Tackling Antimicrobial Resistance: A Sustainable Method for the Removal of Antibiotics from Water
Abstract
1. Introduction
2. Results and Discussion
2.1. Analytical Method
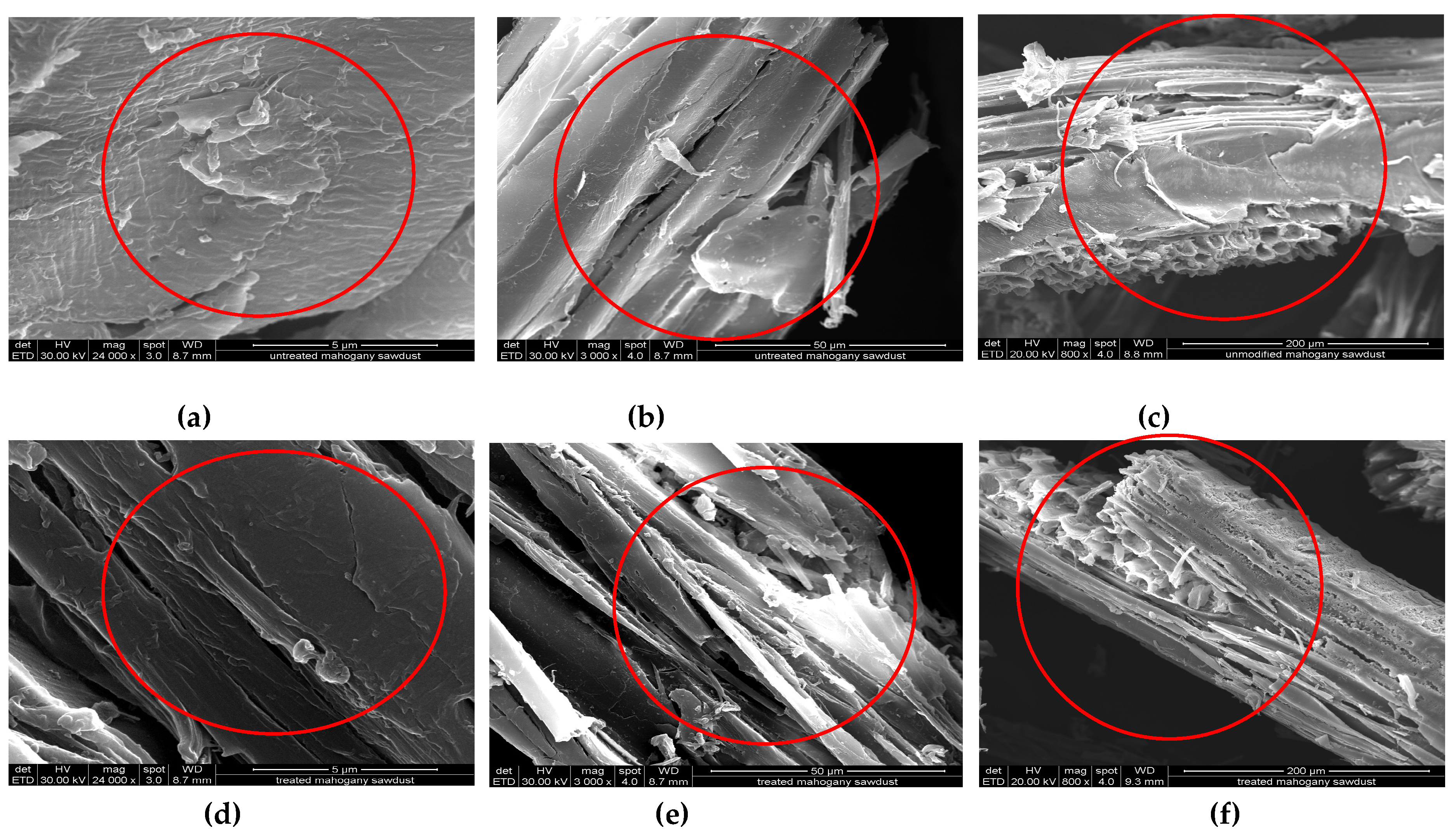
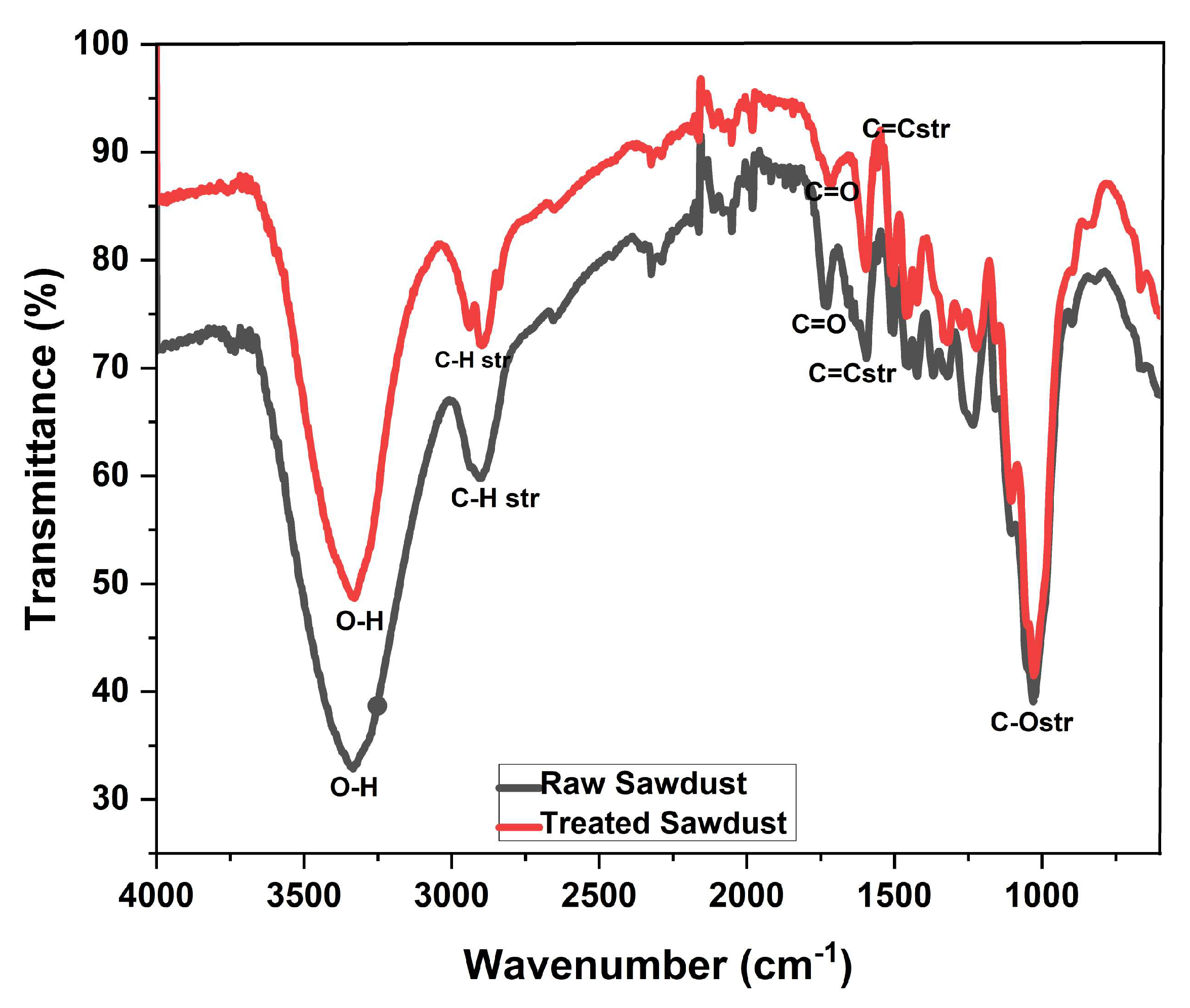
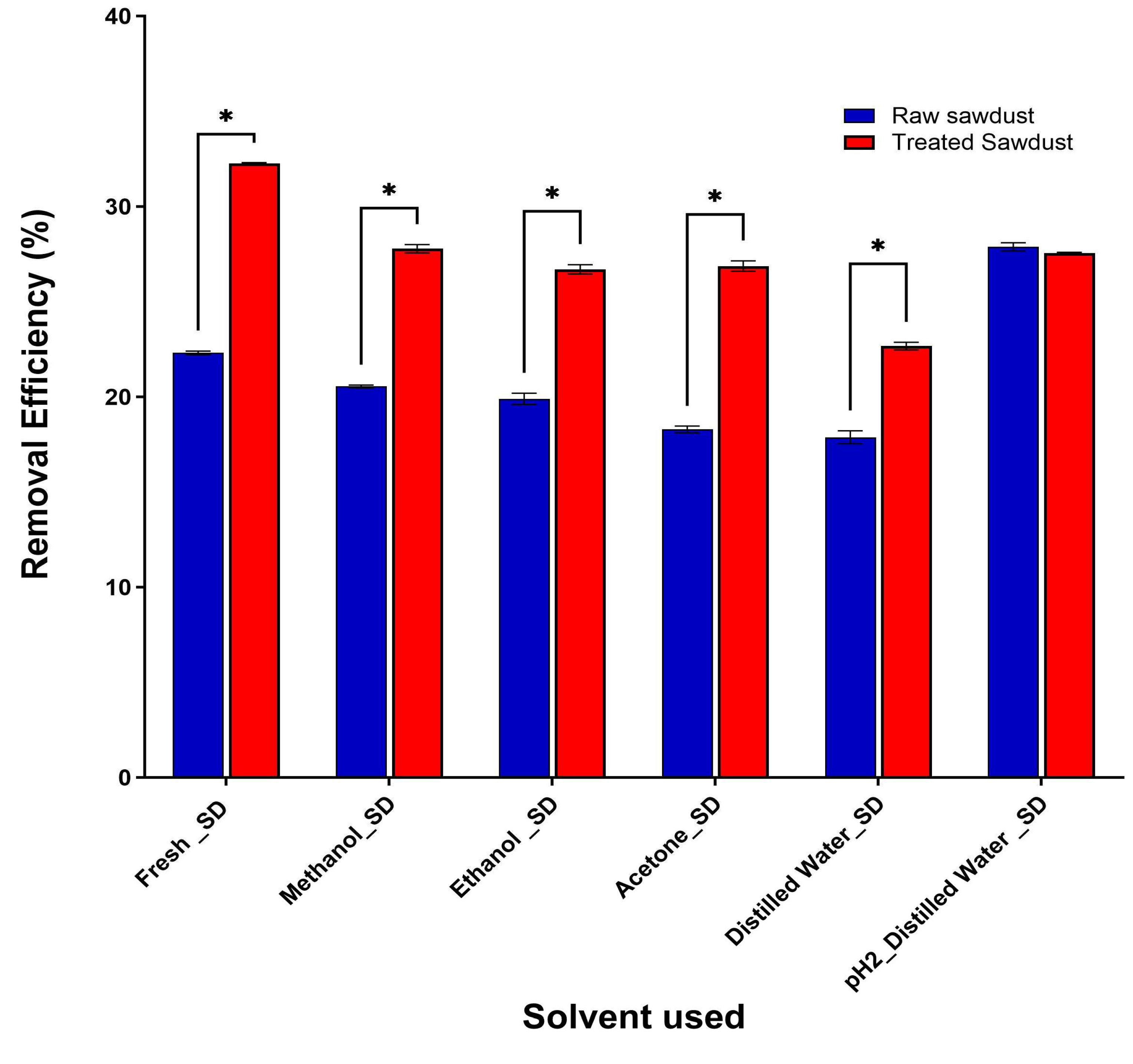
2.2. Characterization of Sawdust
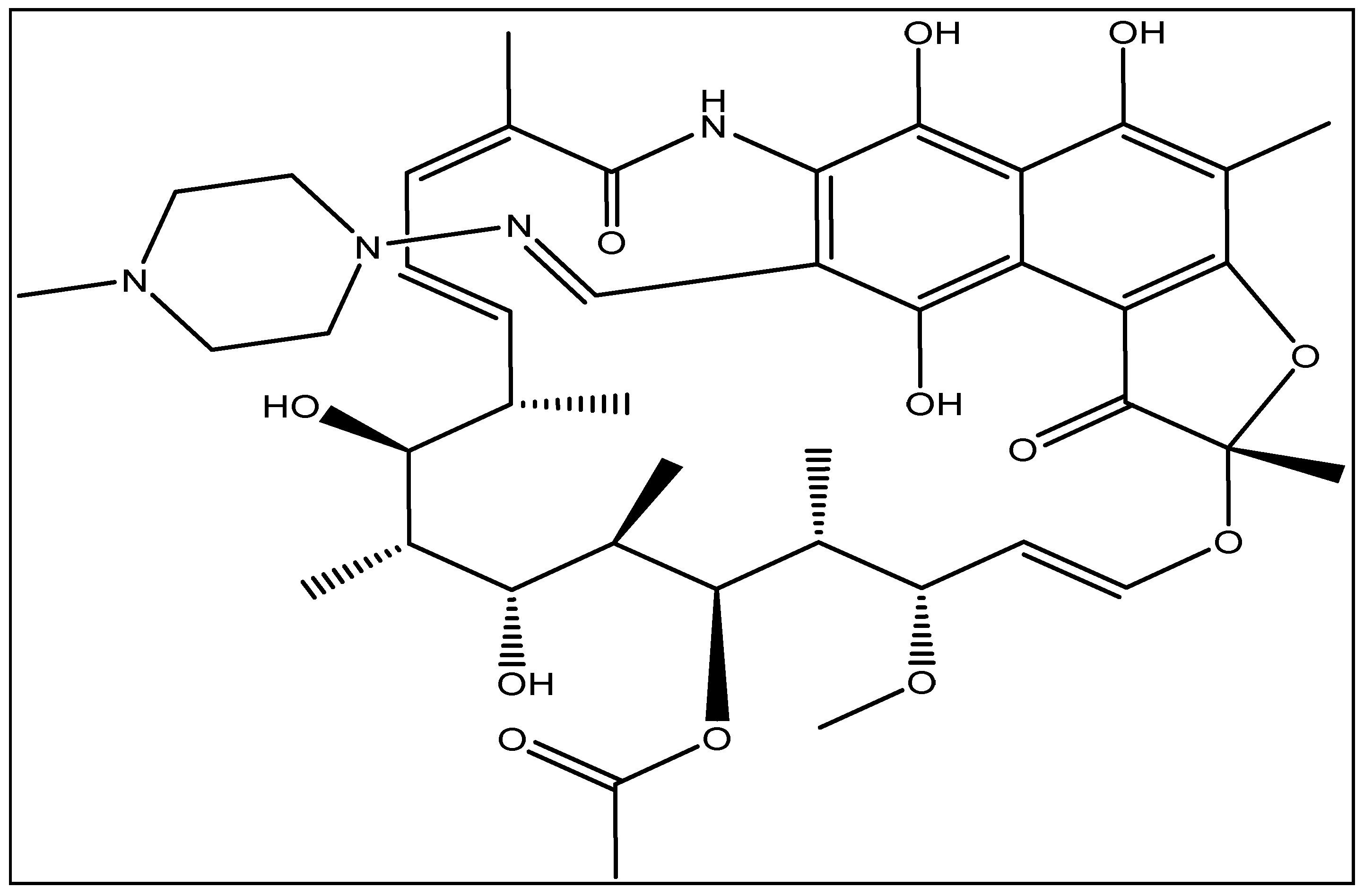
2.3. Adsorption Study of RFA
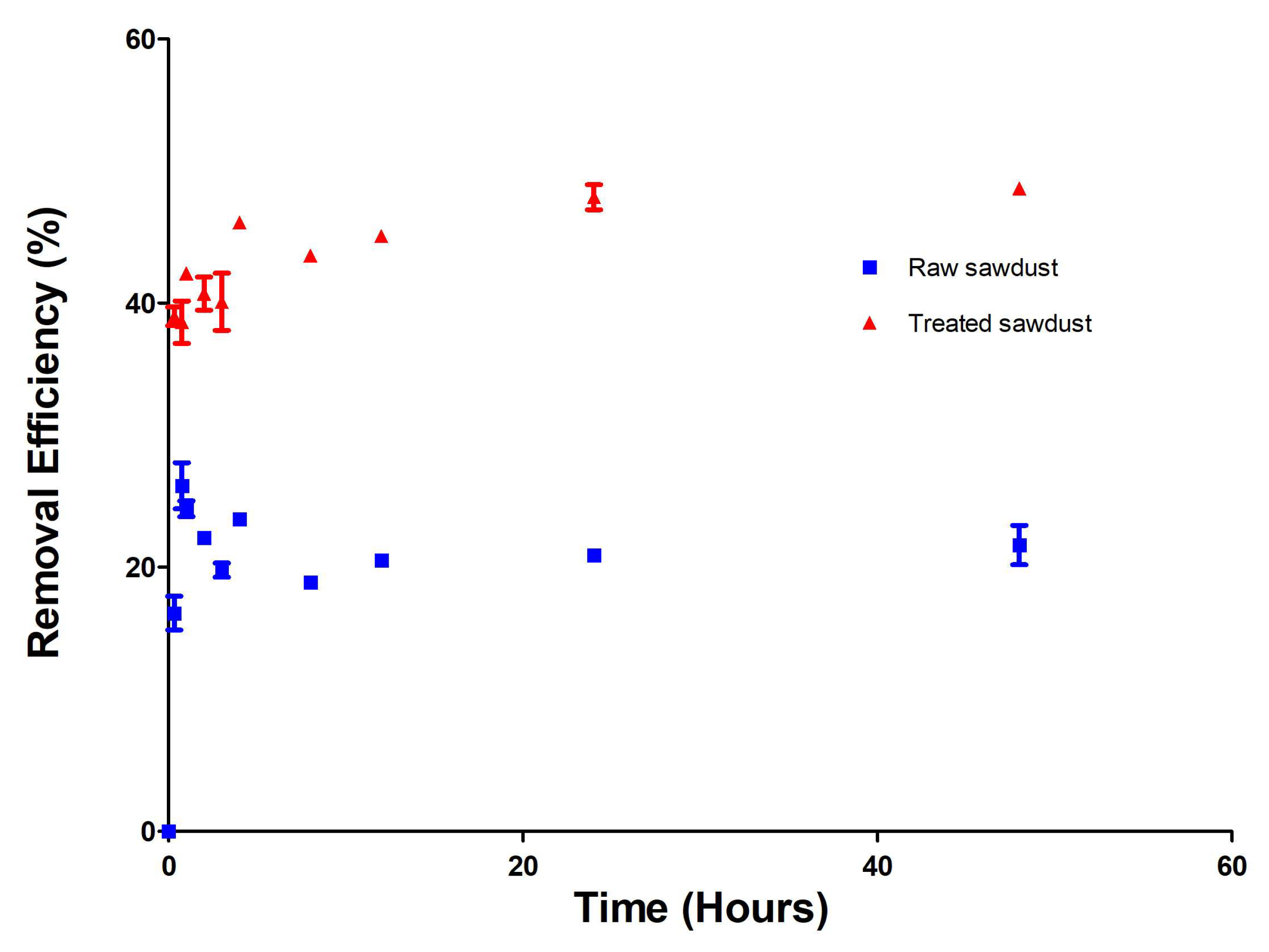
2.3.1. Effect of Contact Time
2.3.2. Effect of Initial RFA Concentration
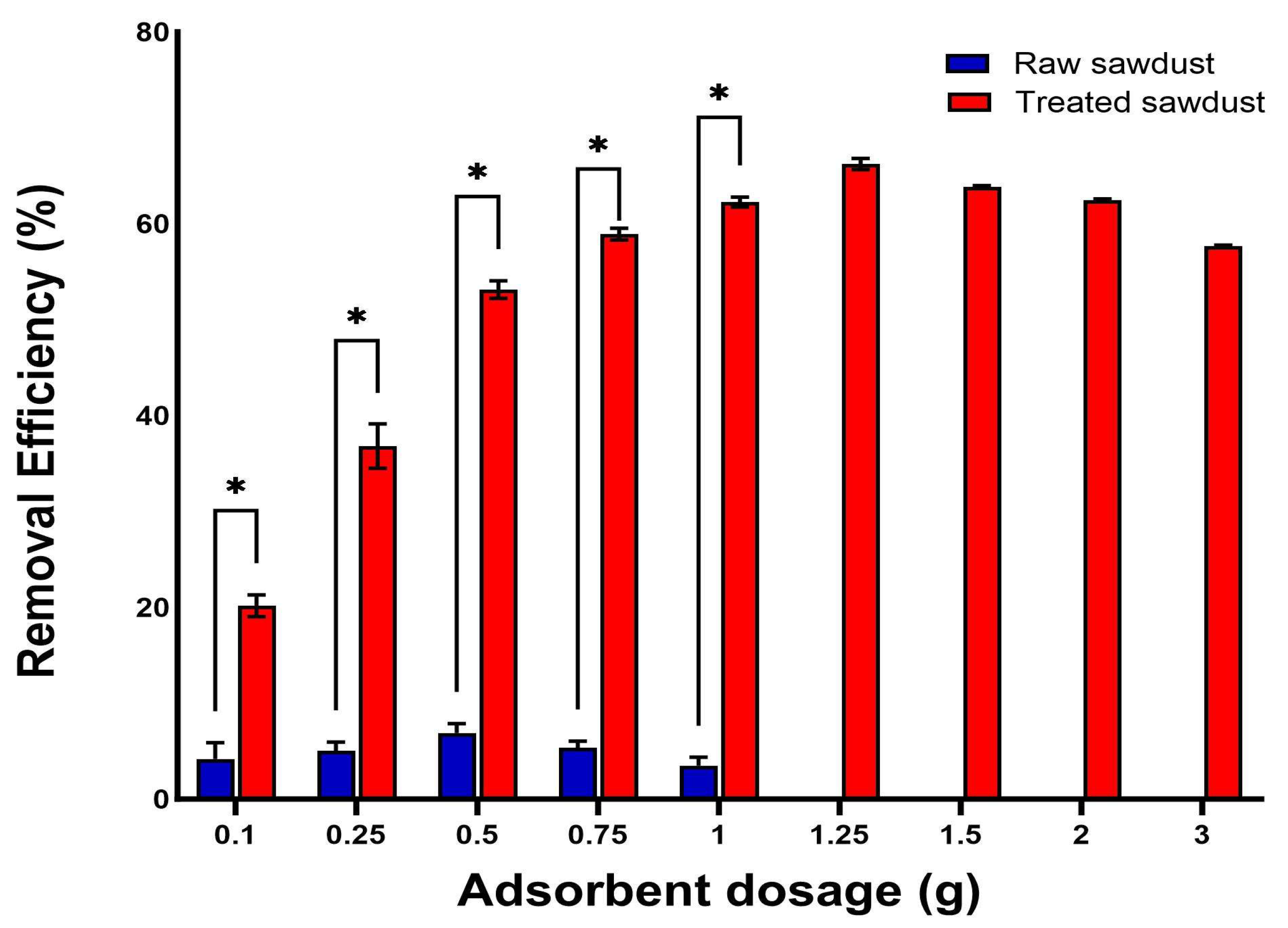
2.3.3. Effect of the Adsorbent Dosage
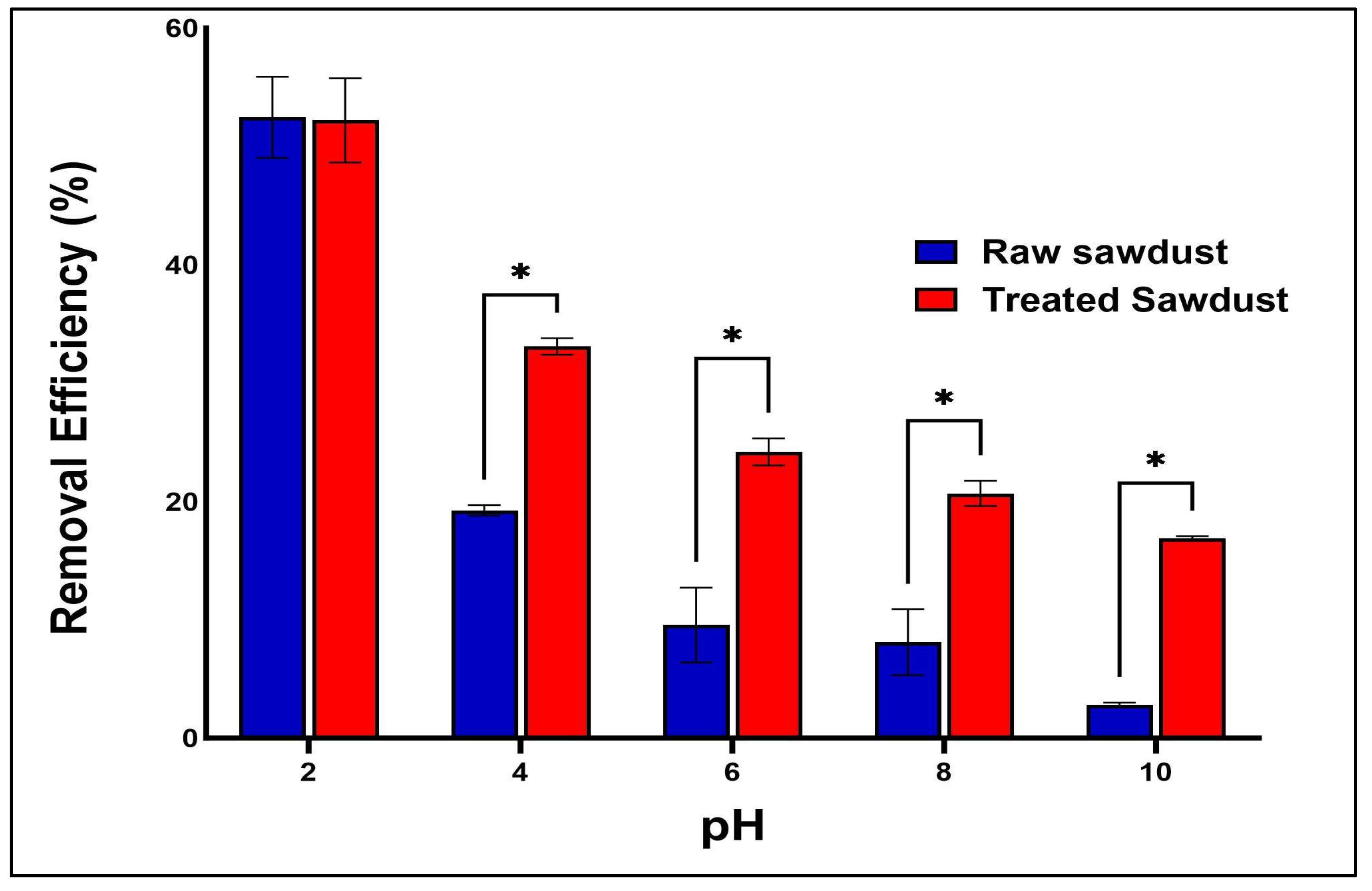
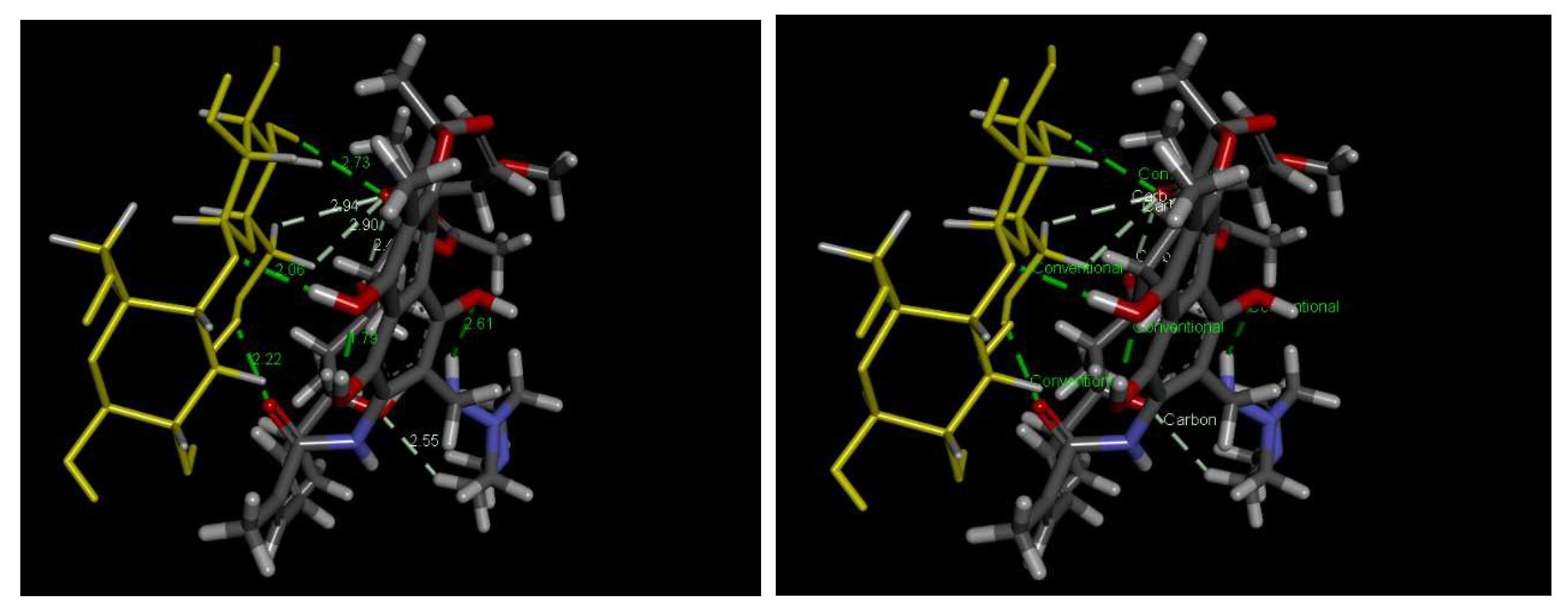
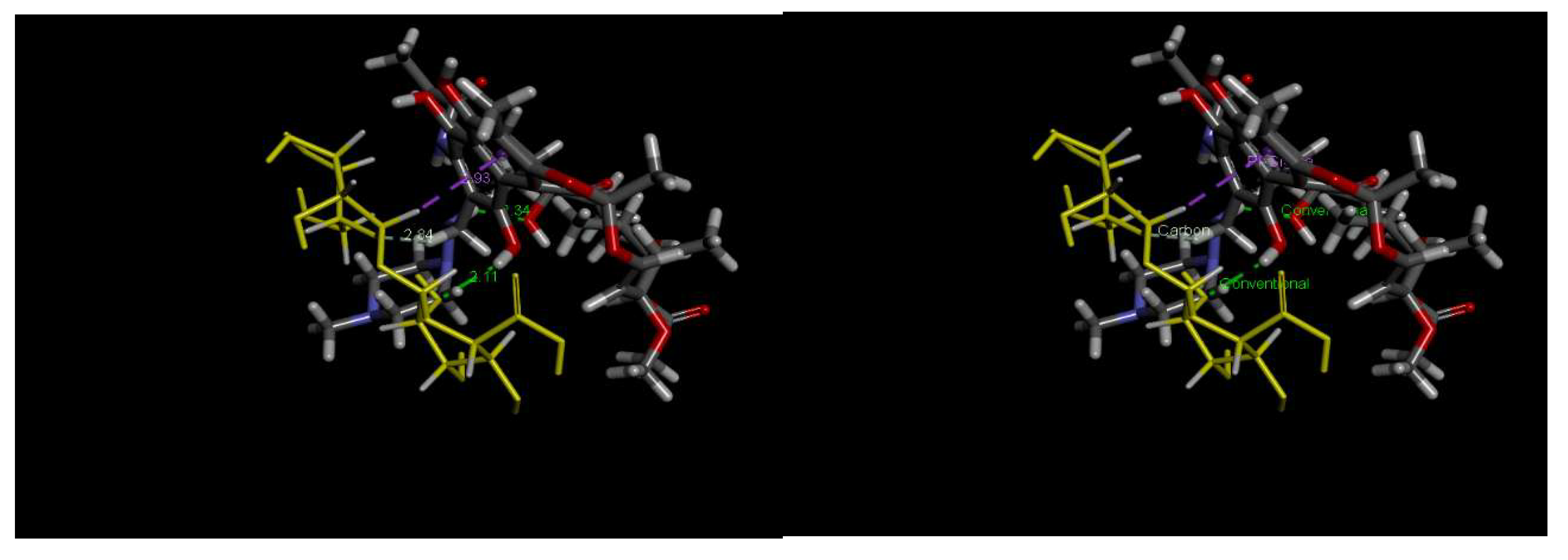
2.3.4. Effect of the pH of RFA Solution and Possible Mechanisms of Adsorption
2.3.5. Effect of Temperature
2.4. Re-Usability of Sawdust
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Method Development
3.2.2. Preparation of the Sawdust
3.3. Characterization of Sawdust
3.4. Adsorption Experiments
3.5. Re-Usability Study
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, K.W.; Michelle Tiong, H.L.; Luang-In, V.; Ma, N.L. An Overview of Antibiotic and Antibiotic Resistance. Environ. Adv. 2023, 11, 100331. [Google Scholar] [CrossRef]
- Kollef, M.H.; Bassetti, M.; Francois, B.; Burnham, J.; Dimopoulos, G.; Garnacho-Montero, J.; Lipman, J.; Luyt, C.-E.; Nicolau, D.P.; Postma, M.J.; et al. The Intensive Care Medicine Research Agenda on Multidrug-Resistant Bacteria, Antibiotics, and Stewardship. Intensive Care Med. 2017, 43, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and Personal Care Products (PPCPs) in the Freshwater Aquatic Environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Akinsanmi, O.; Kaya, S.; Tretsiakova-McNally, S.; Arnscheidt, J.; Coleman, H. Tackling Antimicrobial Resistance: Adsorption of Meropenem and Ciprofloxacin on Lignocellulosic Substrate from Sawdust. In Proceedings of the European Waste Water Management Conference, Birmingham, UK, 16–17 July 2019. [Google Scholar]
- Russell, J.N.; Yost, C.K. Alternative, Environmentally Conscious Approaches for Removing Antibiotics from Wastewater Treatment Systems. Chemosphere 2021, 263, 128177. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Magwira, C.A.; Aneck-Hahn, N.; Taylor, M.B. Fate, Occurrence and Potential Adverse Effects of Antimicrobials Used for Treatment of Tuberculosis in the Aquatic Environment in South Africa. Environ. Pollut. 2019, 254, 112990. [Google Scholar]
- Abbasi, A.R.; Rizvandi, M. Influence of the Ultrasound-Assisted Synthesis of Cu–BTC Metal–Organic Frameworks Nanoparticles on Uptake and Release Properties of Rifampicin. Ultrason. Sonochem. 2018, 40, 465–471. [Google Scholar]
- Mtetwa, H.N.; Amoah, I.D.; Kumari, S.; Bux, F.; Reddy, P. Optimisation of Analytical Methods for Tuberculosis Drug Detection in Wastewater: A Multinational Study. Heliyon 2024, 10, e30720. [Google Scholar] [CrossRef]
- da Silva Duarte, J.L.; Solano, A.M.S.; Arguelho, M.L.P.M.; Tonholo, J.; Martínez-Huitle, C.A.; de Zanta, P.S.C.L. Evaluation of Treatment of Effluents Contaminated with Rifampicin by Fenton, Electrochemical and Associated Processes. J. Water Process Eng. 2018, 22, 250–257. [Google Scholar] [CrossRef]
- Du, J.; Zhao, H.; Chen, J. Simultaneous Determination of 23-Antibiotics in Mariculture Water Using Solid-Phase Extraction and High Performance Liquid Chromatography-Tandem Mass Spectrometry. Chin. J. Chromatogr. 2015, 33, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kais, H.; Mezenner, N.Y.; Trari, M.; Madjene, F. Photocatalytic Degradation of Rifampicin: Influencing Parameters and Mechanism. Russ. J. Phys. Chem. 2019, 93, 2834–2841. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent Advances in the Synthesis and Applications of Graphene-Polymer Nanocomposites. Polym. Chem. 2015, 6. [Google Scholar] [CrossRef]
- Sims, N.; Kasprzyk-Hordern, B. Future Perspectives of Wastewater-Based Epidemiology: Monitoring Infectious Disease Spread and Resistance to the Community Level. Environ. Int. 2020, 139, 105689. [Google Scholar] [CrossRef] [PubMed]
- Mtetwa, H.N.; Amoah, I.D.; Kumari, S.; Bux, F.; Reddy, P. Wastewater-Based Surveillance of Antibiotic Resistance Genes Associated with Tuberculosis Treatment Regimen in KwaZulu Natal, South Africa. Antibiotics 2021, 10, 1362. [Google Scholar] [CrossRef]
- Hao, K.; Suryoprabowo, S.; Song, S.; Kuang, H.; Liu, L. Rapid Detection of Rifampicin in Fish Using Immunochromatographic Strips. Food Agric. Immunol. 2020, 31, 700–710. [Google Scholar] [CrossRef]
- Cai, W.; Weng, X.; Chen, Z. Highly Efficient Removal of Antibiotic Rifampicin from Aqueous Solution Using Green Synthesis of Recyclable Nano-Fe3O4. Environ. Pollut. 2019, 247, 839–846. [Google Scholar] [CrossRef]
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2022, 4, e20. [Google Scholar] [CrossRef]
- Tahvildari, K.; Bigdeli, T. Treatment of Pharmaceutical Wastewater Containing Antibiotic with Oxidation Processes by Metallic Catalysts. Biointerface Res. Appl. Chem. 2019, 9, 3853–3859. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Jabbarvand Behrouz, S.; Vatanpour, V.; Haddadi Gharamaleki, S.; Orooji, Y.; Safarpour, M. Development of MoS2/O-MWCNTs/PES Blended Membrane for Efficient Removal of Dyes, Antibiotic, and Protein. Sep. Purif. Technol. 2022, 280, 119822. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of Antibiotics from Wastewaters by Membrane Technology: Limitations, Successes, and Future Improvements. Sci. Total Environ. 2022, 838, 156010. [Google Scholar] [CrossRef] [PubMed]
- Kais, H.; Yeddou Mezenner, N.; Trari, M. Biosorption of Rifampicin from Wastewater Using Cocoa Shells Product. Sep. Sci. Technol. 2020, 55, 1984–1993. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Q.; Owens, G.; Chen, Z. Fenton-Oxidation of Rifampicin via a Green Synthesized rGO@nFe/Pd Nanocomposite. J. Hazard. Mater. 2021, 402, 123544. [Google Scholar] [CrossRef]
- Yusuf, A.; Giwa, A.; Eniola, J.O.; Amusa, H.K.; Bilad, M.R. Recent Advances in Catalytic Sulfate Radical-Based Approach for Removal of Emerging Contaminants. J. Hazard. Mater. Adv. 2022, 7, 100108. [Google Scholar]
- Ihsanullah, I.; Sajid, M.; Khan, S.; Bilal, M. Aerogel-Based Adsorbents as Emerging Materials for the Removal of Heavy Metals from Water: Progress, Challenges, and Prospects. Sep. Purif. Technol. 2022, 291, 120923. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Eniola, J.O.; Sizirici, B.; Fseha, Y.; Shaheen, J.F.; Aboulella, A.M. Application of Conventional and Emerging Low-Cost Adsorbents as Sustainable Materials for Removal of Contaminants from Water. Env. Sci. Pollut. Res. 2023, 30, 88245–88271. [Google Scholar] [CrossRef]
- Barzegarzadeh, M.; Amini-Fazl, M.S.; Sohrabi, N. Efficient Ultrasound-Assisted Rifampicin Removal Using Cu(BDC)@Wool Biocomposite in Batch Adsorption Column and Fixed Bed. J Inorg. Organomet. Polym. 2024, 34, 207–220. [Google Scholar] [CrossRef]
- Shafaati, M.; Miralinaghi, M.; Shirazi, R.H.S.M.; Moniri, E. The Use of Chitosan/Fe3O4 Grafted Graphene Oxide for Effective Adsorption of Rifampicin from Water Samples. Res. Chem. Intermed. 2020, 46, 5231–5254. [Google Scholar] [CrossRef]
- Lin, Z.; Weng, X.; Owens, G.; Chen, Z. Simultaneous Removal of Pb(II) and Rifampicin from Wastewater by Iron Nanoparticles Synthesized by a Tea Extract. J. Clean. Prod. 2020, 242, 118476. [Google Scholar] [CrossRef]
- Negarestani, M.; Lashkari, A.; Khadir, A.; Mollahosseini, A. Removal of Rifampin by Luffa: A Pharmaceutical Potential in Producing Dye in Water. In Novel Materials for Dye-Containing Wastewater Treatment; Muthu, S.S., Khadir, A., Eds.; Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Springer: Singapore, 2021; pp. 209–229. ISBN 9789811628917. [Google Scholar]
- Adegoke, K.A.; Adesina, O.O.; Okon-Akan, O.A.; Adegoke, O.R.; Olabintan, A.B.; Ajala, O.A.; Olagoke, H.; Maxakato, N.W.; Bello, O.S. Sawdust-Biomass Based Materials for Sequestration of Organic and Inorganic Pollutants and Potential for Engineering Applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100274. [Google Scholar] [CrossRef]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; El Maguana, Y. Efficiency of Sawdust as Low-Cost Adsorbent for Dyes Removal. J. Chem. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Alidadi, H.; Dolatabadi, M.; Davoudi, M.; Barjasteh-Askari, F.; Jamali-Behnam, F.; Hosseinzadeh, A. Enhanced Removal of Tetracycline Using Modified Sawdust: Optimization, Isotherm, Kinetics, and Regeneration Studies. Process Saf. Environ. Prot. 2018, 117, 51–60. [Google Scholar] [CrossRef]
- Delmond, B.; Tretsiakova-McNally, S.; Solan, B.; McDermott, R.; Audoin, A. A Sustainable Method to Reduce Vancomycin Concentrations in Water Using Timber Waste. Water Air Soil Pollut 2023, 234, 81. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Liu, X.; Xu, X.; Duan, W.; Park, J.; Gao, L.; Lu, Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability 2022, 14, 15579. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A Review on Utilization of Wood Biomass as a Sustainable Precursor for Activated Carbon Production and Application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Mzinyane, N.N.; Mnqiwu, K.M.; Moukangoe, K.M. Adsorption of Hexavalent Chromium Ions Using Pine Sawdust Cellulose Fibres. Appl. Sci. 2023, 13, 9798. [Google Scholar] [CrossRef]
- Benyoucef, S.; Harrache, D.; Djaroud, S.; Sail, K.; Gallart-Mateu, D.; de la Guardia, M. Preparation and Characterization of Novel Microstructure Cellulosic Sawdust Material: Application as Potential Adsorbent for Wastewater Treatment. Cellulose 2020, 27, 8169–8180. [Google Scholar] [CrossRef]
- Segovia-Sandoval, S.J.; Ocampo-Pérez, R.; Berber-Mendoza, M.S.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Medellín-Castillo, N.A. Walnut Shell Treated with Citric Acid and Its Application as Biosorbent in the Removal of Zn (II). J. Water Process Eng. 2018, 25, 45–53. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Islam, M.T.; Hernandez, C.; Castro, E.; Katla, S.K.; Kim, H.; Lin, Y.; Curry, M.L.; Gardea-Torresdey, J.; Noveron, J.C. Biomass Conversion of Saw Dust to a Functionalized Carbonaceous Materials for the Removal of Tetracycline, Sulfamethoxazole and Bisphenol A from Water. J. Environ. Chem. Eng. 2018, 6, 4329–4338. [Google Scholar] [CrossRef]
- Negarestani, M.; Farimaniraad, H.; Mollahosseini, A.; Kheradmand, A.; Shayesteh, H. Facile Preparation of Sisal–Fe/Zn Layered Double Hydroxide Bio-Nanocomposites for the Efficient Removal of Rifampin from Aqueous Solution: Kinetic, Equilibrium, and Thermodynamic Studies. Int. J. Phytoremediat. 2023, 25, 586–597. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Yan, B.; Niu, C. Adsorption of Ciprofloxacin from Water by Pretreated Oat Hulls: Equilibrium, Kinetic, and Thermodynamic Studies. Ind. Crops Prod. 2019, 127, 237–250. [Google Scholar] [CrossRef]
- Hamad, M.T.M.H.; El-Sesy, M.E. Adsorptive Removal of Levofloxacin and Antibiotic Resistance Genes from Hospital Wastewater by Nano-Zero-Valent Iron and Nano-Copper Using Kinetic Studies and Response Surface Methodology. Bioresour. Bioprocess. 2023, 10, 1. [Google Scholar] [CrossRef]
- Blanco, S.P.D.M.; Scheufele, F.B.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Marin, P.; Kroumov, A.D.; Borba, C.E. Kinetic, Equilibrium and Thermodynamic Phenomenological Modeling of Reactive Dye Adsorption onto Polymeric Adsorbent. Chem. Eng. J. 2017, 307, 466–475. [Google Scholar]
- Romdhani, M.; Aloulou, W.; Aloulou, H.; Charcosset, C.; Mahouche-Chergui, S.; Carbonnier, B.; Ben Amar, R. Performance Studies of Indigo Dye Removal Using TiO2 Modified Clay and Zeolite Ultrafiltration Membrane Hybrid System. Desalination Water Treat. 2021, 243, 262–274. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of Antibiotic Ciprofloxacin on Carbon Nanotubes: pH Dependence and Thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Al-Musawi, T.J.; Kareem, S.L.; Zarrabi, M.; Al-Ma’abreh, A.M. Simultaneous Adsorption of Tetracycline, Amoxicillin, and Ciprofloxacin by Pistachio Shell Powder Coated with Zinc Oxide Nanoparticles. Arab. J. Chem. 2020, 13, 4629–4643. [Google Scholar] [CrossRef]
- Kwon, T.-N.; Jeon, C. Desorption and Regeneration Characteristics for Previously Adsorbed Indium Ions to Phosphorylated Sawdust. Environ. Eng. Res. 2012, 17, 65–67. [Google Scholar] [CrossRef]
- Tilinca, M.; Hancu, G.; Mircia, E.; Iriminescu, D.; Rusu, A.; Vlad, R.A.; Barabás, E. Simultaneous Determination of Isoniazid and Rifampicin by UV Spectrophotometry. Farmacia 2017, 65, 219–224. [Google Scholar]
- Sayen, S.; Ortenbach-López, M.; Guillon, E. Sorptive Removal of Enrofloxacin Antibiotic from Aqueous Solution Using a Ligno-Cellulosic Substrate from Wheat Bran. J. Environ. Chem. Eng. 2018, 6, 5820–5829. [Google Scholar] [CrossRef]
- Akinsanmi, O.; Coleman, H.; Tretsiakova-McNally, S.; Arnscheidt, J.; Burke, C. Sawdust: A Potential Bio-Adsorbent for Removal of Antibiotics in Contaminated Water. In Proceedings of the Irish Environmental Research Colloquium, Carlow, Ireland, 15–17 April 2019. [Google Scholar]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Mtunzi, F.M.; Adebowale, K.O. Clay-Carbonaceous Material Composites: Towards a New Class of Functional Adsorbents for Water Treatment. Surf. Interfaces 2020, 19, 100506. [Google Scholar] [CrossRef]
- Manfrin, J.; Gonçalves, A.C., Jr.; Schwantes, D.; Conradi, E., Jr.; Zimmermann, J.; Ziemer, G.L. Development of Biochar and Activated Carbon from Cigarettes Wastes and Their Applications in Pb2+ Adsorption. J. Environ. Chem. Eng. 2021, 9, 104980. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, L.; Yuan, G.; Wei, J. Low-Cost Field Production of Biochars and Their Properties. Environ. Geochem. Health 2020, 42, 1569–1578. [Google Scholar]
- Chakraborty, D.; Yurdusen, A.; Mouchaham, G.; Nouar, F.; Serre, C. Large-Scale Production of Metal–Organic Frameworks. Adv. Funct. Mater. 2024, 34, 2309089. [Google Scholar] [CrossRef]








| Concentration, mg/L | Mean, mg/L | Standard Deviation | Recovery | % RSD |
|---|---|---|---|---|
| 25.00 | 25.25 | 0.01 | 100.9–101.1 ± 0.05 | 0.05 |
| Volume-Weighted Mean D (4,3), µm | Surface-Weighted Mean D (3,2), µm | Specific Surface Area, m2/g | Dx10, µm | Dx50, µm | Dx90, µm | |
|---|---|---|---|---|---|---|
| Raw Sawdust | 560 | 403 | 0.0015 | 225 | 479 | 1010 |
| Treated Sawdust | 360 | 190 | 0.0031 | 114 | 318 | 704 |
| Sample | Carbon C % | Hydrogen H % | Nitrogen N % | Sulphur S % |
|---|---|---|---|---|
| Raw Sawdust | 46.70 | 6.36 | <0.3 | <0.3 |
| Treated Sawdust | 47.87 | 6.77 | <0.3 | <0.3 |
| T (K) | 1/T (K−1) | Kd = Qe/Ce | InKd | ΔH (KJ mol−1) | ΔS (J mol−1 K−1) | ΔG0= ΔH − ΔS |
|---|---|---|---|---|---|---|
| 293.15 | 3.41 × 10−03 | 2.98 × 10−02 | −3.51 | 14.53 | 18.95 | −5.54 |
| 298.15 | 3.35 × 10−03 | 2.36 × 10−02 | −3.75 | −5.64 | ||
| 308.15 | 3.25 × 10−03 | 3.00 × 10−02 | −3.51 | −5.83 | ||
| 318.15 | 3.14 × 10−03 | 4.46 × 10−02 | −3.11 | −6.01 |
| T (K) | 1/T (K−1) | Kd = Qe/Ce | InKd | ΔH (KJ mol−1) | ΔS (J mol−1 K−1) | ΔGo = ΔH − TΔS |
|---|---|---|---|---|---|---|
| 293.15 | 3.41 × 10−03 | 1.35 × 10−01 | −2.01 | 20.65 | 53.83 | −15.76 |
| 298.15 | 3.35 × 10−03 | 1.63 × 10−02 | −1.82 | −16.03 | ||
| 308.15 | 3.25 × 10−03 | 1.90 × 10−01 | −1.66 | −16.57 | ||
| 318.15 | 3.14 × 10−03 | 2.73 × 10−01 | −1.30 | −17.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudu, L.; Bhosale, R.C.; Arnscheidt, J.; Tretsiakova-McNally, S.; O’Hagan, B.; Adeyemi, D.K.; Oluseyi, T.; Adams, L.A.; Coleman, H.M. Tackling Antimicrobial Resistance: A Sustainable Method for the Removal of Antibiotics from Water. Antibiotics 2025, 14, 324. https://doi.org/10.3390/antibiotics14030324
Abudu L, Bhosale RC, Arnscheidt J, Tretsiakova-McNally S, O’Hagan B, Adeyemi DK, Oluseyi T, Adams LA, Coleman HM. Tackling Antimicrobial Resistance: A Sustainable Method for the Removal of Antibiotics from Water. Antibiotics. 2025; 14(3):324. https://doi.org/10.3390/antibiotics14030324
Chicago/Turabian StyleAbudu, Lekan, Rutuja C. Bhosale, Joerg Arnscheidt, Svetlana Tretsiakova-McNally, Barry O’Hagan, David K. Adeyemi, Temilola Oluseyi, Luqman A. Adams, and Heather M. Coleman. 2025. "Tackling Antimicrobial Resistance: A Sustainable Method for the Removal of Antibiotics from Water" Antibiotics 14, no. 3: 324. https://doi.org/10.3390/antibiotics14030324
APA StyleAbudu, L., Bhosale, R. C., Arnscheidt, J., Tretsiakova-McNally, S., O’Hagan, B., Adeyemi, D. K., Oluseyi, T., Adams, L. A., & Coleman, H. M. (2025). Tackling Antimicrobial Resistance: A Sustainable Method for the Removal of Antibiotics from Water. Antibiotics, 14(3), 324. https://doi.org/10.3390/antibiotics14030324





