Pseudomonas aeruginosa Isolated from Iranian Children with UTIs. Acta Med. Iran. 2016, 54, 201–210. [Google Scholar]
- Barbosa, T.A. Caracterização Molecular de Bacilos Gram-Negativos Isolados em Unidade de Terapia Intensiva Neonatal e Detecção Fenotípica e Molecular de ESBL e Carbapenemases. Ph.D. Thesis, São Paulo State University, Botucatu, Brazil, 2020. [Google Scholar]
- Ketrin, C.S.; Nilton, L. Epidemiology of extended-spectrum beta-lactamases in Brazil: Clinical impact and implications for agribusiness. J. Bras. Patol. Med. Lab. 2012, 48, 91–99. [Google Scholar]
- Villegas, M.; Blanco, M.G.; Sifuentes-Osornio, J.; Rossi, F. Increasing prevalence of extended spectrum-betalactamase among Gram-negative bacilli in Latin America: 2008 update from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Braz. J. Infect. Dis. 2011, 15, 34–39. [Google Scholar]
- Minarini, L.A.R.; Clímaco, E.C.; Guimarães, D.B.; Ferreira, J.C.; Palazzo, I.C.; Martinez, R.; Darini, A.L. Clonal transmission of ESBL producing Klebsiella spp. at a university hospital in Brazil. Curr. Microbiol. 2008, 56, 587–591. [Google Scholar] [CrossRef]
- Andrade, L.N.; Minarini, L.A.; Pitondo-Silva, A.; Clímaco, E.C.; Palazzo, I.C.; Medeiros, M.I.; Darini, A.L. Determinants of β-lactam resistance in meningitis-causing Enterobacteriaceae in Brazil. Can. J. Microbiol. 2010, 56, 399–407. [Google Scholar] [CrossRef]
- Picão, R.C.; Poirel, L.; Gales, A.C.; Nordmann, P. Further identification of CTX-M-2 extended-spectrum β-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009, 53, 2225–2226. [Google Scholar] [CrossRef]
- Özçerezci, Ö.; Savcı, Ü. Evaluation of Gram-Negative Bacterial Infections Producing Extended Spectrum Beta-Lactamase in Preterm and Term Infants in a Neonatal Intensive Care Unit. J. Pediatr Emerg Intensive Care Med. 2019, 6, 91–97. [Google Scholar] [CrossRef]
- Ghanavati, R.; Emaneini, M.; Kalantar-Neyestanaki, D.; Maraji, A.S.; Dalvand, M.; Beigverdi, R.; Jabalameli, F. Clonal relation and antimicrobial resistance pattern of extended-spectrum β-lactamase- and AmpC β-lactamase producing Enterobacter spp. isolated from different clinical samples in Tehran, Iran. Rev. Soc. Bras. Med. Trop. 2018, 51, 88–93. [Google Scholar] [CrossRef]
- Kanamori, H.; Yano, H.; Hirakata, Y.; Hirotani, A.; Arai, K.; Endo, S.; Ichimura, S.; Ogawa, M.; Shimojima, M.; Aoyagi, T.; et al. Molecular characteristics of extended-spectrum beta-lactamases and qnr determinants in Enterobacter species from Japan. PLoS ONE 2012, 7, e37967. [Google Scholar] [CrossRef]
- Miranda, G.; Kelly, C.; Solorzano, F.; Leanos, B.; Coria, R.; Patterson, J.E. Use of Pulsed-Field Gel Electrophoresis Typing To Study an Outbreak of Infection Due to Serratia marcescens in a Neonatal Intensive Care Unit Guadalupe. J. Clin. Microbiol. 1996, 34, 3138–3141. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Gransden, W.R.; Eykyn, S.J.; Phillips, I.; Rowe, B. Bacteremia dueto Escherichia coli: A study of 861 episodes. Rev. Infect. Dis. 1990, 12, 1008–1018. [Google Scholar] [CrossRef]
- Gibbs, R.S.; Schrag, S.; Schuchat, A. Perinatal infections due to group B Streptococci. Obs. Gynecol. 2004, 104, 1062–1076. [Google Scholar] [CrossRef]
- Friedman, S.; Shah, V.; Ohlsson, A.; Matlow, A.G. Neonatal Escherichia coli infections: Concerns regarding resistance to current therapy. Acta Paediatr. 2000, 89, 686–689. [Google Scholar] [CrossRef]
- Neuwirth, C.; Siebor, E.; Lopez, J.; Pechinot, A.; Kazmierczak, A. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum -lactamase to other members of the family Enterobacteriaceae. J. Clin. Microbiol. 1996, 34, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Högenauer, C.; Langner, C.; Beubler, E.; Lippe, I.T.; Schicho, R.; Gorkiewicz, G.; Krause, R.; Gerstgrasser, N.; Krejs, G.J.; Hinterleitner, T.A. Klebsiella oxytoca as a causative organism of antibiotic-associated haemorrhagic colitis. N. Engl. J. Med. 2006, 355, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Fujita, A.; Kimura, K.; Yokoyama, S.; Jin, W.; Wachino, J.; Yamada, K.; Suematsu, H.; Yamagishi, Y.; Mikamo, H.; Arakawa, Y. Characterization of Piperacillin/Tazobactam-Resistant Klebsiella oxytoca Recovered from a Nosocomial Outbreak. PLoS ONE 2015, 10, e0142366. [Google Scholar] [CrossRef] [PubMed]
- Pavez, M.; Troncoso, C.; Osses, I.; Salazar, R.; Illesca, V.; Reydet, P.; Rodríguez, C.; Chahin, C.; Concha, C.; Barrientos, L. High prevalence of CTX-M-1 group in ESBL-producing Enterobacteriaceae infection in intensive care units in southern Chile. Braz. J. Infect. Dis. 2019, 23, 102–110. [Google Scholar] [CrossRef]
- Aghaei, S.S.; Keykha, M.; Karami, M.; Rahdar, H.A.; Ali Javadi, A.; Takei, E.; Nazari, R. Evaluation and identification of carbapenem resistant Klebsiella pneumoniae isolated from hospitalized patients in Qom City, (Iran). Qom Univ. Med. Sci. J. 2019, 13, 39–47. [Google Scholar] [CrossRef]
- Matthews, S.J.; Lancaster, J.W. Urinary tract infections in the elderly population. Am. J. Geriatr. Pharmacother. 2011, 9, 286–309. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.; Daniil, I.; Sotiropoulos, A.; Balampani, E.; Kokolaki, A.; Bousboulas, S.; Konstantopoulou, S.; Skliros, E.; Petropoulou, D.; Pappas, S. Prevalence of asymptomatic bacteriuria in type 2 diabetic subjects with and without microalbuminuria. BMC Res. Notes 2010, 17, 169. [Google Scholar] [CrossRef]
- Moolenar, R.L.; Crutcher, J.M.; Joaquin, V.H.S.; Sewell, L.V.; Hutwagner, L.C.; Carson, L.A.; Robison, D.A.; Smithee, L.M.K.; Jarvis, W.R. A prolonged outbreak of Pseudomonas aeruginosa in a neonatal intensive care unit: Did staff fingernails play a role in disease transmission. Infect. Control Hosp. Epidemiol. 2000, 21, 80–85. [Google Scholar] [CrossRef]
- Zawacki, A.; O’Rourke, E.; Potter-Bynoe, G.; Macone, A.; Harbarth, S.; Goldmann, D. An outbreak of Pseudomonas aeruginosa pneumonia and bloodstream infection associated with intermittent otitis externa in a healthcare worker. Infect. Control Hosp. Epidemiol. 2004, 25, 1083–1089. [Google Scholar] [CrossRef][Green Version]
- Regev, R.; Dolfin, T.; Zelig, I.; Givoni, S.; Wolach, B. Acinetobacter septicemia: A threat to neonates? Special aspects in a neonatal intensive care unit. Infection 1993, 21, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Presterl, E.; Nadrchal, R.; Winkler, S.; Makristathis, A.; Koller, W.; Rotter, M.L.; Hirschl, A.M. Molecular typing of Acinetobacter baumannii from ten different intensive care units of a university hospital. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D.; Ratner, B.D. Bioinspired implant material befuddle bacteria. ASM News 2004, 70, 232–237. [Google Scholar]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- De Beer, D.; Srinivasan, R.; Stewart, P.S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Env. Microbiol. 1994, 60, 4339–4344. [Google Scholar] [CrossRef]
- Suci, P.A.; Mittelman, M.W.; Yu, F.P.; Geesey, G.G. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 1994, 38, 2125–2133. [Google Scholar] [CrossRef]
- Desta, K.; Woldeamanuel, Y.; Azazh, A.; Mohammod, H.; Desalegn, D.; Shimelis, D.; Gulilat, D.; Lamisso, B.; Makonnen, E.; Worku, A.; et al. High Gastrointestinal Colonization Rate with Extended-Spectrum beta-Lactamase-Producing Enterobacteriaceae in Hospitalized Patients: Emergence of Carbapenemase-Producing K. pneumoniae in Ethiopia. PLoS ONE 2016, 11, e0161685. [Google Scholar] [CrossRef]
- Brady, M.T. Health care-associated infections in the neonatal intensive care unit. Am. J. Infect. Control 2005, 33, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Sarvikivi, E.; Lyytikäinen, O.; Salmenlinna, S.; Vuopio-Varkila, J.; Luukkainen, P.; Tarkka, E.; Saxén, H. Clustering of Serratia marcescens infections in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2004, 25, 723–729. [Google Scholar] [CrossRef]
- Murki, S.; Jonnala, S.; Mohammed, F.; Reddy, A. Restriction of cephalosporins and control of extended spectrum beta-lactamase producing gram negative bacteria in a neonatal intensive care unit. Indian Pediatrics 2010, 47, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, W.R. The epidemiology of colonization. Infect. Control Hosp. Epidemiol. 1996, 17, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária. Critérios Diagnósticos de Infecções Relacionadas à Assistência à Saúde—Neonatologia; Ministério da Saúde: Brasília, Brazil, 2017; 65p. [Google Scholar]
- Turton, J.F.; Woodford, N.; Glover, J.; Yarde, S.; Kaufmann, M.E.; Pitt, T.L. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 2006, 44, 2974–2976. [Google Scholar] [CrossRef]
- Stumpf, A.N.; Roggenkamp, A.; Hoffmann, H. Specificity of enterobacterial repetitive intergenic consensus and repetitive extragenic palindromic polymerase chain reaction for the detection of clonality the Enterobacter cloacae. Diagn. Microbiol. Infect. Dis. 2005, 53, 9–16. [Google Scholar] [CrossRef]
- Thong, K.L.; Lai, M.Y.; Teh, C.S.J.; Chua, K.H. Simultaneous detection of methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa by multiplex PCR. Trop. Biomed. 2011, 28, 21–31. [Google Scholar]
- Jeong, E.S.; Lee, K.S.; Heo, S.H.; Seo, J.H.; Choi, Y.K. Triplex PCR for the simultaneous detection of Pseudomonas aeruginosa, Helicobacter hepaticus and Salmonella typhimurium. Exp. Anim. 2011, 60, 65–70. [Google Scholar] [CrossRef][Green Version]
- Polson, S.W.; Higgins, J.L.; Woodley, C.M. PCR-based Assay for detection of four coral pathogens. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; Volume 7, pp. 247–251. [Google Scholar]
- Belas, R.; Schneider, R.; Melch, M. Characterization of Proteus mirabilis precocious swarming mutants: Identification of rsbA encoding a regulator of warming behavior. J. Bacteriol. 1998, 80, 6126–6139. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, R.T.; De la Cruz, M.A.; Yamamoto, D.; Girón, J.A.; Gomes, T.A.T. Dissection of the role of pili and type 2 and 3 secretion systems in adherence and biofilm formation of an atypical enteropathogenic Escherichia coli strain. Infect. Immun. 2013, 81, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Livermore, D.M. Interpretative Reading: Recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J. Antimicrob. Chemother. 2001, 48, 87–102. [Google Scholar] [CrossRef][Green Version]
- ANVISA. Nota Técnica Nº 01/2013 Medidas de Prevenção e Controle de Infecções por Enterobactérias Multiresistentes; ANVISA: Brasília, Brazil, 2013. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023; ISBN 978-1-68440-170-3 [Print], ISBN 978-1-68440-171-0 [Electronic]. [Google Scholar]
- Toledo, M.R.F.; Fontes, C.F.; Trabulsi, L.R. EPM-modificação do meio de Rugai e Araújo para a realização simultânea dos testes de produção de gás a partir daglicose, H2S, urease e triptofano desaminase. Rev. Microbiol. 1982, 13, 309–315. [Google Scholar]
- Poirel, L.; Naas, T.; Nordmann, P. Diversity, epidemiology, and genetics of class D b-lactamases. Antimicrob. Agents Chemother. 2010, 54, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, S.; Jeya, M.; Perumal, J. blaKPCgene detection in clinical isolates of carbapenem resistant Enterobacteriaceae in a tertiary care hospital. J. Clin. Diag Res. 2013, 7, 2736–2738. [Google Scholar]
- Hunter, S.B.; Vauterin, P.; Lambert-Fair, M.A.; Van Duyne, M.S.; Kubota, K.; Graves, L.; Wrigley, D.; Barrett, T.; Ribot, E. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: Converting the National databases to the new size standard. J. Clin. Microbiol. 2005, 43, 1045–1050. [Google Scholar] [CrossRef]
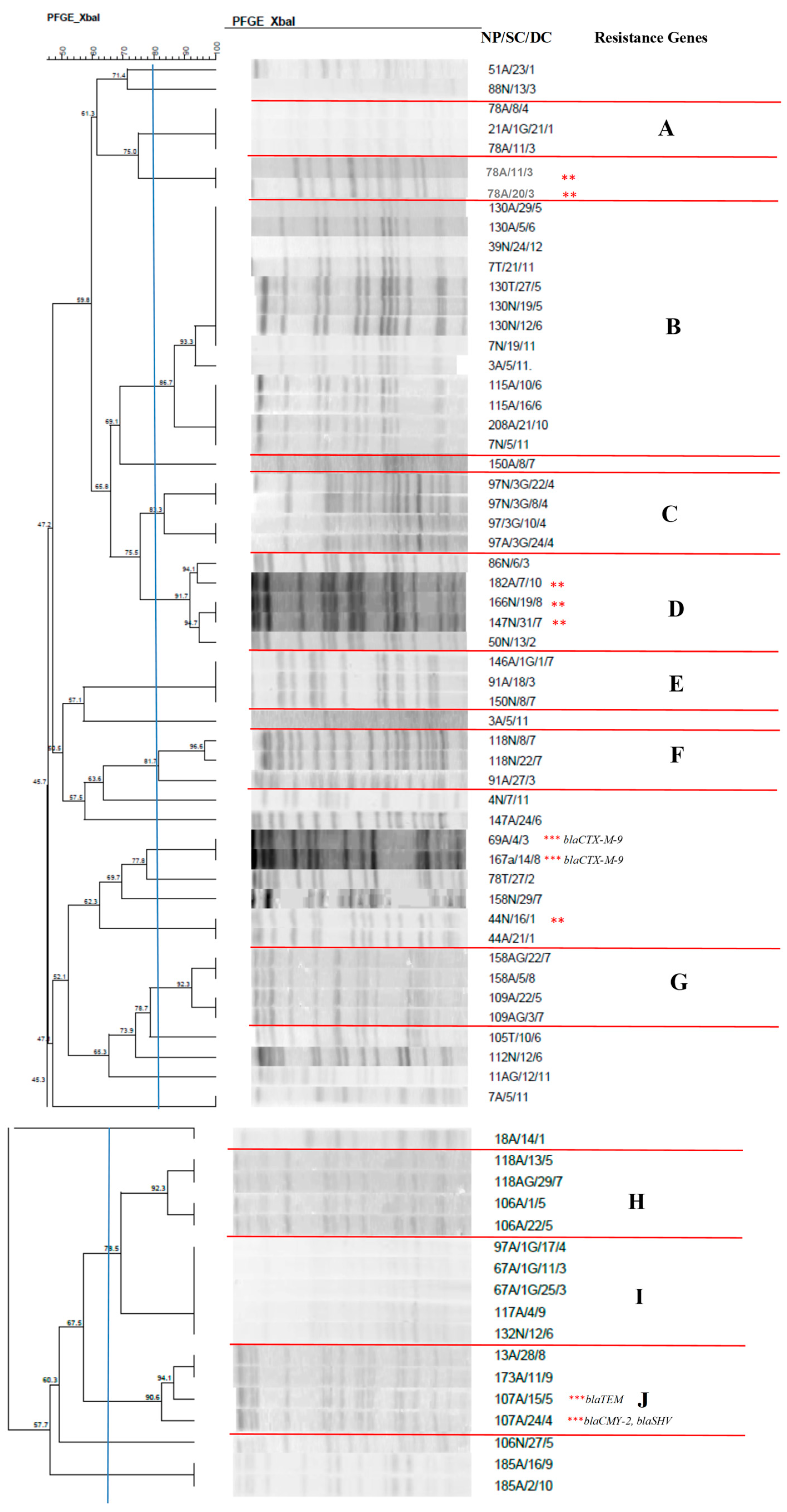
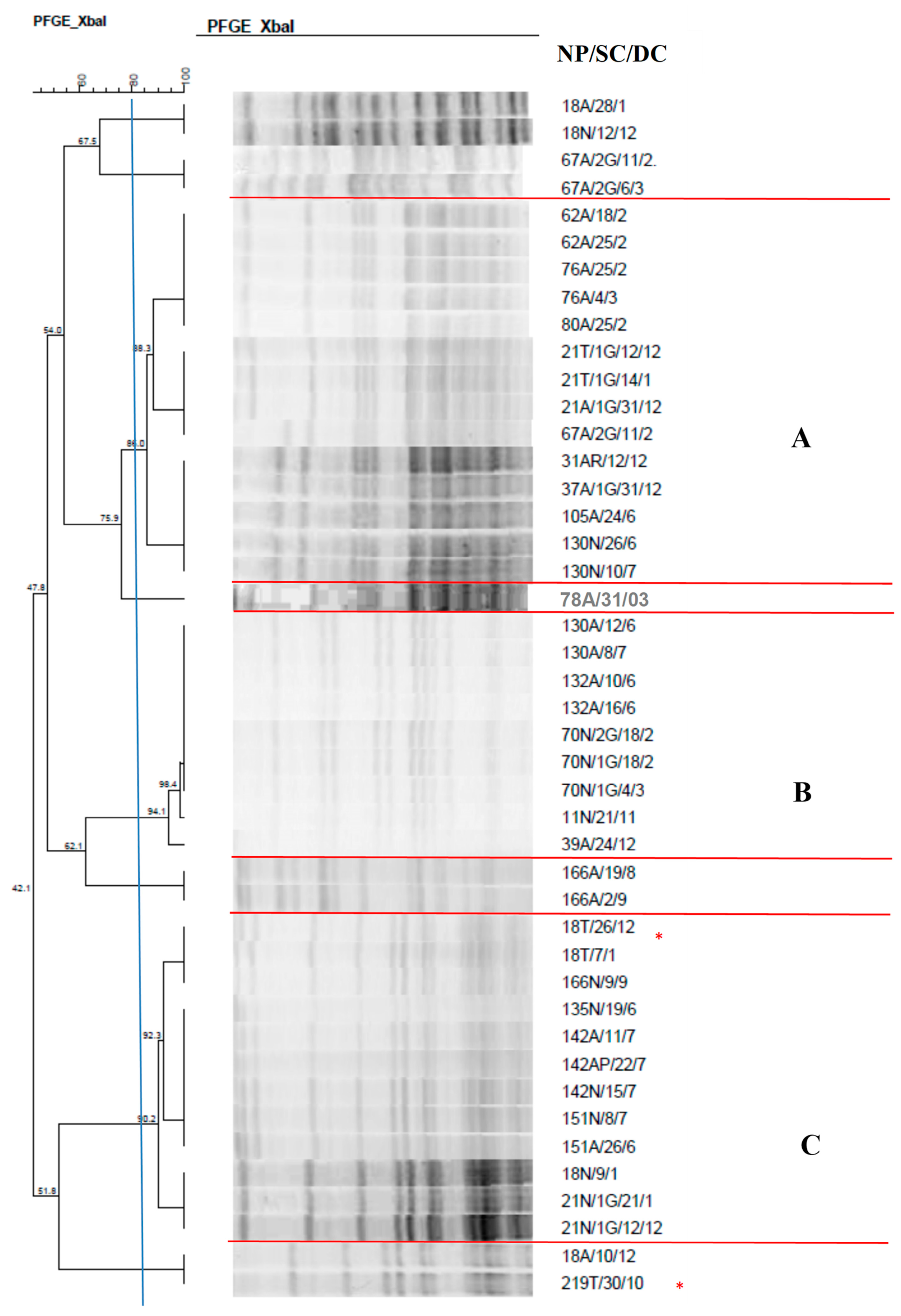


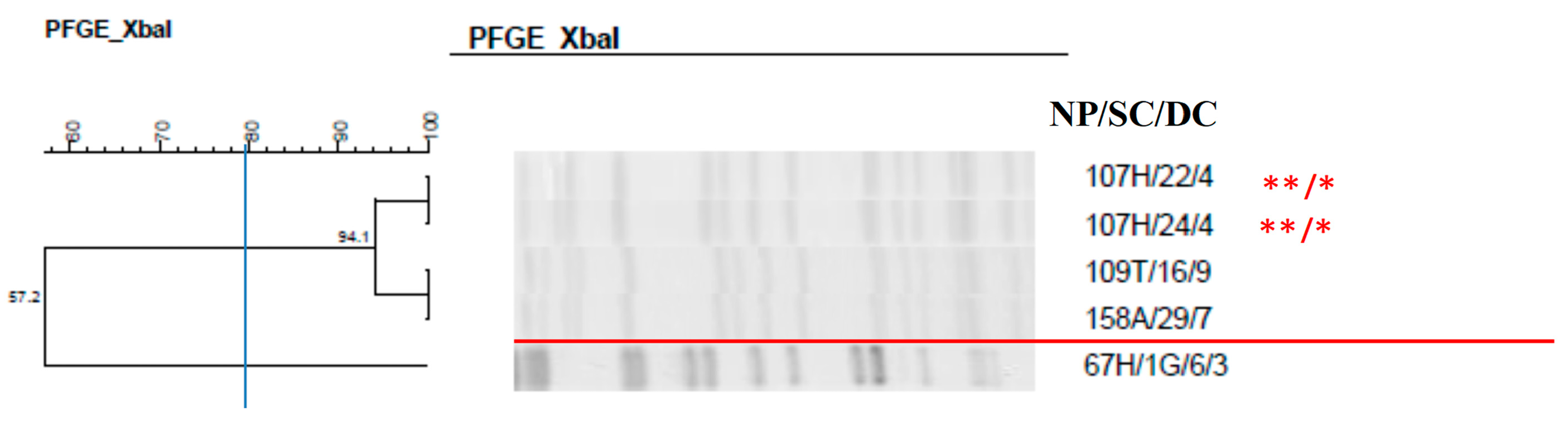

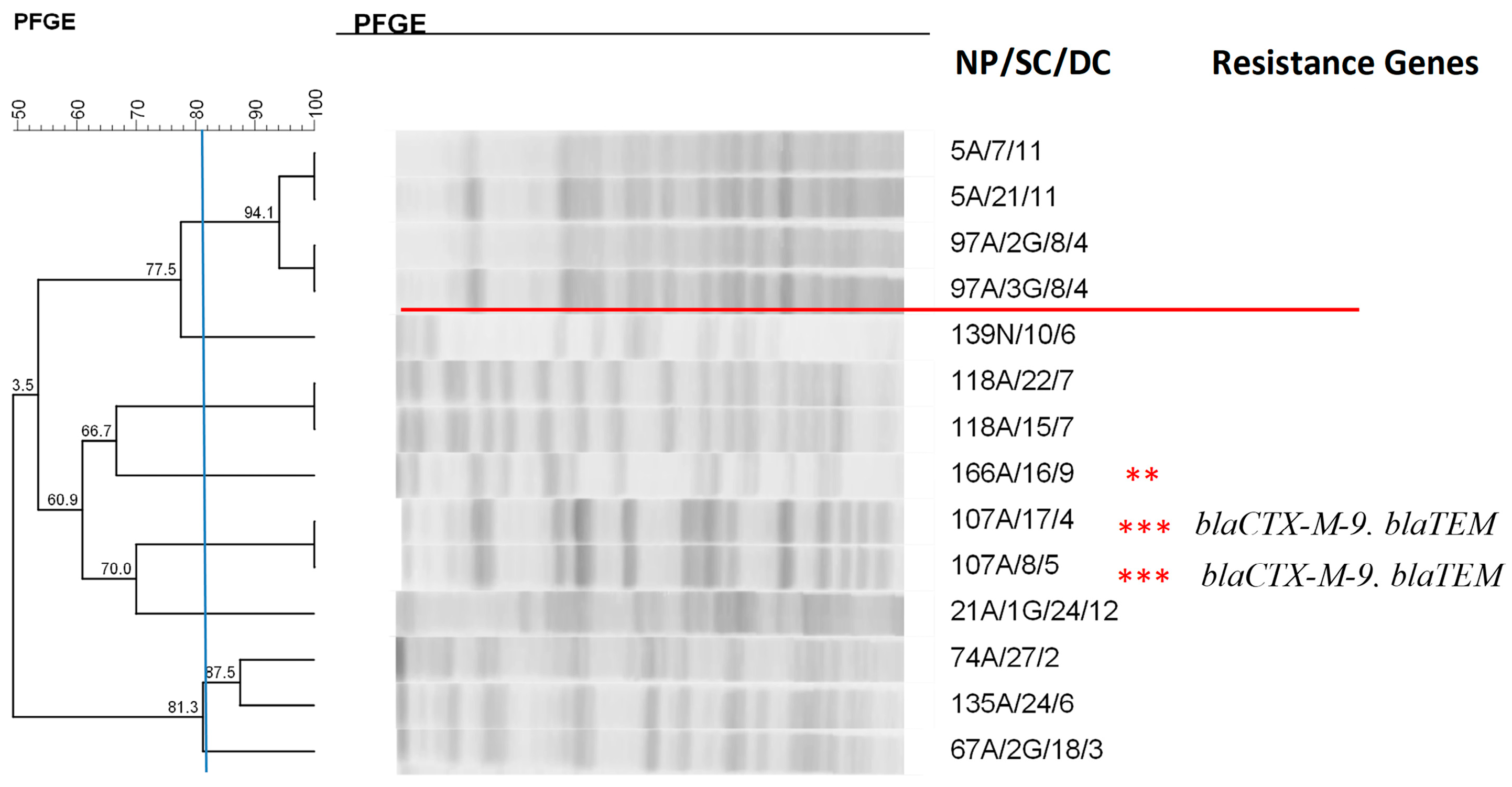

| Total Number of Isolates | Nasal Mucosa | Anal Mucosa | Tracheal Aspirate | |||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | |
| Enterobacter cloacae | 135 | (45) | 30 | (22.2) | 101 | (74.9) | 4 | (2.9) |
| Serratia marcescens | 73 | (24.3) | 16 *** | (21.9) | 53 **** | (72.6) | 4 ** | (5.5) |
| Escherichia coli | 45 | (15) | 14 | (31.1) | 29 | (64.4) | 2 | (4.5) |
| Enterobacter aerogenes * | 17 | (5.7) | 1 | (5.9) | 16 | (94.1) | - | - |
| Klebsiella oxytoca * | 12 | (4) | - | - | 10 */ | (83.3) | 2 *+ | (16.7) |
| Citrobacter freundii * | 7 | (2.3) | - | - | 7 | (100) | - | - |
| Klebsiella pneumoniae | 4 | (1.3) | - | - | 2 *| | (50) | 2 *| | (50) |
| Citrobacter diversus * | 2 | (0.7) | - | - | 2 | (100) | - | - |
| Proteus mirabilis | 2 | (0.7) | - | - | 2 *^ | (100) | - | - |
| Raoultella planticola * | 1 | (0.3) | - | - | 1 | (100) | - | - |
| Raoultella ornithinolytica * | 1 | (0.3) | - | - | 1 | (100) | - | - |
| Cedecea neteri * | 1 | (0.3) | - | - | 1 | (100) | - | - |
| Total | 300 | (100) | 61 | (20.3) | 225 | (75) | 14 | (4.7) |
| Total Number of Isolates | Nasal Mucosa | Anal Mucosa | Tracheal Aspirate | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Pseudomonas aeruginosa | 38 | (76) | 16 | (42.1) | 22 | (57.9) | - | - |
| Acinetobacter baumannii | 10 | (20) | 2 *** | (20) | 7 ** | (70) | 1 ** | (10) |
| Acinetobacter lwoffii * | 2 | (4) | - | - | 2 | (100) | - | - |
| Total | 50 | (100.0) | 18 | (36.0) | 31 | (62) | 1 | (2.0) |
| Isolate | Species | Resistance Genes |
|---|---|---|
| 21A/1G/24/12 | Enterobacter aerogenes | - |
| 107A/17/4 | Enterobacter aerogenes | blaCTX-M-9, blaTEM |
| 107A/8/5 | Enterobacter aerogenes | blaCTX-M-9, blaTEM |
| 166A/16/9 | Enterobacter aerogenes | - |
| 78A/11/3 | Enterobacter cloacae | - |
| 78A/20/3 | Enterobacter cloacae | - |
| 69A/4/3 | Enterobacter cloacae | blaCTX-M-9 |
| 107A/24/4 | Enterobacter cloacae | blaCMY-2, blaSHV |
| 107A/15/5 | Enterobacter cloacae | blaTEM |
| 167A/14/8 | Enterobacter cloacae | blaCTX-M-9 |
| 182A/7/10 | Enterobacter cloacae | - |
| 44N/16/1 | Enterobacter cloacae | - |
| 147N/31/7 | Enterobacter cloacae | - |
| 166N/19/8 | Enterobacter cloacae | - |
| 107H/22/4 | Klebsiella pneumoniae | - |
| 107H/24/4 | Klebsiella pneumoniae | - |
| Predictor | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Male sex | 0.59 (0.10–3.43) | 0.56 | ||
| Birth weight (g × 100) | 0.81 (0.61–1.02) | 0.16 | ||
| Cesarean delivery | 0.94 (0.16–5.63) | 0.95 | ||
| Gestational age (weeks) | 0.80 (0.59–1.09) | 0.16 | ||
| Umbilical catheter | 4.79 (0.55–42.06) | 0.16 | ||
| Peripherally inserted central catheter | 1.81 (0.21–15.84) | 0.59 | ||
| Intravenous catheter | 0.04 (0.00–...) | 0.64 | ||
| Peripheral venous access | 0.20 (0.04–1.13) | 0.07 | ||
| Parenteral nutrition | 33.74 (0.01–...) | 0.38 | ||
| Surgery | 0.59 (0.07–5.25) | 0.64 | ||
| Mechanical ventilation | 20.06 (0.00–...) | 0.54 | ||
| Use of antimicrobials in the first 72 h | 4.99 (0.58–43.39) | 0.15 | ||
| Biofilm | 1.69 (0.99–2.90) | 0.053 | 1.75 (1.10–3.04) | 0.048 |
| ESBL | 42.10 (3.80–463.13) | 0.002 | 46.79 (4.18–523.72) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, T.A.; Bentlin, M.R.; Rugolo, L.M.S.d.S.; Lyra, J.C.; Ferreira, A.M.; Santos, A.C.M.L.d.; Teixeira, N.B.; Medeiros Romero, L.C.; Castelo Branco Fortaleza, C.M.; Ribeiro de Souza da Cunha, M.d.L. Molecular Characterization of Gram-Negative Bacilli Isolated from a Neonatal Intensive Care Unit and Phenotypic and Molecular Detection of ESBL and Carbapenemase. Antibiotics 2025, 14, 342. https://doi.org/10.3390/antibiotics14040342
Barbosa TA, Bentlin MR, Rugolo LMSdS, Lyra JC, Ferreira AM, Santos ACMLd, Teixeira NB, Medeiros Romero LC, Castelo Branco Fortaleza CM, Ribeiro de Souza da Cunha MdL. Molecular Characterization of Gram-Negative Bacilli Isolated from a Neonatal Intensive Care Unit and Phenotypic and Molecular Detection of ESBL and Carbapenemase. Antibiotics. 2025; 14(4):342. https://doi.org/10.3390/antibiotics14040342
Chicago/Turabian StyleBarbosa, Thaís Alves, Maria Regina Bentlin, Lígia Maria Suppo de Souza Rugolo, João César Lyra, Adriano Martison Ferreira, Ana Cláudia Moro Lima dos Santos, Nathalia Bibiana Teixeira, Letícia Calixto Medeiros Romero, Carlos Magno Castelo Branco Fortaleza, and Maria de Lourdes Ribeiro de Souza da Cunha. 2025. "Molecular Characterization of Gram-Negative Bacilli Isolated from a Neonatal Intensive Care Unit and Phenotypic and Molecular Detection of ESBL and Carbapenemase" Antibiotics 14, no. 4: 342. https://doi.org/10.3390/antibiotics14040342
APA StyleBarbosa, T. A., Bentlin, M. R., Rugolo, L. M. S. d. S., Lyra, J. C., Ferreira, A. M., Santos, A. C. M. L. d., Teixeira, N. B., Medeiros Romero, L. C., Castelo Branco Fortaleza, C. M., & Ribeiro de Souza da Cunha, M. d. L. (2025). Molecular Characterization of Gram-Negative Bacilli Isolated from a Neonatal Intensive Care Unit and Phenotypic and Molecular Detection of ESBL and Carbapenemase. Antibiotics, 14(4), 342. https://doi.org/10.3390/antibiotics14040342








