Unlocking the Skin-Protective Effects of Ferulago spp. Essential Oils: A Focus on Antidermatophytic and Wound-Healing Potential
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.2. Minimal Inhibitory and Minimal Lethal Concentrations of Ferulago spp. Essential Oils
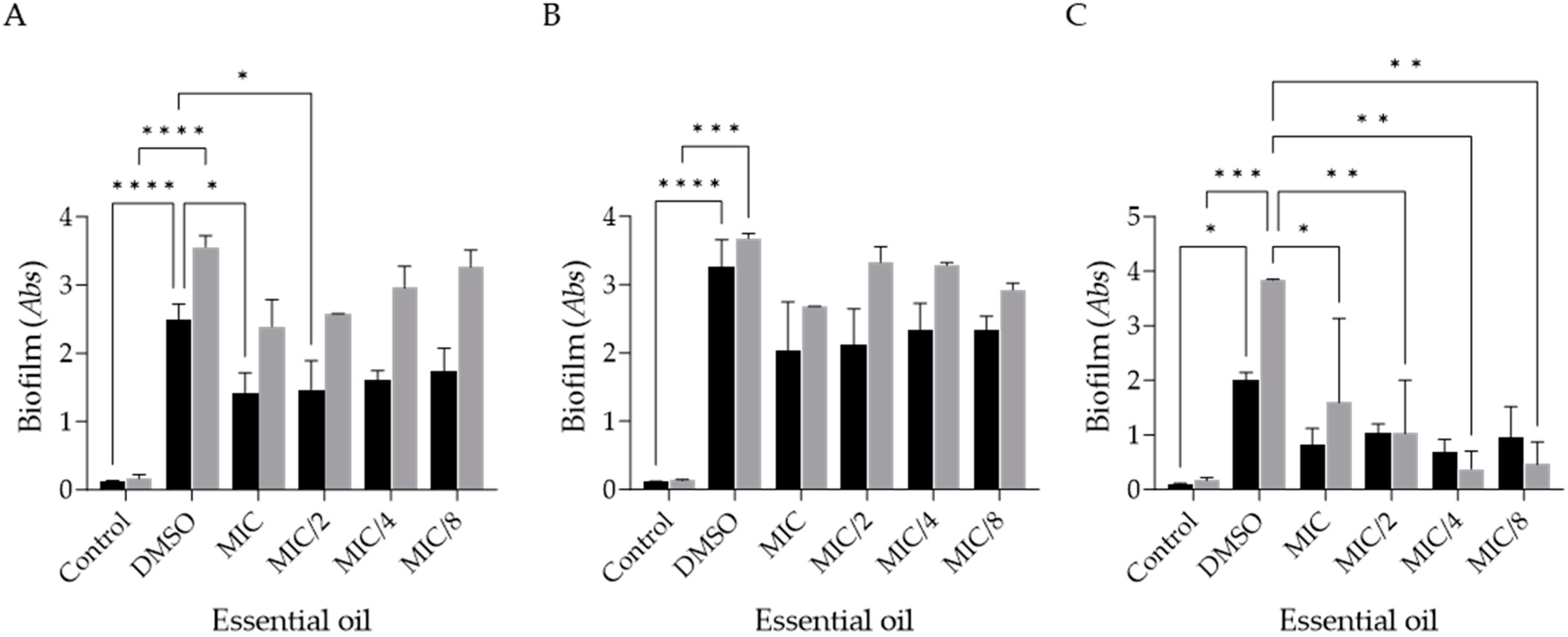
2.3. Effect of Ferulago silaifolia Essential Oil on the Formation of Dermatophyte Biofilms
2.4. Effect of Ferulago silaifolia Essential Oil on the Disruption of Dermatophyte Pre-Formed Biofilms
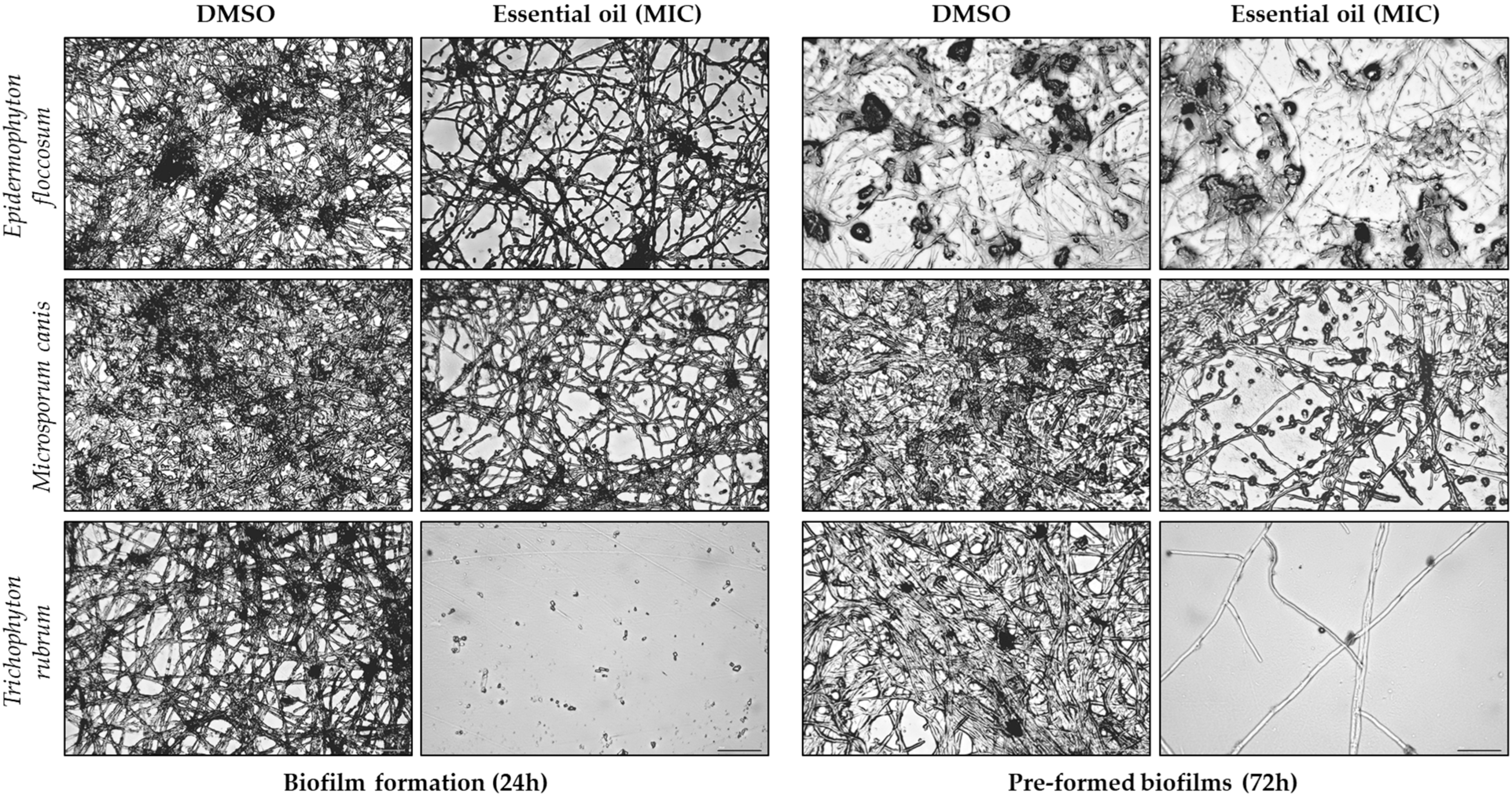
2.5. Morphological Alterations Induced by Ferulago silaifolia Essential Oil on Dermatophytes
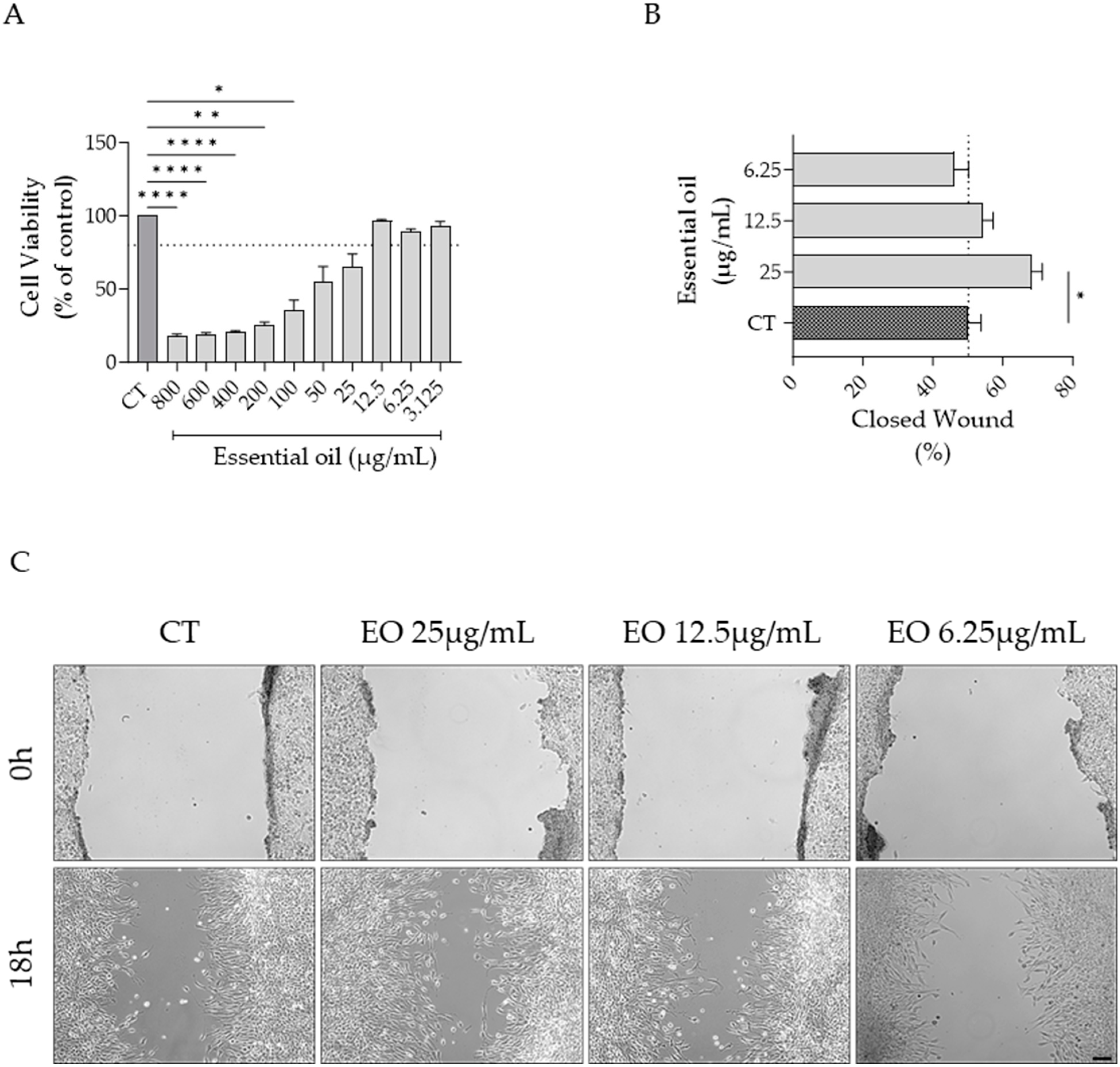
2.6. Effect of Ferulago setifolia and F. silaifolia Essential Oil on Fibrablast Migration
3. Discussion
4. Materials and Methods
4.1. Plant Material and Essential Oils
4.2. Antifungal Effect of Essential Oils on Planktonic Fungi
4.3. Evaluation of Essential Oils’ Effect on Biofilm Formation
- Biofilm mass:
- Extracellular matrix:
4.4. Evaluation of Essential Oils’ Effect on Biofilm Disruption
4.5. Effect of the Essential Oil on Fungal Morphology
4.6. Cell Viability Assay
4.7. Scratch Wound Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIC | Minimal Inhibitory Concentration |
| MLC | Minimal Lethal Concentration |
References
- Gaffi Global Action for Fungal Infections Fungal Disease Frequency. Available online: https://gaffi.org/why/fungi-fungal-infections/ (accessed on 1 December 2024).
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte Resistance to Antifungal Drugs: Mechanisms and Prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Deng, R.; Wang, X.; Li, R. Dermatophyte infection: From fungal pathogenicity to host immune responses. Front. Immunol. 2023, 14, 1285887. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Stewart, C.S. Keratin hydrolysis by dermatophytes. Med. Mycol. 2019, 57, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Jartarkar, S.R.; Patil, A.; Goldust, Y.; Cockerell, C.J.; Schwartz, R.A.; Grabbe, S.; Goldust, M. Pathogenesis, Immunology and Management of Dermatophytosis. J. Fungi 2021, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Mahajan, R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol. Online J. 2016, 7, 77. [Google Scholar] [CrossRef]
- Markantonatou, A.-M.; Samaras, K.; Vyzantiadis, T.-A. Dermatophytic Biofilms: Characteristics, Significance and Treatment Approaches. J. Fungi 2023, 9, 228. [Google Scholar] [CrossRef]
- Aditya, K.G.; Jennifer, E.R.; Melody, C.; Elizabeth, A.C. Dermatophytosis: The Management of Fungal Infections. SKINmed Dermatol. Clin. 2005, 4, 305–310. [Google Scholar] [CrossRef]
- Dabaghian, F.; Aalinezhad, S.; Kesheh, A.R.; Azargashb, N.; Ansari, R.; Ardekani, M.R.S.; Emami, S.A.; Khanavi, M.; Delnavazi, M.R. A review of the ethnomedicinal, phytochemical, and pharmacological properties of the Ferulago genus based on Structure–Activity Relationship (SAR) of coumarins. DARU J. Pharm. Sci. 2024, 32, 825–899. [Google Scholar] [CrossRef]
- Karakaya, S.; Göğer, G.; Kılıç, C.S.; Demirci, B. Türkiye’de Yetişen Ferulago blancheana Post. (Apiaceae) Türünün Toprak Üstü, Çiçek ve Köklerinden Elde Edilen Uçucu Yağların İçeriklerinin ve Antimikrobiyal Aktivitesinin Biyootografi Yöntemiyle Tanımlanması. Turk. J. Pharm. Sci. 2016, 13, 51–59. [Google Scholar] [CrossRef]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M. The ethnobotany, phytochemistry and biological properties of genus Ferulago–A review. J. Ethnopharmacol. 2021, 274, 114050. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, Y.; Delazar, A.; Asnaashari, S.; Asgharian, P. The Genus Ferulago: A Review on Ethnopharmacology, Phytochemistry, and Pharmacology. Iran. J. Pharm. Res. IJPR 2021, 20, 352–377. [Google Scholar] [CrossRef]
- Cecchini, C.; Coman, M.M.; Cresci, A.; Tirillini, B.; Cristalli, G.; Papa, F.; Sagratini, G.; Vittori, S.; Maggi, F. Essential oil from fruits and roots of Ferulago campestris (Besser) Grecescu (Apiaceae): Composition and antioxidant and anti-Candida activity. Flavour Fragr. J. 2010, 25, 493–502. [Google Scholar] [CrossRef]
- Demirci, B.; Dilmaç, E.; Kırcı, D.; Demirci, F.; Kılıç, C.S.; Duman, H.; Gürbüz, İ. Chemical and antimicrobial characterization of essential oils obtained from aerial part, root and fruit of Ferulago longistylis Boiss., an endemic species. Nat. Volatiles Essent. Oils 2020, 7, 18–25. [Google Scholar] [CrossRef]
- Pinto, E.; Hrimpeng, K.; Lopes, G.; Vaz, S.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of Ferulago capillaris essential oil against Candida, Cryptococcus, Aspergillus and dermatophyte species. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1311–1320. [Google Scholar] [CrossRef]
- Kırcı, D.; Demirci, B.; Kılıç, C.S.; Gürbüz, İ.; Duman, H. Essential Oil Composition of Fruits of Eight Ferulago Species Growing in Türkiye and Multivariate Statistical Analyses. Chem. Biodivers. 2023, 20, e202301098. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Izadi, A.; Malek Poor, F.; Hamedi, B. Chemical composition, antioxidant and antibacterial activities of essential oils from Ferulago angulata. Pharm. Biol. 2016, 54, 2515–2520. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature Review on Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities of Essential Oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.; Gonçalves, M.-J.; Hrimpeng, K.; Pinto, J.; Pinto, E. Antifungal activity of the essential oil of Angelica major against Candida, Cryptococcus, Aspergillus and dermatophyte species. J. Nat. Med. 2015, 69, 241–248. [Google Scholar] [CrossRef]
- Nóbrega, J.R.; Silva, D.d.F.; Andrade Júnior, F.P.d.; Sousa, P.M.S.; de Figueiredo, P.T.R.; Cordeiro, L.V.; Lima, E.d.O. Antifungal action of α-pinene against Candida spp. isolated from patients with otomycosis and effects of its association with boric acid. Nat. Prod. Res. 2021, 35, 6190–6193. [Google Scholar] [CrossRef]
- de Barros, D.B.; Lima, L.d.O.e.; Silva, L.A.; Fonseca, M.C.; Diniz-Neto, H.; Rocha, W.P.d.S.; Beltrão, G.V.d.M.; Castellano, L.R.C.; Guerra, F.Q.S.; da Silva, M.V. Efeito antifúngico de α-pineno isolado e em associação com antifúngicos frente às cepas de Candida albicans. Res. Soc. Dev. 2022, 11, e58711427748. [Google Scholar] [CrossRef]
- Alves-Silva, J.; Zuzarte, M.; Cavaleiro, C.; Salgueiro, L. Antibiofilm Effect of Lavandula multifida Essential Oil: A New Approach for Chronic Infections. Pharmaceutics 2023, 15, 2142. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Günbatan, T.; Gürbüz, İ.; Duman, H.; Kılıç, C.S.; İlhan, M. In Vitro Enzyme Inhibitory Activity of Ten Ferulago W. Koch Species Growing in Turkey. Braz. Arch. Biol. Technol. 2022, 65, e22210207. [Google Scholar] [CrossRef]
- HR, J. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard. Second Edition. M38-A2. Clin. Lab. Stand. Inst. 2008, 28, 1–35. [Google Scholar]
- Castelo-Branco, D.d.S.C.M.; de Aguiar, L.; Araújo, G.d.S.; Lopes, R.G.P.; Sales, J.d.A.; Pereira-Neto, W.A.; Pinheiro, A.d.Q.; Paixão, G.C.; Cordeiro, R.d.A.; Sidrim, J.J.C.; et al. In vitro and ex vivo biofilms of dermatophytes: A new panorama for the study of antifungal drugs. Biofouling 2020, 36, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. In Epidermal Cells: Methods and Protocols; Humana: New York, NY, USA, 2019; pp. 225–229. [Google Scholar]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]



| Species | Composition | |
|---|---|---|
| Main Compounds | Amount (%) | |
| F. cassia Boiss. | α-pinene | 61.0 |
| cis-crysanthenyl acetate | 21.3 | |
| α-phellandrene | 4.5 | |
| F. isaurica Peşmen | α-pinene | 17.3 |
| α-phellandrene | 36.5 | |
| limonene | 16.3 | |
| F. setifolia K. Koch | α-pinene | 39.1 |
| 2,3,6-trimethyl benzaldehyde | 35.1 | |
| sabinene | 8.2 | |
| F. silaifolia (Boiss.) Boiss. | α-pinene | 45.4 |
| cis-crysanthenyl acetate | 39.1 | |
| myrcene | 1.7 | |
| F. syriaca Boiss. | myrcene | 16.8 |
| α-phellandrene | 8.8 | |
| cubenol | 9.1 | |
| Dermatophyte Strains | Ferulago silaifolia | Ferulago setifolia | ||
|---|---|---|---|---|
| MIC | MLC | MIC | MLC | |
| Epidermophyton floccosum FF9 | 100 | 100–200 | 100–200 | 200–400 |
| Microsporum canis FF1 | 50 | 100 | 200 | 200–400 |
| Microsporum gypseum CECT 2908 | 200 | 200 | 200 | 400–600 |
| Trichophyton mentagrophytes FF7 | 100–200 | 100–200 | 200 | 200 |
| Trichophyton mentagrophytes var. interdigitale CECT 2958 | 200 | 400 | 200 | 600 |
| Trichophyton rubrum CECT 2794 | 50 | 100 | 50–100 | 100 |
| Trichophyton verrucosum CECT 2992 | 200–400 | 600 | 200 | 400 |
| Species | Local Name | Collection Date | Site of Collection | Accession Number |
|---|---|---|---|---|
| F. cassia (1) | şeytankişnişi | 20 June 2016 | Konya: Beyşehir, Tınaztepe-Bozkır old road, 1st km, serpentine areas, in gladelike areas under Pinus nigra, 1555 m | AEF 28775 |
| F. isaurica (2) | kargıkişnişi | 21 June 2016 | Antalya-Alanya, between Durbannaz-Banlıca, under Pinus brutia forest, calcaerous rocks, 837 m | AEF 28778 |
| F. setifolia (3) | kılkişniş | 14 July 2017 | Erzincan: Üzümlü district, Karakaya Town, between Karakaya-Tekçam Highland, high mountain steppe, 2047 m | AEF 28770 |
| F. silaifolia (4) | ulukişniş | 7 June 2016 | Bursa, Uludağ road, 100 km to National Park, under Castanea sativa Mill. and Pinus nigra forest, 837 m | AEF 28771 |
| F. syriaca (5) | kırkişnişi | 22 June 2016 | Hatay: Harbiye-Şenköy roa, ca. 5th–6th km, around maquis, 452 m | AEF28779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kılıç, C.S.; Catarino, I.; Alves-Silva, J.; Demirci, B.; Kırcı, D.; Salgueiro, L.; Zuzarte, M. Unlocking the Skin-Protective Effects of Ferulago spp. Essential Oils: A Focus on Antidermatophytic and Wound-Healing Potential. Antibiotics 2025, 14, 343. https://doi.org/10.3390/antibiotics14040343
Kılıç CS, Catarino I, Alves-Silva J, Demirci B, Kırcı D, Salgueiro L, Zuzarte M. Unlocking the Skin-Protective Effects of Ferulago spp. Essential Oils: A Focus on Antidermatophytic and Wound-Healing Potential. Antibiotics. 2025; 14(4):343. https://doi.org/10.3390/antibiotics14040343
Chicago/Turabian StyleKılıç, Ceyda Sibel, Inês Catarino, Jorge Alves-Silva, Betül Demirci, Damla Kırcı, Lígia Salgueiro, and Mónica Zuzarte. 2025. "Unlocking the Skin-Protective Effects of Ferulago spp. Essential Oils: A Focus on Antidermatophytic and Wound-Healing Potential" Antibiotics 14, no. 4: 343. https://doi.org/10.3390/antibiotics14040343
APA StyleKılıç, C. S., Catarino, I., Alves-Silva, J., Demirci, B., Kırcı, D., Salgueiro, L., & Zuzarte, M. (2025). Unlocking the Skin-Protective Effects of Ferulago spp. Essential Oils: A Focus on Antidermatophytic and Wound-Healing Potential. Antibiotics, 14(4), 343. https://doi.org/10.3390/antibiotics14040343











