Characterization of Antimicrobial Resistance and Hypervirulent Traits of Klebsiella variicola Isolates Collected in South Korea
Abstract
1. Introduction
2. Results
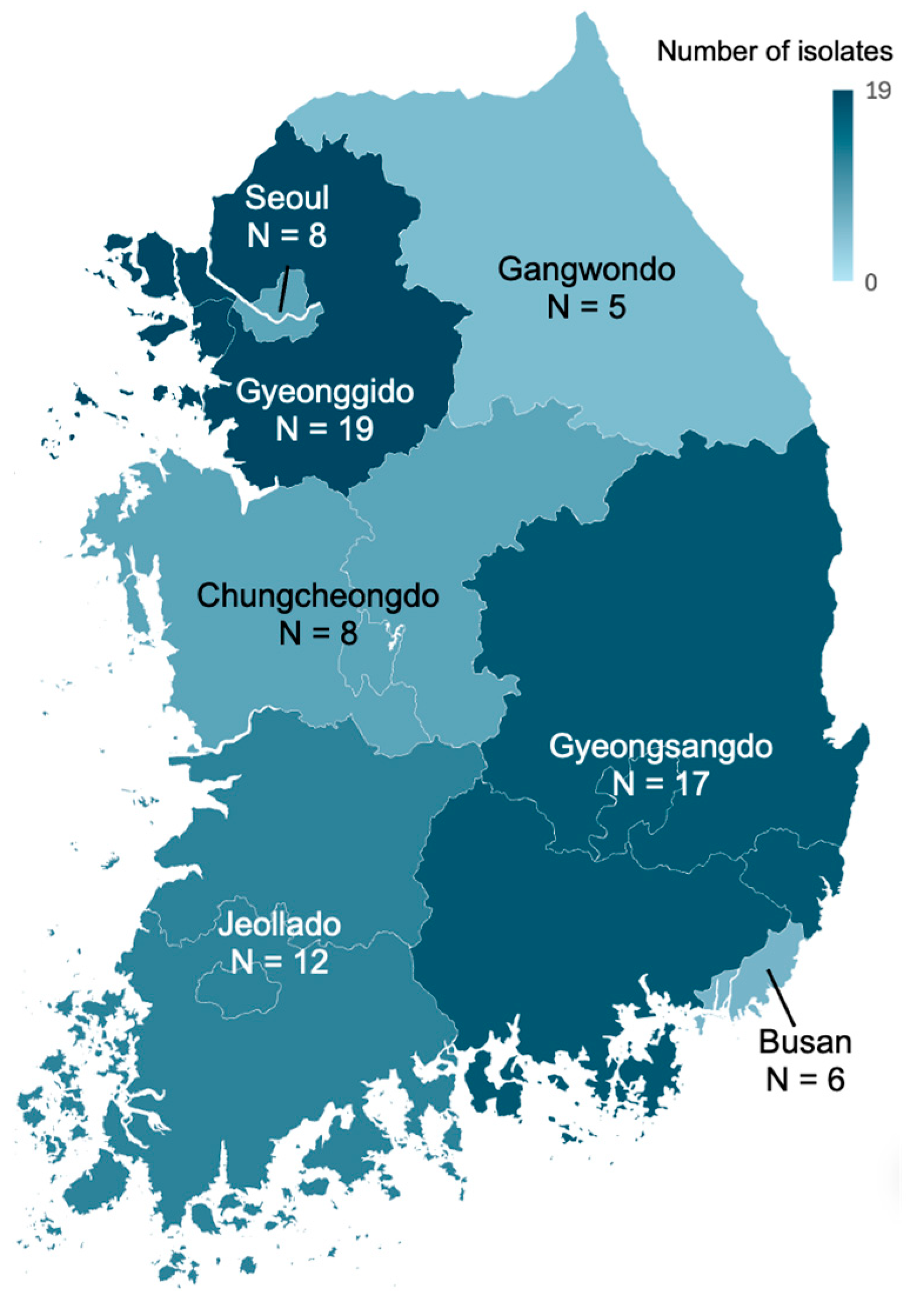
2.1. Collection of K. variicola Isolates
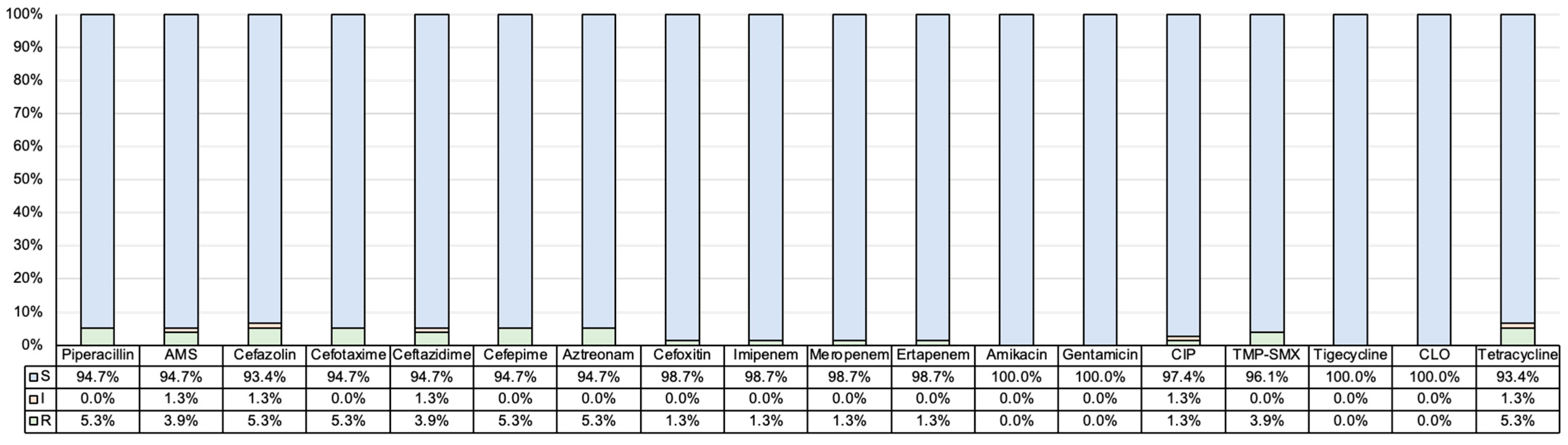
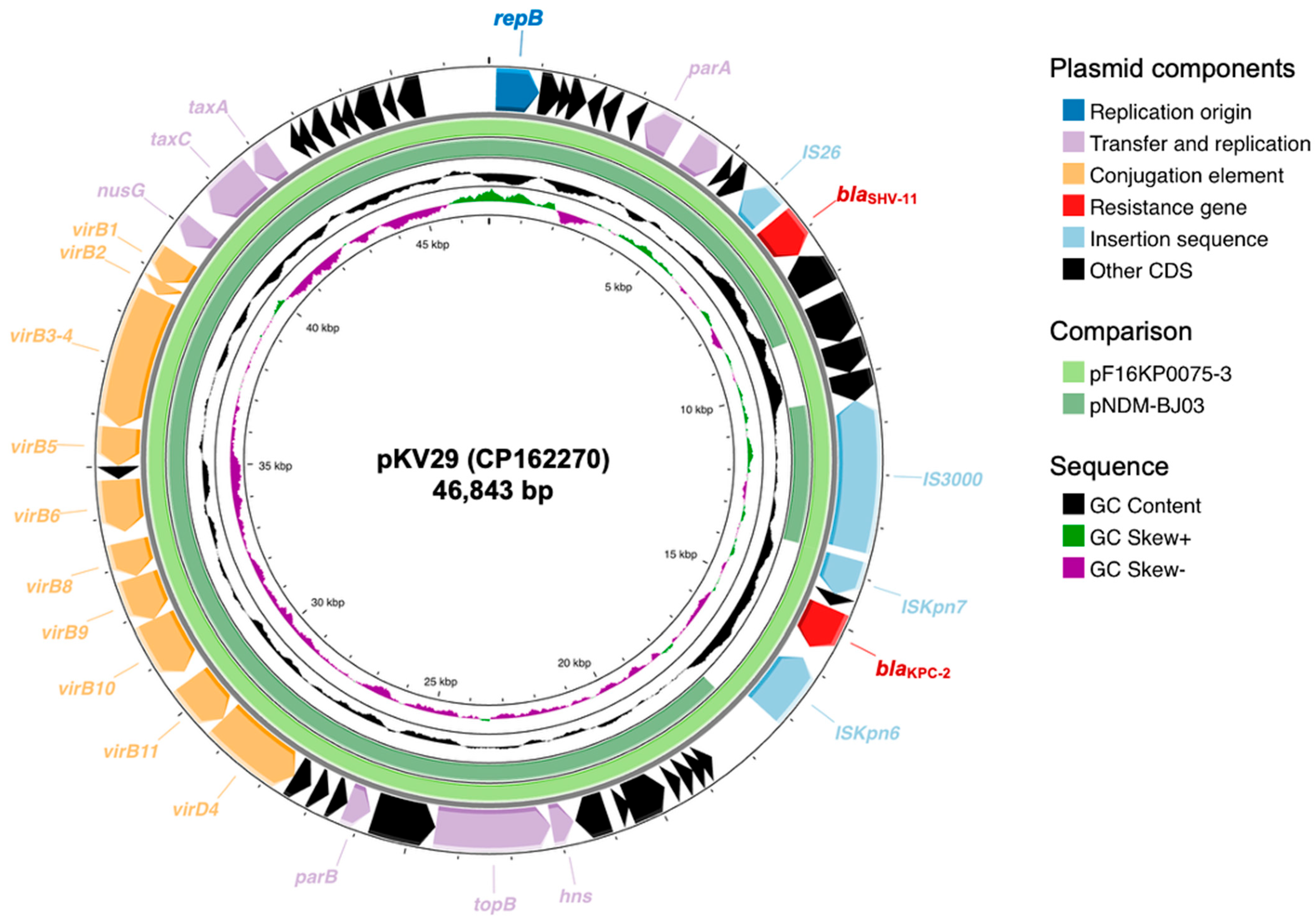
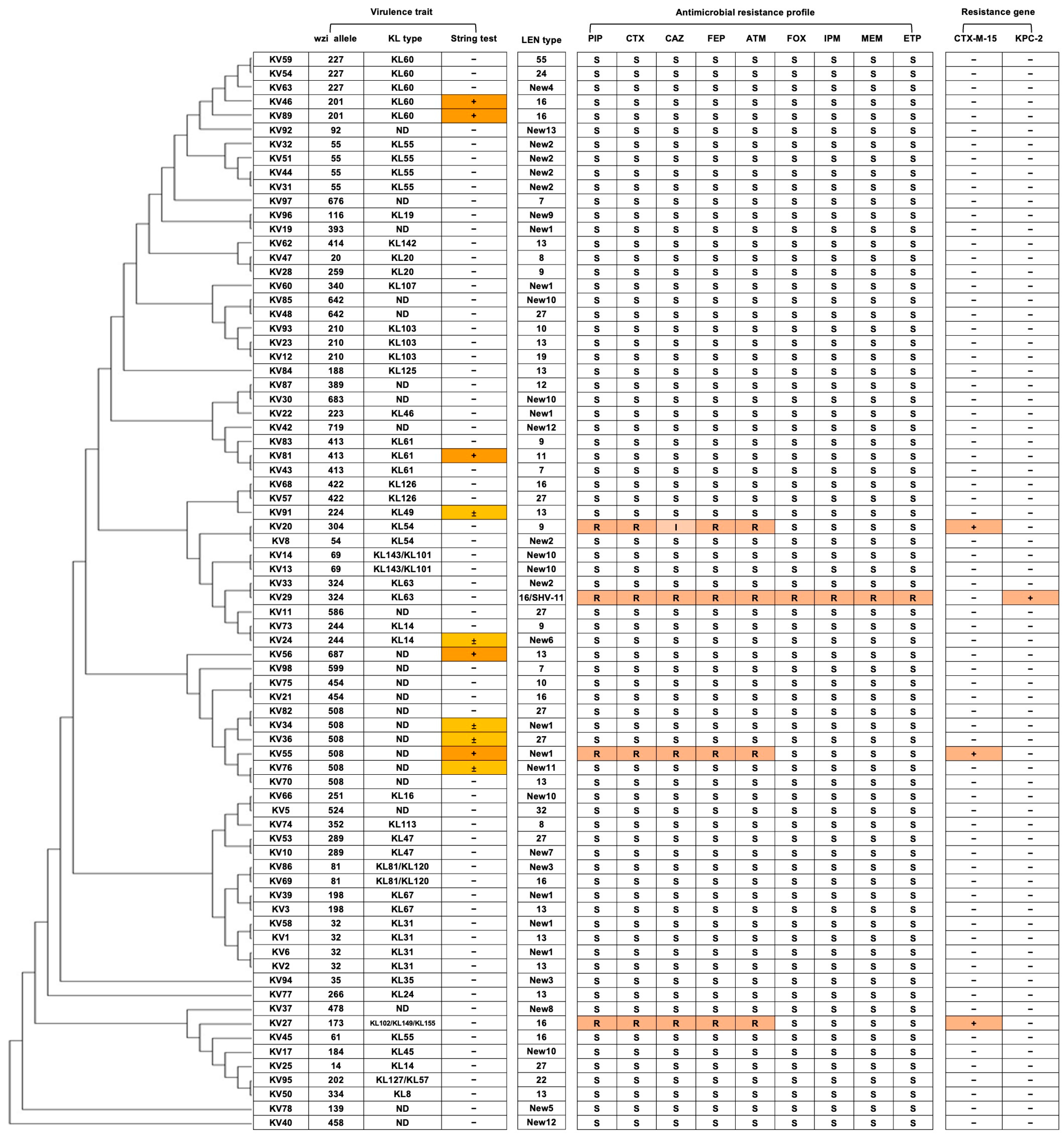
2.2. Antimicrobial Resistance Profiles and β-Lactamase Genotype of K. variicola Isolates
2.3. Capsular Type and Virulence Traits of K. variicola
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Species Identification and Antimicrobial Susceptibility Tests
4.3. β-Lactamase Genotype
4.4. Determination of Hypervirulent Trait
4.5. Whole-Genome Sequencing of K. variicola Isolates
4.6. In Silico Analysis of Whole Genome Sequencing Data
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazmierczak, K.M.; Karlowsky, J.A.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. Epidemiology of Carbapenem Resistance Determinants Identified in Meropenem-Nonsusceptible Enterobacterales Collected as Part of a Global Surveillance Program, 2012 to 2017. Antimicrob. Agents Chemother. 2021, 65, e0200020. [Google Scholar] [CrossRef]
- Kim, D.; Yoon, E.J.; Hong, J.S.; Choi, M.H.; Kim, H.S.; Kim, Y.R.; Kim, Y.A.; Uh, Y.; Shin, K.S.; Shin, J.H.; et al. Major Bloodstream Infection-Causing Bacterial Pathogens and Their Antimicrobial Resistance in South Korea, 2017–2019: Phase I Report from Kor-GLASS. Front. Microbiol. 2021, 12, 799084. [Google Scholar] [CrossRef]
- Gu, D.; Dong, N.; Zheng, Z.; Lin, D.; Huang, M.; Wang, L.; Chan, E.W.; Shu, L.; Yu, J.; Zhang, R.; et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: A molecular epidemiological study. Lancet Infect. Dis. 2018, 18, 37–46. [Google Scholar] [CrossRef]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef]
- Rodrigues, C.; Passet, V.; Rakotondrasoa, A.; Diallo, T.A.; Criscuolo, A.; Brisse, S. Description of Klebsiella africanensis sp. nov., Klebsiella variicola subsp. tropicalensis subsp. nov. and Klebsiella variicola subsp. variicola subsp. nov. Res. Microbiol. 2019, 170, 165–170. [Google Scholar] [CrossRef]
- Rodrigues, C.; Passet, V.; Rakotondrasoa, A.; Brisse, S. Identification of Klebsiella pneumoniae, Klebsiella quasipneumoniae, Klebsiella variicola and Related Phylogroups by MALDI-TOF Mass Spectrometry. Front. Microbiol. 2018, 9, 3000. [Google Scholar] [CrossRef]
- Barrios-Camacho, H.; Aguilar-Vera, A.; Beltran-Rojel, M.; Aguilar-Vera, E.; Duran-Bedolla, J.; Rodriguez-Medina, N.; Lozano-Aguirre, L.; Perez-Carrascal, O.M.; Rojas, J.; Garza-Ramos, U. Molecular epidemiology of Klebsiella variicola obtained from different sources. Sci. Rep. 2019, 9, 10610. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Bonura, E.; Lyski, Z.; Messer, W. The Brief Case: Klebsiella variicola-Identifying the Misidentified. J. Clin. Microbiol. 2019, 57, 1. [Google Scholar] [CrossRef]
- Brusselaers, N.; Monstrey, S.; Snoeij, T.; Vandijck, D.; Lizy, C.; Hoste, E.; Lauwaert, S.; Colpaert, K.; Vandekerckhove, L.; Vogelaers, D.; et al. Morbidity and mortality of bloodstream infections in patients with severe burn injury. Am. J. Crit. Care 2010, 19, e81–e87. [Google Scholar] [CrossRef] [PubMed]
- Long, S.W.; Linson, S.E.; Ojeda Saavedra, M.; Cantu, C.; Davis, J.J.; Brettin, T.; Olsen, R.J. Whole-Genome Sequencing of Human Clinical Klebsiella pneumoniae Isolates Reveals Misidentification and Misunderstandings of Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae. mSphere 2017, 2, 4. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Tang, G.; Lin, J.; Huang, W.; Qiao, F.; Zong, Z. Carbapenem-resistant Isolates of the Klebsiella pneumoniae Complex in Western China: The Common ST11 and the Surprising Hospital-specific Types. Clin. Infect. Dis. 2018, 67, S263–S265. [Google Scholar] [CrossRef]
- Tian, D.; Liu, X.; Chen, W.; Zhou, Y.; Hu, D.; Wang, W.; Wu, J.; Mu, Q.; Jiang, X. Prevalence of hypervirulent and carbapenem-resistant Klebsiella pneumoniae under divergent evolutionary patterns. Emerg. Microbes Infect. 2022, 11, 1936–1949. [Google Scholar] [CrossRef]
- Siu, L.K.; Yeh, K.M.; Lin, J.C.; Fung, C.P.; Chang, F.Y. Klebsiella pneumoniae liver abscess: A new invasive syndrome. Lancet Infect. Dis. 2012, 12, 881–887. [Google Scholar] [CrossRef]
- Yoon, E.J.; Gwon, B.; Liu, C.; Kim, D.; Won, D.; Park, S.G.; Choi, J.R.; Jeong, S.H. Beneficial Chromosomal Integration of the Genes for CTX-M Extended-Spectrum β-Lactamase in Klebsiella pneumoniae for Stable Propagation. mSystems 2020, 5, 5. [Google Scholar] [CrossRef]
- Huang, J.; Deng, S.; Ren, J.; Tu, J.; Ye, M.; Wang, M. Characterization of a blaNDM-1-harboring plasmid from a Salmonella enterica clinical isolate in China. Mol. Med. Rep. 2017, 16, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Ishibashi, N.; Kodana, M.; Tarumoto, N.; Sakai, J.; Kawamura, T.; Takeuchi, S.; Taji, Y.; Ebihara, Y.; Ikebuchi, K.; et al. Clinical characteristics in blood stream infections caused by Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae: A comparative study, Japan, 2014–2017. BMC Infect. Dis. 2019, 19, 946. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ramos, U.; Martínez-Romero, E.; Silva-Sánchez, J. SHV-type extended-spectrum beta-lactamase (ESBL) are encoded in related plasmids from enterobacteria clinical isolates from Mexico. Salud Publica Mex. 2007, 49, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Olson, R.; Fang, C.T.; Stoesser, N.; Miller, M.; MacDonald, U.; Hutson, A.; Barker, J.H.; La Hoz, R.M.; Johnson, J.R. Identification of Biomarkers for Differentiation of Hypervirulent Klebsiella pneumoniae from Classical K. pneumoniae. J. Clin. Microbiol. 2018, 56, 9. [Google Scholar] [CrossRef]
- Rodríguez-Medina, N.; Martínez-Romero, E.; De la Cruz, M.A.; Ares, M.A.; Valdovinos-Torres, H.; Silva-Sánchez, J.; Lozano-Aguirre, L.; Martínez-Barnetche, J.; Andrade, V.; Garza-Ramos, U. A Klebsiella variicola Plasmid Confers Hypermucoviscosity-Like Phenotype and Alters Capsule Production and Virulence. Front. Microbiol. 2020, 11, 579612. [Google Scholar] [CrossRef]
- Rodrigues, C.; Sousa, C.; Lopes, J.A.; Novais, Â.; Peixe, L. A Front Line on Klebsiella pneumoniae Capsular Polysaccharide Knowledge: Fourier Transform Infrared Spectroscopy as an Accurate and Fast Typing Tool. mSystems 2020, 5, 2. [Google Scholar] [CrossRef]
- CLSI. Performance standards for antimicrobial susceptibility testing. Clin. Lab. Stand. Inst. 2016, 35, 16–38. [Google Scholar]
- Fonseca, E.L.; Ramos, N.D.; Andrade, B.G.; Morais, L.L.; Marin, M.F.; Vicente, A.C. A one-step multiplex PCR to identify Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae in the clinical routine. Diagn. Microbiol. Infect. Dis. 2017, 87, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Testing ECoAS. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance, Version 2.0; 2017. Available online: https://aurosan.de/images/mediathek/servicematerial/EUCAST_detection_of_resistance_mechanisms.pdf (accessed on 16 September 2025).
- Kim, D.; Park, B.Y.; Choi, M.H.; Yoon, E.J.; Lee, H.; Lee, K.J.; Park, Y.S.; Shin, J.H.; Uh, Y.; Shin, K.S.; et al. Antimicrobial resistance and virulence factors of Klebsiella pneumoniae affecting 30 day mortality in patients with bloodstream infection. J. Antimicrob. Chemother. 2019, 74, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
| Variable | Total (n = 76) | Male (n = 21) | Female (n = 55) | p-Value |
|---|---|---|---|---|
| Old age (>60) | 53 (69.7) | 17 (81.0) | 34 (61.8) | 0.189 |
| Regions | 0.432 | |||
| Gyeonggido | 19 (25) | 4 (19.0) | 15 (27.3) | |
| Gyeongsangdo | 17 (22.4) | 3 (14.3) | 14 (25.5) | |
| Jeollado | 12 (15.8) | 4 (19.0) | 8 (14.5) | |
| Chungcheongdo | 8 (10.5) | 2 (9.5) | 6 (10.9) | |
| Seoul | 8 (10.5) | 5 (23.8) | 3 (5.5) | |
| Busan | 6 (7.9) | 2 (9.5) | 4 (7.3) | |
| Gangwondo | 5 (6.6) | 1 (4.8) | 4 (7.3) | |
| Type of hospital | 0.032 | |||
| General hospital | 42 (55.3) | 16 (76.2) | 26 (47.3) | |
| Clinic | 20 (26.3) | 2 (9.5) | 18 (32.7) | |
| Nursing hospital | 4 (5.3) | 1 (4.8) | 3 (5.5) | |
| Others | 10 (13.2) | 2 (9.5) | 8 (14.5) | |
| Specimen | <0.001 | |||
| Urine | 40 (52.6) | 3 (14.3) | 37 (67.3) | |
| Abscess | 17 (22.4) | 8 (28.6) | 9 (16.4) | |
| Blood | 6 (7.9) | 5 (23.8) | 1 (1.8) | |
| Sputum | 5 (6.6) | 4 (19.0) | 1 (1.8) | |
| Others | 8 (10.5) | 1 (4.8) | 7 (12.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, D.; Bae, H.G.; Jang, W.-J.; Jeong, S.H.; Lee, K. Characterization of Antimicrobial Resistance and Hypervirulent Traits of Klebsiella variicola Isolates Collected in South Korea. Antibiotics 2025, 14, 1046. https://doi.org/10.3390/antibiotics14101046
Lee D, Kim D, Bae HG, Jang W-J, Jeong SH, Lee K. Characterization of Antimicrobial Resistance and Hypervirulent Traits of Klebsiella variicola Isolates Collected in South Korea. Antibiotics. 2025; 14(10):1046. https://doi.org/10.3390/antibiotics14101046
Chicago/Turabian StyleLee, Dokun, Dokyun Kim, Hye Gyung Bae, Won-Jong Jang, Seok Hoon Jeong, and Kyungwon Lee. 2025. "Characterization of Antimicrobial Resistance and Hypervirulent Traits of Klebsiella variicola Isolates Collected in South Korea" Antibiotics 14, no. 10: 1046. https://doi.org/10.3390/antibiotics14101046
APA StyleLee, D., Kim, D., Bae, H. G., Jang, W.-J., Jeong, S. H., & Lee, K. (2025). Characterization of Antimicrobial Resistance and Hypervirulent Traits of Klebsiella variicola Isolates Collected in South Korea. Antibiotics, 14(10), 1046. https://doi.org/10.3390/antibiotics14101046





