Beyond the Spotlight: Enterobacter spp. as Overlooked Carbapenemase Producers in Europe
Abstract
1. Introduction
2. Taxonomy and Clinical Relevance of Enterobacter spp.
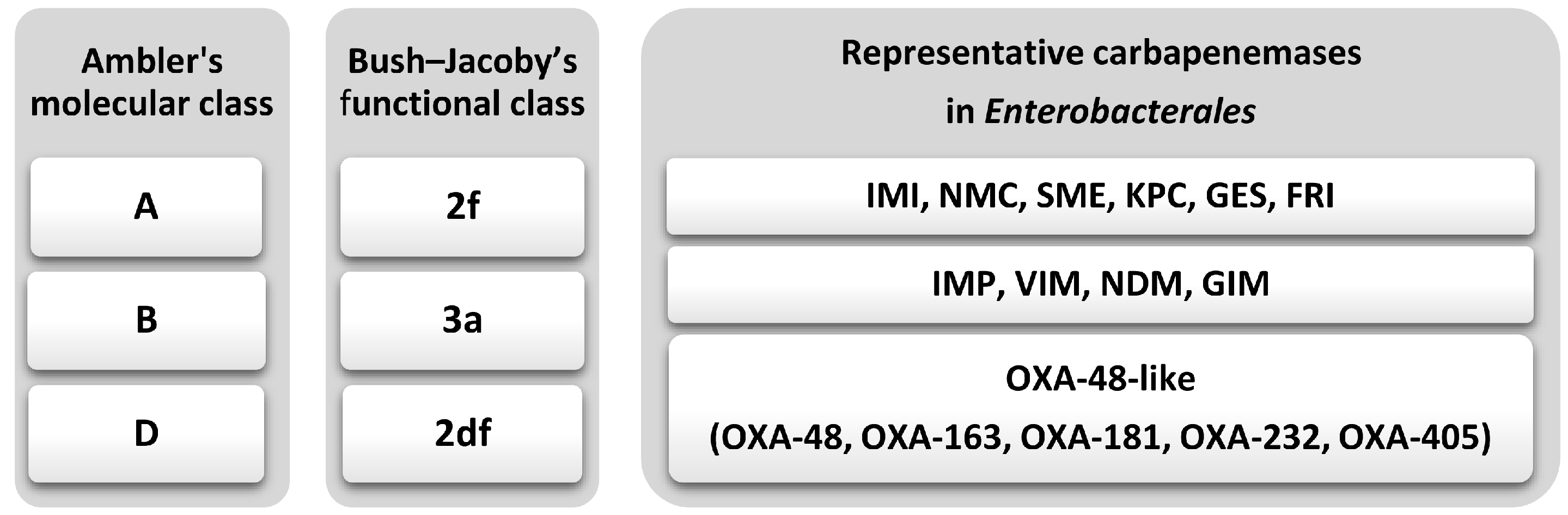
3. Carbapenemases: Classification and Genetic Context
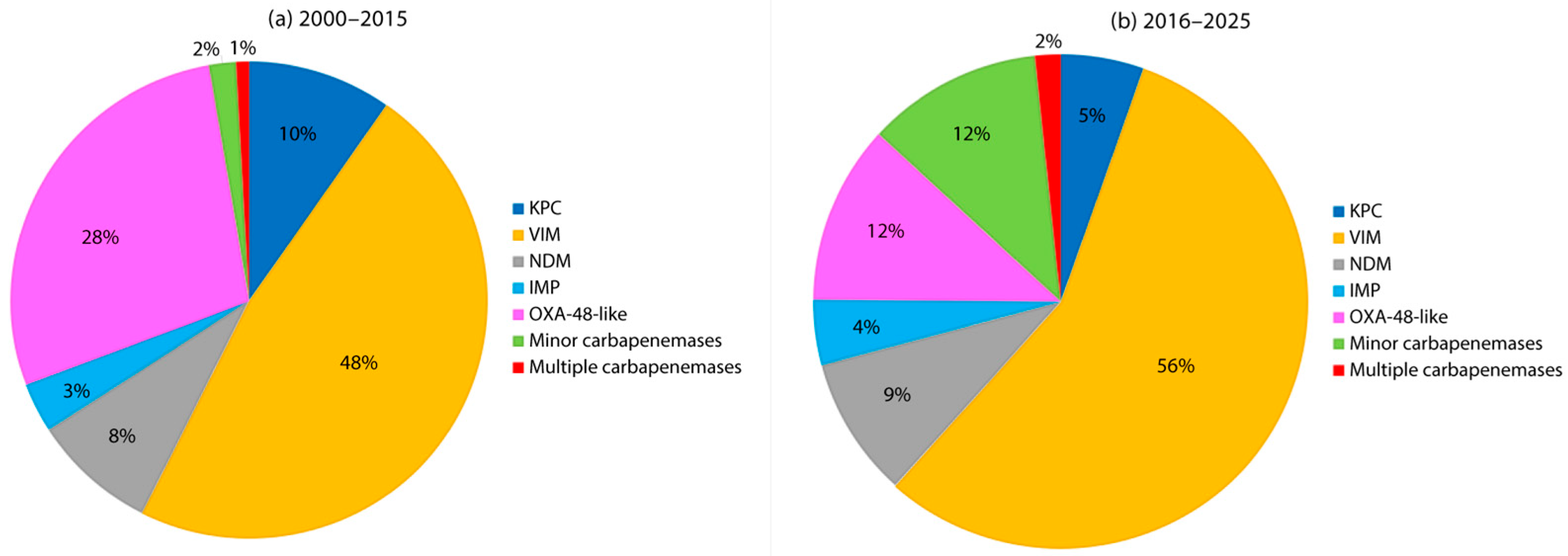
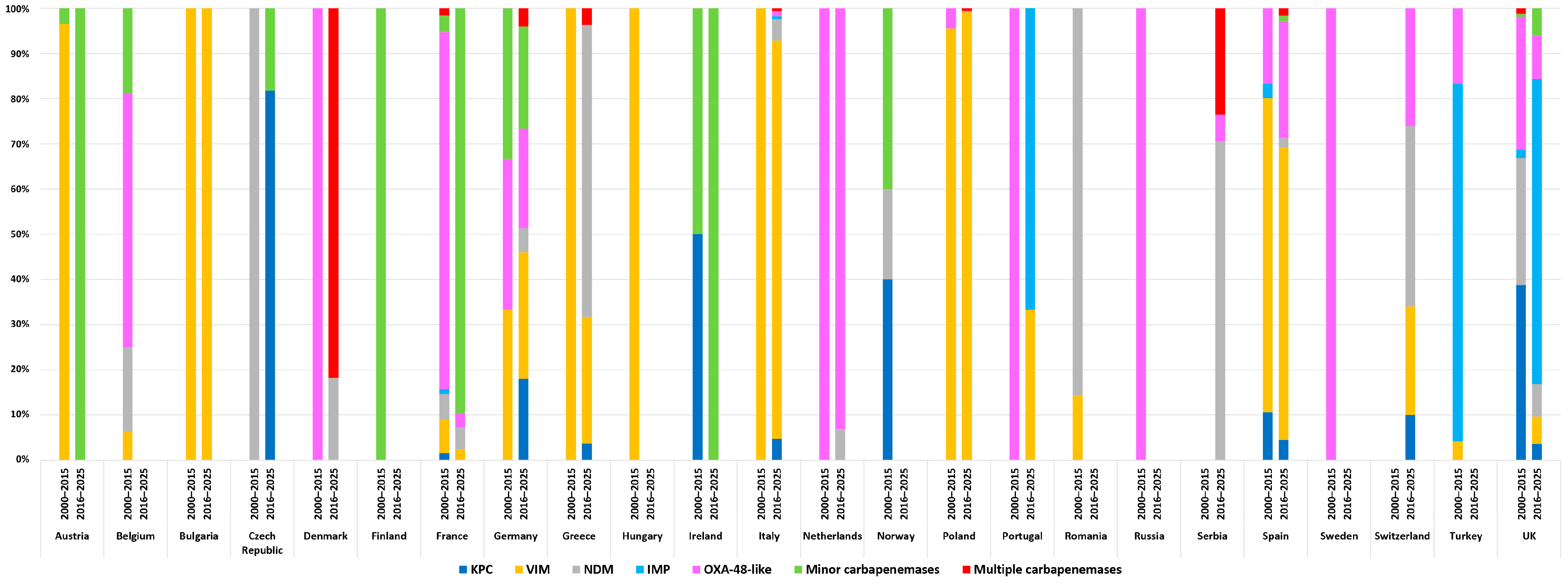
4. Epidemiology of Carbapenemase-Producing Enterobacter spp. in Europe
4.1. IMI-like Enzymes
4.2. KPC
4.3. GES
4.4. FRI
4.5. IMP
4.6. VIM
4.7. NDM
4.8. GIM
4.9. OXA-48 Like
4.10. Multiple Carbapenemases
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet Lond. Engl. 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- WHO. Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 31 July 2025).
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- CDC. 2019 Antibiotic Resistance Threats Report. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 31 July 2025).
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 31 July 2025).
- Davin-Regli, A.; Lavigne, J.-P.; Pagès, J.-M. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin. Microbiol. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef]
- Mezzatesta, M.L.; Gona, F.; Stefani, S. Enterobacter cloacae complex: Clinical Impact and Emerging Antibiotic Resistance. Future Microbiol. 2012, 7, 887–902. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Paauw, A.; Caspers, M.P.M.; Schuren, F.H.J.; Leverstein-van Hall, M.A.; Delétoile, A.; Montijn, R.C.; Verhoef, J.; Fluit, A.C. Genomic Diversity within the Enterobacter cloacae complex. PLoS ONE 2008, 3, e3018. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P. Intragenomic Heterogeneity between Multiple 16S Ribosomal RNA Operons in Sequenced Bacterial Genomes. FEMS Microbiol. Lett. 2003, 228, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Roggenkamp, A. Population Genetics of the Nomenspecies Enterobacter cloacae. Appl. Environ. Microbiol. 2003, 69, 5306–5318. [Google Scholar] [CrossRef]
- Wu, W.; Feng, Y.; Zong, Z. Precise Species Identification for Enterobacter: A Genome Sequence-Based Study with Reporting of Two Novel Species, Enterobacter quasiroggenkampii sp. nov. and Enterobacter quasimori sp. nov. mSystems 2020, 5, e00527-20. [Google Scholar] [CrossRef]
- Besser, J.; Carleton, H.A.; Gerner-Smidt, P.; Lindsey, R.L.; Trees, E. Next-Generation Sequencing Technologies and Their Application to the Study and Control of Bacterial Infections. Clin. Microbiol. Infect. 2018, 24, 335–341. [Google Scholar] [CrossRef]
- Tenover, F.C.; Tickler, I.A. Genomic Analysis of Enterobacter Species Isolated from Patients in United States Hospitals. Antibiotics 2024, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-Resistant Enterobacter cloacae complex Emerging as a Global, Diversifying Threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef]
- Chudejova, K.; Rotova, V.; Skalova, A.; Medvecky, M.; Adamkova, V.; Papagiannitsis, C.C.; Hrabak, J. Emergence of Sequence Type 252 Enterobacter cloacae Producing GES-5 Carbapenemase in a Czech Hospital. Diagn. Microbiol. Infect. Dis. 2018, 90, 148–150. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase–Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.J.; Stoesser, N.; Sheppard, A.E.; Abuoun, M.; Fowler, P.; Swann, J.; Quan, T.P.; Griffiths, D.; Vaughan, A.; Morgan, M.; et al. Reconciling the Potentially Irreconcilable? Genotypic and Phenotypic Amoxicillin-Clavulanate Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2020, 64, e02026-19. [Google Scholar] [CrossRef]
- Pacios, O.; Fernández-García, L.; Bleriot, I.; Blasco, L.; Ambroa, A.; López, M.; Ortiz-Cartagena, C.; González de Aledo, M.; Fernández-Cuenca, F.; Oteo-Iglesias, J.; et al. Adaptation of Clinical Isolates of Klebsiella pneumoniae to the Combination of Niclosamide with the Efflux Pump Inhibitor Phenyl-Arginine-β-Naphthylamide (PaβN): Co-Resistance to Antimicrobials. J. Antimicrob. Chemother. 2022, 77, 1272–1281. [Google Scholar] [CrossRef]
- Livermore, D.M. Beta-Lactamases in Laboratory and Clinical Resistance. Clin. Microbiol. Rev. 1995, 8, 557–584. [Google Scholar] [CrossRef]
- Takei, K.; Ogawa, M.; Sakata, R.; Kanamori, H. Epidemiological Characteristics of Carbapenem-Resistant Enterobacterales in Japan: A Nationwide Analysis of Data from a Clinical Laboratory Center (2016–2022). Pathogens 2023, 12, 1246. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The Versatile Beta-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Ambler, R.P. The Structure of Beta-Lactamases. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1980, 289, 321–331. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of Beta-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Al Hassan, S.; Shaker, W.; El Hajjar, R.; Allam, S.; Bou Zerdan, M.; Naji, A.; Zeineddine, N. Carbapenemase Inhibitors: Updates on Developments in 2021. J. Clin. Med. Res. 2022, 14, 251–259. [Google Scholar] [CrossRef]
- Rasmussen, B.A.; Bush, K. Carbapenem-Hydrolyzing Beta-Lactamases. Antimicrob. Agents Chemother. 1997, 41, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, R.A.; Jousset, A.B.; Emeraud, C.; Oueslati, S.; Dortet, L.; Naas, T. Genetic Diversity, Biochemical Properties, and Detection Methods of Minor Carbapenemases in Enterobacterales. Front. Med. 2020, 7, 616490. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Doi, Y. The Global Epidemiology of Carbapenemase-Producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem Resistance in Enterobacteriaceae: Here Is the Storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef]
- Ding, L.; Shen, S.; Chen, J.; Tian, Z.; Shi, Q.; Han, R.; Guo, Y.; Hu, F. Klebsiella pneumoniae Carbapenemase Variants: The New Threat to Global Public Health. Clin. Microbiol. Rev. 2023, 36, e0000823. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Bonomo, R.A. “Stormy Waters Ahead”: Global Emergence of Carbapenemases. Front. Microbiol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Alvisi, G.; Curtoni, A.; Fonnesu, R.; Piazza, A.; Signoretto, C.; Piccinini, G.; Sassera, D.; Gaibani, P. Epidemiology and Genetic Traits of Carbapenemase-Producing Enterobacterales: A Global Threat to Human Health. Antibiotics 2025, 14, 141. [Google Scholar] [CrossRef]
- Eichenberger, E.M.; Thaden, J.T. Epidemiology and Mechanisms of Resistance of Extensively Drug Resistant Gram-Negative Bacteria. Antibiotics 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Osano, E.; Arakawa, Y.; Wacharotayankun, R.; Ohta, M.; Horii, T.; Ito, H.; Yoshimura, F.; Kato, N. Molecular Characterization of an Enterobacterial Metallo Beta-Lactamase Found in a Clinical Isolate of Serratia marcescens That Shows Imipenem Resistance. Antimicrob. Agents Chemother. 1994, 38, 71–78. [Google Scholar] [CrossRef]
- Taggar, G.; Attiq Rheman, M.; Boerlin, P.; Diarra, M.S. Molecular Epidemiology of Carbapenemases in Enterobacteriales from Humans, Animals, Food and the Environment. Antibiotics 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a New Metallo-Beta-Lactamase Gene, bla(NDM-1), and a Novel Erythromycin Esterase Gene Carried on a Unique Genetic Structure in Klebsiella pneumoniae Sequence Type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Peirano, G.; Kock, M.M.; Strydom, K.-A.; Matsumura, Y. The Global Ascendency of OXA-48-Type Carbapenemases. Clin. Microbiol. Rev. 2019, 33, e00102-19. [Google Scholar] [CrossRef]
- Nordmann, P.; Mariotte, S.; Naas, T.; Labia, R.; Nicolas, M.H. Biochemical Properties of a Carbapenem-Hydrolyzing Beta-Lactamase from Enterobacter cloacae and Cloning of the Gene into Escherichia coli. Antimicrob. Agents Chemother. 1993, 37, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; D’Andrea, M.M.; Di Pilato, V.; Viaggi, B.; Torricelli, F.; Rossolini, G.M. Characterization of a Novel Putative Xer-Dependent Integrative Mobile Element Carrying the blaNMC-A Carbapenemase Gene, Inserted into the Chromosome of Members of the Enterobacter cloacae complex. Antimicrob. Agents Chemother. 2015, 59, 6620–6624. [Google Scholar] [CrossRef]
- Österblad, M.; Kirveskari, J.; Hakanen, A.J.; Tissari, P.; Vaara, M.; Jalava, J. Carbapenemase-Producing Enterobacteriaceae in Finland: The First Years (2008–11). J. Antimicrob. Chemother. 2012, 67, 2860–2864. [Google Scholar] [CrossRef]
- Boo, T.W.; O’Connell, N.; Power, L.; O’Connor, M.; King, J.; McGrath, E.; Hill, R.; Hopkins, K.L.; Woodford, N. First Report of IMI-1-Producing Colistin-Resistant Enterobacter Clinical Isolate in Ireland, March 2013. Euro Surveill. 2013, 18, 20548. [Google Scholar] [CrossRef]
- Farrell, M.L.; Chueiri, A.; Maguire, M.; Kovářová, A.; Miliotis, G.; O’Connor, L.; McDonagh, F.; Duane, S.; Cormican, M.; Devane, G.; et al. Longitudinal Carriage of Antimicrobial Resistant Enterobacterales in Healthy Individuals in Ireland—Assessing the Impact of Recreational Water Use on Duration of Carriage. Sci. Total Environ. 2023, 905, 167100. [Google Scholar] [CrossRef]
- Naas, T.; Cattoen, C.; Bernusset, S.; Cuzon, G.; Nordmann, P. First Identification of blaIMI-1 in an Enterobacter cloacae Clinical Isolate from France. Antimicrob. Agents Chemother. 2012, 56, 1664–1665. [Google Scholar] [CrossRef]
- Emeraud, C.; Girlich, D.; Deschamps, M.; Rezzoug, I.; Jacquemin, A.; Jousset, A.B.; Lecolant, S.; Locher, L.; Birer, A.; Naas, T.; et al. IMI-Type Carbapenemase-Producing Enterobacter cloacae complex, France and Overseas Regions, 2012–2022. Emerg. Infect. Dis. 2024, 30, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Miltgen, G.; Bonnin, R.A.; Avril, C.; Benoit-Cattin, T.; Martak, D.; Leclaire, A.; Traversier, N.; Roquebert, B.; Jaffar-Bandjee, M.-C.; Lugagne, N.; et al. Outbreak of IMI-1 Carbapenemase-Producing Colistin-Resistant Enterobacter cloacae on the French Island of Mayotte (Indian Ocean). Int. J. Antimicrob. Agents 2018, 52, 416–420. [Google Scholar] [CrossRef]
- Blanco-Martín, T.; Guzmán-Puche, J.; Riazzo, C.; Gasca-Santiyán, M.; Hernández-García, M.; Cantón, R.; Torre-Cisneros, J.; Herrera, C.; Martínez-Martínez, L. Phenotypic and Molecular Characterization of an Enterobacter ludwigii Clinical Isolate Carrying a Plasmid-Mediated blaIMI-6 Gene. Microbiol. Spectr. 2023, 11, e04620-22. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá González, R.; Iglesias Llorente, L.; Fernández-Paniagua, E.Á.; Alonso Acero, L.; Monserrat Blázquez, T.; Horcajada, I.; Florén Zabala, L.F. Nosocomial Cluster of Patients Infected with Imipenemase-1-Producing Enterobacter ludwigii. J. Med. Microbiol. 2024, 73, 001919. [Google Scholar] [CrossRef]
- Samuelsen, Ø.; Overballe-Petersen, S.; Bjørnholt, J.V.; Brisse, S.; Doumith, M.; Woodford, N.; Hopkins, K.L.; Aasnæs, B.; Haldorsen, B.; Sundsfjord, A.; et al. Molecular and Epidemiological Characterization of Carbapenemase-Producing Enterobacteriaceae in Norway, 2007 to 2014. PLoS ONE 2017, 12, e0187832. [Google Scholar] [CrossRef]
- Rotova, V.; Papagiannitsis, C.C.; Chudejova, K.; Medvecky, M.; Skalova, A.; Adamkova, V.; Hrabak, J. First Description of the Emergence of Enterobacter asburiae Producing IMI-2 Carbapenemase in the Czech Republic. J. Glob. Antimicrob. Resist. 2017, 11, 98–99. [Google Scholar] [CrossRef]
- Hartl, R.; Kerschner, H.; Gattringer, R.; Lepuschitz, S.; Allerberger, F.; Sorschag, S.; Ruppitsch, W.; Apfalter, P. Whole-Genome Analysis of a Human Enterobacter mori Isolate Carrying a blaIMI-2 Carbapenemase in Austria. Microb. Drug Resist. 2019, 25, 94–96. [Google Scholar] [CrossRef]
- Toner, G.; Russell, C.D.; Hamilton, F.; Templeton, K.; Laurenson, I.F. Phenotypic and Molecular Detection Methods for Carbapenemase-Producing Organisms and Their Clinical Significance at Two Scottish Tertiary Care Hospitals. J. Med. Microbiol. 2019, 68, 560–565. [Google Scholar] [CrossRef]
- Petrella, S.; Ziental-Gelus, N.; Mayer, C.; Renard, M.; Jarlier, V.; Sougakoff, W. Genetic and Structural Insights into the Dissemination Potential of the Extremely Broad-Spectrum Class A Beta-Lactamase KPC-2 Identified in an Escherichia coli Strain and an Enterobacter cloacae Strain Isolated from the Same Patient in France. Antimicrob. Agents Chemother. 2008, 52, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Vaux, S.; Carbonne, A.; Thiolet, J.M.; Jarlier, V.; Coignard, B. RAISIN and Expert Laboratories Groups Emergence of Carbapenemase-Producing Enterobacteriaceae in France, 2004 to 2011. Euro Surveill. 2011, 16, 19880. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Cuzon, G.; Ponties, V.; Nordmann, P. Trends in Carbapenemase-Producing Enterobacteriaceae, France, 2012 to 2014. Euro Surveill. 2017, 22, 30461. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.; Saez, D.; Bautista, V.; Fernández-Romero, S.; Hernández-Molina, J.M.; Pérez-Vázquez, M.; Aracil, B.; Campos, J. Spanish Collaborating Group for the Antibiotic Resistance Surveillance Program Carbapenemase-Producing Enterobacteriaceae in Spain in 2012. Antimicrob. Agents Chemother. 2013, 57, 6344–6347. [Google Scholar] [CrossRef]
- Pena, I.; Picazo, J.J.; Rodríguez-Avial, C.; Rodríguez-Avial, I. Carbapenemase-Producing Enterobacteriaceae in a Tertiary Hospital in Madrid, Spain: High Percentage of Colistin Resistance among VIM-1-Producing Klebsiella pneumoniae ST11 Isolates. Int. J. Antimicrob. Agents 2014, 43, 460–464. [Google Scholar] [CrossRef]
- Villa, J.; Viedma, E.; Brañas, P.; Orellana, M.A.; Otero, J.R.; Chaves, F. Multiclonal Spread of VIM-1-Producing Enterobacter cloacae Isolates Associated with In624 and In488 Integrons Located in an IncHI2 Plasmid. Int. J. Antimicrob. Agents 2014, 43, 451–455. [Google Scholar] [CrossRef]
- Oteo, J.; Pérez-Vázquez, M.; Bautista, V.; Ortega, A.; Zamarrón, P.; Saez, D.; Fernández-Romero, S.; Lara, N.; Ramiro, R.; Aracil, B.; et al. The Spread of KPC-Producing Enterobacteriaceae in Spain: WGS Analysis of the Emerging High-Risk Clones of Klebsiella pneumoniae ST11/KPC-2, ST101/KPC-2 and ST512/KPC-3. J. Antimicrob. Chemother. 2016, 71, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.; Hernández-García, M.; Del Campo, R.; Martínez-García, L.; Gijón, D.; Morosini, M.I.; Ruiz-Garbajosa, P.; Cantón, R. Emergence and Persistence over Time of Carbapenemase-Producing Enterobacter Isolates in a Spanish University Hospital in Madrid, Spain (2005–2018). Microb. Drug Resist. 2021, 27, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, N.; Phan, H.T.T.; Seale, A.C.; Aiken, Z.; Thomas, S.; Smith, M.; Wyllie, D.; George, R.; Sebra, R.; Mathers, A.J.; et al. Genomic Epidemiology of Complex, Multispecies, Plasmid-Borne blaKPC Carbapenemase in Enterobacterales in the United Kingdom from 2009 to 2014. Antimicrob. Agents Chemother. 2020, 64, e02244-19. [Google Scholar] [CrossRef]
- Findlay, J.; Hopkins, K.L.; Alvarez-Buylla, A.; Meunier, D.; Mustafa, N.; Hill, R.; Pike, R.; McCrae, L.-X.; Hawkey, P.M.; Woodford, N. Characterization of Carbapenemase-Producing Enterobacteriaceae in the West Midlands Region of England: 2007-14. J. Antimicrob. Chemother. 2017, 72, 1054–1062. [Google Scholar] [CrossRef]
- Kraftova, L.; Finianos, M.; Studentova, V.; Chudejova, K.; Jakubu, V.; Zemlickova, H.; Papagiannitsis, C.C.; Bitar, I.; Hrabak, J. Evidence of an Epidemic Spread of KPC-Producing Enterobacterales in Czech Hospitals. Sci. Rep. 2021, 11, 15732. [Google Scholar] [CrossRef]
- Yao, Y.; Falgenhauer, L.; Rezazadeh, Y.; Falgenhauer, J.; IncN Study Group; Imirzalioglu, C.; Chakraborty, T. Predominant Transmission of KPC-2 Carbapenemase in Germany by a Unique IncN Plasmid Variant Harboring a Novel Non-Transposable Element (NTE KPC-Y). Microbiol. Spectr. 2024, 12, e0256423. [Google Scholar] [CrossRef]
- Fasciana, T.; Antonelli, A.; Bianco, G.; Lombardo, D.; Codda, G.; Roscetto, E.; Perez, M.; Lipari, D.; Arrigo, I.; Galia, E.; et al. Multicenter Study on the Prevalence of Colonization Due to Carbapenem-Resistant Enterobacterales Strains before and during the First Year of COVID-19, Italy 2018-2020. Front. Public Health 2023, 11, 1270924. [Google Scholar] [CrossRef]
- O’Connor, C.; Cormican, M.; Boo, T.W.; McGrath, E.; Slevin, B.; O’Gorman, A.; Commane, M.; Mahony, S.; O’Donovan, E.; Powell, J.; et al. An Irish Outbreak of New Delhi Metallo-β-Lactamase (NDM)-1 Carbapenemase-Producing Enterobacteriaceae: Increasing but Unrecognized Prevalence. J. Hosp. Infect. 2016, 94, 351–357. [Google Scholar] [CrossRef]
- Izdebski, R.; Baraniak, A.; Herda, M.; Fiett, J.; Bonten, M.J.M.; Carmeli, Y.; Goossens, H.; Hryniewicz, W.; Brun-Buisson, C.; Gniadkowski, M.; et al. MLST Reveals Potentially High-Risk International Clones of Enterobacter cloacae. J. Antimicrob. Chemother. 2015, 70, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ljungquist, O.; Haldorsen, B.; Pöntinen, A.K.; Janice, J.; Josefsen, E.H.; Elstrøm, P.; Kacelnik, O.; Sundsfjord, A.; Samuelsen, Ø.; Handal, N.; et al. Nationwide, Population-Based Observational Study of the Molecular Epidemiology and Temporal Trend of Carbapenemase-Producing Enterobacterales in Norway, 2015 to 2021. Euro Surveill. 2023, 28, 2200774. [Google Scholar] [CrossRef]
- Finianos, M.; Kraftova, L.; Papagiannitsis, C.C.; Adamkova, V.; Hrabak, J.; Bitar, I. Genomic Characterisation of Three GES-Producing Enterobacterales Isolated in the Czech Republic. J. Glob. Antimicrob. Resist. 2022, 29, 116–119. [Google Scholar] [CrossRef]
- Ellington, M.J.; Davies, F.; Jauneikaite, E.; Hopkins, K.L.; Turton, J.F.; Adams, G.; Pavlu, J.; Innes, A.J.; Eades, C.; Brannigan, E.T.; et al. A Multispecies Cluster of GES-5 Carbapenemase-Producing Enterobacterales Linked by a Geographically Disseminated Plasmid. Clin. Infect. Dis. 2020, 71, 2553–2560. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Karlowsky, J.A.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. Epidemiology of Carbapenem Resistance Determinants Identified in Meropenem-Nonsusceptible Enterobacterales Collected as Part of a Global Surveillance Program, 2012 to 2017. Antimicrob. Agents Chemother. 2021, 65, e0200020. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Poirel, L.; Abbas, S.; Oueslati, S.; Nordmann, P. Genetic and Biochemical Characterization of FRI-1, a Carbapenem-Hydrolyzing Class A β-Lactamase from Enterobacter cloacae. Antimicrob. Agents Chemother. 2015, 59, 7420–7425. [Google Scholar] [CrossRef] [PubMed]
- Meunier, D.; Findlay, J.; Doumith, M.; Godoy, D.; Perry, C.; Pike, R.; Gronthoud, F.; Shryane, T.; Poirel, L.; Welfare, W.; et al. FRI-2 Carbapenemase-Producing Enterobacter cloacae complex in the UK. J. Antimicrob. Chemother. 2017, 72, 2478–2482. [Google Scholar] [CrossRef]
- Schauer, J.; Gatermann, S.G.; Marschal, M.; Pfennigwerth, N. Genetic and Biochemical Characterization of FRI-3, a Novel Variant of the Ambler Class A Carbapenemase FRI-1. J. Antimicrob. Chemother. 2019, 74, 2891–2894. [Google Scholar] [CrossRef]
- Deshpande, L.M.; Jones, R.N.; Fritsche, T.R.; Sader, H.S. Occurrence and Characterization of Carbapenemase-Producing Enterobacteriaceae: Report from the SENTRY Antimicrobial Surveillance Program (2000–2004). Microb. Drug Resist. 2006, 12, 223–230. [Google Scholar] [CrossRef]
- Shet, V.; Gouliouris, T.; Brown, N.M.; Turton, J.F.; Zhang, J.; Woodford, N. IMP Metallo-β-Lactamase-Producing Clinical Isolates of Enterobacter cloacae in the UK. J. Antimicrob. Chemother. 2011, 66, 1408–1409. [Google Scholar] [CrossRef]
- Oteo, J.; Ortega, A.; Bartolomé, R.; Bou, G.; Conejo, C.; Fernández-Martínez, M.; González-López, J.J.; Martínez-García, L.; Martínez-Martínez, L.; Merino, M.; et al. Prospective Multicenter Study of Carbapenemase-Producing Enterobacteriaceae from 83 Hospitals in Spain Reveals High In Vitro Susceptibility to Colistin and Meropenem. Antimicrob. Agents Chemother. 2015, 59, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Izdebski, R.; Biedrzycka, M.; Urbanowicz, P.; Żabicka, D.; Gniadkowski, M. Genome-Based Epidemiologic Analysis of VIM/IMP Carbapenemase-Producing Enterobacter spp., Poland. Emerg. Infect. Dis. 2023, 29, 1618–1626. [Google Scholar] [CrossRef]
- Menozzi, I.; Scaltriti, E.; Secci, B.; Dodi, A.; Lazzarotto, T.; Pongolini, S.; Foschi, C.; Ambretti, S. First Report of an Enterobacter cloacae ST837 Resistant to Cefiderocol Co-Harbouring blaIMP-19 and mcr-4.3 Resistance Genes in Italy. J. Glob. Antimicrob. Resist. 2025, 42, 37–41. [Google Scholar] [CrossRef]
- Manageiro, V.; Cano, M.; Furtado, C.; Iglesias, C.; Reis, L.; Vieira, P.; Teixeira, A.; Martins, C.; Veloso, I.; Machado, J.; et al. Genomic and Epidemiological Insight of an Outbreak of Carbapenemase-Producing Enterobacterales in a Portuguese Hospital with the Emergence of the New KPC-124. J. Infect. Public Health 2024, 17, 386–395. [Google Scholar] [CrossRef]
- Matsumura, Y.; Peirano, G.; Bradford, P.A.; Motyl, M.R.; DeVinney, R.; Pitout, J.D.D. Genomic Characterization of IMP and VIM Carbapenemase-Encoding Transferable Plasmids of Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 3034–3038. [Google Scholar] [CrossRef]
- Gacar, G.G.; Midilli, K.; Kolayli, F.; Ergen, K.; Gundes, S.; Hosoglu, S.; Karadenizli, A.; Vahaboglu, H. Genetic and Enzymatic Properties of Metallo-β-Lactamase VIM-5 from a Clinical Isolate of Enterobacter cloacae. Antimicrob. Agents Chemother. 2005, 49, 4400–4403. [Google Scholar] [CrossRef]
- Galani, I.; Souli, M.; Chryssouli, Z.; Orlandou, K.; Giamarellou, H. Characterization of a New Integron Containing bla(VIM-1) and aac(6’)-IIc in an Enterobacter cloacae Clinical Isolate from Greece. J. Antimicrob. Chemother. 2005, 55, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Souli, M.; Kontopidou, F.V.; Papadomichelakis, E.; Galani, I.; Armaganidis, A.; Giamarellou, H. Clinical Experience of Serious Infections Caused by Enterobacteriaceae Producing VIM-1 Metallo-Beta-Lactamase in a Greek University Hospital. Clin. Infect. Dis. 2008, 46, 847–854. [Google Scholar] [CrossRef]
- Koratzanis, E.; Souli, M.; Galani, I.; Chryssouli, Z.; Armaganidis, A.; Giamarellou, H. Epidemiology and Molecular Characterisation of Metallo-β-Lactamase-Producing Enterobacteriaceae in a University Hospital Intensive Care Unit in Greece. Int. J. Antimicrob. Agents 2011, 38, 390–397. [Google Scholar] [CrossRef]
- Koumaki, V.; Voudanta, E.; Michelaki, A.; Orfanidou, M.; Vagiakou, E.; Vrioni, G.; Tsakris, A. Changing Epidemiology of Carbapenemases Among Carbapenem-Resistant Enterobacterales in a Greek Tertiary Care Hospital in Athens, 2020 to 2023. Antibiotics 2025, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Tato, M.; Coque, T.M.; Ruíz-Garbajosa, P.; Pintado, V.; Cobo, J.; Sader, H.S.; Jones, R.N.; Baquero, F.; Cantón, R. Complex Clonal and Plasmid Epidemiology in the First Outbreak of Enterobacteriaceae Infection Involving VIM-1 Metallo-Beta-Lactamase in Spain: Toward Endemicity? Clin. Infect. Dis. 2007, 45, 1171–1178. [Google Scholar] [CrossRef]
- Treviño, M.; Moldes, L.; Martínez-Lamas, L.; Varón, C.; Regueiro, B.J. Carbapenem-Resistant Enterobacter cloacae and the Emergence of Metallo-Beta-Lactamase-Producing Strains in a Third-Level Hospital (Santiago de Compostela, NW Spain). Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Piedra-Carrasco, N.; Bartolomé, R.; Quintero-Zarate, J.N.; Larrosa, N.; Cornejo-Sánchez, T.; Prats, G.; Garcillán-Barcia, M.P.; de la Cruz, F.; González-Lopéz, J.J. Role of IncHI2 Plasmids Harbouring blaVIM-1, blaCTX-M-9, aac(6′)-Ib and qnrA Genes in the Spread of Multiresistant Enterobacter cloacae and Klebsiella pneumoniae Strains in Different Units at Hospital Vall d’Hebron, Barcelona, Spain. Int. J. Antimicrob. Agents 2012, 39, 514–517. [Google Scholar] [CrossRef]
- Cobo, F.; Reguera-Márquez, J.A.; Marín-Rodríguez, J.A.; Martín-Pérez, F.J.; Pérez-Palacios, P.; Recacha, E.; Navarro-Marí, J.M. A 5-Year Study of Bloodstream Infections Caused by Carbapenemase-Producing Gram-Negative Bacilli in Southern Spain. Rev. Esp. Quimioter. 2024, 37, 472–478. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Luzzaro, F.; Migliavacca, R.; Mugnaioli, C.; Pini, B.; De Luca, F.; Perilli, M.; Pollini, S.; Spalla, M.; Amicosante, G.; et al. First Countrywide Survey of Acquired Metallo-β-Lactamases in Gram-Negative Pathogens in Italy. Antimicrob. Agents Chemother. 2008, 52, 4023–4029. [Google Scholar] [CrossRef]
- Aschbacher, R.; Pagani, L.; Doumith, M.; Pike, R.; Woodford, N.; Spoladore, G.; Larcher, C.; Livermore, D.M. Metallo-β-Lactamases among Enterobacteriaceae from Routine Samples in an Italian Tertiary-Care Hospital and Long-Term Care Facilities during 2008. Clin. Microbiol. Infect. 2011, 17, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Aschbacher, R.; Giani, T.; Corda, D.; Conte, V.; Arena, F.; Pasquetto, V.; Scalzo, K.; Nicoletti, M.; Rossolini, G.M.; Pagani, E. Carbapenemase-Producing Enterobacteriaceae during 2011-12 in the Bolzano Area (Northern Italy): Increasing Diversity in a Low-Endemicity Setting. Diagn. Microbiol. Infect. Dis. 2013, 77, 354–356. [Google Scholar] [CrossRef]
- Cascio, A.; Mezzatesta, M.L.; Odierna, A.; Di Bernardo, F.; Barberi, G.; Iaria, C.; Stefani, S.; Giordano, S. Extended-Spectrum Beta-Lactamase-Producing and Carbapenemase-Producing Enterobacter cloacae Ventriculitis Successfully Treated with Intraventricular Colistin. Int. J. Infect. Dis. 2014, 20, 66–67. [Google Scholar] [CrossRef]
- Errico, G.; Gagliotti, C.; Monaco, M.; Masiero, L.; Gaibani, P.; Ambretti, S.; Landini, M.P.; D’Arezzo, S.; Di Caro, A.; Parisi, S.G.; et al. Colonization and Infection Due to Carbapenemase-Producing Enterobacteriaceae in Liver and Lung Transplant Recipients and Donor-Derived Transmission: A Prospective Cohort Study Conducted in Italy. Clin. Microbiol. Infect. 2019, 25, 203–209. [Google Scholar] [CrossRef]
- De Pascale, G.; Cortegiani, A.; Rinaldi, M.; Antonelli, M.; Cattaneo, S.; Cecconi, M.; Cuffaro, R.; Dalfino, L.; Di Biase, F.; Donati, A.; et al. Incidence of Hospital-Acquired Infections Due to Carbapenem-Resistant Enterobacterales and Pseudomonas aeruginosa in Critically Ill Patients in Italy: A Multicentre Prospective Cohort Study. Crit. Care Lond. Engl. 2025, 29, 32. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Cuzon, G.; Nordmann, P. Dissemination of Carbapenemase-Producing Enterobacteriaceae in France, 2012. J. Antimicrob. Chemother. 2014, 69, 623–627. [Google Scholar] [CrossRef]
- Zujić Atalić, V.; Bedenić, B.; Kocsis, E.; Mazzariol, A.; Sardelić, S.; Barišić, M.; Plečko, V.; Bošnjak, Z.; Mijač, M.; Jajić, I.; et al. Diversity of Carbapenemases in Clinical Isolates of Enterobacteriaceae in Croatia--the Results of a Multicentre Study. Clin. Microbiol. Infect. 2014, 20, O894–O903. [Google Scholar] [CrossRef] [PubMed]
- Bedenić, B.; Sardelić, S.; Luxner, J.; Bošnjak, Z.; Varda-Brkić, D.; Lukić-Grlić, A.; Mareković, I.; Frančula-Zaninović, S.; Krilanović, M.; Šijak, D.; et al. Molecular Characterization of Class b Carbapenemases in Advanced Stage of Dissemination and Emergence of Class d Carbapenemases in Enterobacteriaceae from Croatia. Infect. Genet. Evol. 2016, 43, 74–82. [Google Scholar] [CrossRef]
- Heller, I.; Grif, K.; Orth, D. Emergence of VIM-1-Carbapenemase-Producing Enterobacter cloacae in Tyrol, Austria. J. Med. Microbiol. 2012, 61, 567–571. [Google Scholar] [CrossRef]
- Juhász, E.; Jánvári, L.; Tóth, Á.; Damjanova, I.; Nobilis, A.; Kristóf, K. Emergence of VIM-4- and SHV-12-Producing Enterobacter cloacae in a Neonatal Intensive Care Unit. Int. J. Med. Microbiol. 2012, 302, 257–260. [Google Scholar] [CrossRef]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a New Antibiotic Resistance Mechanism in India, Pakistan, and the UK: A Molecular, Biological, and Epidemiological Study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Jain, A.; Hopkins, K.L.; Turton, J.; Doumith, M.; Hill, R.; Loy, R.; Meunier, D.; Pike, R.; Livermore, D.M.; Woodford, N. NDM Carbapenemases in the United Kingdom: An Analysis of the First 250 Cases. J. Antimicrob. Chemother. 2014, 69, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Ciesielczuk, H.; Nelson, S.M.; Wilks, M.; Cummins, M.N. A Point Prevalence Study to Determine the Inpatient Rate of Carbapenemase-Producing Organisms at a Large London NHS Trust. J. Hosp. Infect. 2020, 104, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, P.; Bouchahrouf, W.; de Castro, R.R.; Deplano, A.; Berhin, C.; Piérard, D.; Denis, O.; Glupczynski, Y. Emergence of NDM-1-Producing Enterobacteriaceae in Belgium. Antimicrob. Agents Chemother. 2011, 55, 3036–3038. [Google Scholar] [CrossRef]
- Huang, T.D.; Bogaerts, P.; Berhin, C.; Jans, B.; Deplano, A.; Denis, O.; Glupczynski, Y. Rapid Emergence of Carbapenemase-Producing Enterobacteriaceae Isolates in Belgium. Euro Surveill. 2011, 16, 19900. [Google Scholar] [CrossRef] [PubMed]
- Davido, B.; Moussiegt, A.; Dinh, A.; Bouchand, F.; Matt, M.; Senard, O.; Deconinck, L.; Espinasse, F.; Lawrence, C.; Fortineau, N.; et al. Germs of Thrones—Spontaneous Decolonization of Carbapenem-Resistant Enterobacteriaceae (CRE) and Vancomycin-Resistant Enterococci (VRE) in Western Europe: Is This Myth or Reality? Antimicrob. Resist. Infect. Control 2018, 7, 100. [Google Scholar] [CrossRef]
- Petrosillo, N.; Vranić-Ladavac, M.; Feudi, C.; Villa, L.; Fortini, D.; Barišić, N.; Bedenić, B.; Ladavac, R.; D’Arezzo, S.; Andrašević, A.T.; et al. Spread of Enterobacter cloacae Carrying blaNDM-1, blaCTX-M-15, blaSHV-12 and Plasmid-Mediated Quinolone Resistance Genes in a Surgical Intensive Care Unit in Croatia. J. Glob. Antimicrob. Resist. 2016, 4, 44–48. [Google Scholar] [CrossRef]
- Brkić, S.; Božić, D.; Stojanović, N.; Vitorović, T.; Topalov, D.; Jovanović, M.; Stepanović, M.; Ćirković, I. Antimicrobial Susceptibility and Molecular Characterization of Carbapenemase-Producing Enterobacter spp. Community Isolates in Belgrade, Serbia. Microb. Drug Resist. 2020, 26, 378–384. [Google Scholar] [CrossRef]
- Gartzonika, K.; Politi, L.; Mavroidi, A.; Tsantes, A.G.; Spanakis, N.; Priavali, E.; Vrioni, G.; Tsakris, A. High Prevalence of Clonally Related ST182 NDM-1-Producing Enterobacter cloacae complex Clinical Isolates in Greece. Int. J. Antimicrob. Agents 2023, 62, 106837. [Google Scholar] [CrossRef]
- Tavoschi, L.; Forni, S.; Porretta, A.; Righi, L.; Pieralli, F.; Menichetti, F.; Falcone, M.; Gemignani, G.; Sani, S.; Vivani, P.; et al. Prolonged Outbreak of New Delhi Metallo-Beta-Lactamase-Producing Carbapenem-Resistant Enterobacterales (NDM-CRE), Tuscany, Italy, 2018 to 2019. Euro Surveill. 2020, 25, 2000085. [Google Scholar] [CrossRef]
- Villa, J.; Carretero, O.; Viedma, E.; Lora-Tamayo, J.; Mingorance, J.; Chaves, F. Emergence of NDM-7-Producing Multi-Drug-Resistant Enterobacter hormaechei Sequence Type ST-78 in Spain: A High-Risk International Clone. Int. J. Antimicrob. Agents 2019, 53, 533–534. [Google Scholar] [CrossRef]
- Ramette, A.; Gasser, M.; Nordmann, P.; Zbinden, R.; Schrenzel, J.; Perisa, D.; Kronenberg, A. Temporal and Regional Incidence of Carbapenemase-Producing Enterobacterales, Switzerland, 2013 to 2018. Euro Surveill. 2021, 26, 1900760. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, C.N.; Porsbo, L.J.; Sydenham, T.V.; Hansen, S.G.K.; Steinke, K.; Larsen, S.L.; Helgason, K.O.; Hansen, F.; Karstensen, K.T.; Henius, A.E.; et al. Contaminated Dicloxacillin Capsules as the Source of an NDM-5/OXA-48-Producing Enterobacter hormaechei ST79 Outbreak, Denmark and Iceland, 2022 and 2023. Euro Surveill. 2023, 28, 2300108. [Google Scholar] [CrossRef]
- Hamprecht, A.; Poirel, L.; Göttig, S.; Seifert, H.; Kaase, M.; Nordmann, P. Detection of the Carbapenemase GIM-1 in Enterobacter cloacae in Germany. J. Antimicrob. Chemother. 2013, 68, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Carrër, A.; Poirel, L.; Yilmaz, M.; Akan, Ö.A.; Feriha, C.; Cuzon, G.; Matar, G.; Honderlick, P.; Nordmann, P. Spread of OXA-48-Encoding Plasmid in Turkey and Beyond. Antimicrob. Agents Chemother. 2010, 54, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Ros, A.; Carrër, A.; Fortineau, N.; Carricajo, A.; Berthelot, P.; Nordmann, P. Cross-Border Transmission of OXA-48-Producing Enterobacter cloacae from Morocco to France. J. Antimicrob. Chemother. 2011, 66, 1181–1182. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like Carbapenemases: The Phantom Menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef]
- Pantel, A.; Boutet-Dubois, A.; Jean-Pierre, H.; Marchandin, H.; Sotto, A.; Lavigne, J.-P.; CARB-LR group. French Regional Surveillance Program of Carbapenemase-Producing Gram-Negative Bacilli: Results from a 2-Year Period. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2285–2292. [Google Scholar] [CrossRef]
- Nicolas-Chanoine, M.-H.; Vigan, M.; Laouénan, C.; Robert, J. “E-carb Study Group” Risk Factors for Carbapenem-Resistant Enterobacteriaceae Infections: A French Case-Control-Control Study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 383–393. [Google Scholar] [CrossRef]
- Fernández, J.; Montero, I.; Martínez, Ó.; Fleites, A.; Poirel, L.; Nordmann, P.; Rodicio, M.R. Dissemination of Multiresistant Enterobacter cloacae Isolates Producing OXA-48 and CTX-M-15 in a Spanish Hospital. Int. J. Antimicrob. Agents 2015, 46, 469–474. [Google Scholar] [CrossRef]
- Glupczynski, Y.; Huang, T.-D.; Bouchahrouf, W.; Rezende de Castro, R.; Bauraing, C.; Gérard, M.; Verbruggen, A.-M.; Deplano, A.; Denis, O.; Bogaerts, P. Rapid Emergence and Spread of OXA-48-Producing Carbapenem-Resistant Enterobacteriaceae Isolates in Belgian Hospitals. Int. J. Antimicrob. Agents 2012, 39, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Rondinaud, E.; Nordmann, P. Intercontinental Spread of OXA-48 Beta-Lactamase-Producing Enterobacteriaceae over a 11-Year Period, 2001 to 2011. Euro Surveill. 2013, 18, 20549. [Google Scholar] [CrossRef]
- Bogaerts, P.; Naas, T.; Saegeman, V.; Bonnin, R.A.; Schuermans, A.; Evrard, S.; Bouchahrouf, W.; Jove, T.; Tande, D.; de Bolle, X.; et al. OXA-427, a New Plasmid-Borne Carbapenem-Hydrolysing Class D β-Lactamase in Enterobacteriaceae. J. Antimicrob. Chemother. 2017, 72, 2469–2477. [Google Scholar] [CrossRef]
- Pfeifer, Y.; Schlatterer, K.; Engelmann, E.; Schiller, R.A.; Frangenberg, H.R.; Stiewe, D.; Holfelder, M.; Witte, W.; Nordmann, P.; Poirel, L. Emergence of OXA-48-Type Carbapenemase-Producing Enterobacteriaceae in German Hospitals. Antimicrob. Agents Chemother. 2012, 56, 2125–2128. [Google Scholar] [CrossRef]
- Katchanov, J.; Asar, L.; Klupp, E.-M.; Both, A.; Rothe, C.; König, C.; Rohde, H.; Kluge, S.; Maurer, F.P. Carbapenem-Resistant Gram-Negative Pathogens in a German University Medical Center: Prevalence, Clinical Implications and the Role of Novel β-Lactam/β-Lactamase Inhibitor Combinations. PLoS ONE 2018, 13, e0195757. [Google Scholar] [CrossRef]
- Boutin, S.; Welker, S.; Gerigk, M.; Miethke, T.; Heeg, K.; Nurjadi, D. Molecular Surveillance of Carbapenem-Resistant Enterobacterales in Two Nearby Tertiary Hospitals to Identify Regional Spread of High-Risk Clones in Germany, 2019–2020. J. Hosp. Infect. 2024, 149, 126–134. [Google Scholar] [CrossRef]
- Findlay, J.; Hopkins, K.L.; Loy, R.; Doumith, M.; Meunier, D.; Hill, R.; Pike, R.; Mustafa, N.; Livermore, D.M.; Woodford, N. OXA-48-like Carbapenemases in the UK: An Analysis of Isolates and Cases from 2007 to 2014. J. Antimicrob. Chemother. 2017, 72, 1340–1349. [Google Scholar] [CrossRef]
- Dimou, V.; Dhanji, H.; Pike, R.; Livermore, D.M.; Woodford, N. Characterization of Enterobacteriaceae Producing OXA-48-like Carbapenemases in the UK. J. Antimicrob. Chemother. 2012, 67, 1660–1665. [Google Scholar] [CrossRef]
- Brehony, C.; McGrath, E.; Brennan, W.; Tuohy, A.; Whyte, T.; Brisse, S.; Maiden, M.; Jolley, K.; Morris, D.; Cormican, M. An MLST Approach to Support Tracking of Plasmids Carrying OXA-48-like Carbapenemase. J. Antimicrob. Chemother. 2019, 74, 1856–1862. [Google Scholar] [CrossRef]
- Samuelsen, Ø.; Hansen, F.; Aasnæs, B.; Hasman, H.; Lund, B.A.; Leiros, H.-K.S.; Lilje, B.; Janice, J.; Jakobsen, L.; Littauer, P.; et al. Dissemination and Characteristics of a Novel Plasmid-Encoded Carbapenem-Hydrolyzing Class D β-Lactamase, OXA-436, Found in Isolates from Four Patients at Six Different Hospitals in Denmark. Antimicrob. Agents Chemother. 2018, 62, e01260-17. [Google Scholar] [CrossRef]
- Izdebski, R.; Baraniak, A.; Zabicka, D.; Machulska, M.; Urbanowicz, P.; Fiett, J.; Literacka, E.; Bojarska, K.; Kozinska, A.; Zieniuk, B.; et al. Enterobacteriaceae Producing OXA-48-like Carbapenemases in Poland, 2013-January 2017. J. Antimicrob. Chemother. 2018, 73, 620–625. [Google Scholar] [CrossRef]
- Majewski, P.; Wieczorek, P.; Sacha, P.T.; Frank, M.; Juszczyk, G.; Ojdana, D.; Kłosowska, W.; Wieczorek, A.; Sieńko, A.; Michalska, A.D.; et al. Emergence of OXA-48 Carbapenemase-Producing Enterobacter cloacae ST89 Infection in Poland. Int. J. Infect. Dis. 2014, 25, 107–109. [Google Scholar] [CrossRef]
- Hallbäck, E.T.; Johnning, A.; Myhrman, S.; Studahl, M.; Hentz, E.; Elfvin, A.; Adlerberth, I. Outbreak of OXA-48-Producing Enterobacteriaceae in a Neonatal Intensive Care Unit in Western Sweden. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 597–605. [Google Scholar] [CrossRef]
- Bedenić, B.; Slade, M.; Starčević, L.Ž.; Sardelić, S.; Vranić-Ladavac, M.; Benčić, A.; Zujić Atalić, V.; Bogdan, M.; Bubonja-Šonje, M.; Tomić-Paradžik, M.; et al. Epidemic Spread of OXA-48 Beta-Lactamase in Croatia. J. Med. Microbiol. 2018, 67, 1031–1041. [Google Scholar] [CrossRef]
- Smit, P.W.; van Tienen, C.; Landman, F.; Zagers, S.; den Drijver, M.; Burggraaf, A.; Notermans, D.W.; Damen, M.; Hendrickx, A.P.A.; Jamin, C. Diversification of blaOXA-48-Harbouring Plasmids among Carbapenemase-Producing Enterobacterales, 11 Years after a Large Outbreak in a General Hospital in the Netherlands. Microb. Genomics 2025, 11, 001335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.-B.; Dai, L.-T.; Zhang, Q.-K.; Zhong, Y.-X.; Liu, W.-T.; Yang, L.; Chen, D.-Q. Global Emergence of Double and Multi-Carbapenemase Producing Organisms: Epidemiology, Clinical Significance, and Evolutionary Benefits on Antimicrobial Resistance and Virulence. Microbiol. Spectr. 2024, 12, e0000824. [Google Scholar] [CrossRef]
- Cabello, M.; Hernández-García, M.; Maruri-Aransolo, A.; Michelena, M.; Pérez-Viso, B.; Ponce-Alonso, M.; Cantón, R.; Ruiz-Garbajosa, P. Occurrence of Multi-Carbapenemase-Producing Enterobacterales in a Tertiary Hospital in Madrid (Spain): A New Epidemiologic Scenario. J. Glob. Antimicrob. Resist. 2024, 38, 281–291. [Google Scholar] [CrossRef]
- Jayol, A.; Poirel, L.; Dortet, L.; Nordmann, P. National Survey of Colistin Resistance among Carbapenemase-Producing Enterobacteriaceae and Outbreak Caused by Colistin-Resistant OXA-48-Producing Klebsiella pneumoniae, France, 2014. Euro Surveill. 2016, 21, 30339. [Google Scholar] [CrossRef] [PubMed]
- Bošnjak, Z.; Hasman, H.; Hansen, F.; Hammerum, A.M.; Roer, L.; Jurić, I.; Budimir, A. Co-Occurrence of Triple Carbapenemase Genes, blaVIM-2, blaNDM-1, and blaOXA-48 in Enterobacter hormaechei Clinical Isolates -First Report from Croatia. J. Chemother. 2025, 37, 10–14. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirkovic, I.; Brkic, S. Beyond the Spotlight: Enterobacter spp. as Overlooked Carbapenemase Producers in Europe. Antibiotics 2025, 14, 1045. https://doi.org/10.3390/antibiotics14101045
Cirkovic I, Brkic S. Beyond the Spotlight: Enterobacter spp. as Overlooked Carbapenemase Producers in Europe. Antibiotics. 2025; 14(10):1045. https://doi.org/10.3390/antibiotics14101045
Chicago/Turabian StyleCirkovic, Ivana, and Snezana Brkic. 2025. "Beyond the Spotlight: Enterobacter spp. as Overlooked Carbapenemase Producers in Europe" Antibiotics 14, no. 10: 1045. https://doi.org/10.3390/antibiotics14101045
APA StyleCirkovic, I., & Brkic, S. (2025). Beyond the Spotlight: Enterobacter spp. as Overlooked Carbapenemase Producers in Europe. Antibiotics, 14(10), 1045. https://doi.org/10.3390/antibiotics14101045





