Enteric Pathogens in Wild Boars Across the European Union: Prevalence and Antimicrobial Resistance Within a One Health Framework
Abstract
1. Introduction
2. Materials and Methods
3. Silent Carriers at the Human–Wildlife Interface: Enteric Pathogens in European Wild Boars
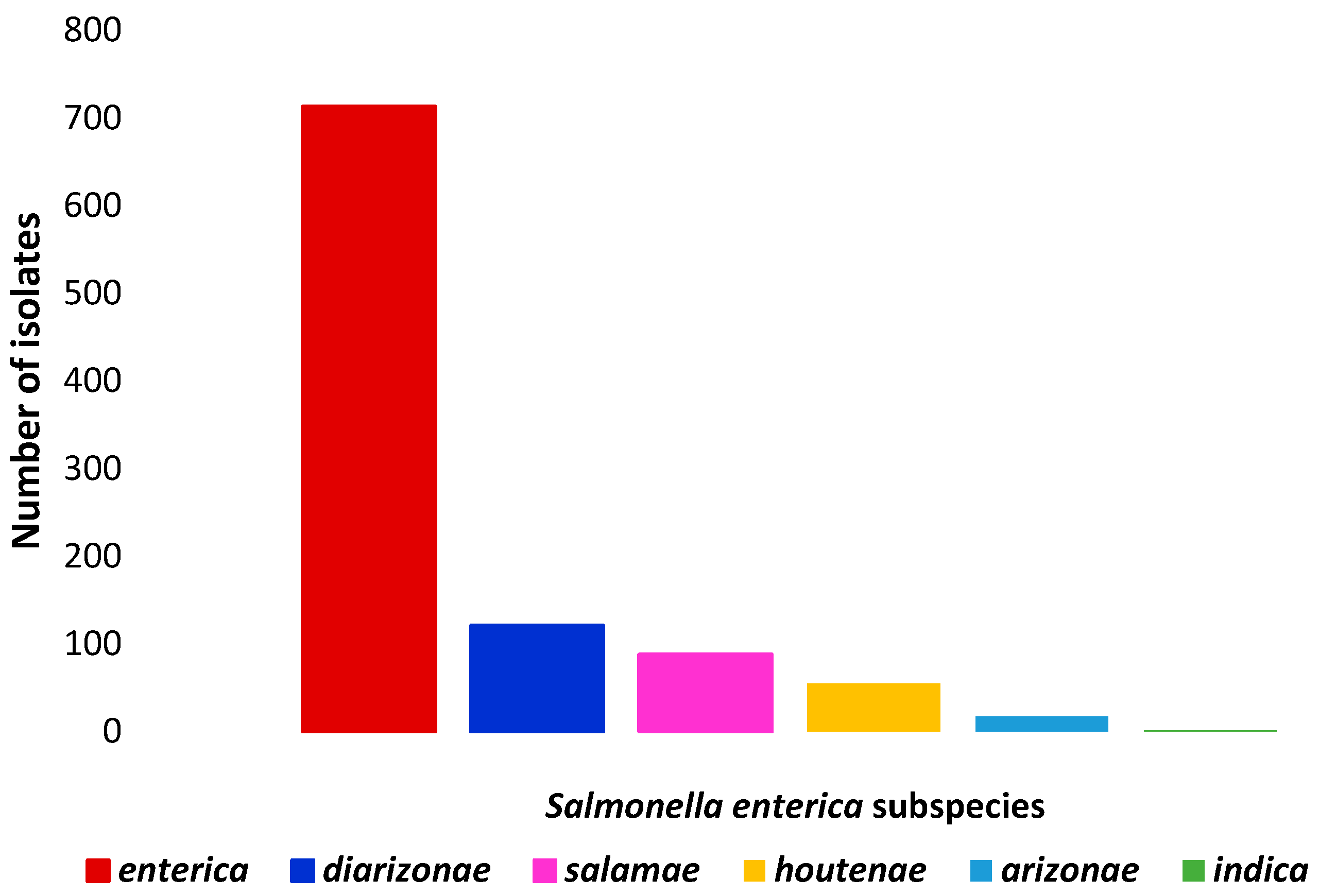
3.1. Prevalence and Diversity of Salmonella spp.
| Country | Sampling Period | Source | Positive/Total (%) | Comments on Detection Method | Ref. |
|---|---|---|---|---|---|
| Portugal | 2005–2006 | Feces (rectum content) | 17/77 (22.1) | Culture | [34] |
| Switzerland | 2007–2008 | Feces (not specified) | 0/73 | RT–PCR | [33] |
| 0/73 | Culture (for RT-PCR positive samples) | ||||
| Tonsils | 19/153 (35.8) | RT-PCR | |||
| 8/153 (5.2) | Culture (for RT-PCR positive samples) | ||||
| Italy | 2007–2010 | Feces (intestinal content) | 326/1313 (24.8) | Culture | [36] |
| Spain | 2007–2011 | Feces (rectum content) | 66/214 (30.8) | Culture | [35] |
| Spain | 2009–2011 | Feces (rectum content) | 1/574 (0.17) | Culture | [37] |
| Carcass surface | 5/585 (0.8) | ||||
| Italy | 2010–2012 | Feces (colon content) | 54/499 (10.8) | Culture | [38] |
| Blood (serum) | 255/383 (66.5) | Enzyme-Linked Immunosorbent Assay | |||
| Spain | 2010–2015 | Feces (colon content) | 25/838 (2.9) | Culture | [43] |
| Lymph nodes (sub-mandibular) | 21/415 (5.1) | ||||
| Tonsils | 40/214 (18.7) | ||||
| Italy | 2012–2013 | Muscle (diaphragm or leg) | 7/194 (3.6) | Enzyme-Linked Fluorescent Assay followed by culture for positive samples | [61] |
| Portugal | 2013–2014 | Feces (rectum content) | 1/21 (4.7) | Culture | [39] |
| Serbia | 2013–2014 | Feces (rectum content) | 13/425 (3.1) | Culture | [50] |
| Carcass surface | 4/425 (0.9) | ||||
| Skin surface | 1/425 (0.2) | ||||
| Italy | 2013–2017 | Liver | 260/4335 (6) | PCR followed by culture for positive samples (results referred to cultural positivity) | [62] |
| Sweden | 2014–2016 | Feces (not specified) | 7/90 (7.8) | RT-PCR preceded by culture (results referred to PCR positivity) | [40] |
| Lymph nodes (mesenteric) | 9/90 (10) | ||||
| Lymph nodes (sub-mandibular) | 3/25 (12) | ||||
| Tonsils | 20/136 (14.7) | ||||
| Denmark | 2014–2016 | Feces (rectum content) | 0/115 | Culture | [44] |
| Spain | 2015–2016 | Feces (rectal swabs) | 4/130 (3.1) | Culture | [13] |
| Serbia | 2015 | Carcass surface Skin surface | 4/210 (1.9) 3/210 (1.4) | Culture | [63] |
| Germany | 2016 | Feces (not specified) | 13/552 (2.4) | Culture | [47] |
| Finland | 2016 | Blood (serum) | 69/181 (38) | Enzyme-Linked Immunosorbent Assay | [64] |
| Organs (kidney and spleen) | 6/130 (4.6) | RT-PCR followed by culture for positive samples (results referred to PCR positivity) | |||
| Italy | 2016–2017 | Feces (caecum content) | 4/57 (7) | Culture | [41] |
| Lymph nodes (mesenteric) | 2/57 (3.5) | ||||
| Carcass surface | 0/30 | ||||
| Italy | 2016–2019 | Feces (colon content) | 32/90 (35.6) | Culture | [46] |
| Lymph nodes (mesenteric) | 16/90 (17.8) | ||||
| Carcass surface | 1/90 (1.1) | ||||
| Italy | 2017–2018 | Feces (not specified) | 30/189 (15.9) | Culture | [42] |
| Lymph nodes (mesenteric) | 6/189 (3.2) | ||||
| Italy | 2017–2020 | Feces (not specified) | Not reported | Culture (previous study) | [48] |
| Lymph nodes (mesenteric) | |||||
| Carcass surface | |||||
| Italy | 2018–2019 | Carcass (excision method) | 3/120 (2.5) | Culture | [65] |
| Italy | 2018–2023 | Feces (rectum content) | 1/280 (0.3) | Culture | [49] |
| Carcass surface | 5/280 (1.8) | Enzyme-Linked Fluorescent Assay | |||
| Liver | 3/280 (1.1) | Enzyme-Linked Fluorescent Assay | |||
| Italy | 2018–2020 | Feces (rectal swabs) | 7/287 (2.4) | Culture | [45] |
| Spleen | 6/287 (2) | ||||
| Liver | 5/287 (1.7) | ||||
| Italy | 2019 | Tonsils | 0/36 | Culture | [66] |
| Carcass surface | 0/36 | ||||
| Meat (forearm area) | 1/36 (2.8) | ||||
| Italy | 2020 | Carcass surface | 5/64 (7.8) | Culture | [67] |
| Lymph nodes (mesenteric) | 5/64 (7.8) | ||||
| Italy | 2020–2022 | Feces (colon content) | 3/66 (4.5) | Culture | [11] |
| Lymph nodes (mesenteric) | 0/66 | ||||
| Carcass surface | 0/49 |
3.2. Unveiling Y. enterocolitica: Prevalence Trends and Bio-Serotypes
| Country | Sampling Period | Source | Pos/Tot (%) | Comments on Detection Method | Ref. |
|---|---|---|---|---|---|
| Switzerland | 2007–2008 | Feces (not specified) | 4/73 (5.5) | RT-PCR | [33] |
| 1/73 (1.3) | Culture (direct plating of RT-PCR positive samples) | ||||
| Tonsils | 26/73 (36) | Real-Time PCR | |||
| 6/73 (8) | Culture (direct plating of RT-PCR positive samples) | ||||
| Italy | 2008–2010 | Carcass surface | 3/251 (1.2) | Culture (cold enrichment) | [83] |
| Spain | 2009–2012 | Tonsils | 24/72 (33.3) | RT-PCR followed by cultural (direct plating of RT-PCR enrichment of RT-PCR positive samples) | [78] |
| Poland | 2012–2013 | Feces (rectal swabs) | 40/151 (26.5) | Culture (warm and cold enrichment) | [75] |
| Germany | 2012–2013 | Tonsils | 19/111 (17.1) | Culture (warm enrichment) | [70] |
| Italy | 2012–2013 | Muscle (diaphragm and leg) | 34/230 (14.8) | Culture (cold enrichment) | [61] |
| Czech Republic | 2013–2014 | Meat juice from diaphragm | 89/135 (81.9) | Enzyme-Linked Immunosorbent Assay (antibodies anti-Yersinia spp.) | [84] |
| Poland | 2013–2014 | Feces (rectal swabs) | 110/434 (25.3) | Culture (warm and cold enrichment) | [74] |
| Italy | 2013–2018 | Liver | 126/4890 (2.6) | Culture (warm enrichment) | [85] |
| Italy | 2014–2015 | Muscle (Longissimus dorsi) | 0/22 | Culture (cold enrichment) | [86] |
| Sweden | 2014–2016 | Feces | 4/90 (4.4) | RT-PCR | [40] |
| Lymph nodes (mesenteric) | 6/67 (6.7) | ||||
| Lymph nodes (sub-mandibular) | 3/25 (12) | ||||
| Tonsils | 19/136 (14) | ||||
| Italy | 2015–2018 | Feces (not specified) | 0/107 | RT-PCR followed by culture (warm enrichment) for positive samples | [76] |
| Finland | 2016 | Blood (serum) | 102/181 (56) | Enzyme-Linked Immunosorbent Assay (antibodies anti-Yersinia spp.) | [64] |
| Organs (kidney, spleen) | 22/130 (17) | RT-PCR followed by culture (warm enrichment) for positive samples | |||
| Italy | 2017 | Muscle (shoulder area) | 2/22 (9) | RT-PCR followed by culture (cold enrichment) for positive samples | [87] |
| Italy | 2017–2019 | Feces | 19/305 (6.2) | Culture (warm and cold enrichment) | [67] |
| Lymph nodes (mesenteric) | 10/305 (3.3) | ||||
| Italy | 2018–2020 | Feces (rectal swabs) | 54/287 (18.8) | Culture (cold enrichment) | [45] |
| Italy | 2019 | Carcass surface | 12/36 (33.3) | Culture (warm enrichment) | [66] |
| Tonsils | 9/36 (25) | ||||
| Meat (forearm area) | 10/36 (27) | ||||
| Italy | 2020 | Lymph nodes (mesenteric) | 0/64 | Culture (cold enrichment) | [79] |
| Carcass surface | 0/64 | ||||
| Italy | 2020–2022 | Feces (colon content) | 18/66 (27.3) | Culture (cold enrichment) | [13] |
| Lymph nodes (mesenteric) | 3/66 (4.5) | ||||
| Carcass surface | 3/49 (6.1) |
3.3. Campylobacter spp. Occurrence and Epidemiological Insights
| Country | Sampling Period | Source | Pos/Tot (%) | Campylobacter Species Identified (%) | Ref. |
|---|---|---|---|---|---|
| Germany | 2006–2007 | Muscle (various carcass area) | 3/127 (2.4) | C. coli (66.6), C. jejuni (33.3) | [94] |
| Spain | 2009–2011 | Feces (rectal content) | 188/287 (66) | C. spp. (one C. jejuni) | [37] |
| Spain | 2010–2011 | Feces (rectal content) | 10/41(24.4) | C. coli (21.1), other thermophilic species (79.9) | [35] |
| Spain | 2011–2012 | Feces (intestine or rectum) | 49/126 (38.9) | C. lanianae (69.4), C coli (16.3), C. jejuni (4.11), others (10.2) | [89] |
| Italy | 2012–2019 | Feces (not specified) Liver Muscle | Not reported | C. coli (91.7), C. jejuni (8.3) | [95] |
| Italy | 2016 | Feces (caecum content) | 29/56 (51.8) | Not investigated | [41] |
| Lymph nodes (mesenteric) | 0/56 | ||||
| Carcass surface | 5/30 (16.7) | ||||
| Spain | 2015–2016 | Feces (rectal swabs) | 79/130 (60.8) | C. lanienae (46.2), C. coli (16.2), C. hyointestinalis (0.8) | [13] |
| Italy | 2018–2019 | Feces (rectal swabs) | 78/183 (42.6) | C. coli (48), C. lanianae (42), C. jejuni (6), C. hyointestinalis (4) | [90] |
| Carcass surface | 10/55 (18.2) | ||||
| Liver | 9/187 (4.81) | ||||
| Bile | 3/152 (1.9) | ||||
| Italy | 2019 | Feces (rectal content) | 38/76 (50) | C. lanienae (40.8), C. hyointestinalis (14.5), C. coli (7.9), C. jejuni (1.3), C. fetus (1.3) | [14] |
| Italy | 2019 | Carcass surface | 4/36 (11.1) | C. coli (33), C. jejuni (33), other species (33) | [66] |
| Meat (leg) | 2/36 (5.5) | ||||
| Italy | 2019–2020 | Meat (leg) | 0/28 | [87] |
3.4. Prevalence and Diversity of STEC
| Country | Sampling Period | Source | Pos/Tot (%) | STEC Serogroups (%) and Others Pathotypes Identified (%) | Ref. |
|---|---|---|---|---|---|
| Portugal | 2009–2010 | Feces (rectal swabs) | 22/262 (8.4) | non-O157 (8.4), O157:H7 (0.38) | [100] |
| Spain | 2009–2011 | Frozen meat | 1/36 (2.7) | non-O157 (100%) | [109] |
| Meat products | 2/21 (9.5) | ||||
| Spain | 2009–2011 | Feces (rectal content) | 4/117 (3.4) | Detection method only for O157:H7 | [106] |
| Sweden | 2010–2011 | Feces (not specified) | 0/88 | Detection method only for O157:H7 | [105] |
| Lymph nodes (mesenteric) | 0/56 | ||||
| Tonsils | 0/175 | ||||
| Spain | 2009–2011 | Feces (rectal content) Carcass surface | 11/301 (4) 12/310 (4) | non-O157 (100%) | [37] |
| Portugal | 2013–2014 | Feces (rectal content) | 1/21 (4.8) | non-O157 (100%) | [103] |
| Spain | 2013–2015 | Feces (not specified) | 3/90 (3.3) | non O157 (100%) E. coli EPEC (3.3) | [101] |
| Germany | 2016 | Feces (not specified) | 37/536 (6.9) | O157:H7 (8.3) non-O157 (91.7) | [47] |
| Poland | 2017–2018 | Feces (rectal swabs) | 64/152 (28.3) | non-O157 (92.1), O157 (7.9) E. coli EPEC (17.11) | [102] |
| Portugal | 2017–2019 | Feces (environment or rectal content) | 8/56 (14) | non-O157 (100%) | [12] |
| Italy | 2018–2019 | Feces (rectal swabs) | 13/200 (6.5) | Serogroups not reported EHEC (6.3), EAEC (5.7), aEPEC (3.4), and unspecific pathotypes also identified | [107] |
| Italy | 2019–2020 | Meat (forearm area) | 12/28 (42.8) | Serogroups not reported | [66] |
| Switzerland | 2021 | Meat (not specified) | 3/25 (12) | non O157 (100%) | [110] |
| Switzerland | 2022–2023 | Feces (colon content) | 13/59 (22) | non O157 (100%) | [104] |
3.5. Wild Boar as Carriers of Enteric Pathogens
3.6. Focus on the Risk of Meat Contamination; Presence of Zoonotic Pathogens on Carcass Surface, Organs, and Meat
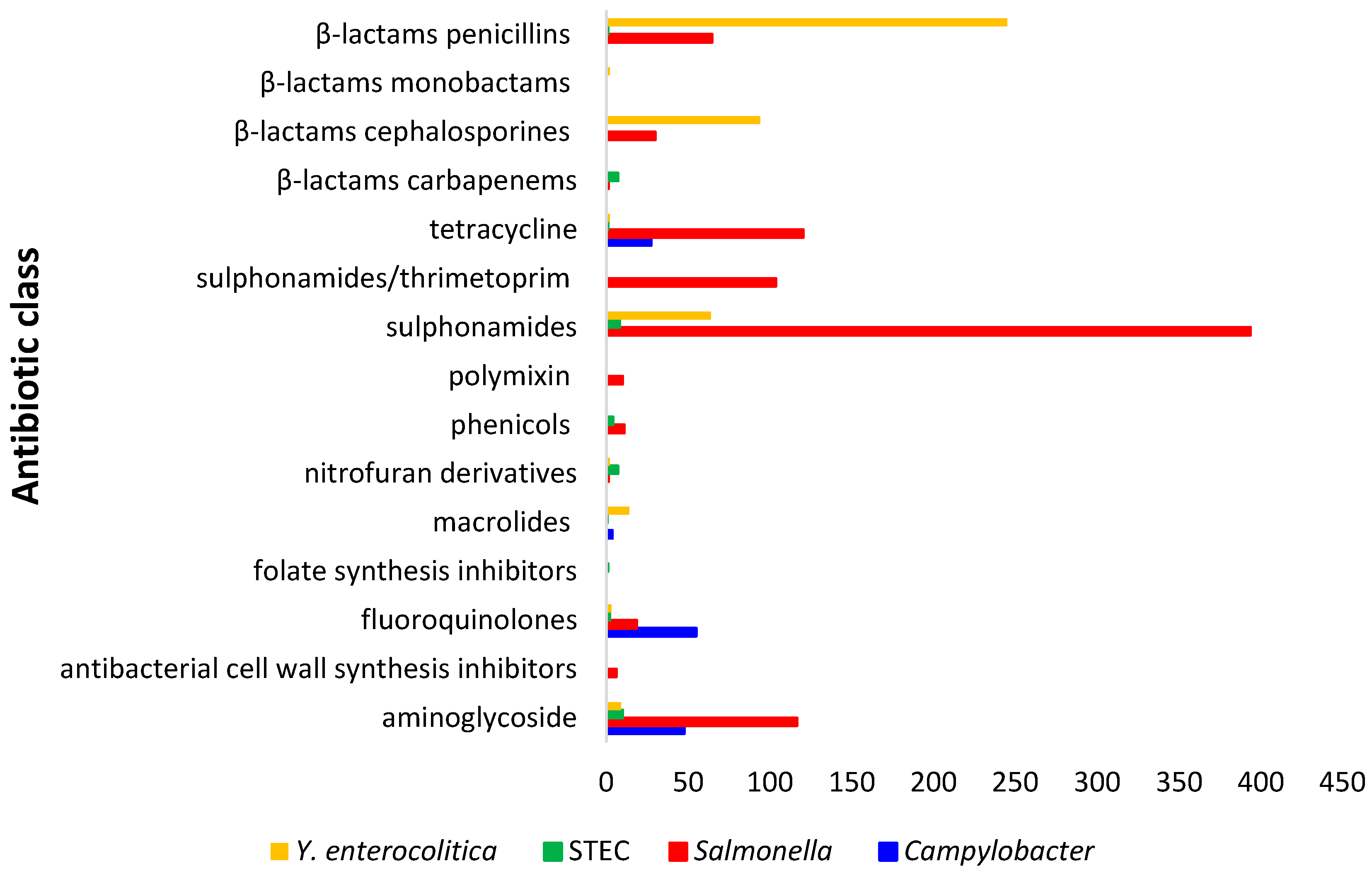
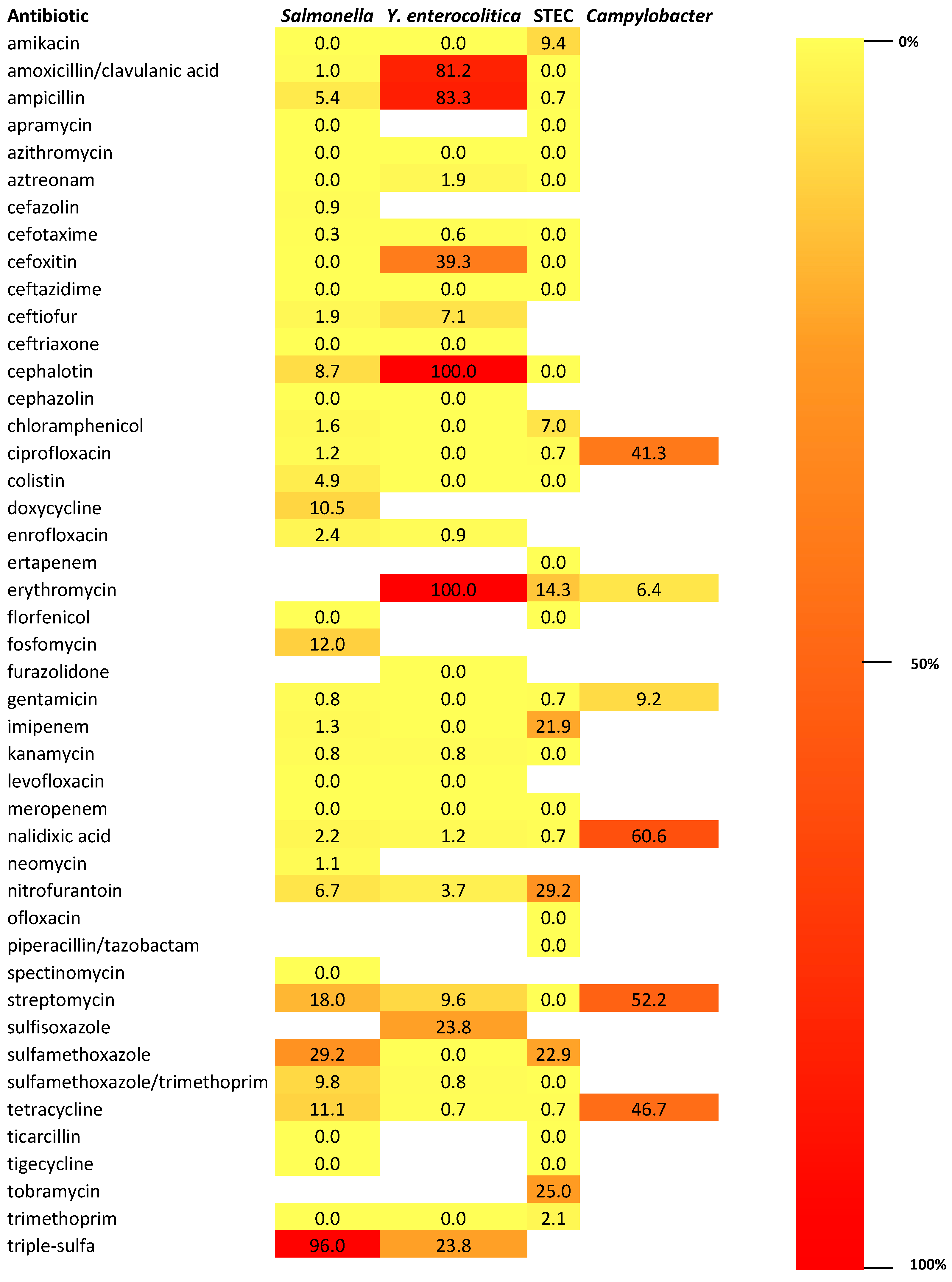
4. Antimicrobial Resistance Profiles of Enteric Pathogens Detected from Wild Boars in European Union
4.1. AMR Patterns in Salmonella Isolates
AMR in Salmonella Isolates Against Critically Important and High Important Antibiotics
4.2. AMR Patterns in Y. enterocolitica Isolates
4.3. AMR Patterns in STEC Isolates
4.4. AMR Patterns in Campylobacter spp. Isolates
5. Future Perspective in AMR: Studying the Fecal Resistome
6. From Forest to City: The Wild Boar as an Example of Synurbization
7. Conclusions and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Geisser, H.; Reyer, H.U. The influence of food and temperature on population density of wild boar Sus scrofa in the Thurgau (Switzerland). J. Zool. 2005, 267, 89–96. [Google Scholar] [CrossRef]
- Holland, E.P.; Burrow, J.F.; Dytham, C.; Aegerter, J.N. Modelling with uncertainty: Introducing a probabilistic framework to predict animal population dynamics. Ecol. Model. 2009, 220, 1203–1217. [Google Scholar] [CrossRef]
- Tack, J. Wild Boar (Sus scrofa) Populations in Europe: A Scientific Review of Population Trends and Implications for Management; European Landowners’ Organization: Brussels, Belgium, 2018; p. 56. [Google Scholar]
- Tinoco Torres, R.; Fernandes, J.; Carvalho, J.; Cunha, M.V.; Caetano, T.; Mendo, S.; Serrano, E.; Fonseca, C. Wild boar as a reservoir of antimicrobial resistance. Sci. Total Environ. 2020, 717, 135001. [Google Scholar] [CrossRef] [PubMed]
- Podgórski, T.; Borowik, T.; Lyjak, M.; Wozniakowski, G. Spatial epidemiology of African swine fever: Host, landscape and anthropogenic drivers of disease occurrence in wild boar. Prev. Vet. Med. 2020, 177, 104691. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.G.; Rose, N.; Hayes, B.; Hammami, P.; Baubet, E.; Desvaux, S.; Andraud, M. Effects of habitat fragmentation and hunting activities on African swine fever dynamics among wild boar populations. Prev. Vet. Med. 2022, 208, 105750. [Google Scholar] [CrossRef]
- Correa Lopes, B.; Roth, M.; Vidaletti, M.; Loiko, M.R.; da Silva Andrade, J.; Gisler Maciel, A.L.; Doyle, R.L.; Cavalheiro Bertagnolli, A.; Oliveira Rodrigues, R.; Driemeier, D.; et al. Investigation of Mycobacterium bovis and Metastrongylus sp. co-infection and its relationship to tuberculosis lesions’ occurrence in wild boars. Comp. Immunol. Microbiol. Infect. Dis. 2021, 77, 101674. [Google Scholar] [CrossRef]
- Martins Ruano, Z.; Mateus, T.L.; Vieira-Pinto, M. An insight into brucellosis in wild boar and domestic pigs in Europe: A systematic review. J. Infect. Public Health 2025, 18, 102691. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; et al. African swine fever in wild boar in Europe—A review. Viruses 2022, 13, 1717. [Google Scholar] [CrossRef]
- Siddi, G.; Piras, F.; Meloni, M.P.; Gymoese, P.; Torpdahl, M.; Fredriksson-Ahomaa, M.; Migoni, M.; Cabras, D.; Cuccu, M.; De Santis, E.P.L.; et al. Hunted wild boars in Sardinia: Prevalence, antimicrobial resistance and genomic analysis of Salmonella and Yersinia enterocolitica. Foods 2024, 13, 65. [Google Scholar] [CrossRef]
- Dias, D.; Costa, S.; Fonseca, C.; Baraúna, R.; Caetano, T.; Mendo, S. Pathogenicity of Shiga toxin-producing Escherichia coli (STEC) from wildlife: Should we care? Sci. Total Environ. 2022, 812, 152324. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Marín, M.; López-Olvera, J.R.; Ayats, T.; Fernández-Aguilar, X.; Lavín, S.; Mentaberre, G.; Cerdà-Cuéllar, M. Zoonotic Campylobacter spp. and Salmonella spp. carried by wild boars in a metropolitan area: Occurrence, antimicrobial susceptibility and public health relevance. Sci. Total Environ. 2022, 822, 153444. [Google Scholar] [CrossRef]
- Kerkhof, P.J.; Peruzy, M.F.; Murru, N.; Houf, K. Wild boars as reservoir for Campylobacter and Arcobacter. Vet. Microbiol. 2022, 270, 109462. [Google Scholar] [CrossRef]
- Tayh, G.; Srairi, S.; Selmi, R.; Chehida, F.B.; Mamlouk, A.; Daaloul-Jedidi, M.; Messadi, L. Risk for public health of multiresistant Shiga toxin-producing Escherichia coli (STEC) in wild boar (Sus scrofa) in Tunisia. Microb. Pathog. 2025, 201, 107366. [Google Scholar] [CrossRef]
- Seinige, D.; von Altrock, A.; Kehrenberg, C. Genetic diversity and antibiotic susceptibility of Staphylococcus aureus isolates from wild boars. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 7–12. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Gamal, M.M. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Tripartite AMR Project: Working Together to Fight Antimicrobial Resistance. 2023. Available online: https://rr-americas.woah.org/en/projects/ue-ram/ (accessed on 5 November 2025).
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2022–2023. EFSA J. 2025, 23, e9237. [Google Scholar] [CrossRef] [PubMed]
- WHO. Critically Important Antimicrobials for Human Medicine: 6th Revision; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Doyle, C.; Wall, K.; Fanning, S.; McMahon, B.J. Making sense of sentinels: Wildlife as the One Health bridge for environmental antimicrobial resistance surveillance. J. Appl. Microbiol. 2025, 136, lxaf017. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Hughes, J.M. Critical importance of a One Health approach to antimicrobial resistance. EcoHealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Martinez, J.L.; Aracil-Gisbert, S.; Lanza, V.S. Gene transmission in the One Health microbiosphere and the channels of antimicrobial resistance. Front. Microbiol. 2019, 10, 2892. [Google Scholar] [CrossRef]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef]
- Vezeau, N.; Kahn, L. Current understanding and knowledge gaps regarding wildlife as reservoirs of antimicrobial resistance. Am. J. Vet. Res. 2024, 85, 1–9. [Google Scholar] [CrossRef]
- Bonnedahl, J.; Järhult, J.D. Antibiotic resistance in wild birds. Upsala J. Med. Sci. 2014, 119, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Vittecoq, M.; Godreuil, S.; Prugnolle, F.; Durand, P.; Brazier, L.; Renaud, N.; Arnal, A.; Aberkane, S.; Jean-Pierre, H.; Gauthier-Clerc, M.; et al. Antimicrobial resistance in wildlife: An emerging issue. Trends Microbiol. 2016, 24, 448–459. [Google Scholar] [CrossRef]
- Unger, F.; Eisenberg, T.; Prenger-Berninghoff, E.; Leidner, U.; Semmler, T.; Ewers, C. Phenotypic and genomic characterization of ESBL- and AmpC-β-lactamase-producing Enterobacterales isolates from imported healthy reptiles. Antibiotics 2024, 13, 1230. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Abreu, R.; Serrano, I.; Such, R.; Garcia-Vila, E.; Quirós, S.; Cunha, E.; Tavares, L.; Oliveira, M. Resistant Escherichia coli isolated from wild mammals from two rescue and rehabilitation centers in Costa Rica: Characterization and public health relevance. Sci. Rep. 2024, 14, 8039. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Salmonella and Food. U.S. Department of Health and Human Services, 2023. Available online: https://www.cdc.gov/salmonella/ (accessed on 5 November 2025).
- Paulsen, P.; Smulders, F.J.M.; Hilbert, F. Salmonella in meat from hunted game: A central European perspective. Food Res. Int. 2012, 45, 609–616. [Google Scholar] [CrossRef]
- Wacheck, S.; Fredriksson-Ahomaa, M.; König, M.; Stolle, A.; Stephan, R. Wild Boars as an important reservoir for foodborne pathogens. Foodborne Pathog. Dis. 2010, 7, 307–312. [Google Scholar] [CrossRef]
- Vieira-Pinto, M.; Morais, L.; Caleja, C.; Themudo, P.; Torres, C.; Igrejas, G.; Poeta, P.; Martins, C. Salmonella sp. in game (Sus scrofa and Oryctolagus cuniculus). Foodborne Pathog. Dis. 2011, 8, 739–740. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, N.; Casas-Díaz, E.; Porrero, C.M.; Mateos, A.; Domínguez, L.; Lavín, S.; Serrano, E. Food-Borne zoonotic pathogens and antimicrobial resistance of indicator bacteria in urban wild boars in Barcelona, Spain. Vet. Microbiol. 2013, 167, 686–689. [Google Scholar] [CrossRef]
- Chiari, M.; Zanoni, M.; Tagliabue, S.; Lavazza, A.; Alborali, L.G. Salmonella serotypes in wild boars (Sus scrofa) hunted in northern Italy. Acta Vet. Scand. 2013, 55, 42. [Google Scholar] [CrossRef]
- Díaz-Sánchez, S.; Sánchez, S.; Herrera-León, S.; Porrero, C.; Blanco, J.; Dahbi, G.; Blanco, J.E.; Mora, A.; Mateo, R.; Hanning, I.; et al. Prevalence of shiga toxin-producing Escherichia coli, Salmonella spp. and Campylobacter spp. in large game animals intended for consumption: Relationship with management practices and livestock influence. Vet. Microbiol. 2013, 163, 274–281. [Google Scholar] [CrossRef]
- Zottola, T.; Montagnaro, S.; Magnapera, C.; Sasso, S.; De Martino, L.; Bragagnolo, A.; D’Amici, L.; Condoleo, R.; Pisanelli, G.; Iovane, G.; et al. Prevalence and antimicrobial susceptibility of Salmonella in European wild boar (Sus scrofa): Latium region, Italy. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 161–168. [Google Scholar] [CrossRef]
- Dias, D.; Torres, R.T.; Kronvall, G.; Fonseca, C.; Mendo, S.; Caetano, T. Assessment of antibiotic resistance of Escherichia coli isolates and screening of Salmonella spp. in wild ungulates from Portugal. Res. Microbiol. 2015, 166, 584–593. [Google Scholar] [CrossRef]
- Sannö, A.; Rosendal, T.; Aspán, A.; Backhans, A.; Jacobson, M. Distribution of enteropathogenic Yersinia spp. and Salmonella spp. in the Swedish wild boar population, and assessment of risk factors that may affect their prevalence. Acta Vet. Scand. 2018, 60, 40. [Google Scholar] [CrossRef]
- Stella, S.; Tirloni, E.; Castelli, E.; Colombo, F.; Bernardi, C. Microbiological evaluation of carcasses of wild boar hunted in a hill area of northern Italy. J. Food Prot. 2018, 81, 1519–1525. [Google Scholar] [CrossRef]
- Bonardi, S.; Bolzoni, L.; Zanoni, R.G.; Morganti, M.; Corradi, M.; Gilioli, S.; Pongolini, S. Limited exchange of Salmonella among domestic pigs and wild boars in Italy. EcoHealth 2019, 16, 420–428. [Google Scholar] [CrossRef]
- Gil Molino, M.; García Sánchez, A.; Risco Pérez, D.; Gonçalves Blanco, P.; Quesada Molina, A.; Rey Pérez, J.; Martín Cano, F.E.; Cerrato Horrillo, R.; Hermoso-de-Mendoza Salcedo, J.; Fernández Llario, P. Prevalence of Salmonella spp. in tonsils, mandibular lymph nodes and faeces of wild boar from Spain and genetic relationship between isolates. Transbound. Emerg. Dis. 2019, 66, 1218–1226. [Google Scholar] [CrossRef]
- Petersen, H.H.; Takeuchi-Storm, N.; Enemark, H.L.; Nielsen, S.T.; Larsen, G.; Chriél, M. Surveillance of important bacterial and parasitic infections in Danish wild boars (Sus scrofa). Acta Vet. Scand. 2020, 62, 41. [Google Scholar] [CrossRef]
- Cilia, G.; Turchi, B.; Fratini, F.; Bilei, S.; Bossù, T.; De Marchis, M.L.; Cerri, D.; Pacini, M.I.; Bertelloni, F. Prevalence, virulence and antimicrobial susceptibility of Salmonella spp., Yersinia enterocolitica and Listeria monocytogenes in European wild boar (Sus. scrofa) hunted in Tuscany (central Italy). Pathogens 2021, 10, 93. [Google Scholar] [CrossRef]
- Piras, F.; Spanu, V.; Siddi, G.; Gymoese, P.; Spanu, C.; Cibin, V.; Schjørring, S.; De Santis, E.P.L.; Scarano, C. Whole-genome sequencing analysis of highly prevalent Salmonella serovars in wild boars from a national park in Sardinia. Food Control 2021, 130, 108247. [Google Scholar] [CrossRef]
- Plaza-Rodríguez, C.; Alt, K.; Grobbel, M.; Hammerl, J.A.; Irrgang, A.; Szabo, I.; Stingl, K.; Schuh, E.; Wiehle, L.; Pfefferkorn, B.; et al. Wildlife as sentinels of antimicrobial resistance in Germany? Front. Vet. Sci. 2021, 7, 627821. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, L.; Bonardi, S.; Tansini, C.; Scaltriti, E.; Menozzi, I.; Morganti, M.; Conter, M.; Pongolini, S. Different roles of wild boars and livestock in Salmonella transmission to humans in Italy. EcoHealth 2023, 20, 122–132. [Google Scholar] [CrossRef]
- Altissimi, C.; Primavilla, S.; Roila, R.; Gavaudan, S.; Morandi, B.; Di Lullo, S.; Coppini, M.; Baldinelli, C.; Cai, D.; Branciari, R.; et al. Salmonella in wild boar meat: Prevalence and risk assessment in central Italy (Umbria and Marche region). Foods 2024, 13, 1156. [Google Scholar] [CrossRef] [PubMed]
- Petrović, J.; Mirčeta, J.; Velhner, M.; Stojanov, I.; Ratajac, R.; Prodanov-Radulović, J. Salmonella in wild boars (Sus scrofa): Influence of hunting and dressing procedures on meat safety. Arch. Vet. Med. 2024, 17, 51–67. [Google Scholar] [CrossRef]
- Beloeil, P.A.; Chauvin, C.; Proux, K.; Rose, N.; Queguiner, S.; Eveno, E.; Houdayer, C.; Rose, V.; Fravalo, P.; Madec, F. Longitudinal serological responses to Salmonella enterica of growing pigs in a subclinically infected herd. Prev. Vet. Med. 2003, 60, 207–226. [Google Scholar] [CrossRef]
- Ivanek, R.; Österberg, J.; Gautam, R.; Sternberg Lewerin, S. Salmonella fecal shedding and immune responses are dose- and serotype-dependent in pigs. PLoS ONE 2012, 7, e34660. [Google Scholar] [CrossRef]
- Berends, B.R.; van Knapen, F.; Snijders, J.M.A.; Mossel, D.A.A. Identification and quantification of risk factors in animal management and transport regarding Salmonella spp. in pigs. Int. J. Food Microbiol. 1996, 44, 207–217. [Google Scholar] [CrossRef]
- Kranker, S.; Dahl, J.; Wingstrand, A. Bacteriological and serological examination and risk factor analysis of Salmonella occurrence in sow herds, including risk factors for high Salmonella seroprevalence in receiver finishing herds. Berl. Munch. Tierarztl. Wochenschr. 2001, 114, 350–352. [Google Scholar]
- EFSA BIOHAZ Panel. Scientific opinion on a quantitative microbiological risk assessment of Salmonella in slaughter and breeder pigs. EFSA J. 2010, 8, 1547. [Google Scholar] [CrossRef]
- Ainslie-Garcia, M.H.; Farzan, A.; Newman, J.E.; Friendship, R.M.; Lillie, B.N. Salmonella fecal shedding in pigs from birth to market and its association with the presence of Salmonella in palatine tonsils and submandibular lymph nodes at slaughter. Can. J. Vet. Res. 2018, 82, 249–255. [Google Scholar] [PubMed]
- Soliani, L.; Rugna, G.; Prosperi, A.; Chiapponi, C.; Luppi, A. Salmonella infection in pigs: Disease, prevalence, and a link between swine and human health. Pathogens 2023, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.P.; Cowled, B.; Galea, F.; Garner, M.G.; Laffan, S.W.; Marsh, I.; Negus, K.; Sarre, S.D.; Woolnough, A.P. Salmonella infection in a remote, isolated wild pig population. Vet. Microbiol. 2013, 162, 921–929. [Google Scholar] [CrossRef]
- Gaffuri, A.; Holmes, J.P. Salmonella infections. In Infectious Diseases of Wild Mammals and Birds in Europe; Gavier-Widén, D., Duff, J.P., Meredith, A., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 398–407. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, N.; Mentaberre, G.; Porrero, C.M.; Serrano, E.; Mateos, A.; López-Martín, J.M.; Lavín, S.; Domínguez, L. Effect of cattle on Salmonella carriage, diversity and antimicrobial resistance in free-ranging wild boar (Sus scrofa) in northeastern Spain. PLoS ONE 2012, 7, e51614. [Google Scholar] [CrossRef]
- Flores Rodas, E.M.; Bogdanova, T.; Bossù, T.; Pecchi, S.; Tomassetti, F.; De Santis, P.; Tolli, R.; Condoleo, R.; Greco, S.; Brozzi, A.; et al. Microbiological assessment of freshly-shot wild boars meat in Lazio Region, Viterbo Territory: A preliminary study. Ital. J. Food Saf. 2014, 3, 1711. [Google Scholar] [CrossRef]
- Razzuoli, E.; Listorti, V.; Martini, I.; Migone, L.; Decastelli, L.; Mignone, W.; Berio, E.; Battistini, R.; Ercolini, C.; Serracca, L.; et al. Prevalence and antimicrobial resistances of Salmonella spp. isolated from wild boars in Liguria Region, Italy. Pathogens 2021, 10, 568. [Google Scholar] [CrossRef]
- Mirceta, J.; Petrovic, J.; Malesevic, M.; Blagojevic, B.; Antic, D. Assessment of microbial carcass contamination of hunted wild boars. Eur. J. Wildl. Res. 2017, 63, 37–44. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; London, L.; Skrzypczak, T.; Kantala, T.; Laamanen, I.; Biström, M.; Maunula, L.; Gadd, T. Foodborne zoonoses common in hunted wild boars. EcoHealth 2020, 17, 512–522. [Google Scholar] [CrossRef]
- Ranucci, D.; Roila, R.; Onofri, A.; Cambiotti, F.; Primavilla, S.; Miraglia, D.; Andoni, E.; Di Cerbo, A.; Branciari, R. Improving hunted wild boar carcass hygiene: Roles of different factors involved in the harvest phase. Foods 2021, 10, 1548. [Google Scholar] [CrossRef]
- Peruzy, M.F.; Murru, N.; Smaldone, G.; Proroga, Y.T.R.; Cristiano, D.; Fioretti, A.; Anastasio, A. Hygiene evaluation and microbiological hazards of hunted wild boar carcasses. Food Control 2022, 135, 108782. [Google Scholar] [CrossRef]
- Bonardi, S.; Tansini, C.; Cacchioli, A.; Soliani, L.; Poli, L.; Lamperti, L.; Gilioli, S. Enterobacteriaceae and Salmonella contamination of wild boar (Sus scrofa) carcasses: Comparison between different sampling strategies. Eur. J. Wildl. Res. 2021, 67, 88. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; Grant, T.H.; Robins-Browne, R.M. Pathogenicity of Yersinia enterocolitica biotype 1A. FEMS Microbiol. Lett. 2003, 38, 127–137. [Google Scholar] [CrossRef]
- Fàbrega, A.; Vila, J. Yersinia enterocolitica: Pathogenesis, virulence and antimicrobial resistance. Enferm. Infecc. Microbiol. Clin. 2012, 30, 24–32. [Google Scholar] [CrossRef]
- Von Altrock, A.; Seinige, D.; Kehrenberg, C. Yersinia enterocolitica isolates from wild boars hunted in Lower Saxony, Germany. Appl. Environ. Microbiol. 2015, 81, 4835–4840. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; Gerhardt, M.; Stolle, A. High bacterial contamination of pig tonsils at slaughter. Meat Sci. 2009, 83, 334–336. [Google Scholar] [CrossRef]
- Fois, F.; Piras, F.; Torpdahl, M.; Mazza, R.; Ladu, D.; Consolati, S.G.; Spanu, C.; Scarano, C.; De Santis, E.P.L. Prevalence, bioserotyping and antibiotic resistance of pathogenic Yersinia enterocolitica detected in pigs at slaughter in Sardinia. Int. J. Food Microbiol. 2018, 283, 1–6. [Google Scholar] [CrossRef]
- Arrausi-Subiza, M.; Ibabe, J.C.; Atxaerandio, R.; Juste, R.A.; Barral, M. Evaluation of different enrichment methods for pathogenic Yersinia species detection by real-time PCR. BMC Vet. Res. 2014, 10, 192. [Google Scholar] [CrossRef]
- Syczyło, K.; Platt-Samoraj, A.; Bancerz-Kisiel, A.; Szczerba-Turek, A.; Pajdak-Czaus, J.; Łabuć, S.; Szweda, W. The prevalence of Yersinia enterocolitica in game animals in Poland. PLoS ONE 2018, 13, e0195136. [Google Scholar] [CrossRef]
- Bancerz-Kisiel, A.; Platt-Samoraj, A.; Szczerba-Turek, A.; Syczyło, K.; Szweda, W. The first pathogenic Yersinia enterocolitica bioserotype 4/O:3 strain isolated from a hunted wild boar (Sus scrofa) in Poland. Epidemiol. Infect. 2015, 143, 2758–2765. [Google Scholar] [CrossRef]
- Carella, E.; Romano, A.; Domenis, L.; Robetto, S.; Spedicato, R.; Guidetti, C.; Orusa, R. Characterisation of Yersinia enterocolitica strains isolated from wildlife in the northwestern Italian Alps. J. Vet. Res. 2022, 66, 141. [Google Scholar] [CrossRef]
- Delibato, E.; Ventola, E.; Lovari, S.; Farneti, S.; Finazzi, G.; Owczarek, S.; Stefano, B. Molecular characterization of Yersinia enterocolitica strains to evaluate virulence-associated genes. Ann. Ist. Super. Sanità 2023, 59, 280–285. [Google Scholar] [CrossRef]
- Arrausi-Subiza, M.; Gerrikagoitia, X.; Alvarez, V.; Ibabe, J.C.; Barral, M. Prevalence of Yersinia enterocolitica and Yersinia pseudotuberculosis in wild boars in the Basque Country, northern Spain. Acta Vet. Scand. 2016, 58, 4. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S.; Brémont, S.; Vismarra, A.; Poli, I.; Diegoli, G.; Bolzoni, L.; Corradi, M.; Gilioli, S.; Le Guern, A.S. Is Yersinia bercovieri surpassing Yersinia enterocolitica in wild boars (Sus scrofa)? EcoHealth 2020, 17, 388–392. [Google Scholar] [CrossRef]
- Slee, K.J.; Skilbeck, N.W. Epidemiology of Yersinia pseudotuberculosis and Y. enterocolitica infections in sheep in Australia. J. Clin. Microbiol. 1992, 30, 712–715. [Google Scholar] [CrossRef]
- Nikolaou, K.; Hensel, A.; Bartling, C.; Tomaso, H.; Arnold, T.; Rösler, U.; Ganter, M.; Petry, T.; Neubauer, H. Prevalence of anti-Yersinia outer protein antibodies in goats in Lower Saxony. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 17–24. [Google Scholar] [CrossRef]
- Piras, F.; Spanu, C.; Sanna, R.; Siddi, G.; Mocci, A.M.; Demontis, M.; Meloni, M.P.; Spanu, V.; De Santis, E.P.L.; Scarano, C. Detection, virulence genes and antimicrobial resistance of Yersinia enterocolitica in sheep and goat raw milk. Int. Dairy J. 2021, 117, 105011. [Google Scholar] [CrossRef]
- Avagnina, A.; Nucera, D.; Grassi, M.A.; Ferroglio, E.; Dalmasso, A.; Civera, T. The microbiological conditions of carcasses from large game animals in Italy. Meat Sci. 2012, 91, 266–271. [Google Scholar] [CrossRef]
- Lorencová, A.; Babák, V.; Lamká, J. Serological prevalence of enteropathogenic Yersinia spp. in pigs and wild boars from different production systems in the Moravian region, Czech Republic. Foodborne Pathog. Dis. 2016, 13, 275–279. [Google Scholar] [CrossRef]
- Modesto, P.; De Ciucis, C.G.; Vencia, W.; Pugliano, M.C.; Mignone, W.; Berio, E.; Masotti, C.; Ercolini, C.; Serracca, L.; Andreoli, T.; et al. Evidence of antimicrobial resistance and presence of pathogenicity genes in Yersinia enterocolitica isolate from wild boars. Pathogens 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Balloni, S.; Altomonte, I.; Martini, M.; Nuvoloni, R.; Cecchi, F.; Pedonese, F.; Salari, F.; Santana Da Silva, A.M.; Torracca, B.; et al. Fatty acid and microbiological profile of the meat (Longissimus dorsi muscle) of wild boar (Sus scrofa scrofa) hunted in Tuscany. Ital. J. Anim. Sci. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Peruzy, M.F.; Cristiano, D.; Delibato, E.; D’Alessio, N.; Proroga, Y.T.R.; Capozza, R.L.; Rippa, A.; Murru, N. Presence of enteric bacterial pathogens in meat samples of wild boar hunted in Campania Region, Southern Italy. Ital. J. Food Saf. 2022, 11, 9967. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Xu, B.; Dong, Q.; Jia, K.; Lin, Z.; Wang, X.; Liu, Y.; Qin, X. Prevalence, antibiotic resistance, resistance and virulence determinants of Campylobacter jejuni in China: A systematic review and meta-analysis. One Health 2025, 20, 100990. [Google Scholar] [CrossRef]
- Carbonero, A.; Paniagua, J.; Torralbo, A.; Arenas-Montes, A.; Borge, C.; García-Bocanegra, I. Campylobacter infection in wild artiodactyl species from southern Spain: Occurrence, risk factors and antimicrobial susceptibility. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 115–121. [Google Scholar] [CrossRef]
- Ziomek, M.; Gondek, M.; Torracca, B.; Marotta, F.; Garofolo, G.; Wieczorek, K.; Michalak, K.; Fratini, F.; Pedonese, F. Occurrence of Campylobacter in faeces, livers and carcasses of wild boars hunted in Tuscany (Italy) and evaluation of MALDI-TOF MS for the identification of Campylobacter species. Foods 2023, 12, 778. [Google Scholar] [CrossRef]
- Abe, T.; Haga, S.; Yokoyama, K.; Watanabe, N. An outbreak of Campylobacter jejuni subsp. jejuni infection via tap water. Jpn. J. Infect. Dis. 2008, 61, 327. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.; Thomas, D.R.; Smith, R.M.; Nehaul, L.; Ribeiro, C.D.; Brown, A.G.; Salmon, R.L. A community outbreak of Campylobacter jejuni infection from a chlorinated public water supply. Epidemiol. Infect. 2007, 135, 1151–1158. [Google Scholar] [CrossRef]
- Gonzalez, M.; Hanninen, M.L. Effect of temperature and antimicrobial resistance on survival of Campylobacter jejuni in well water: Application of the Weibull model. J. Appl. Microbiol. 2012, 113, 284–293. [Google Scholar] [CrossRef]
- Atanassova, V.; Apelt, J.; Reich, F.; Klein, G. Microbiological quality of freshly shot game in Germany. Meat Sci. 2008, 78, 414–419. [Google Scholar] [CrossRef]
- Marotta, F.; Di Marcantonio, L.; Janowicz, A.; Pedonese, F.; Di Donato, G.; Ardelean, A.; Nuvoloni, R.; Di Giannatale, E.; Garofolo, G. Genotyping and antibiotic resistance traits in Campylobacter jejuni and coli from pigs and wild boars in Italy. Front. Cell. Infect. Microbiol. 2020, 10, 592512. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 1989, 2, 15–38. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Pathogenicity assessment of Shiga toxin-producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA J. 2020, 18, e05967. [Google Scholar] [CrossRef]
- Frank, E.; Bonke, R.; Drees, N.; Heurich, M.; Märtlbauer, E.; Gareis, M. Shiga toxin-producing Escherichia coli (STEC) shedding in a wild roe deer population. Vet. Microbiol. 2019, 239, 108479. [Google Scholar] [CrossRef] [PubMed]
- Amézquita-López, B.A.; Soto-Beltrán, M.; Lee, B.G.; Yambao, J.C.; Quiñones, B. Isolation, genotyping and antimicrobial resistance of Shiga toxin-producing Escherichia coli. J. Microbiol. Immunol. Infect. 2018, 51, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; López, C.; Dhabi, G.; López-Beceiro, A.M.; Fidalgo, L.E.; Díaz, E.A.; Martinez-carrasco, C.; Mamani, R.; Herrera, A.; Blanco, J.E.; et al. Seropathotypes, phylogroups, Stx subtypes, and intimin types of wildlife-carried, Shiga toxin-producing Escherichia coli strains with the same characteristics as human-pathogenic isolates. Appl. Environ. Microbiol. 2012, 78, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.A.; Mora, A.; Díaz, D.; Blanco, M.; González-Barrio, D.; Ruiz-Fons, F.; Simon, C.; Blanco, J.; Torres, C. Occurrence and characterization of stx and/or eae-positive Escherichia coli isolated from wildlife, including a typical EPEC strain from a wild boar. Vet. Microbiol. 2017, 207, 69–73. [Google Scholar] [CrossRef]
- Szczerba-Turek, A.; Socha, P.; Bancerz-Kisiel, A.; Platt-Samoraj, A.; Lipczynska-Ilczuk, K.; Siemionek, J.; Kończyk, K.; Terech-Majewska, E.; Szweda, W. Pathogenic potential to humans of Shiga toxin-producing Escherichia coli isolated from wild boars in Poland. Int. J. Food Microbiol. 2019, 300, 8–13. [Google Scholar] [CrossRef]
- Dias, D.; Caetano, T.; Torres, R.T.; Fonseca, C.; Mendo, S. Shiga toxin-producing Escherichia coli in wild ungulates. Sci. Total Environ. 2019, 651, 203–209. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Barmettler, K.; Stevens, M.J.A.; Cernela, N. Shiga toxin-producing Escherichia coli isolated from hunted wild boar (Sus scrofa) in Switzerland. Schweiz. Arch. Tierheilkd 2024, 166, 131–140. [Google Scholar]
- Sannö, A.; Aspán, A.; Hestvik, G.; Jacobson, M. Presence of Salmonella spp., Yersinia enterocolitica, Yersinia pseudotuberculosis and Escherichia coli O157:H7 in wild boars. Epidemiol. Infect. 2014, 142, 2542–2547. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, N.; Porrero, M.C.; Mentaberre, G.; Serrano, E.; Mateos, A.; Cabal, A.; Domínguez, L.; Lavín, S. Escherichia coli O157:H7 in wild boars (Sus scrofa) and Iberian ibex (Capra pyrenaica) sharing pastures with free-ranging livestock in a natural environment in Spain. Vet. Q. 2015, 35, 102–106. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cilia, G.; Bogi, S.; Ebani, V.V.; Turini, L.; Nuvoloni, R.; Cerri, D.; Fratini, F.; Turchi, B. Pathotypes and antimicrobial susceptibility of Escherichia coli isolated from wild boar (Sus. scrofa) in Tuscany. Animals 2020, 10, 744. [Google Scholar] [CrossRef]
- Siddi, G.; Piras, F.; Gymoese, P.; Torpdahl, M.; Meloni, M.P.; Cuccu, M.; Migoni, M.; Cabras, D.; Fredriksson-Ahomaa, M.; De Santis, E.P.; et al. Pathogenic profile and antimicrobial resistance of Escherichia coli, Escherichia marmotae and Escherichia ruysiae detected from hunted wild boars in Sardinia (Italy). Int. J. Food Microbiol. 2024, 421, 110790. [Google Scholar] [CrossRef]
- Díaz-Sánchez, S.; Sánchez, S.; Sánchez, M.; Herrera-León, S.; Hanning, I.; Vidal, D. Detection and characterization of Shiga toxin-producing Escherichia coli in game meat and ready-to-eat meat products. Int. J. Food Microbiol. 2012, 160, 179–182. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Treier, A.; Stevens, M.J.; Stephan, R. Whole genome sequence-based characterisation of Shiga toxin-producing Escherichia coli isolated from game meat originating from several European countries. Sci. Rep. 2023, 13, 3247. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O. Microbiological conditions of meats from large game animals and birds. Meat Sci. 2007, 77, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Vezeau, N.; Kahn, L.H. Spread and mitigation of antimicrobial resistance at the wildlife-urban and wildlife-livestock interfaces. J. Am. Vet. Med. Assoc. 2024, 262, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, N.; Castillo-Contreras, R.; Casas-Díaz, E.; Morellet, N.; Porrero, M.C.; Molina-Vacas, G.; Torres, R.T.; Fonseca, C.; Mentaberre, G.; Domínguez, L.; et al. Carriage of antibiotic-resistant bacteria in urban versus rural wild boars. Eur. J. Wildl. Res. 2018, 64, 1–9. [Google Scholar] [CrossRef]
- Dolejská, M.; Literák, I. Wildlife is overlooked in the epidemiology of medically important antibiotic-resistant bacteria. Antimicrob. Agents Chemother. 2019, 63, e01167-19. [Google Scholar] [CrossRef]
- Altissimi, C.; Noé-Nordberg, C.; Ranucci, D.; Paulsen, P. Presence of foodborne bacteria in wild boar and wild boar meat—A literature survey for the period 2012–2022. Foods 2023, 12, 1689. [Google Scholar] [CrossRef]
- Weber, R.E.; Fleige, C.; Layer, F.; Neumann, B.; Kresken, M.; Werner, G. Determination of a tentative epidemiological cut-off value (ECOFF) for dalbavancin and Enterococcus faecium. Antibiotics 2021, 10, 915. [Google Scholar] [CrossRef]
- Akwongo, C.J.; Borrelli, L.; Houf, K.; Fioretti, A.; Peruzy, M.F.; Murru, N. Antimicrobial resistance in wild game mammals: A glimpse into the contamination of wild habitats in a systematic review and meta-analysis. BMC Vet. Res. 2025, 21, 14. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). ECDC/EFSA/EMA second joint report on the integrated analysis of antimicrobial consumption (AMC) and antimicrobial resistance (AMR) in bacteria from humans and food-producing animals. EFSA J. 2017, 15, 4872. [Google Scholar] [CrossRef]
- Gil-Molino, M.; Gonçalves, P.; Risco, D.; Martín-Cano, F.E.; García, A.; Rey, J.; Quesada, A. Dissemination of antimicrobial-resistant isolates of Salmonella spp. in wild boars and its relationship with management practices. Transbound. Emerg. Dis. 2022, 69, e1488–e1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Shen, J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kastoris, A.C.; Kapaskelis, A.M.; Karageorgopoulos, D.E. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: A systematic review. Lancet Infect. Dis. 2010, 10, 43–50. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). M100 Performance Standards for Antimicrobial Susceptibility Testing A CLSI Supplement for Global Application, 28th ed.; CLSI supplementM100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters 2020. Version 10.0. 2020. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 10 November 2025).
- Fredriksson-Ahomaa, M.; Wacheck, S.; Bonke, R.; Stephan, R. Different enteropathogenic Yersinia strains found in wild boars and domestic pigs. Foodborne Pathog. Dis. 2011, 8, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Mir, N.A.; Dev, K.; Khan, I.A. Dynamics of antibiotic resistance with special reference to Shiga toxin-producing Escherichia coli infections. J. Appl. Microbiol. 2018, 125, 1228–1237. [Google Scholar] [CrossRef]
- Mühlen, S.; Dersch, P. Treatment strategies for infections with Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2020, 10, 169. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, 6007. [Google Scholar] [CrossRef]
- Benavides, J.A.; Salgado-Caxito, M.; Torres, C.; Godreuil, S. Public health implications of antimicrobial resistance in wildlife at the One Health interface. Med. Sci. Forum 2024, 25, 1. [Google Scholar] [CrossRef]
- Luniak, M. Synurbization: Adaptation of animal wildlife to urban development. In Proceedings of the 4th International Symposium on Urban Wildlife Conservation, Tucson, AZ, USA, 1–5 May 1999; Shaw, W.S., Harris, L.K., VanDruff, L., Eds.; University of Arizona: Tucson, AZ, USA, 2004; pp. 50–55. [Google Scholar]
- Ruiz-Ponsell, L.; Monastiri, A.; Lopez-Roig, M.; Sauleda, S.; Bes, M.; Mentaberre, G.; Escobar-González, M.; Costafreda, M.I.; López-Olvera, J.R.; Serra-Cobo, J. Endemic maintenance of human-related hepatitis E virus strains in synurbic wild boars, Barcelona Metropolitan Area, Spain. Sci. Total Environ. 2024, 955, 176871. [Google Scholar] [CrossRef]
- Conejero, C.; González-Crespo, C.; Fatjó, J.; Castillo-Contreras, R.; Serrano, E.; Lavín, S.; Mentaberre, G.; López-Olvera, J.R. Between conflict and reciprocal habituation: Human–wild boar coexistence in urban areas. Sci. Total Environ. 2024, 936, 173258. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Carvalho, J.; Serrano, E.; Mentaberre, G.; Fernández-Aguilar, X.; Colom, A.; González-Crespo, C.; Lavín, S.; López-Olvera, J.R. Urban wild boars prefer fragmented areas with food resources near natural corridors. Sci. Total Environ. 2018, 615, 282–288. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Mentaberre, G.; Fernández-Aguilar, X.; Conejero, C.; Colom-Cadena, A.; Ráez-Bravo, A.; González-Crespo, C.; Espunyes, J.; Lavín, S.; López-Olvera, J.R. Wild boar in the city: Phenotypic responses to urbanisation. Sci. Total Environ. 2021, 773, 145593. [Google Scholar] [CrossRef]
- Conejero, C.; Castillo-Contreras, R.; González-Crespo, C.; Serrano, E.; Mentaberre, G.; Lavín, S.; López-Olvera, J.R. Past experiences drive citizen perception of wild boar in urban areas. Mamm. Biol. 2019, 96, 68–72. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Magen, L.; Birtles, R.; Varela-Castro, L.; Hall, J.L.; Conejero, C.; Aguilar, X.F.; Colom-Cadena, A.; Lavín, S.; Mentaberre, G.; et al. Ticks on wild boar in the metropolitan area of Barcelona (Spain) are infected with spotted fever group rickettsiae. Transbound. Emerg. Dis. 2021, 68, 3223–3231. [Google Scholar] [CrossRef]
- Darwich, L.; Seminati, C.; López-Olvera, J.R.; Vidal, A.; Aguirre, L.; Cerdá, M.; Garcias, B.; Valldeperes, M.; Castillo-Contreras, R.; Migura-Garcia, L.; et al. Detection of beta-lactam-resistant Escherichia coli and toxigenic Clostridioides difficile strains in wild boars foraging in an anthropization gradient. Animals 2021, 11, 1585. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Thomas, V.; Simjee, S.; Godinho, K.; Schiessl, B.; Klein, U.; Butty, P.; Vallé, M.; Marion, H.; Shryock, T.R. Pan-European monitoring of susceptibility to human-use antimicrobial agents in enteric bacteria isolated from healthy food-producing animals. J. Antimicrob. Chemother. 2012, 67, 638–665. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Simjee, S.; El Garch, F.; Moyaert, H.; Rose, M.; Youala, M.; Dry, M. Antimicrobial susceptibility of enterococci recovered from healthy cattle, pigs and chickens in nine EU countries (EASSA Study) to critically important antibiotics. Vet. Microbiol. 2018, 216, 168–175. [Google Scholar] [CrossRef] [PubMed]
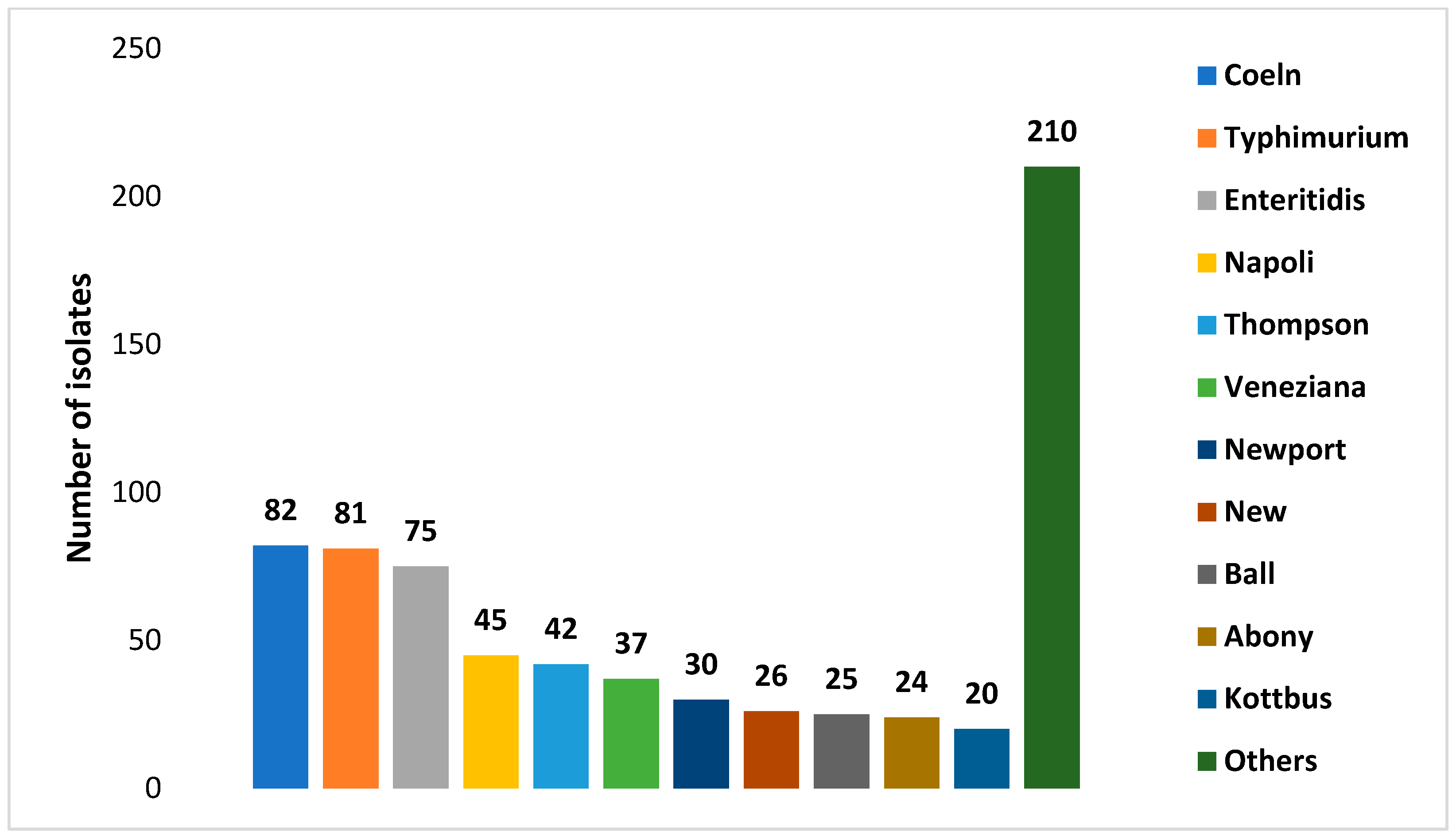
| Source | Salmonella spp. | Y. enterocolitica | STEC | Campylobacter spp. |
|---|---|---|---|---|
| Feces | 0–35.6 | 0–27.3 | 0–28.3 | 24.4–66 |
| Lymph nodes | 0–17.8 | 0–12 | 0 | 0 |
| Tonsils | 0–35.8 | 8–33.3 | 0 | n.i. |
| Carcass surface | 0–7.8 | 0–33.3 | 4 | 11.1–18.2 |
| Muscle/meat | 2.8–3.6 | 0–27 | 2.7–42.8 | 0–2.4 |
| Liver | 1.7–6 | 2.6 | n.i. | 4.81 |
| Other organs (kidneys, spleen) | 2–4.6 | 17 | n.i. | n.i. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piras, F.; Siddi, G.; De Santis, E.P.L.; Scarano, C. Enteric Pathogens in Wild Boars Across the European Union: Prevalence and Antimicrobial Resistance Within a One Health Framework. Antibiotics 2025, 14, 1246. https://doi.org/10.3390/antibiotics14121246
Piras F, Siddi G, De Santis EPL, Scarano C. Enteric Pathogens in Wild Boars Across the European Union: Prevalence and Antimicrobial Resistance Within a One Health Framework. Antibiotics. 2025; 14(12):1246. https://doi.org/10.3390/antibiotics14121246
Chicago/Turabian StylePiras, Francesca, Giuliana Siddi, Enrico Pietro Luigi De Santis, and Christian Scarano. 2025. "Enteric Pathogens in Wild Boars Across the European Union: Prevalence and Antimicrobial Resistance Within a One Health Framework" Antibiotics 14, no. 12: 1246. https://doi.org/10.3390/antibiotics14121246
APA StylePiras, F., Siddi, G., De Santis, E. P. L., & Scarano, C. (2025). Enteric Pathogens in Wild Boars Across the European Union: Prevalence and Antimicrobial Resistance Within a One Health Framework. Antibiotics, 14(12), 1246. https://doi.org/10.3390/antibiotics14121246






