Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Turkeys in Hungary (2022–2023)
Abstract
1. Introduction
2. Results
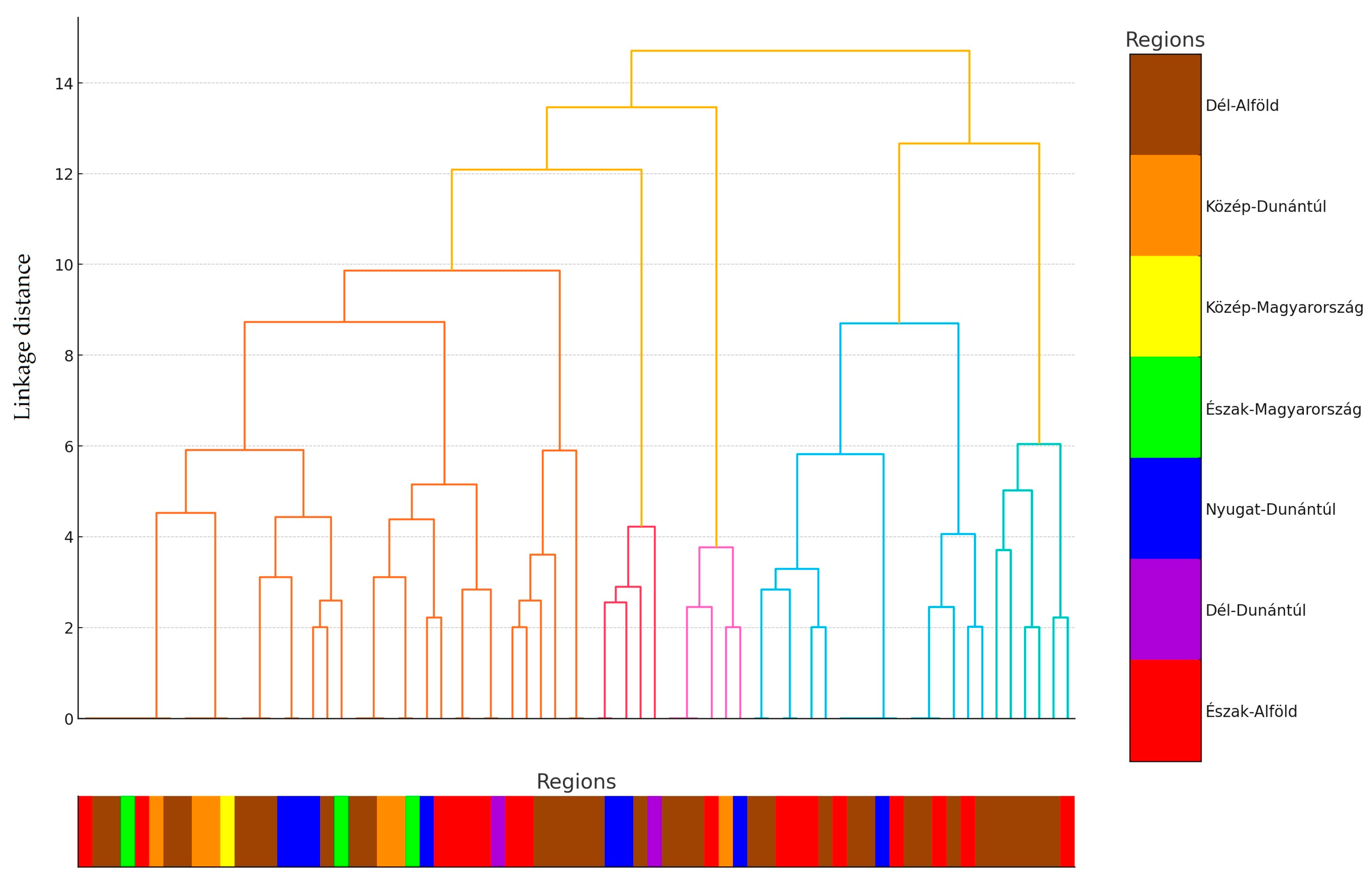
2.1. Regional Distribution and Origin of Samples Received
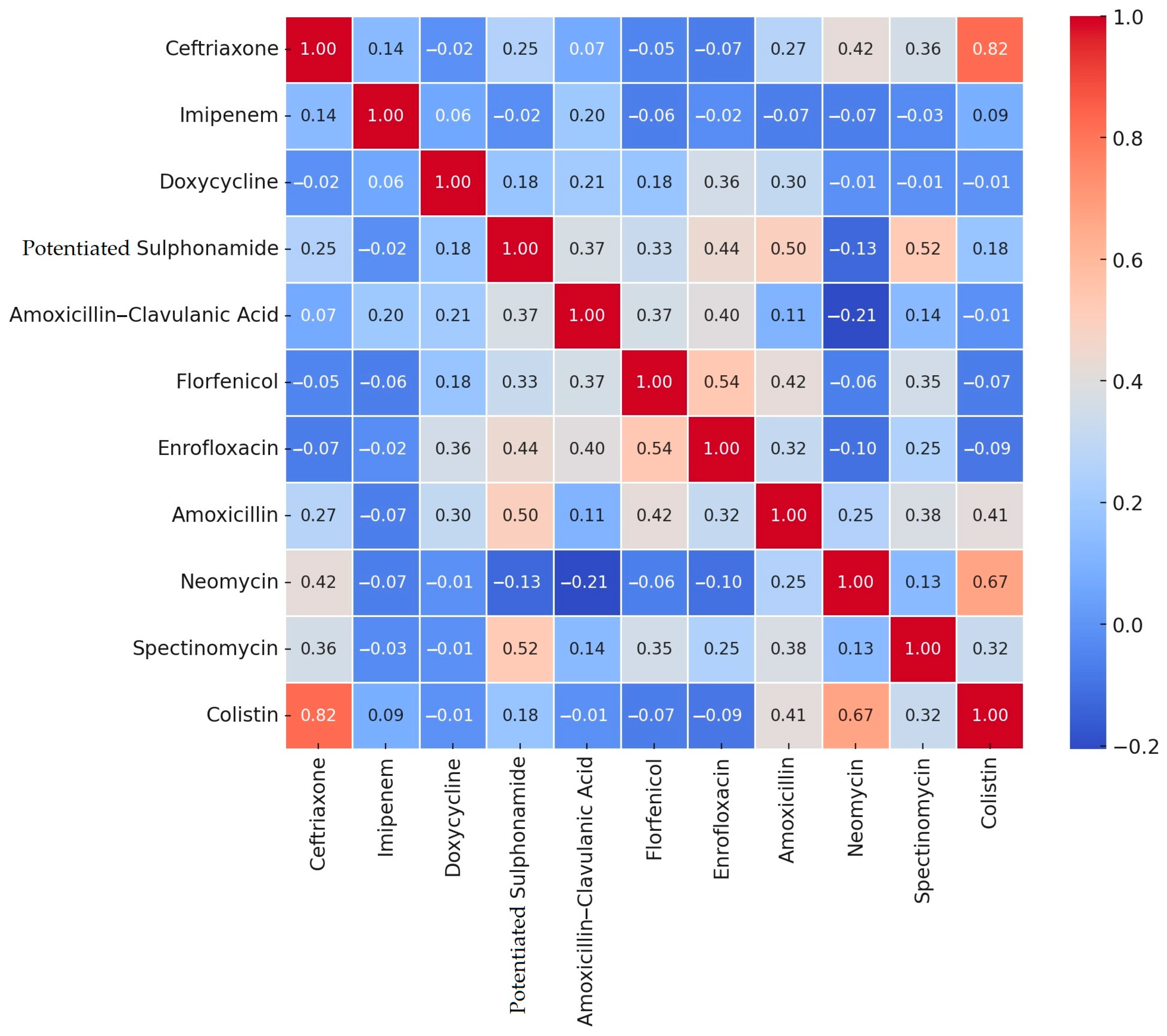
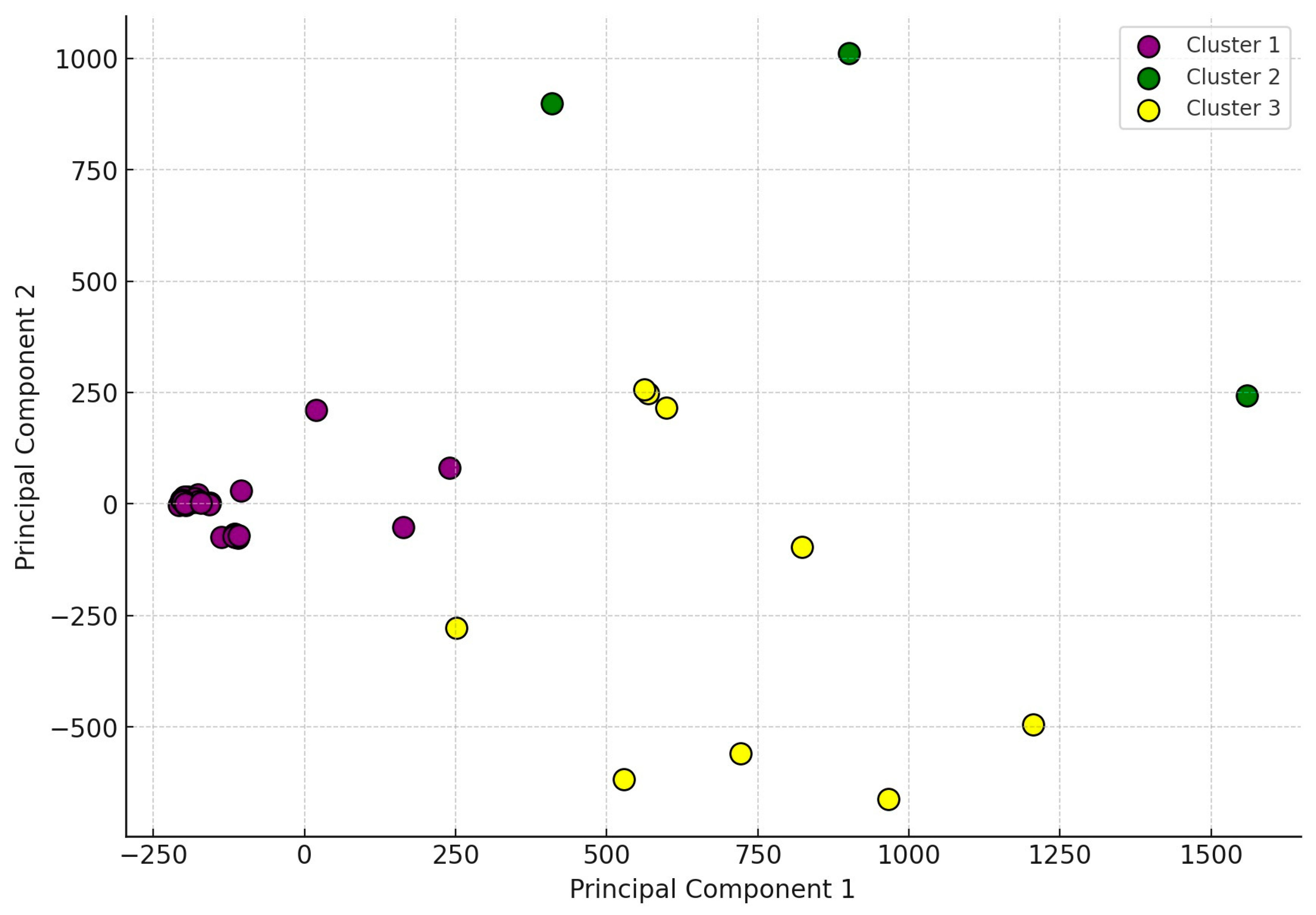
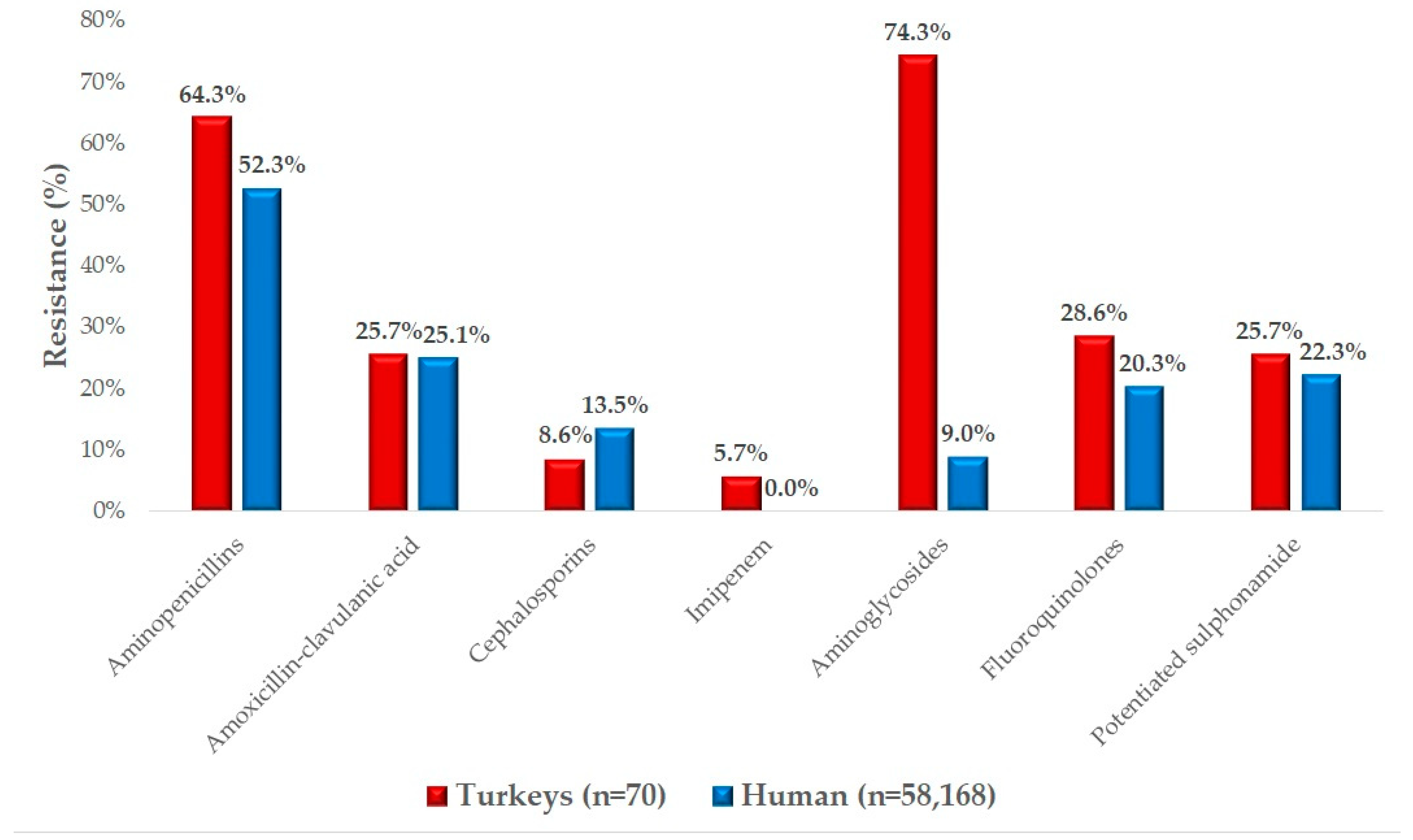
2.2. Antimicrobial Susceptibility Testing
3. Discussion
4. Materials and Methods
4.1. The Origin of Strains and Human Data
4.2. Minimum Inhibitory Concentration (MIC) Determination
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelband, H.; Laxminarayan, R. Tackling Antimicrobial Resistance at Global and Local Scales. Trends Microbiol. 2015, 23, 524–526. [Google Scholar] [CrossRef]
- Kong, L.-C.; Guo, X.; Wang, Z.; Gao, Y.-H.; Jia, B.-Y.; Liu, S.-M.; Ma, H.-X. Whole Genome Sequencing of an ExPEC That Caused Fatal Pneumonia at a Pig Farm in Changchun, China. BMC Vet. Res. 2017, 13, 169. [Google Scholar] [CrossRef]
- Adorján, A.; Makrai, L.; Könyves, L.; Tóth, I. Enteropathogenic Escherichia coli (EPEC): Short Literature Summary. MÁL 2021, 143, 429–438. [Google Scholar]
- Christensen, H.; Bachmeier, J.; Bisgaard, M. New Strategies to Prevent and Control Avian Pathogenic Escherichia coli (APEC). Avian Pathol. 2021, 50, 370–381. [Google Scholar] [CrossRef]
- Sagi, S.; Konduru, B.; Parida, M. Heterologous Expression of Intimin and IpaB Fusion Protein in Lactococcus lactis and Its Mucosal Delivery Elicit Protection against Pathogenicity of Escherichia coli O157 and Shigella flexneri in a Murine Model. Int. Immunopharmacol. 2020, 85, 106617. [Google Scholar] [CrossRef] [PubMed]
- Day, M.; Doumith, M.; Jenkins, C.; Dallman, T.J.; Hopkins, K.L.; Elson, R.; Godbole, G.; Woodford, N. Antimicrobial Resistance in Shiga Toxin-Producing Escherichia coli Serogroups O157 and O26 Isolated from Human Cases of Diarrhoeal Disease in England, 2015. J. Antimicrob. Chemother. 2017, 72, 145–152. [Google Scholar] [CrossRef]
- Carey, C.M.; Kostrzynska, M.; Ojha, S.; Thompson, S. The Effect of Probiotics and Organic Acids on Shiga-Toxin 2 Gene Expression in Enterohemorrhagic Escherichia coli O157:H7. J. Microbiol. Methods 2008, 73, 125–132. [Google Scholar] [CrossRef]
- Jørgensen, F.; McLauchlin, J.; Verlander, N.Q.; Aird, H.; Balasegaram, S.; Chattaway, M.A.; Dallman, T.; Herdman, M.T.; Hoban, A.; Lai, S.; et al. Levels and Genotypes of Salmonella and Levels of Escherichia coli in Frozen Ready-to-Cook Chicken and Turkey Products in England Tested in 2020 in Relation to an Outbreak of S. Enteritidis. Int. J. Food Microbiol. 2022, 369, 109609. [Google Scholar] [CrossRef]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Jouy, E.; Haenni, M.; Lupo, A.; Madec, J.-Y.; Leblond, A.; Gay, E. Co-Resistance to Amoxicillin and Tetracycline as an Indicator of Multidrug Resistance in Escherichia coli Isolates from Animals. Front. Microbiol. 2019, 10, 2288. [Google Scholar] [CrossRef]
- Leekitcharoenphon, P.; Johansson, M.H.K.; Munk, P.; Malorny, B.; Skarżyńska, M.; Wadepohl, K.; Moyano, G.; Hesp, A.; Veldman, K.T.; Bossers, A.; et al. Genomic Evolution of Antimicrobial Resistance in Escherichia coli. Sci. Rep. 2021, 11, 15108. [Google Scholar] [CrossRef]
- Alhababi, D.A.; Eltai, N.O.; Nasrallah, G.K.; Farg, E.A.; Al Thani, A.A.; Yassine, H.M. Antimicrobial Resistance of Commensal Escherichia coli Isolated from Food Animals in Qatar. Microb. Drug Resist. 2020, 26, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Mencía-Ares, O.; Argüello, H.; Puente, H.; Gómez-García, M.; Manzanilla, E.G.; Álvarez-Ordóñez, A.; Carvajal, A.; Rubio, P. Antimicrobial Resistance in Commensal Escherichia coli and Enterococcus spp. Is Influenced by Production System, Antimicrobial Use, and Biosecurity Measures on Spanish Pig Farms. Porc. Health Manag. 2021, 7, 27. [Google Scholar] [CrossRef]
- Landman, W.J.M.; van Eck, J.H.H. The Incidence and Economic Impact of the Escherichia coli Peritonitis Syndrome in Dutch Poultry Farming. Avian Pathol. 2015, 44, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. Horizontal Gene Exchange in Environmental Microbiota. Front. Microbiol. 2011, 2, 158. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.; Roshdy, H.; Samir, A.H.; Hamed, E.A. Antibiotic Resistance and Extended-Spectrum β-Lactamase in Escherichia coli Isolates from Imported 1-Day-Old Chicks, Ducklings, and Turkey Poults. Vet. World 2020, 13, 1037–1044. [Google Scholar] [CrossRef]
- Feng, A.; Akter, S.; Leigh, S.A.; Wang, H.; Pharr, G.T.; Evans, J.; Branton, S.L.; Landinez, M.P.; Pace, L.; Wan, X.-F. Genomic Diversity, Pathogenicity and Antimicrobial Resistance of Escherichia coli Isolated from Poultry in the Southern United States. BMC Microbiol. 2023, 23, 15. [Google Scholar] [CrossRef]
- De Oliveira, A.L.; Newman, D.M.; Sato, Y.; Noel, A.; Rauk, B.; Nolan, L.K.; Barbieri, N.L.; Logue, C.M. Characterization of Avian Pathogenic Escherichia coli (APEC) Associated with Turkey Cellulitis in Iowa. Front. Vet. Sci. 2020, 7, 380. [Google Scholar] [CrossRef]
- Zanella, A.; Alborali, G.L.; Bardotti, M.; Candotti, P.; Guadagnini, P.F.; Martino, P.A.; Stonfer, M. Severe Escherichia coli O111 Septicaemia and Polyserositis in Hens at the Start of Lay. Avian Pathol. 2000, 29, 311–317. [Google Scholar] [CrossRef]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/ pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-Resistant Escherichia coli from Retail Poultry Meat with Different Antibiotic Use Claims. BMC Microbiol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Tuska-Szalay, B.; Kovács, L.; Jerzsele, Á. In Vitro Efficacy of Hungarian Propolis against Bacteria, Yeast, and Trichomonas gallinae Isolated from Pigeons—A Possible Antibiotic Alternative? Resources 2023, 12, 101. [Google Scholar] [CrossRef]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Schrijver, R.; Stijntjes, M.; Rodríguez-Baño, J.; Tacconelli, E.; Rajendran, N.B.; Voss, A. Review of Antimicrobial Resistance Surveillance Programmes in Livestock and Meat in EU with Focus on Humans. Clin. Microbiol. Infect. 2018, 24, 577–590. [Google Scholar] [CrossRef]
- Aguirre, L.; Vidal, A.; Seminati, C.; Tello, M.; Redondo, N.; Darwich, L.; Martín, M. Antimicrobial Resistance Profile and Prevalence of Extended-Spectrum Beta-Lactamases (ESBL), AmpC Beta-Lactamases and Colistin Resistance (Mcr) Genes in Escherichia coli from Swine between 1999 and 2018. Porc. Health Manag. 2020, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017. EFSA J. 2019, 17, e05598. [Google Scholar] [CrossRef]
- Guarner, J. Introduction: One Health and Emerging Infectious Diseases. Semin. Diagn. Pathol. 2019, 36, 143–145. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Phillips, C.; Chapman, B.; Agunos, A.; Carson, C.A.; Parmley, E.J.; Reid-Smith, R.J.; Smith, B.A.; Murphy, C.P. A Scoping Review of Factors Potentially Linked with Antimicrobial-Resistant Bacteria from Turkeys (iAM.AMR Project). Epidemiol. Infect. 2022, 150, e153. [Google Scholar] [CrossRef]
- MacKinnon, M.C.; Sargeant, J.M.; Pearl, D.L.; Reid-Smith, R.J.; Carson, C.A.; Parmley, E.J.; McEwen, S.A. Evaluation of the Health and Healthcare System Burden Due to Antimicrobial-Resistant Escherichia coli Infections in Humans: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- McCrackin, M.A.; Helke, K.L.; Galloway, A.M.; Poole, A.Z.; Salgado, C.D.; Marriott, B.P. Effect of Antimicrobial Use in Agricultural Animals on Drug-Resistant Foodborne Campylobacteriosis in Humans: A Systematic Literature Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2115–2132. [Google Scholar] [CrossRef]
- Murphy, C.P.; Carson, C.; Smith, B.A.; Chapman, B.; Marrotte, J.; McCann, M.; Primeau, C.; Sharma, P.; Parmley, E.J. Factors Potentially Linked with the Occurrence of Antimicrobial Resistance in Selected Bacteria from Cattle, Chickens and Pigs: A Scoping Review of Publications for Use in Modelling of Antimicrobial Resistance (IAM.AMR Project). Zoonoses Public Health 2018, 65, 957–971. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Shrestha, R.D.; Agunos, A.; Gow, S.P.; Deckert, A.E.; Varga, C. Associations between Antimicrobial Resistance in Fecal Escherichia coli Isolates and Antimicrobial Use in Canadian Turkey Flocks. Front. Microbiol. 2022, 13, 954123. [Google Scholar] [CrossRef]
- Suwono, B.; Eckmanns, T.; Kaspar, H.; Merle, R.; Zacher, B.; Kollas, C.; Weiser, A.A.; Noll, I.; Feig, M.; Tenhagen, B.-A. Cluster Analysis of Resistance Combinations in Escherichia coli from Different Human and Animal Populations in Germany 2014–2017. PLoS ONE 2021, 16, e0244413. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Gow, S.P.; Léger, D.F.; Carson, C.A.; Deckert, A.E.; Bosman, A.L.; Loest, D.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial Use and Antimicrobial Resistance Indicators-Integration of Farm-Level Surveillance Data from Broiler Chickens and Turkeys in British Columbia, Canada. Front. Vet. Sci. 2019, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Boulianne, M.; Arsenault, J.; Daignault, D.; Archambault, M.; Letellier, A.; Dutil, L. Drug Use and Antimicrobial Resistance among Escherichia coli and Enterococcus spp. Isolates from Chicken and Turkey Flocks Slaughtered in Quebec, Canada. Can. J. Vet. Res. 2016, 80, 49–59. [Google Scholar] [PubMed]
- Cook, A.; Reid-Smith, R.; Irwin, R.; McEwen, S.A.; Valdivieso-Garcia, A.; Ribble, C. Antimicrobial Resistance in Campylobacter, Salmonella, and Escherichia coli Isolated from Retail Turkey Meat from Southern Ontario, Canada. J. Food Prot. 2009, 72, 473–481. [Google Scholar] [CrossRef]
- Grobbel, M.; Hammerl, J.A.; Alt, K.; Irrgang, A.; Kaesbohrer, A.; Tenhagen, B.-A. Comparison of Antimicrobial Resistances in Escherichia coli from Conventionally and Organic Farmed Poultry from Germany. Antibiotics 2022, 11, 1282. [Google Scholar] [CrossRef]
- Houang, E.T.; Greenwood, D. Aminoglycoside Cross-Resistance Patterns of Gentamicin-Resistant Bacteria. J. Clin. Pathol. 1977, 30, 738–744. [Google Scholar] [CrossRef]
- Campbell, K.L. Sulphonamides: Updates on Use in Veterinary Medicine. Vet. Dermatol. 1999, 10, 205–215. [Google Scholar] [CrossRef]
- Nobili, G.; La Bella, G.; Basanisi, M.G.; Damato, A.M.; Coppola, R.; Migliorelli, R.; Rondinone, V.; Leekitcharoenphon, P.; Bortolaia, V.; La Salandra, G. Occurrence and Characterisation of Colistin-Resistant Escherichia coli in Raw Meat in Southern Italy in 2018–2020. Microorganisms 2022, 10, 1805. [Google Scholar] [CrossRef]
- Mesa-Varona, O.; Kaspar, H.; Grobbel, M.; Tenhagen, B.-A. Phenotypical Antimicrobial Resistance Data of Clinical and Non-Clinical Escherichia coli from Poultry in Germany between 2014 and 2017. PLoS ONE 2020, 15, e0243772. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Chen, W.; Liu, Z.; Zhao, Q.; Yang, H.; Sun, Z.; Chen, X.; Li, J. Prevalence and Characteristics of Mcr-1-Producing Escherichia coli in Three Kinds of Poultry in Changsha, China. Front. Microbiol. 2022, 13, 840520. [Google Scholar] [CrossRef]
- Gambi, L.; Rossini, R.; Menandro, M.L.; Franzo, G.; Valentini, F.; Tosi, G.; D’Incau, M.; Fiorentini, L. Virulence Factors and Antimicrobial Resistance Profile of Escherichia coli Isolated from Laying Hens in Italy. Animals 2022, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, A.; Giovanardi, D.; Dotto, G.; Grilli, G.; Montesissa, C.; Boldrin, C.; Salata, C.; Giacomelli, M. Antimicrobial Resistance and Class 1 and 2 Integrons in Escherichia coli from Meat Turkeys in Northern Italy. Avian Pathol. 2014, 43, 396–405. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Hafez, H.M.; Ramadan, H.; Tomaso, H.; Braun, S.D.; Ehricht, R.; Diezel, C.; Gary, D.; Engelmann, I.; et al. Occurrence, Phenotypic and Molecular Characteristics of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Healthy Turkeys in Northern Egypt. Antibiotics 2022, 11, 1075. [Google Scholar] [CrossRef]
- Barnácz, F.; Kerek, Á.; Csirmaz, B.; Román, I.L.; Gál, C.; Horváth, Á.; Hajduk, E.; Szabó, Á.; Jerzsele, Á.; Kovács, L. The Status of Antimicrobial Resistance in Domestic Poultry with Different Breeding Purposes in Hungary between 2022–2023. Magy. Állatorvosok Lapja 2024, 146, 339–356. [Google Scholar] [CrossRef]
- EFSA. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2014. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4380 (accessed on 22 September 2023).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2016. EFSA J. 2018, 16, e05182. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017/2018. EFSA J. 2020, 18, e06007. [Google Scholar] [CrossRef]
- Authority, E.F.S.; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- EFSA. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2021–2022. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8583 (accessed on 27 November 2024).
- Brătfelan, D.O.; Tabaran, A.; Colobatiu, L.; Mihaiu, R.; Mihaiu, M. Prevalence and Antimicrobial Resistance of Escherichia coli Isolates from Chicken Meat in Romania. Animals 2023, 13, 3488. [Google Scholar] [CrossRef]
- Carmona-Cartaya, Y.; Hidalgo-Benito, M.; Borges-Mateus, L.M.; Pereda-Novales, N.; González-Molina, M.K.; Quiñones-Pérez, D. Community-Acquired Uropathogenic Escherichia coli, Antimicrobial Susceptibility, and Extended-Spectrum Beta-Lactamase Detection. MEDICC Rev. 2022, 24, 20–25. [Google Scholar] [CrossRef]
- Jańczak, D.; Górecki, P.; Stryjek, R.; Zasada, A. Multidrug Resistance of Escherichia coli Isolated from the Urinary Bladder of Dogs and Cats with Suspected Urinary Tract Infections. Ann. Agric. Env. Med. 2024, 31, 178–184. [Google Scholar] [CrossRef]
- Brown, S.A. Fluoroquinolones in Animal Health. J. Vet. Pharmacol. Ther. 1996, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.C.; Lang, R.; Stokes, W. How I Manage Bacterial Prostatitis. Clin. Microbiol. Infect. 2023, 29, 32–37. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume CLSI Standards M07. [Google Scholar]
- Dubraska, D.-C.; Claire, B. VET01SEd7—Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Heck, D.W.; Moshagen, M. RRreg: Correlation and Regression Analyses for Randomized Response Data. J. Stat. Softw. 2022, 85, 1–29. [Google Scholar]
- Revelle, W. Hierarchical Cluster Analysis And The Internal Structure Of Tests. Multivar. Behav. Res. 1979, 14, 57–74. [Google Scholar] [CrossRef]
- Vogt, W.; Nagel, D. Cluster Analysis in Diagnosis. Clin. Chem. 1992, 38, 182–198. [Google Scholar]
- Ben Salem, K.; Ben Abdelaziz, A. Principal Component Analysis (PCA). Tunis. Med. 2021, 99, 383–389. [Google Scholar]

| Antibiotic | 1 BP * | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | 2 ECOFF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | µg/mL | ||||||||||||||||||||||||
| Enrofloxacin | 1 2 | 4 | 16 | 1 | 2 | 5 | 13 | 9 | 1 | 3 | 3 | 11 | 1 | 1 | 0.5 | 16 | 0.125 | ||||||||
| 5.7% | 22.9% | 1.4% | 2.9% | 7.1% | 18.6% | 12.9% | 1.4% | 4.3% | 4.3% | 15.7% | 1.4% | 1.4% | |||||||||||||
| Colistin | 2 | 1 | 0 | 0 | 5 | 3 | 5 | 8 | 9 | 19 | 15 | 5 | 0.5 | 1 | 2 | ||||||||||
| 1.4% | 0.0% | 0.0% | 7.1% | 4.3% | 7.1% | 11.4% | 12.9% | 27.1% | 21.4% | 7.1% | |||||||||||||||
| Ceftriaxone | 1 4 | 5 | 19 | 21 | 8 | 3 | 5 | 2 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 2 | 0.06 | 1 | 0.125 | |||||
| 7.1% | 27.1% | 30.0% | 11.4% | 4.3% | 7.1% | 2.9% | 1.4% | 0.0% | 0.0% | 5.7% | 0.0% | 0.0% | 0.0% | 0.0% | 2.9% | ||||||||||
| Imipenem | 1 4 | 1 | 4 | 10 | 26 | 11 | 4 | 10 | 4 | 0.25 | 2 | 0.5 | |||||||||||||
| 1.4% | 5.7% | 14.3% | 37.1% | 15.7% | 5.7% | 14.3% | 5.7% | ||||||||||||||||||
| 3 Potentiated sulphonamide | 1 4 | 2 | 3 | 6 | 10 | 14 | 15 | 2 | 0 | 11 | 0 | 2 | 5 | 8 | 128 | 0.5 | |||||||||
| 2.9% | 4.3% | 8.6% | 14.3% | 20.0% | 21.4% | 2.9% | 0.0% | 15.7% | 0.0% | 2.9% | 7.1% | ||||||||||||||
| Doxycycline | 1 16 | 5 | 4 | 11 | 9 | 10 | 19 | 9 | 2 | 1 | 8 | 32 | 8 | ||||||||||||
| 7.1% | 5.7% | 15.7% | 12.9% | 14.3% | 27.1% | 12.9% | 2.9% | 1.4% | |||||||||||||||||
| Florfenicol | 1 16 | 3 | 30 | 17 | 5 | 11 | 0 | 2 | 2 | 16 | 64 | 16 | |||||||||||||
| 4.3% | 42.9% | 24.3% | 7.1% | 15.7% | 0.0% | 2.9% | 2.9% | ||||||||||||||||||
| Amoxicillin | 1 32 | 1 | 0 | 4 | 14 | 5 | 1 | 29 | 0 | 1 | 5 | 3 | 7 | 32 | 512 | 8 | |||||||||
| 1.4% | 0.0% | 5.7% | 20.0% | 7.1% | 1.4% | 41.4% | 0.0% | 1.4% | 7.1% | 4.3% | 10.0% | ||||||||||||||
| 4 Amoxicillin–clavulanic acid | 32 | 6 | 1 | 2 | 9 | 13 | 21 | 17 | 1 | 16 | 32 | 8 | |||||||||||||
| 8.6% | 1.4% | 2.9% | 12.9% | 18.6% | 30.0% | 24.3% | 1.4% | ||||||||||||||||||
| Neomycin | 32 | 1 | 2 | 2 | 1 | 12 | 39 | 10 | 0 | 0 | 3 | 32 | 64 | 8 | |||||||||||
| 1.4% | 2.9% | 2.9% | 1.4% | 17.1% | 55.7% | 14.3% | 0.0% | 0.0% | 4.3% | ||||||||||||||||
| Spectinomycin | 128 | 1 | 4 | 44 | 12 | 4 | 2 | 2 | 1 | 32 | 128 | 64 | |||||||||||||
| 1.4% | 5.7% | 62.9% | 17.1% | 5.7% | 2.9% | 2.9% | 1.4% | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerzsele, Á.; Szabó, Á.; Barnácz, F.; Csirmaz, B.; Kovács, L.; Kerek, Á. Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Turkeys in Hungary (2022–2023). Antibiotics 2025, 14, 338. https://doi.org/10.3390/antibiotics14040338
Jerzsele Á, Szabó Á, Barnácz F, Csirmaz B, Kovács L, Kerek Á. Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Turkeys in Hungary (2022–2023). Antibiotics. 2025; 14(4):338. https://doi.org/10.3390/antibiotics14040338
Chicago/Turabian StyleJerzsele, Ákos, Ábel Szabó, Franciska Barnácz, Bence Csirmaz, László Kovács, and Ádám Kerek. 2025. "Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Turkeys in Hungary (2022–2023)" Antibiotics 14, no. 4: 338. https://doi.org/10.3390/antibiotics14040338
APA StyleJerzsele, Á., Szabó, Á., Barnácz, F., Csirmaz, B., Kovács, L., & Kerek, Á. (2025). Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Turkeys in Hungary (2022–2023). Antibiotics, 14(4), 338. https://doi.org/10.3390/antibiotics14040338







