High Prevalence of hefA Efflux Pump Overexpression in Isolates of Helicobacter pylori Resistant to Clarithromycin
Abstract
1. Introduction
2. Results
2.1. Clarithromycin Susceptibility
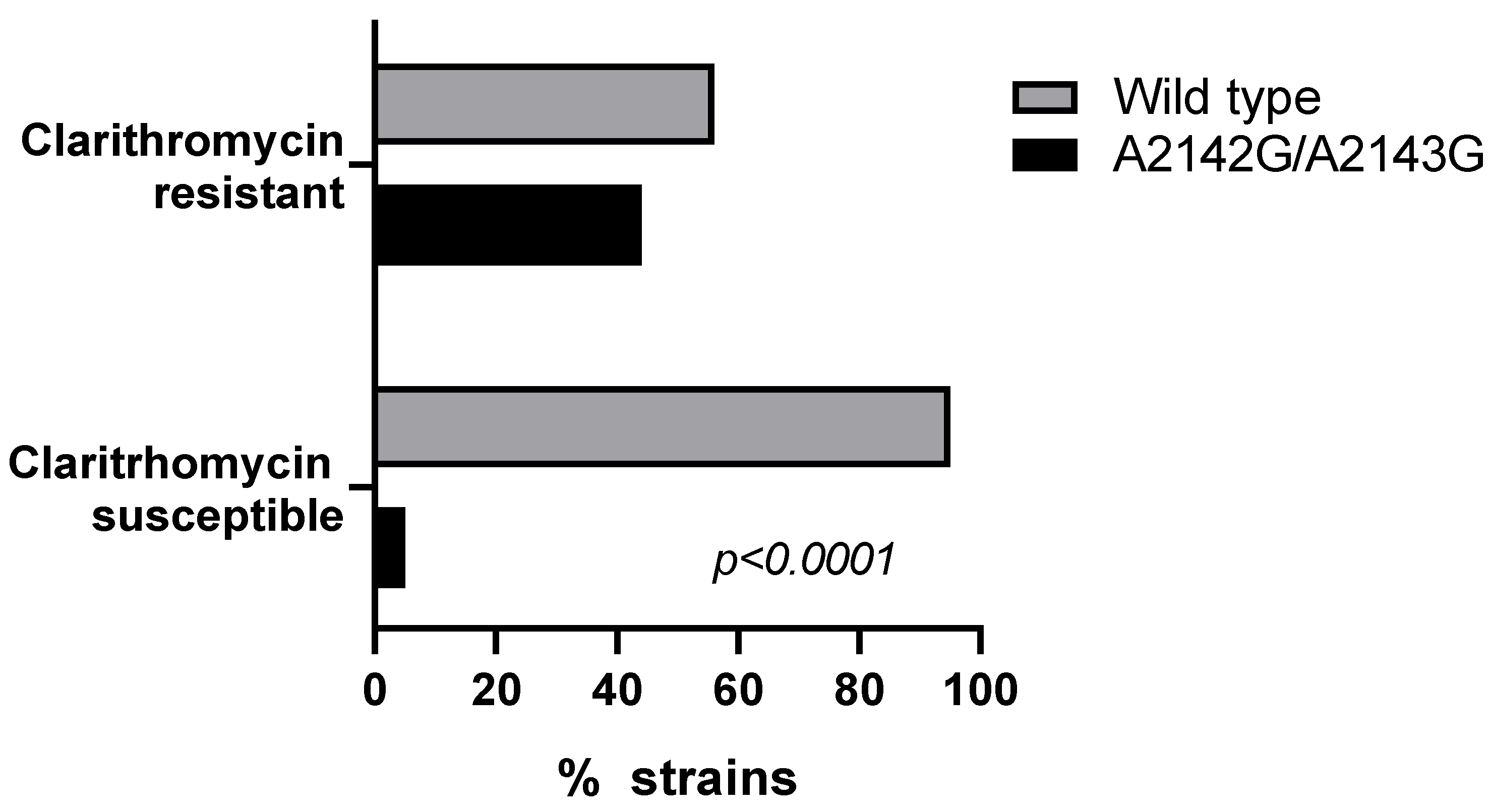
2.2. 23S rRNA Mutations (A2142G, A2143G)
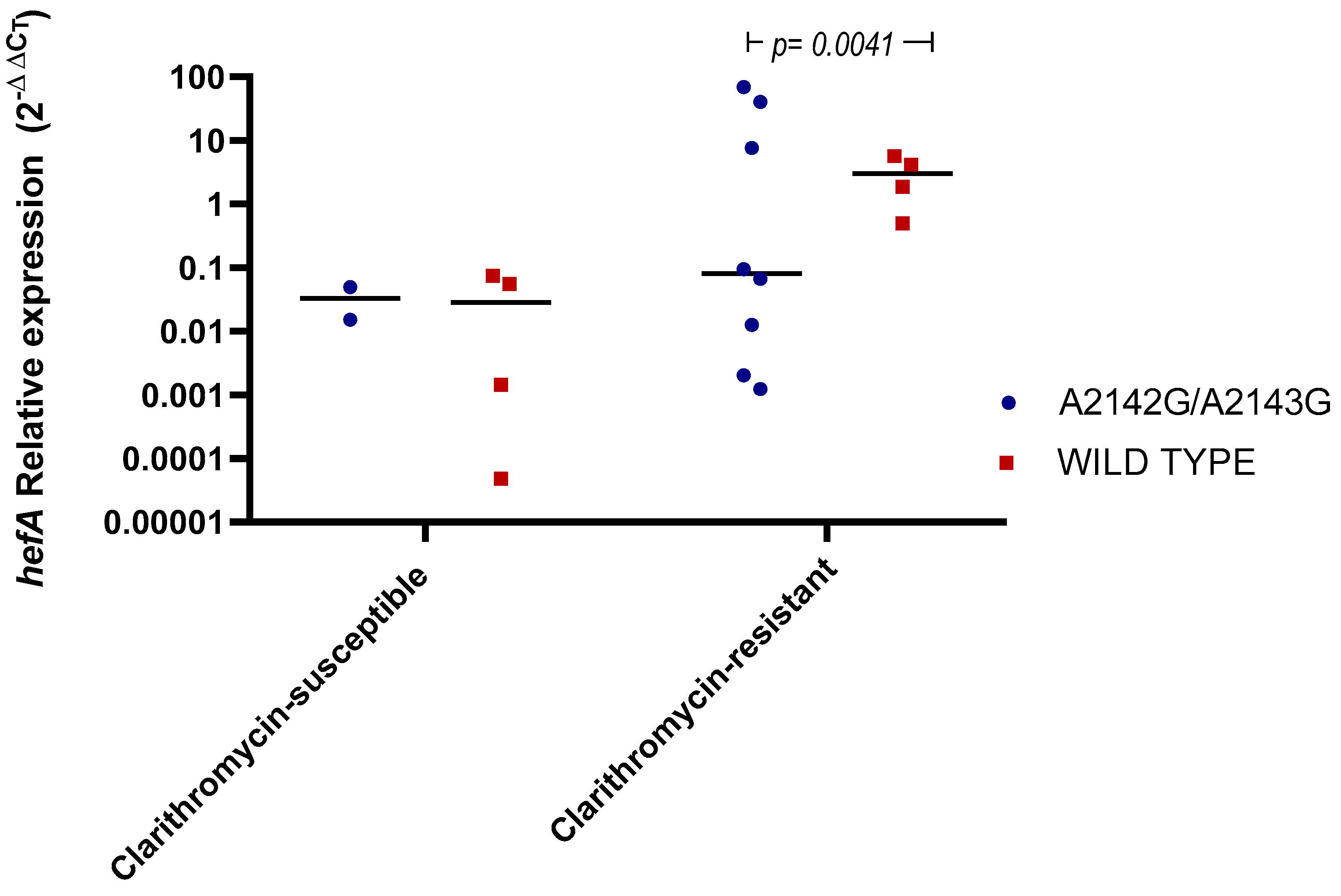
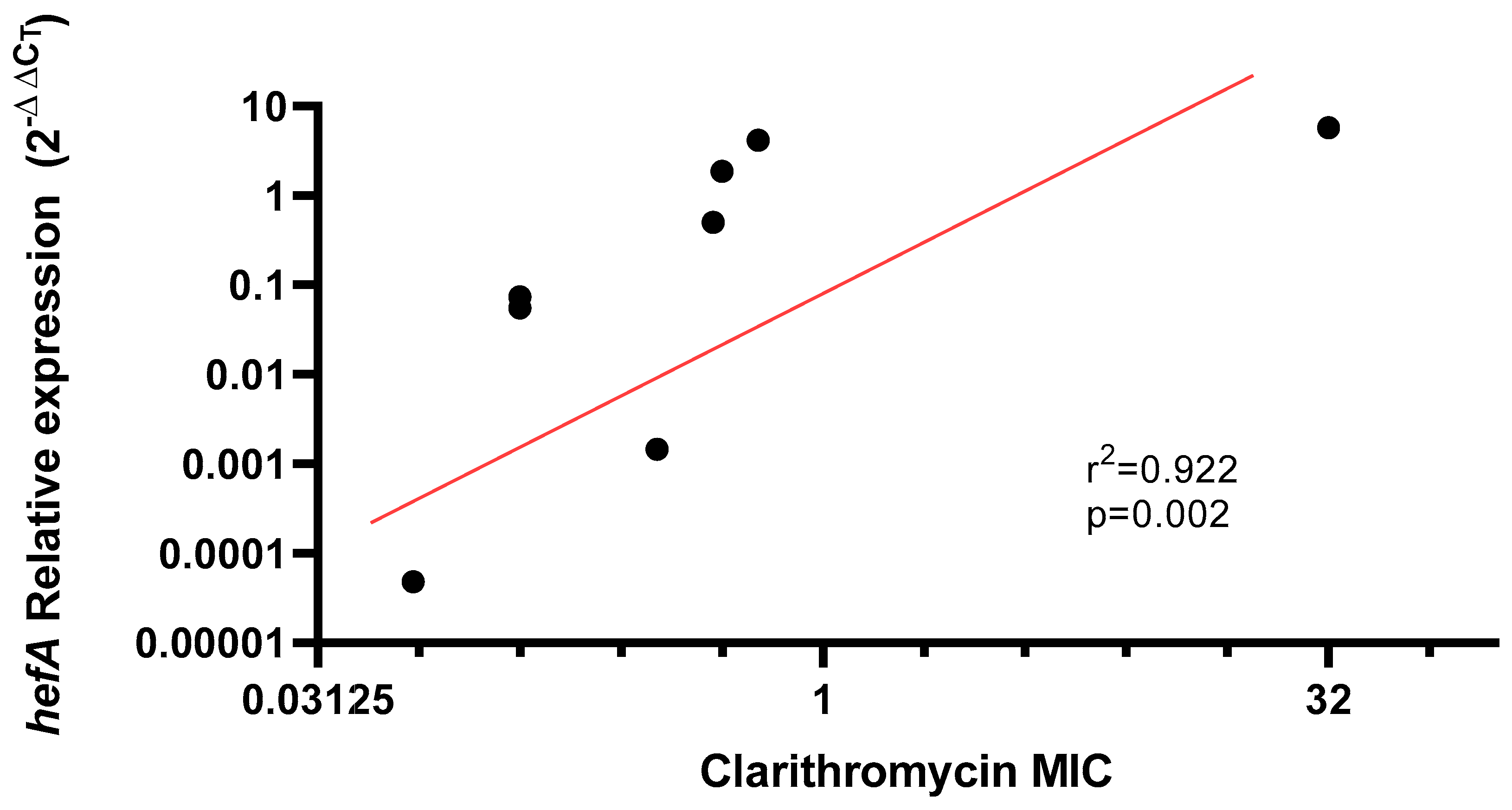
2.3. hefA Gene Expression
3. Discussion
4. Materials and Methods
4.1. Patients Recruitment and Obtention of Biological Samples
4.2. Culture and Phenotypic Identification of H. pylori
4.3. Antibiotic Susceptibility Testing
4.4. DNA Extraction from Pure Culture
4.5. Molecular Confirmation of H. pylori
4.6. RFLP Analysis for A2142G and A2143G Mutations
4.7. 23S rRNA Gene Sequencing
4.8. hefA Gene Relative Expression
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RFLP | Restriction fragment length polymorphism |
| WHO | World Health Organization |
| ARG | antibiotic resistance genes |
| H. pylori | Helicobacter pylori |
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.M.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori world gastroenterology organization global guideline. J. Clin. Gastroenterol. 2023, 57, 111–126. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Forman, D.; Hunt, R.; Moayyedi, P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst. Rev. 2020, 7, CD005583. [Google Scholar] [CrossRef]
- Kocsmár, É.; Buzás, G.M.; Szirtes, I.; Kocsmár, I.; Kramer, Z.; Szijártó, A.; Fadgyas-Freyler, P.; Szénás, K.; Rugge, M.; Fassan, M.; et al. Primary and secondary clarithromycin resistance in Helicobacter pylori and mathematical modeling of the role of macrolides. Nat. Commun. 2021, 12, 2255. [Google Scholar] [CrossRef]
- Fischbach, L.; Evans, E.L. Meta-analysis: The effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment. Pharmacol. Ther. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, X.; Liu, X.; Song, Y.; Song, C.; Wu, S.; An, Y.; Yuan, R.; Wan, Y.; Xie, Y. The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: A systematic review and meta-analysis. Helicobacter 2020, 25, e12714. [Google Scholar] [CrossRef]
- Versalovic, J.; Shortridge, D.; Kibler, K.; Griffy, M.V.; Beyer, J.; Flamm, R.K.; Tanaka, S.K.; Graham, D.Y. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 1996, 40, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Alfaresi, M.S.; Eltom, A.A.; Alshaikh, S.S.; Buchel, E.H.; Saeed, L.S.; Aljenaibi, M.M. Molecular prevalence of point mutations conferring resistance to clarithromycin in Helicobacter pylori in the United Arab Emirates. Saudi Med. J. 2005, 26, 763–766. [Google Scholar] [PubMed]
- Hu, Y.; Zhang, M.; Lu, B.; Dai, J. Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter 2016, 21, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Suzuki, H.; Nishizawa, T.; Tsugawa, H.; Muraoka, H.; Saito, Y.; Matsuzaki, J.; Hibi, T. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J. Gastroenterol. Hepatol. 2010, 25, S75–S79. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Oporto, M.; Pavez, M.; Troncoso, C.; Cerda, A.; Hofmann, E.; Sierralta, A.; Rios, E.; Coppelli, L.; Barrientos, L. Prevalence of infection and antibiotic susceptibility of Helicobacter pylori: An evaluation in public and private health systems of southern Chile. Pathogens 2019, 8, 226. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O´Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Parra-Sepúlveda, C.; Merino, J.S.; Sáez-Carrillo, K.; González, C.; García-Cancino, A. Antibiotic resistance surveillance of Helicobacter pylori at the Biobío region (Chile) in a decade. Arq. Gastroenterol. 2019, 56, 361–366. [Google Scholar] [CrossRef]
- Arenas, A.; Serrano, C.; Quiñones, L.; Harris, P.; Sandoval, M.; Lavanderos, M.; Sepúlveda, R.; Maquilón, S.; Echeverría, A.; Ríos, C.; et al. High prevalence of clarithromycin resistance and effect on Helicobacter pylori eradication in a population from Santiago, Chile: Cohort study and meta-analysis. Sci. Rep. 2019, 9, 20070. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Mégraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—The Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Villalón, A.; Reyes, D.; Ortiz, J.; Gándara, V.; Díaz, L.; Chahuán, J.; Pizarro, M.; Riquelme, A. Tratamiento y manejo de la infección por Helicobacter pylori. Gastroenterol. Latinoam. 2020, 31, 136–146. [Google Scholar] [CrossRef]
- Lubell, Y.; Turner, P.; Ashley, E.A.; White, N.J. Susceptibility of bacterial isolates from community-acquired infections in sub-Saharan Africa and Asia to macrolide antibiotics. Trop. Med. Int. Health 2011, 16, 1192–1205. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Liu, Y.; Lu, S.; Qin, P.; Guo, X.; Bi, B.; Wang, L.; Xi, B.; Wu, F.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Hadzhiyski, P.; Gergova, R.; Markovska, R. Evolution of Helicobacter pylori resistance to antibiotics: A topic of increasing concern. Antibiotics 2023, 12, 332. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; An, Y.; Li, Z.; Liu, D.; Yong, X. The crosstalk between efflux pump and resistance gene mutation in Helicobacter pylori. Gut Microbes 2024, 16, 2379439. [Google Scholar] [CrossRef] [PubMed]
- Albasha, A.M.; Elnosh, M.M.; Osman, E.H.; Zeinalabdin, D.M.; Fadl, A.A.; Ali, M.A.; Altayb, H.N. Helicobacter pylori 23S rRNA gene A2142G, A2143G, T2182C, and C2195T mutations associated with clarithromycin resistance detected in Sudanese patients. BMC Microbiol. 2021, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P. Helicobacter pylori efflux pumps: A double-edged sword in antibiotic resistance and biofilm formation. Int. J. Mol. Sci. 2024, 25, 12222. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.M.; James, C.E.; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008, 6, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Pavez, M.; Vieira, C.; de Araujo, M.R.; Cerda, A.; de Almeida, L.M.; Lincopan, N.; Mamizuka, E.M. Molecular mechanisms of membrane impermeability in clinical isolates of Enterobacteriaceae exposed to imipenem selective pressure. Int. J. Antimicrob. Agents 2016, 48, 78–85. [Google Scholar] [CrossRef]
- Dam, S.; Pagès, J.M.; Masi, M. Stress responses, outer membrane permeability control and antimicrobial resistance in Enterobacteriaceae. Microbiology 2018, 164, 260–267. [Google Scholar] [CrossRef]
- Kinana, A.D.; Vargiu, A.V.; Nikaido, H. Effect of site-directed mutations in multidrug efflux pump AcrB examined by quantitative efflux assays. Biochem. Biophys. Res. Commun. 2016, 480, 552–557. [Google Scholar] [CrossRef]
- Alfaray, R.I.; Saruuljavkhlan, B.; Fauzia, K.A.; Torres, R.C.; Thorell, K.; Dewi, S.R.; Kryukov, K.A.; Matsumoto, T.; Akada, J.; Vilaichone, R.; et al. Global antimicrobial resistance gene study of Helicobacter pylori: Comparison of detection tools, ARG and efflux pump gene analysis, worldwide epidemiological distribution, and information related to the antimicrobial-resistant phenotype. Antibiotics 2023, 12, 1118. [Google Scholar] [CrossRef]
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef]
- Marin, G.H.; Giangreco, L.; Dorati, C.; Mordujovich, P.; Boni, S.; Mantilla-Ponte, H.; Alfonso Arvez, M.J.; Lopez Peña, M.; Aldunate González, M.F.; Ching Fung, S.M.; et al. Antimicrobial consumption in Latin American countries: First steps of a long road ahead. J. Prim. Care Community Health 2022, 13, 21501319221082346. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. 2025. Available online: https://www.eucast.org (accessed on 25 January 2025).
- Wormwood, T.; Parra, Á.; Bresky, G.; Madariaga, J.A.; Häberle, S.; Flores, J.; Bernal, G. Prevalencia de cepas cagA-positivo en la región de Coquimbo, determinada mediante nested-qPCR en muestras fecales. Rev. Méd. Chile 2018, 146, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Background | Patients with Clarithromycin Resistant Strains n = 36 | Patients with Clarithromycin Sensitive Strains n = 66 | p Value |
|---|---|---|---|
| Age (years) | 47.2 ± 13.7 | 49.2 ± 12.2 | 0.466 |
| Sex (female) | 20 (55.56%) | 49 (74.24%) | 0.054 |
| Urban residence | 32 (88.9%) | 43 (64.7%) | 0.012 *ƚ |
| Education level ≤ 12 years | 20 (57.1%) | 46 (74.2%) | 0.084 |
| Public health insurance | 28 (80%) | 60 (92.3%) | 0.071 |
| Family group ≥ 5 members | 6 (17.1%) | 7 (10.8%) | 0.366 |
| Smoker | 7 (19.4%) | 24 (36.9%) | 0.068 |
| Drinker | 17 (47.2%) | 35(53.8%) | 0.524 |
| Previous treatment for H. pylori | 12 (33.3%) | 5 (7.6%) | 0.001 *ƚƚ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas, M.; Ortega, C.; Gómez, K.; Cerda, A.; Sepúlveda, R.; Lara, C.; Bustamante, L.; Garcia, D.; Coppelli, L.; Hofmann, E.; et al. High Prevalence of hefA Efflux Pump Overexpression in Isolates of Helicobacter pylori Resistant to Clarithromycin. Antibiotics 2025, 14, 1044. https://doi.org/10.3390/antibiotics14101044
Villegas M, Ortega C, Gómez K, Cerda A, Sepúlveda R, Lara C, Bustamante L, Garcia D, Coppelli L, Hofmann E, et al. High Prevalence of hefA Efflux Pump Overexpression in Isolates of Helicobacter pylori Resistant to Clarithromycin. Antibiotics. 2025; 14(10):1044. https://doi.org/10.3390/antibiotics14101044
Chicago/Turabian StyleVillegas, Marcela, Catalina Ortega, Krishna Gómez, Alvaro Cerda, Rolando Sepúlveda, Christian Lara, Luis Bustamante, Daniela Garcia, Luis Coppelli, Edmundo Hofmann, and et al. 2025. "High Prevalence of hefA Efflux Pump Overexpression in Isolates of Helicobacter pylori Resistant to Clarithromycin" Antibiotics 14, no. 10: 1044. https://doi.org/10.3390/antibiotics14101044
APA StyleVillegas, M., Ortega, C., Gómez, K., Cerda, A., Sepúlveda, R., Lara, C., Bustamante, L., Garcia, D., Coppelli, L., Hofmann, E., Sierralta, A., & Pavez, M. (2025). High Prevalence of hefA Efflux Pump Overexpression in Isolates of Helicobacter pylori Resistant to Clarithromycin. Antibiotics, 14(10), 1044. https://doi.org/10.3390/antibiotics14101044






