Antimicrobial Activity of Honey and Propolis from Alba County, Romania
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Two-Way ANOVA Test
3.2. Pearson Correlation
4. Materials and Methods
- Analysis of Honey and Propolis
- Preparation of Aqueous Propolis Extracts
- Microorganism Cultures
- Determination of the Antimicrobial Properties of Honey and Propolis Extracts
- Minimum Inhibitory Concentrations (MICs) of Honey and Propolis Extracts
- Minimum Bactericidal/Fungicidal Concentrations (MBCs/MFCs) of Honey and Propolis Extracts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bee Culture Magazine, Honey Bee Immune System, Metabolic Pathway Discovered,—USDA Agricultural Research Service. 23 January 2024. Available online: https://www.beeculture.com/honey-bee-immune-system/ (accessed on 4 July 2024).
- Nader, R.A.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive Products as Antibacterial Agents: A Review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Vică, M.L.; Glevitzky, M.; Dumitrel, G.A.; Junie, L.M.; Popa, M. Antibacterial activity of different natural honeys from Transylvania, Romania. J. Environ. Sci. Health B 2014, 49, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Vică, M.L.; Glevitzky, M.; Dumitrel, G.-A.; Bostan, R.; Matei, H.V.; Kartalska, Y.; Popa, M. Qualitative Characterization and Antifungal Activity of Romanian Honey and Propolis. Antibiotics 2022, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.; Roberts, A.; Cooper, R.; Jenkins, R. A review of selected bee products as potential anti-bacterial, anti-fungal, and anti-viral agents. Med. Res. Arch. 2016, 4, 1–20. [Google Scholar]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Premratanachai, P.; Chanchao, C. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef]
- Delaplane, K.S. Bee Products: Properties, Applications, and Apitherapy. Am. Entomol. 1999, 45, 125–126. [Google Scholar] [CrossRef][Green Version]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering. Pharmaceutics 2022, 14, 750. [Google Scholar] [CrossRef]
- Weis, W.A.; Ripari, N.; Conte, F.L.; da Silva Honorio, M.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An Overview about Apitherapy and its Clinical Applications. Phytomed. Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Pavlova, T.; Stamatovska, V.; Kalevska, T.; Dimov, I.; Nakov, G. Quality Characteristics of Honey: A Review. Proceed. Univ. Ruse 2019, 57, 31–37. [Google Scholar]
- Ball, D.W. The Chemical Composition of Honey. J. Chem. Educ. 2007, 84, 10. [Google Scholar] [CrossRef]
- Cara, M.C.; Dumitrel, G.A.; Glevitzky, M.; Mischie, C.; Silaghi-Perju, D. Thermal Degradation of Streptomycin Residues in Honey during Storage. Food Technol. Biotechnol. 2013, 51, 429–433. [Google Scholar]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Brudzynski, K. Honey as an Ecological Reservoir of Antibacterial Compounds Produced by Antagonistic Microbial Interactions in Plant Nectars, Honey and Honey Bee. Antibiotics 2021, 10, 551. [Google Scholar] [CrossRef]
- Nayik, G.; Shah, T.; Muzaffar, K.; Wani, S.; Gull, A.; Majid, I.; Bhat, F. Honey: Its history and religious significance: A review. Univers. J. Pharm. 2014, 3, 5–8. [Google Scholar]
- Al-Jabri, A.A. Honey, milk and antibiotics. Afr. J. Biotechnol. 2005, 4, 1580–1587. [Google Scholar]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Zumla, A.; Lulat, A. Honey—A remedy rediscovered. J. R. Soc. Med. 1989, 82, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [PubMed]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid. Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed]
- Kartal, M.; Yıldız, S.; Kaya, S. Antimicrobial activity of propolis samples from two different regions of Anatolia. J. Ethnopharmacol. 2003, 86, 69–73. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Polysaccharides/propolis composite as promising materials with biomedical and packaging applications: A review. Biomass Convers. Biorefin. 2024, 14, 4555–4565. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Altabbal, S.; Athamnah, K.; Rahma, A.; Wali, A.F.; Eid, A.H.; Iratni, R.; Al Dhaheri, Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 2023, 16, 450. [Google Scholar] [CrossRef]
- Albu, A.; Radu-Rusu, C.-G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality Assessment of Raw Honey Issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- European Commission—Honey. Detailed Information on Honey Production in the European Union. Available online: https://agriculture.ec.europa.eu/farming/animal-products/honey_en (accessed on 15 July 2024).
- Nichițean, A.L.; Constantinescu-Aruxandei, D.; Oancea, F. Health promoting quality of the Romanian honey. Sci. Bull. Ser. F Biotechnol. 2021, XXV, 95–103. [Google Scholar]
- Bora, F.D.; Andrecan, A.F.; Călugăr, A.; Bunea, C.I.; Popescu, M.; Petrescu-Mag, I.V.; Bunea, A. Comprehensive Elemental Profiling of Romanian Honey: Exploring Regional Variance, Honey Types, and Analyzed Metals for Sustainable Apicultural and Environmental Practices. Foods 2024, 13, 1253. [Google Scholar] [CrossRef]
- Zugravu, A. Guide to Apicultural Potential, Climate Conditions, Air and Soil Quality in the Black Sea Basin. 2020. Available online: https://blacksea-cbc.net/wp-content/uploads/2020/12/BSB136_ITM-BEE-BSB_Guide-to-apicultural-potential-climate-conditions-air-and-soil-quality-in-the-Black-Sea-Basin_EN.pdf (accessed on 28 September 2024).
- Isopescu, R.D.; Josceanu, A.M.; Colta, T.; Spulber, R. Romanian Honey: Characterization and Classification. In Honey Analysis; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Nicolson, S. Bee food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- European Parliament. EU Honey Market in Figures (Infographic). Available online: https://www.europarl.europa.eu/topics/ro/article/20180222STO98435/piata-mierii-din-ue-in-cifre-infografic (accessed on 7 July 2024).
- National Institute of Statistics, INSE. Available online: https://insse.ro (accessed on 14 July 2024).
- Alba County Regional Directorate of Statistics. Statistical Yearbook of Alba County, 2023 Edition, No. 939/22.03.2023. Available online: https://alba.insse.ro/wp-content/uploads/2023/09/Anuar-Judetul-Alba-2023-1.pdf (accessed on 14 July 2024).
- Cioba, L.G. Significant Decrease in Honey Production; Forbes Romania: Bucarest, Romania, 2024; Available online: https://www.forbes.ro/scadere-semnificativa-a-productiei-de-miere-406203 (accessed on 16 July 2024).
- Popescu, N.; Meica, S. Bee Products and Their Chemical Analysis; Diacon CORESI Press: Bucharest, Romanian, 1997; p. 125. (In Romanian) [Google Scholar]
- Codex Stan 12-1981; Codex Standards for Honey. Rev. 1 (1987), Rev. 2 (2001). Codex Alimentarius Commission: Rome, Italy, 2001.
- EU Council. Council Directive 2001/110/CE Concerning Honey. Off. J. Eur. Communities 2002, L10, 47–52. [Google Scholar]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863. [Google Scholar] [CrossRef]
- Israili, Z.H. Antimicrobial Properties of Honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Veiga, R.S.; De Mendonça, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Lagareiro Netto, A.A.; Marcucci, M.C. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Heghedűş-Mîndru, R.C.; Glevitzky, I.; Matei, H.V.; Balici, S.; Popa, M.; Teodoru, C.A. Potential Effects of Romanian Propolis Extracts against Pathogen Strains. Int. J. Environ. Res. Public Health 2022, 19, 2640. [Google Scholar] [CrossRef]
- Romanian Standard SR 784-3; Honey. Part 3: Analysis Methods. Romanian Standards Association, ASRO: Bucharest, Romania, 2009.
- Romanian Standard SR 784-1; Honey. Part 1: Quality Requirements at Delivery by Producers. Romanian Standards Association, ASRO: Bucharest, Romania, 2009.
- Ebrahimi, Y.; Ramírez-Coronel, A.A.; Al-Dhalimy, A.M.B.; Alfilm, R.H.C.; Al-Hassan, M.; Obaid, R.F.; Alameri, A.A.; Rastiani, F.; Yousef, K.; Shokri, S. Contamination of honey products by Clostridium botulinum spores and fungi along with their effects on human health. Casp. J. Environ. Sci. 2023, 20, 1143–1148. [Google Scholar]
- Romanian Standard SR 784-2; Honey. Part 2: Quality Requirements at Sale. Romanian Standards Association, ASRO: Bucharest, Romania, 2009.
- Iancu, R.M.; Oprean, L.; Tița, M.A.; Lengyel, E.; Codoi, V.; Boicean, A.; Schneider, A.O. Physical-chemical analysis and antibiotic content of polyflora honey in Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca-Anim. Sci. Biotechnol. 2012, 69, 255–260. [Google Scholar]
- Popa, M.; Vică, M.; Axinte, R.; Glevitzky, M.; Varvara, S. Correlations on the microbiological and physical-chemical characteristics of different types of honey. J. Environ. Prot. Ecol. 2009, 10, 1113–1121. [Google Scholar]
- Marchini, L.C.; Moretti, A.C.; Otsuki, I.P.; Sodré, G. Physicochemical Composition of Apis Mellifera Honey Samples from São Paulo State, Brazil. Quim. Nova 2007, 30, 1653–1657. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, K.; Oddo, L.P. Physico-chemical methods for the characterization of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piazza, M.G.; Sabatini, A.G.; Accorti, M. Characterization of unifloral honeys. Apidologie 1995, 26, 453–465. [Google Scholar] [CrossRef]
- Fallico, B.; Zappala, M.; Arena, E.; Verzera, A. Effects of heating on honey HMF content during storage. Food Chem. 2004, 85, 553–558. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Med. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Tomás-Barberán, F.A. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J. Agric. Food Chem. 2000, 48, 1498–1502. [Google Scholar] [CrossRef]
- Kaskonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographical origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants: Structures, Bioavailability, and Evidence of Protective Effects against Human Chronic Diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Todorova, M.; Topuzova, M.; Gineva, G.; Yanakieva, V.; Ivanov, I.; Petkova, N. Comparative Study on Physicochemical, Antioxidant and Antimicrobial Properties of Propolis Collected from Different Regions of Bulgaria. J. Apic. Sci. 2023, 67, 37–56. [Google Scholar] [CrossRef]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.A.; Rebouças Silva, J.; da Silva, D.F.; da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- El Menyiy, N.; Bakour, M.; El Ghouizi, A.; El Guendouz, S.; Lyoussi, B. Influence of Geographic Origin and Plant Source on Physicochemical Properties, Mineral Content, and Antioxidant and Antibacterial Activities of Moroccan Propolis. Int. J. Food Sci. 2021, 2, 12. [Google Scholar] [CrossRef]
- Cibanal, I.; Fernández, L.A.; Krepper, G.; Pellegrini, C.; Gallez, L. Advances in the Development of a Biofungicide: Physical-Chemical Characterization and Antifungal Activity of Propolis. Agrocienc. Urug. 2019, 23, 99–108. [Google Scholar]
- Bankova, V.; Christov, R.; Stoev, G.; Popov, S. Determination of phenolics from propolis by capillary gas chromatography. J. Chromatogr. A 1992, 607, 150–153. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of bioactive phenolic compounds in propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical origin and chemical composition of Brazilian propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef]
- Salatino, A.; Teixeira, É.W.; Negri, G.; Message, D. Origin and chemical variation of Brazilian propolis. Evid. Based Complement. Alternat. Med. 2005, 2, 33–38. [Google Scholar] [CrossRef]
- Bankova, V.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Coll, F.V. Phenolic composition of propolis from China and from South America. Z. Leb.-Forsch. A 1994, 199, 91–93. [Google Scholar] [CrossRef]
- Marcucci, M.C. Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Park, Y.K.; Ikegaki, M.; Alencar, S.M.; Aguiar, C.L. Classification of Brazilian propolis by physicochemical methods and biological activity. Arzneimittel-Forschung 2000, 50, 785–790. [Google Scholar]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity. Chem. Cent. J. 2021, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- AOAC. 978.18 Water Activity. In Official Methods of Analysis of the Association of Official Analytical Chemist, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Mărghitaş, L.A. Bees and Their Products; Ceres Publishing: Bucharest, Romania, 2008; pp. 280–282. (In Romanian) [Google Scholar]
- Beykaya, M. Determination of physicochemical properties of raw honey samples. Prog. Nutr. 2021, 23, e2021020. [Google Scholar]
- Bogdanov, S. Harmonized Methods of the European Honey Commission. International Honey Commission. 2009. Available online: www.bee-hexagon.net (accessed on 12 May 2024).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 29, 10–18. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.K. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Vica, M.L.; Glevitzky, I.; Glevitzky, M.; Siserman, C.V.; Matei, H.V.; Teodoru, C.A. Antibacterial Activity of Propolis Extracts from the Central Region of Romania against Neisseria gonorrhoeae. Antibiotics 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing. 2020. Available online: https://clsi.org/media/3481/m100ed30_sample.pdf (accessed on 27 February 2024).
- dos Santos, L.; Hochheim, S.; Boeder, A.M.; Kroger, A.; Tomazzoli, M.M.; Dal Pai Neto, R.; Maraschin, M.; Guedes, A.; de Cordova, C.M. Chemical characterization, antioxidant, cytotoxic and antibacterial activity of propolis extracts and isolated compounds from the Brazilian stingless bees Melipona quadrifasciata and Tetragonisca angustula. J. Apic. Res. 2017, 56, 543–558. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. EUCAST Guidance Documents. Available online: https://www.eucast.org/eucastguidancedocuments (accessed on 7 March 2024).
| Year | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|---|---|
| Beehives in Romania, pcs | 1354 | 1350 | 1392 | 1437 | 1602 | 1689 | 1843 | 1879 | 1903 |
| Production in Romania, t | 26,678 | 18,040 | 27,893 | 21,202 | 30,177 | 29,162 | 25,269 | 30,724 | 30,831 |
| Production in Alba County, t | 679 | 546 | 997 | 757 | 822 | 936 | 1105 | 1076 | 1090 |
| Sample | Moisture Content (%) | Water Activity, aw | Ash Content (%) | pH | Acidity Meq/kg | HMF mg/100 g | Phenols (mg GAE/100 g) | Flavonoids (mg QE/100 g) |
|---|---|---|---|---|---|---|---|---|
| I H | 15.23 ± 0.18 | 0.578 ± 0.013 | 0.20 ± 0.03 | 3.87 ± 0.34 | 20.8 ± 0.6 | 1.39 ± 0.06 | 82.14 ± 0.26 | 4.26 ± 0.18 |
| II H | 18.06 ± 0.35 | 0.582 ± 0.024 | 0.41 ± 0.06 | 4.02 ± 0.16 | 24.6 ± 0.9 | 1.13 ± 0.12 | 70.77 ± 0. 52 | 2.57 ± 0.05 |
| III H | 17.44 ± 0.42 | 0.577 ± 0.030 | 0.29 ± 0.02 | 4.14 ± 0.19 | 16.5 ± 0.7 | 0.47 ± 0.10 | 98.49 ± 1.03 | 5.35 ± 0.12 |
| IV H | 16.67 ± 0.20 | 0.591 ± 0.019 | 0.36 ± 0.07 | 3.98 ± 0.50 | 18.0 ± 0.2 | 1.21 ± 0.07 | 63.51 ± 0.28 | 3.29 ± 0.16 |
| V H | 14.51 ± 0.11 | 0.569 ± 0.027 | 0.45 ± 0.04 | 3.85 ± 0.22 | 14.3 ± 0.5 | 1.08 ± 0.09 | 64.20 ± 0.47 | 3.40 ± 0.31 |
| Sample | Moisture Content (%) | Water Activity, aw | Ash Content (%) | Phenols (mg GAE/g) | Flavonoids (mg QE/g) |
|---|---|---|---|---|---|
| I P | 4.38 ± 0.1 | 0.62 ± 0.20 | 3.21 ± 0.06 | 180.4 ± 4.28 | 76.22 ± 0.17 |
| II P | 5.07 ± 0.2 | 0.65 ± 0.14 | 2.48 ± 0.04 | 152.9 ± 3.71 | 71.53 ± 0.16 |
| III P | 6.31 ± 0.1 | 0.71 ± 0.12 | 3.02 ± 0.07 | 174.3 ± 5.02 | 80.72 ± 0.25 |
| IV P | 4.95 ± 0.1 | 0.66 ± 0.08 | 2.62 ± 0.08 | 149.2 ± 2.50 | 68.33 ± 0.14 |
| V P | 5.26 ± 0.1 | 0.70 ± 0.11 | 2.79 ± 0.05 | 165.6 ± 2.13 | 70.48 ± 0.30 |
| Microbial Strain | Sample | Ciprofloxacin 5 µg | ||||
|---|---|---|---|---|---|---|
| I H | II H | III H | IV H | V H | ||
| E. coli | 0 | 11 | 14 | 8 | 0 | 29 |
| S. typhimurium | 0 | 12 | 15 | 9 | 7 | 29 |
| S. enteritidis | 0 | 7 | 8 | 7 | 0 | 27 |
| S. anatum | 0 | 8 | 7 | 0 | 0 | 28 |
| S. choleraesuis | 0 | 10 | 10 | 0 | 7 | 28 |
| P. aeruginosa | 13 | 18 | 17 | 14 | 13 | 25 |
| P. fluorescens | 14 | 16 | 18 | 16 | 16 | 24 |
| S. aureus | 19 | 19 | 20 | 21 | 21 | 30 |
| S. epidermidis | 17 | 17 | 17 | 19 | 18 | 29 |
| B. cereus | 0 | 8 | 9 | 0 | 10 | 30 |
| B. subtilis | 17 | 18 | 19 | 11 | 12 | 30 |
| L. monocytogenes | 10 | 8 | 10 | 8 | 8 | 24 |
| Microbial Strain | Sample | Ciprofloxacin 5 µg | ||||
|---|---|---|---|---|---|---|
| I P | II P | III P | IV P | V P | ||
| E. coli | 18 | 27 | 32 | 22 | 18 | 29 |
| S. typhimurium | 18 | 26 | 30 | 25 | 24 | 29 |
| S. enteritidis | 15 | 19 | 25 | 17 | 15 | 27 |
| S. anatum | 17 | 26 | 22 | 16 | 19 | 28 |
| S. choleraesuis | 15 | 24 | 28 | 17 | 21 | 28 |
| P. aeruginosa | 28 | 32 | 29 | 27 | 27 | 25 |
| P. fluorescens | 27 | 33 | 33 | 28 | 29 | 24 |
| S. aureus | 22 | 27 | 26 | 30 | 27 | 30 |
| S. epidermidis | 26 | 24 | 20 | 31 | 29 | 29 |
| B. cereus | 24 | 26 | 27 | 24 | 29 | 30 |
| B. subtilis | 25 | 29 | 29 | 23 | 23 | 30 |
| L. monocytogenes | 31 | 29 | 30 | 28 | 28 | 24 |
| Fungal Strain | Sample | Voriconazole 1 µg | ||||
|---|---|---|---|---|---|---|
| I H | II H | III H | IV H | V H | ||
| C. albicans | 0 | 10 | 10 | 8 | 8 | 37 |
| A. niger | 8 | 9 | 10 | 10 | 9 | 45 |
| A. flavus | 9 | 9 | 10 | 10 | 10 | 43 |
| P. chrysogenum | 10 | 8 | 11 | 8 | 12 | 18 |
| R. stolonifer | 12 | 9 | 9 | 8 | 8 | 16 |
| F. oxysporum | 9 | 10 | 11 | 11 | 11 | 29 |
| A. alternata | 7 | 11 | 7 | 8 | 0 | 16 |
| Fungal Strain | Sample | Voriconazole 1 µg | ||||
|---|---|---|---|---|---|---|
| I P | II P | III P | IV P | V P | ||
| C. albicans | 17 | 21 | 22 | 18 | 19 | 37 |
| A. niger | 15 | 23 | 26 | 25 | 21 | 45 |
| A. flavus | 17 | 25 | 27 | 24 | 23 | 43 |
| P. chrysogenum | 23 | 16 | 25 | 18 | 27 | 18 |
| R. stolonifer | 25 | 21 | 19 | 19 | 20 | 16 |
| F. oxysporum | 21 | 22 | 28 | 27 | 25 | 29 |
| A. alternata | 20 | 26 | 25 | 16 | 17 | 16 |
| Parameter | Value | UM | Limit | ||
|---|---|---|---|---|---|
| RO | EU | Codex | |||
| Water | 20 | 20 | 21 | % | Maximum |
| Ash | 0.5 | 0.5 | 0.6 | % | Maximum |
| Sucrose | 5 | 5 | 5 | g/100 g | Maximum |
| Fructose and glucose content (sum of both) | 70 | 60 | 65 | g/100 g | Minimum |
| Free acid, ml NaOH sol 1N % | 40 | 50 | 40 | Meq/kg | Maximum |
| Water-insoluble content | 0.1 | 0.1 | 0.1 | g/100 g | Maximum |
| Electrical conductivity | - | 0.8 | - | mS/cm | Maximum |
| Diastase activity | 10.9 | 8 | 8 | Schade scale | Minimum |
| HMF | 1.5 * | 40 | 40 | mg/100 g honey | Maximum |
| Strains | Source of Variance | df | SS | MS | F-Ratio calc. | F0.05 prob. | Remark (prob > F) |
|---|---|---|---|---|---|---|---|
| Bacterial | Between samples, H | 4 | 315.07 | 28.64 | 3.09 | 2.01 | Significant |
| Between samples, P | 238.57 | 21.69 | 2.14 | 2.01 | Significant | ||
| Between strains, H | 11 | 1911.78 | 477.95 | 51.53 | 2.58 | Significant | |
| Between strains, H | 750.53 | 187.63 | 18.48 | 2.58 | Significant | ||
| Errors, H | - | 408.13 | - | - | - | - | |
| Errors, P | - | 446.63 | - | - | - | - | |
| Total, H | 44 | 9.28 | - | - | - | - | |
| Total, P | 10.15 | - | - | - | - | ||
| Fungal | Between samples, H | 4 | 16.86 | 2.81 | 0.46 | 2.51 | Not significant |
| Between samples, P | 89.03 | 14.84 | 1.22 | 2.51 | Not significant | ||
| Between strains, H | 6 | 59.49 | 14.87 | 2.41 | 2.78 | Not significant | |
| Between strains, P | 88.00 | 22.00 | 1.8 | 2.78 | Not significant | ||
| Errors, H | - | 147.94 | - | - | - | - | |
| Errors, P | - | 292.57 | - | - | - | - | |
| Total, H | 24 | 6.16 | - | - | - | - | |
| Total, P | 12.19 | - | - | - | - |
| Bacterial Strains | Honey | Propolis | ||
|---|---|---|---|---|
| Flavonoids | Phenols | Flavonoids | Phenols | |
| E. coli | 0.637 | 0.966 | 0.460 | 0.957 |
| S. typhimurium | 0.695 | 0.775 | 0.713 | 0.728 |
| S. enteritidis | 0.613 | 0.911 | 0.184 | 0.550 |
| S. anatum | 0.055 | 0.817 | 0.250 | −0.274 |
| S. choleraesuis | 0.136 | 0.464 | 0.051 | 0.362 |
| P. aeruginosa | 0.175 | 0.855 | 0.411 | −0.477 |
| P. fluorescens | 0.667 | 0.672 | 0.277 | 0.133 |
| S. aureus | 0.776 | −0.287 | 0.682 | 0.479 |
| S. epidermis | 0.721 | −0.315 | 0.157 | −0.068 |
| B. cereus | 0.167 | 0.158 | −0.461 | 0.199 |
| B. subtilis | −0.519 | 0.621 | 0.191 | −0.095 |
| L. monocytogenes | −0.539 | 0.287 | −0.406 | −0.267 |
| Fungal Strains | Honey | Propolis | ||
|---|---|---|---|---|
| Flavonoids | Phenols | Flavonoids | Phenols | |
| C. albicans | 0.721 | 0.528 | 0.182 | 0.299 |
| A. niger | 0.950 | 0.591 | 0.568 | 0.699 |
| A. flavus | 0.789 | 0.026 | 0.449 | 0.582 |
| P. chrysogenum | −0.185 | −0.365 | −0.917 | 0.353 |
| R. stolonifer | −0.909 | −0.106 | −0.399 | −0.757 |
| F. oxysporum | 0.912 | 0.194 | 0.142 | 0.981 |
| A. alternata | −0.047 | 0.705 | 0.144 | −0.201 |
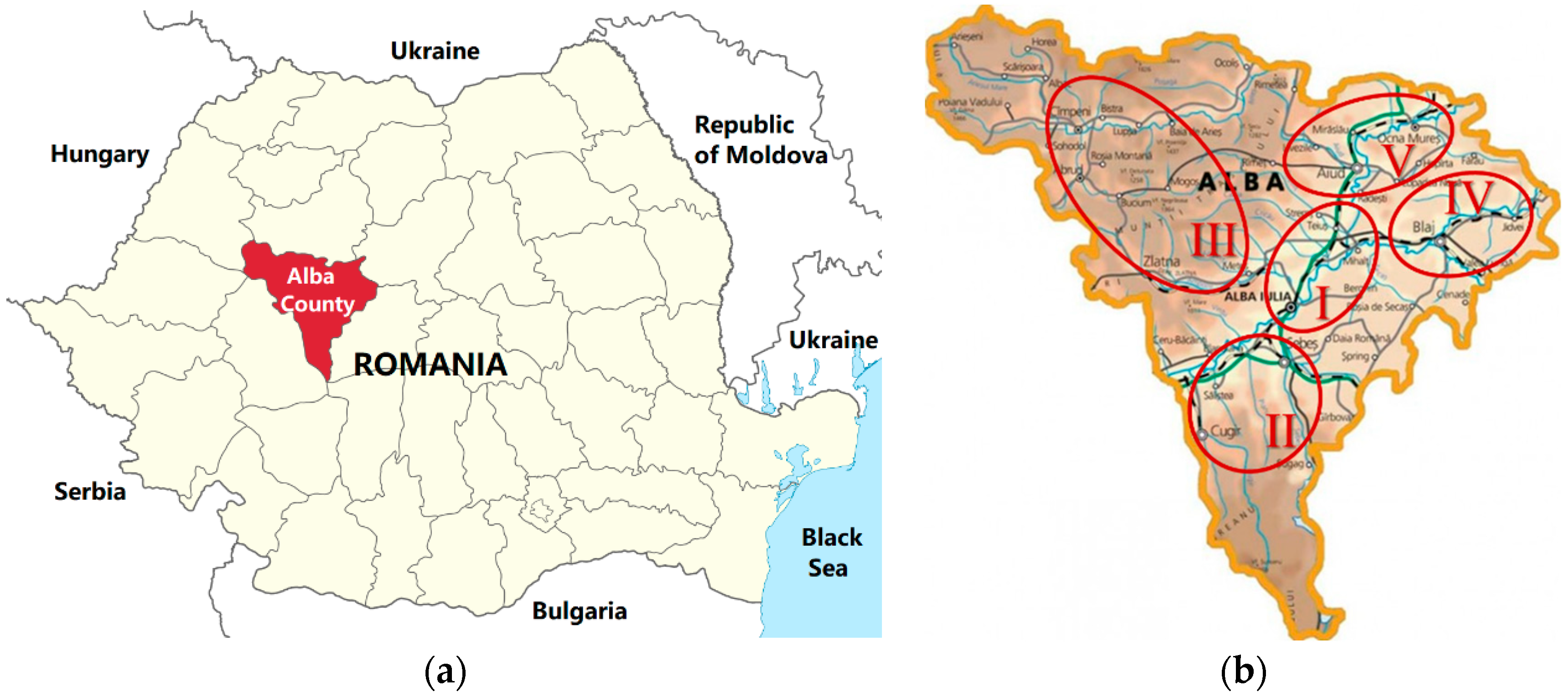
| Sample | Apiary Location Area | Geographical Origin | Latitude | Longitude | Landforms |
|---|---|---|---|---|---|
| I | Alba Iulia—Teiuș | Sântimbru | 46°07′36″ N | 23°37′38″ E | Plain |
| II | Sebeș—Cugir | Șibot | 45°56′27″ N | 23°20′10″ E | Sub-mountainous |
| III | Cîmpeni—Zlatna | Abrud | 46°16′53″ N | 23°03′39″ E | Mountainous |
| IV | Blaj | Valea Lungă | 46°07′52″ N | 24°04′48″ E | Hilly |
| V | Aiud—Ocna Mureș | Ciumbrud | 46°18′29″ N | 23°45′44″ E | Hilly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vică, M.L.; Glevitzky, M.; Dumitrel, G.-A.; Popa, M.; Glevitzky, I.; Teodoru, C.A. Antimicrobial Activity of Honey and Propolis from Alba County, Romania. Antibiotics 2024, 13, 952. https://doi.org/10.3390/antibiotics13100952
Vică ML, Glevitzky M, Dumitrel G-A, Popa M, Glevitzky I, Teodoru CA. Antimicrobial Activity of Honey and Propolis from Alba County, Romania. Antibiotics. 2024; 13(10):952. https://doi.org/10.3390/antibiotics13100952
Chicago/Turabian StyleVică, Mihaela Laura, Mirel Glevitzky, Gabriela-Alina Dumitrel, Maria Popa, Ioana Glevitzky, and Cosmin Adrian Teodoru. 2024. "Antimicrobial Activity of Honey and Propolis from Alba County, Romania" Antibiotics 13, no. 10: 952. https://doi.org/10.3390/antibiotics13100952
APA StyleVică, M. L., Glevitzky, M., Dumitrel, G.-A., Popa, M., Glevitzky, I., & Teodoru, C. A. (2024). Antimicrobial Activity of Honey and Propolis from Alba County, Romania. Antibiotics, 13(10), 952. https://doi.org/10.3390/antibiotics13100952







