Plasma and Muscle Pharmacokinetics of Ceftriaxone in Nile Tilapia (Oreochromis niloticus) After Different Administration Routes
Abstract
1. Introduction
2. Results
2.1. HPLC Method
2.2. Animals
2.3. Plasma Pharmacokinetic Parameters
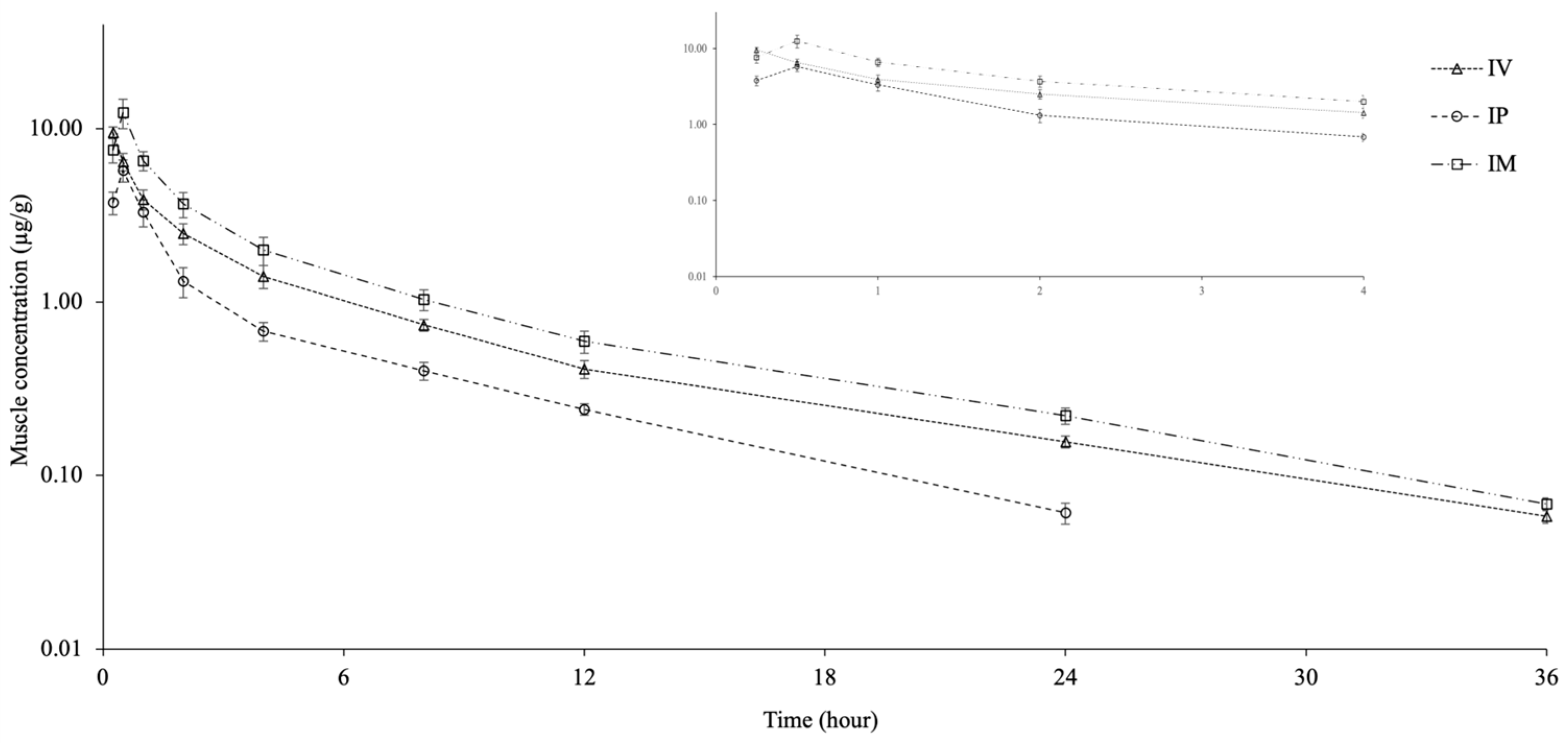
2.4. Muscle Pharmacokinetic Parameters
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Experimental Design
4.4. Ceftriaxone Analysis
4.5. Pharmacokinetic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Global Aquaculture Production 1950–2023. 2025. Available online: https://www.fao.org/statistics/events/events-detail/global-production.-march-2025-update/en (accessed on 15 November 2025).
- Department of Agriculture—Bureau of Fisheries and Aquatic Resources. The Philippine Tilapia Industry Roadmap (2022–2025). 2022. Available online: http://www.pcaf.da.gov.ph (accessed on 15 November 2025).
- Bardhan, A.; Abraham, T.J.; Sar, T.K.; Rajisha, R.; Panda, S.K.; Patil, P.K. Pharmacokinetics and residues of florfenicol in Nile tilapia (Oreochromis niloticus) post-oral gavage. Environ. Toxicol. Pharmacol. 2024, 108, 104471. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Groumellec, M.L.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Corum, O.; Er, A.; Corum, D.D.; Atik, O.; Uney, K. Pharmacokinetics and bioavailability of ceftriaxone in brown trout (Salmo trutta fario) after intravenous and intramuscular administration. Aquaculture 2019, 500, 272–277. [Google Scholar] [CrossRef]
- Lamb, H.M.; Ormrod, D.; Scott, L.J.; Figgitt, D.P. Ceftriaxone: An update of its use in the management of community-acquired and nosocomial infections. Drugs 2002, 62, 1041–1089. [Google Scholar] [CrossRef] [PubMed]
- Rawls, S.M.; Daroff, R.B.; Aminoff, M.J. Encyclopedia of the Neurological Sciences; Antibiotics, β-Lactam; Academic Press: Cambridge, MA, USA, 2014; pp. 207–209. [Google Scholar]
- Sharma, B.; Chalikwar, R.; Bhalerao, S.; Gondane, A.A.; Pawar, D.; Sharma, A. Cefotaxime versus ceftriaxone: A comprehensive comparative review. Cureus 2024, 16, e69146. [Google Scholar] [CrossRef] [PubMed]
- Karungamye, P.; Rugaika, A.; Mtei, K.; Machunda, R. A review of methods for removal of ceftriaxone from wastewater. J. Xenobiot. 2022, 12, 223–235. [Google Scholar] [CrossRef]
- EMA. 2019. Available online: https://www.ema.europa.eu/en/documents/other/answer-request-european-commission-updating-scientific-advice-impact-public-health-and-animal-health-use-antibiotics-animals-categorisation-antimicrobials_en.pdf (accessed on 30 November 2025).
- Ceftriaxone Sodium for Injection. 2025. Available online: https://www.advacarepharma.com/en/veterinary/ceftriaxone-sodium-for-injection (accessed on 17 November 2025).
- Ceftron-Vet® Injection. 2025. Available online: https://www.squarepharma.com.bd/product-details.php?pid=480 (accessed on 17 November 2025).
- Mohammed, E.A.H.; Kovács, B.; Kuunya, R.; Mustafa, E.O.A.; Abbo, A.S.H.; Pál, K. Antibiotic resistance in aquaculture: Challenges, trends analysis, and alternative approaches. Antibiotics 2025, 14, 598. [Google Scholar] [CrossRef]
- Soo Seo, J.; Kwon, M.G.; Youn Hwang, J.; Don Hwang, S.; Kim, D.H.; Bae, J.S.; Park, K.H.; Lee, J.H. Estimation of pharmacological properties of ceftiofur, an injectable cephalosporin antibiotic, for treatment of streptococcosis in cultured olive flounder Paralichthys olivaceus. Aquac. Res. 2021, 52, 831–841. [Google Scholar] [CrossRef]
- Terzi, E.; Corum, O.; Bilen, S.; Kenanoglu, O.N.; Atik, O.; Uney, K. Pharmacokinetics of danofloxacin in rainbow trout after different routes of administration. Aquaculture 2020, 520, 734984. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.C. Amoxicillin-florfenicol combination reduces mortality in olive flounder (Paralichthys olivaceus) experimentally infected by Streptococcus iniae. Aquac. Res. 2015, 46, 2300–2304. [Google Scholar] [CrossRef]
- Lim, J.W.; Jung, M.H.; Jung, S.J.; Kim, D.H.; Park, K.H.; Kang, S.Y. The efficacy of amoxicillin sodium against streptococcosis in cultured olive flounder Paralichthys olivaceus and its pharmacokinetics. J. Vet. Pharmacol. Ther. 2017, 40, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, E.; Alexander, M.E.; Snellgrove, D.; Henriquez, F.L.; Sloman, K.A. Current disease treatments for the ornamental pet fish trade and their associated problems. Rev. Aquac. 2025, 17, e12948. [Google Scholar] [CrossRef]
- Yucel, N.; Aslim, B.; Beyatli, Y. Prevalence and resistance to antibiotics for Aeromonas species isolated from retail fish in Turkey. J. Food Qual. 2005, 28, 313–324. [Google Scholar] [CrossRef]
- Plumb, J.A.; Hanson, L.A. Tilapia bacterial diseases. In Health Maintenance and Principal Microbial Diseases of Cultured Fishes, 3rd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 445–463. [Google Scholar]
- Corum, O.; Uney, K.; Terzi, E.; Durna Corum, D.; Coskun, D.; Altan, F.; Elmas, M. Effects of temperature on the pharmacokinetics, tissue residues, and withdrawal times of doxycycline in rainbow trout (Oncorhynchus mykiss) following oral administration. Vet. Sci. 2023, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Uney, K.; Corum, O.; Durna Corum, D.; Coskun, D.; Sakin, F.; Elmas, M. Pharmacokinetics and plasma protein binding of flunixin in rainbow trout (Oncorhynchus mykiss). J. Vet. Pharmacol. Ther. 2025, 48, 103–109. [Google Scholar] [CrossRef]
- Corum, O.; Durna Corum, D.; Marin, P.; Yildirim, O.; Terzi, E.; Gonzales, R.C.; Arriesgado, D.M.; Navarro, N.V.; Bilen, S.; Sonmez, A.Y.; et al. Pharmacokinetics and bioavailability of cefuroxime in Nile tilapia (Oreochromis niloticus). Vet. Res. Commun. 2026, 50, 18. [Google Scholar] [CrossRef]
- Durna Corum, D.; Corum, O.; Terzi, E.; Coskun, D.; Bilen, S.; Cetin, G.; Uney, K. Pharmacokinetics of cefquinome in rainbow trout (Oncorhynchus mykiss) after intravascular, intraperitoneal, and oral administrations. J. Vet. Pharmacol. Ther. 2022, 45, 578–583. [Google Scholar] [CrossRef]
- Khalil, W.F.; Shaheen, H.M.; Abdou, R.H. Ceftiofur pharmacokinetics in Nile tilapia Oreochromis niloticus after intracardiac and intramuscular administrations. Dis. Aquat. Organ. 2016, 121, 29–35. [Google Scholar] [CrossRef]
- Shan, Q.; Zhu, X.; Liu, S.; Bai, Y.; Ma, L.; Yin, Y.; Zheng, G. Pharmacokinetics of cefquinome in tilapia (Oreochromis niloticus) after a single intramuscular or intraperitoneal administration. J. Vet. Pharmacol. Ther. 2015, 38, 601–605. [Google Scholar] [CrossRef]
- Poapolathep, S.; Giorgi, M.; Chaiyabutr, N.; Klangkaew, N.; Phaochoosak, N.; Wongwaipairote, T.; Poapolathep, A. Pharmacokinetics of ceftriaxone in freshwater crocodiles (Crocodylus siamensis) after intramuscular administration at two dosages. J. Vet. Pharmacol. Ther. 2020, 43, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Ferran, A.; Bousquet-Melou, A. Species differences in pharmacokinetics and pharmacodynamics. In Comparative and Veterinary Pharmacology; Cunningham, F., Elliott, J., Lees, P., Eds.; Springer: Cham, Switzerland, 2010. [Google Scholar]
- Toutain, P.L.; Bousquet-Melou, A. Plasma clearance. J. Vet. Pharmacol. Ther. 2004, 27, 415–425. [Google Scholar] [CrossRef]
- Toutain, P.L.; Bousquet-Mélou, A. Volumes of distribution. J. Vet. Pharmacol. Ther. 2004, 27, 441–453. [Google Scholar] [CrossRef]
- Jadot, L.; Judong, A.; Canivet, J.L.; Lorenzo-Villalba, N.; Damas, P. Ceftriaxone-induced Encephalopathy: A Pharmacokinetic Approach. Eur. J. Case Rep. Intern. Med. 2021, 8, 003011. [Google Scholar] [CrossRef]
- Corum, D.D.; Corum, O.; Altan, F.; Faki, H.E.; Bahcivan, E.; Er, A.; Uney, K. Pharmacokinetics of ceftriaxone following single ascending intravenous doses in sheep. Small Rumin. Res. 2018, 169, 108–112. [Google Scholar] [CrossRef]
- Ringger, N.C.; Brown, M.P.; Kohlepp, S.J.; Gronwall, R.R.; Merritt, K. Pharmacokinetics of ceftriaxone in neonatal foals. Equine Vet. J. 1998, 30, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Johal, B.; Srivastava, A.K. Disposition kinetics and dosage regimen of ceftriaxone in crossbred calves (short communication). Acta Vet. Hung. 1999, 47, 243–248. [Google Scholar] [CrossRef]
- Corum, O.; Durna Corum, D.; Marin, P.; Acar, O.F.; Aksoy, M.; Uney, K. Pharmacokinetics, bioavailability and plasma protein binding of tolfenamic acid in rainbow trout (Oncorhynchus mykiss). Vet. Med. Sci. 2024, 10, e1533. [Google Scholar] [CrossRef] [PubMed]
- Goudah, A.; Shin, H.C.; Shim, J.H.; Abd El-Aty, A.M. Characterization of the relationship between serum and milk residue disposition of ceftriaxone in lactating ewes. J. Vet. Pharmacol. Ther. 2006, 29, 307–312. [Google Scholar] [CrossRef]
- Ismail, M.M. Pharmacokinetics, urinary and mammary excretion of ceftriaxone in lactating goats. J. Vet. Med. 2005, 52, 354–358. [Google Scholar] [CrossRef]
- Kleinow, K.M.; Margaret, O.J.; Lech, J.J. Drug pharmacokinetics and metabolism in food-producing fish and crustaceans: Methods and examples. In Xenobiotics and Food-Producing Animals; ACS Symposium Series; Hutson, D.H., Hawkins, D.R., Paulson, G.D., Struble, C.B., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 98–130. [Google Scholar]
- Henneberger, L.; Klüver, N.; Mühlenbrink, M.; Escher, B. Trout and human plasma protein binding of selected pharmaceuticals informs the fish plasma model. Environ. Toxicol. Chem. 2020, 41, 559–568. [Google Scholar] [CrossRef]
- Rairat, T.; Hsieh, C.Y.; Thongpiam, W.; Sung, C.H.; Chou, C.C. Temperature—Dependent pharmacokinetics of florfenicol in Nile tilapia (Oreochromis niloticus) following single oral and intravenous administration. Aquaculture 2019, 503, 483–488. [Google Scholar] [CrossRef]
- Van Ginneken, V.T.; Nouws, J.F.M.; Grondel, J.L.; Driessens, F.; Degen, M. Pharmacokinetics of sulphadimidine in carp (Cyprinus carpio L.) and rainbow trout (Salmo gairdneri Richardson) acclimated at two different temperature levels. Vet. Q. 1991, 13, 88–96. [Google Scholar] [CrossRef] [PubMed]
- EMA 2006. EMEA/CHMP/212746/2006. Available online: https://www.ema.europa.eu/en/documents/referral/ceftriaxone-tyrol-pharma-article-29-referral-annex-i-ii-iii_en.pdf (accessed on 19 November 2025).
- Mandal, T.K.; Sar, T.K.; Das, S.K.; Chakraborty, A.K. Pharmacokinetics of ceftriaxone in carbontetrachloride-induced hepatopathic and uranyl nitrate-induced nephropathic goats following single dose intravenous administration. Drug Metab. Lett. 2008, 2, 23–28. [Google Scholar]
- Stoeckel, K.; Trueb, V.; Dubach, U.C.; McNamara, P.J. Effect of probenecid on the elimination and protein binding of ceftriaxone. Eur. J. Clin. Pharmacol. 1988, 34, 151–156. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Roy, A.D.; Ingebrigtsen, K. Xenobiotic excretion in fish with aglomerular kidneys. Mar. Ecol. Prog. Ser. 1996, 136, 303–304. [Google Scholar] [CrossRef]
- Nambiar, S.P.; Pillai, D.; Nair, S.N.; Krishnan, R. The role of cytochrome P450 in fish health and metabolism: A vital enzyme system. J. Fish Health 2025, 5, 289–316. [Google Scholar] [CrossRef]
- Cerveny, D.; Fick, J.; Klaminder, J.; McCallum, E.S.; Bertram, M.G.; Castillo, N.A.; Brodin, T. Water temperature affects the biotransformation and accumulation of a psychoactive pharmaceutical and its metabolite in aquatic organisms. Environ. Int. 2021, 155, 106705. [Google Scholar] [CrossRef]
- Rebuelto, M.; Albarellos, G.; Ambros, L.; Kreil, V.; Montoya, L.; Bonafine, R.; Otero, P.; Hallu, R. Pharmacokinetics of ceftriaxone administered by the intravenous, intramuscular or subcutaneous routes to dogs. J. Vet. Pharmacol. Ther. 2002, 25, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Bowser, P.R.; Babish, J.G. Clinical pharmacology and efficacy of fluoroquinolones in fish. Annu. Rev. Fish Dis. 1991, 1, 63–66. [Google Scholar] [CrossRef]
- Toutain, P.L.; Bousquet-Mélou, A. Bioavailability and its assessment. J. Vet. Pharmacol. Ther. 2004, 27, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.D.M.; Martins, E.S.; Peccinini, R.G.; Rosa, G.S.; Guerra, S.T.; Ribeiro, M.G.; Santos, B.; Garcia, H.D.M.; Watanabe, M.J.; Takahira, R.K.; et al. Plasma and peritoneal ceftriaxone concentrations after intraperitoneal administration in horses with septic peritonitis. J. Equine Vet. Sci. 2021, 96, 103310. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Del Castillo, J.R.; Bousquet-Mélou, A. The pharmacokinetic–pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef] [PubMed]
- CLSI. 2020. Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 20 November 2025).
- Vasuntrarak, K.; Wittayalertpanya, S.; Wongtavatchai, J.; Suanpairintr, N. Pharmacokinetics and pharmacokinetic/pharmacodynamic-based dosing regimens of long-acting oxytetracycline in Nile tilapia (Oreochromis niloticus) broodstock to minimize selection of drug resistance. Aquaculture 2022, 557, 738302. [Google Scholar] [CrossRef]

| Parameter | IV | IP | IM |
|---|---|---|---|
| t1/2λz (h) | 5.27 | 5.61 | 5.44 |
| AUC0–36 (h * µg/mL) | 113.05 | 75.79 | 114.72 |
| AUC0–∞ (h * µg/mL) | 113.57 | 76.22 | 115.36 |
| AUCextrap (%) | 0.46 | 0.56 | 0.55 |
| MRT0–∞ (h) | 3.85 | 4.40 | 4.75 |
| ClT (L/h/kg) | 0.22 | - | - |
| Vdarea (L/kg) | 1.67 | - | - |
| Vdss (L/kg) | 0.85 | - | - |
| Cmax (µg/mL) | - | 37.71 ± 3.12 | 40.51 ± 2.77 |
| C0.25h (µg/mL) | 57.37 ± 4.01 | - | - |
| Tmax (h) | - | 0.25 | 0.50 |
| F (%) | - | 67.04 | 101.48 |
| Parameter | IV | IP | IM |
|---|---|---|---|
| t1/2λz (h) | 7.27 | 5.79 | 7.36 |
| AUC0–last (h * µg/g) | 25.13 | 12.88 | 33.63 |
| AUC0–∞ (h * µg/g) | 25.74 | 13.39 | 34.36 |
| AUCextrap (%) | 2.38 | 3.80 | 2.11 |
| Cmax (µg/g) | 9.49 ± 0.75 | 5.71 ± 0.85 | 12.24 ± 2.41 |
| Tmax (h) | 0.25 | 0.50 | 0.50 |
| AUC0–∞muscle/AUC0–∞plasma | 0.23 | 0.18 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín, P.; Corum, O.; Corum, D.D.; Badillo, E.; Yuste, M.T.; Yildirim, O.; Terzi, E.; Gonzales, R.C.; Arriesgado, D.M.; Navarro, V.R.; et al. Plasma and Muscle Pharmacokinetics of Ceftriaxone in Nile Tilapia (Oreochromis niloticus) After Different Administration Routes. Antibiotics 2025, 14, 1253. https://doi.org/10.3390/antibiotics14121253
Marín P, Corum O, Corum DD, Badillo E, Yuste MT, Yildirim O, Terzi E, Gonzales RC, Arriesgado DM, Navarro VR, et al. Plasma and Muscle Pharmacokinetics of Ceftriaxone in Nile Tilapia (Oreochromis niloticus) After Different Administration Routes. Antibiotics. 2025; 14(12):1253. https://doi.org/10.3390/antibiotics14121253
Chicago/Turabian StyleMarín, Pedro, Orhan Corum, Duygu Durna Corum, Elena Badillo, María Teresa Yuste, Onder Yildirim, Ertugrul Terzi, Ruby C. Gonzales, Dan M. Arriesgado, Victor R. Navarro, and et al. 2025. "Plasma and Muscle Pharmacokinetics of Ceftriaxone in Nile Tilapia (Oreochromis niloticus) After Different Administration Routes" Antibiotics 14, no. 12: 1253. https://doi.org/10.3390/antibiotics14121253
APA StyleMarín, P., Corum, O., Corum, D. D., Badillo, E., Yuste, M. T., Yildirim, O., Terzi, E., Gonzales, R. C., Arriesgado, D. M., Navarro, V. R., & Uney, K. (2025). Plasma and Muscle Pharmacokinetics of Ceftriaxone in Nile Tilapia (Oreochromis niloticus) After Different Administration Routes. Antibiotics, 14(12), 1253. https://doi.org/10.3390/antibiotics14121253








