Thai Medicinal Flowers as Natural Antioxidants and Antibacterial Agents Against Pathogenic Enteric Bacteria: A Comparative Study of Mesua ferrea, Mammea siamensis, and Clitoria ternatea
Abstract
1. Introduction
2. Results
2.1. Flower Extraction
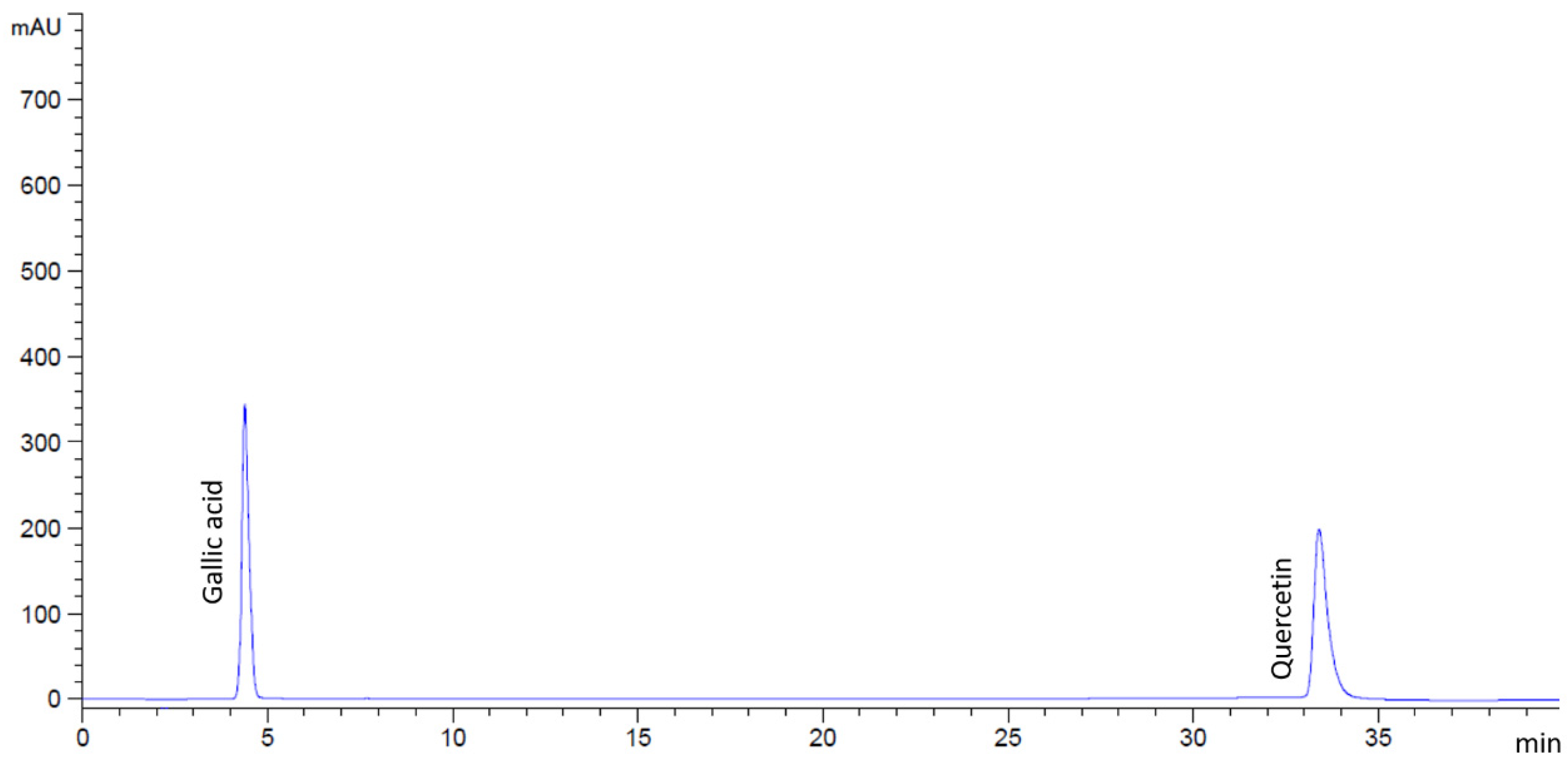
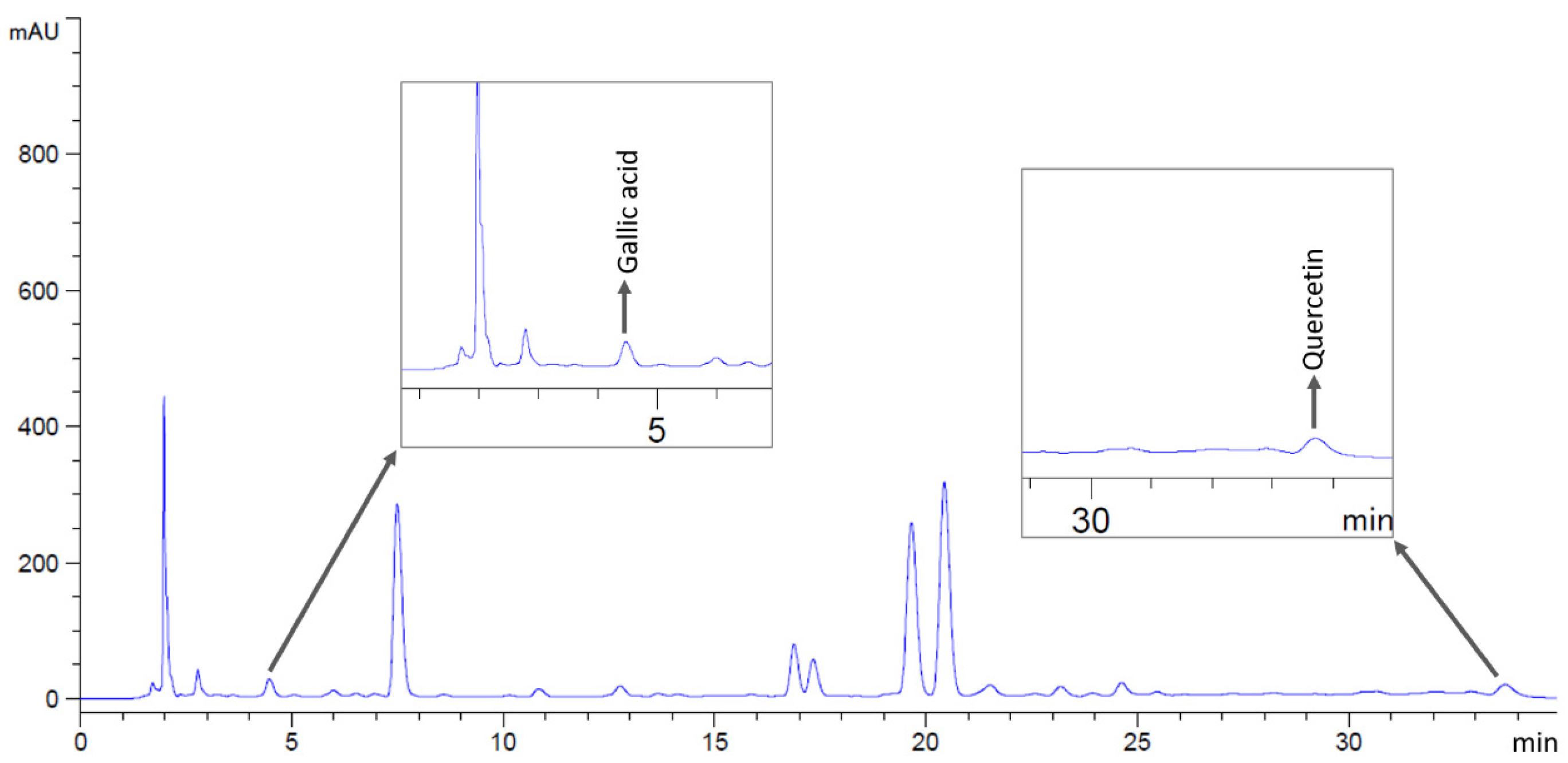
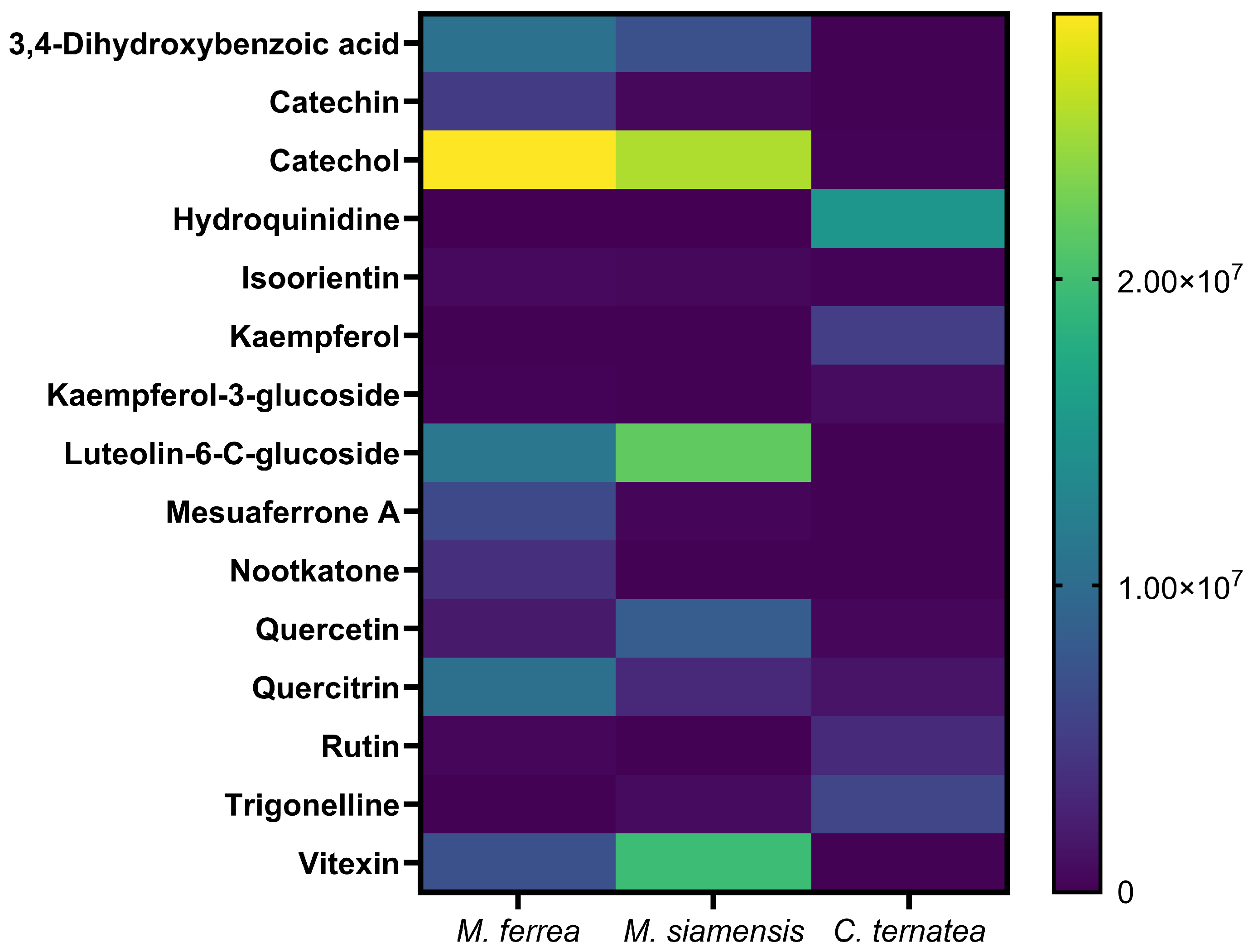
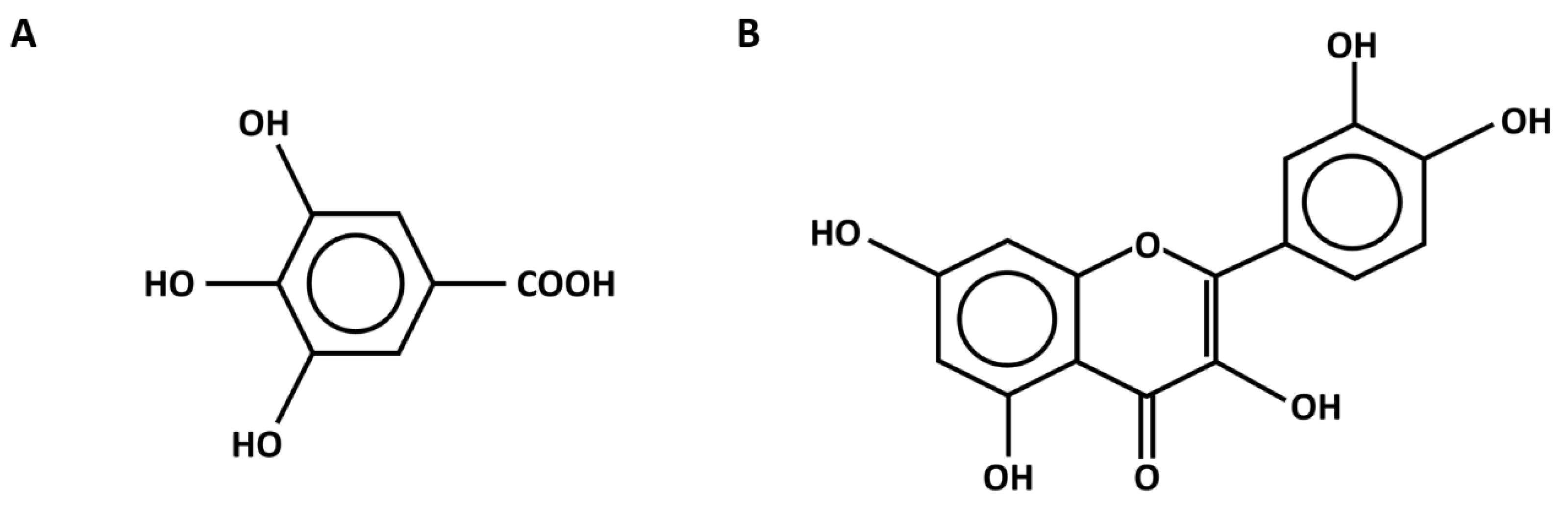
2.2. Phytochemical Compounds in Flower Extracts
2.3. Total Phenolic and Flavonoid Contents in Flower Extracts
2.4. Antioxidant Activities of Flower Extracts
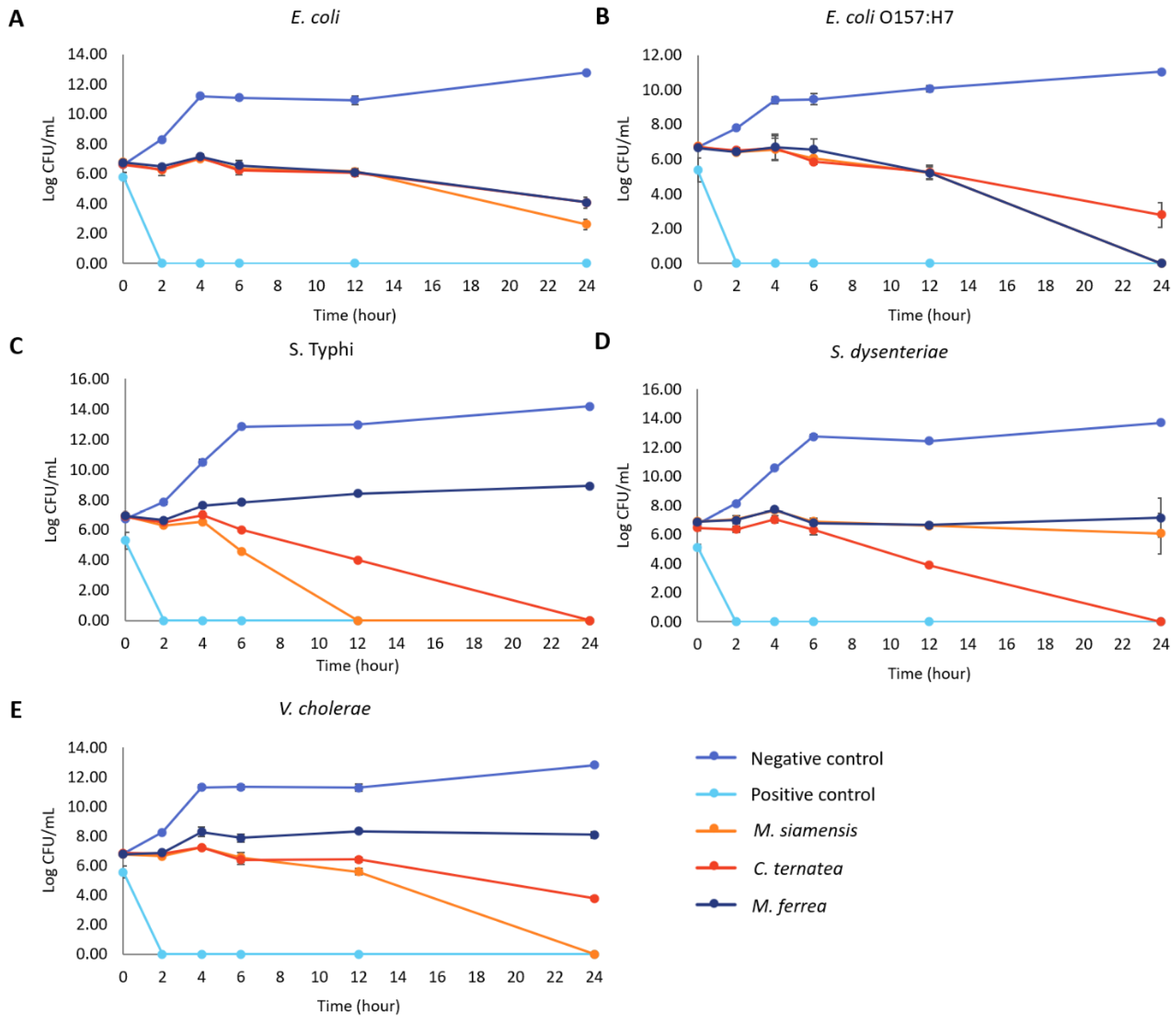
2.5. Antibacterial Activities of Flower Extracts
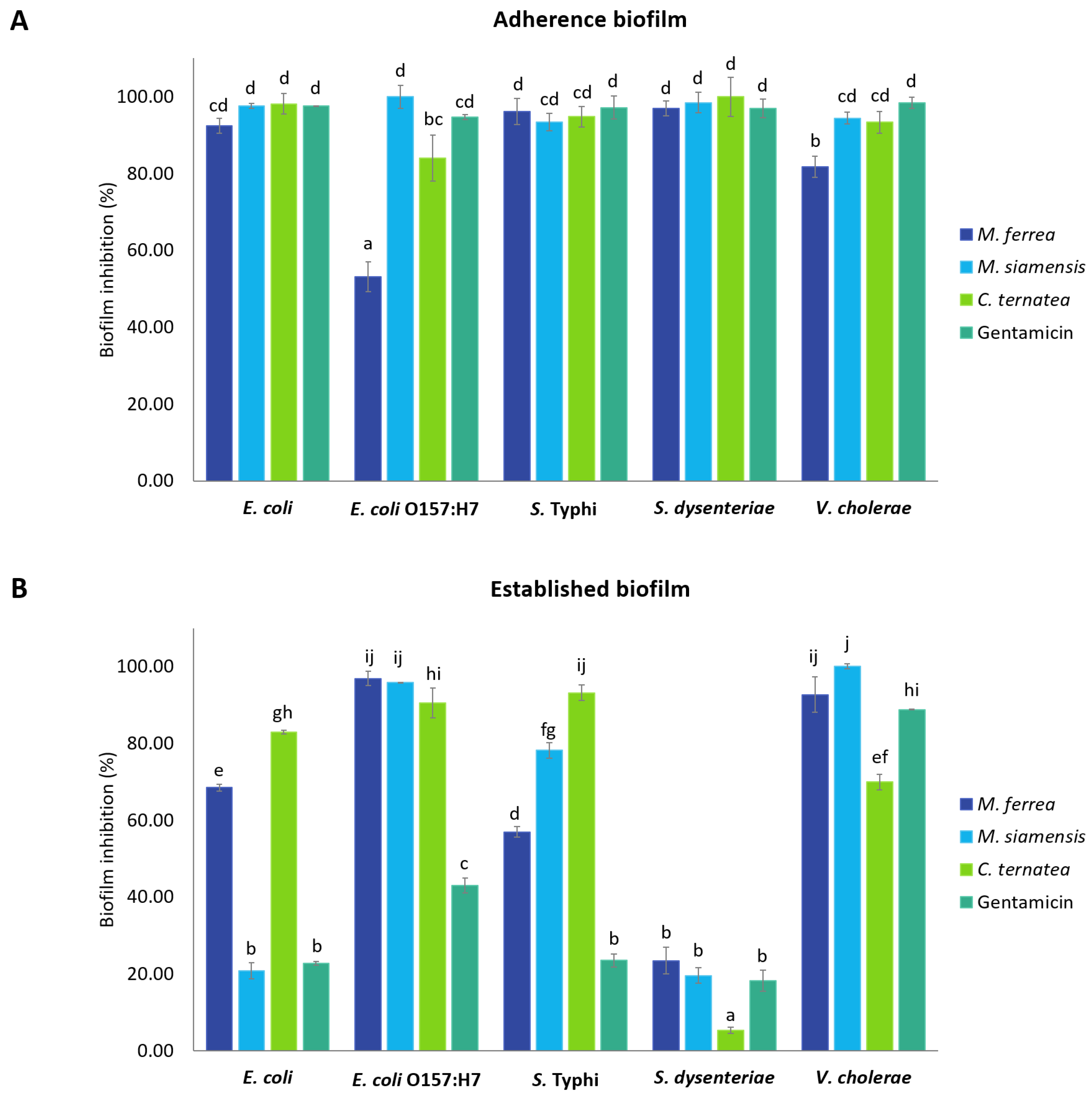
2.6. Antibiofilm Activity of Flower Extracts
2.7. Antibacterial Adhesion Activity of Flower Extracts on Caco-2 Cells
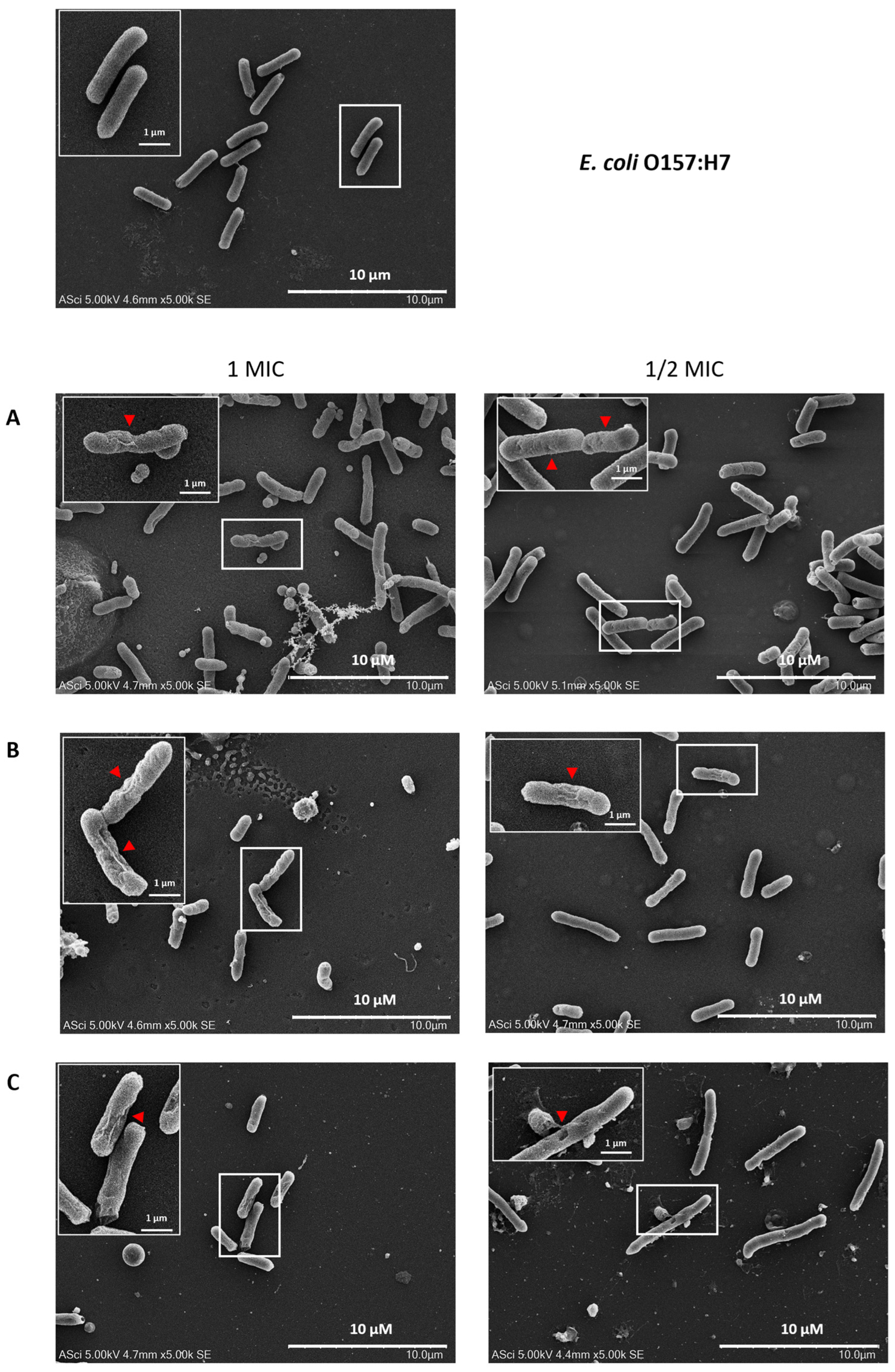
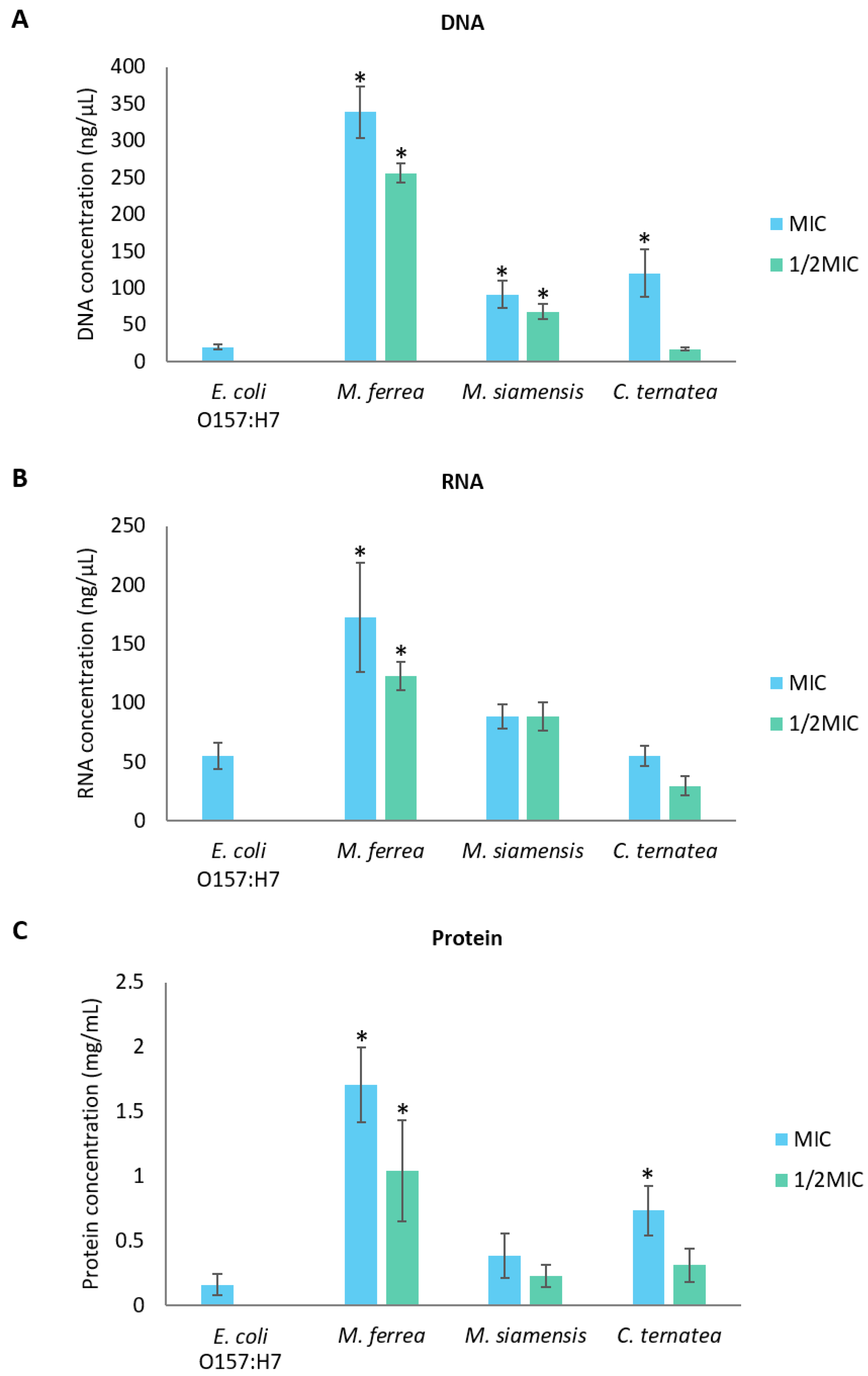
2.8. Effects of Flower Extracts on the Cellular Structure of E. coli O157:H7
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Flower Samples and Extraction Procedure
4.3. Identification of Bioactive Compounds in Flower Extracts by HPLC Analysis
4.4. Identification of Bioactive Compounds in Flower Extracts by LC-MS Analysis
4.5. Determination of Total Phenolic and Flavonoid Contents in Flower Extracts
4.6. Antioxidant Activities
4.6.1. DPPH Radical Scavenging Assay
4.6.2. ABTS Radical Cation Decolorization Assay
4.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
4.7. Antibacterial Activities
4.7.1. Bacterial Strains
4.7.2. Agar Well Diffusion Method
4.7.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.7.4. Time–Kill Assay
4.8. Anti-Biofilm Assay
4.8.1. Effects on Adherence Biofilm
4.8.2. Effects on Established Biofilm
4.9. Antibacterial Adhesion Activity on Caco-2 Cells
4.9.1. Cell Culture and Cytotoxicity by Flower Extracts
4.9.2. Determination of Antibacterial Adhesion by Flower Extracts
4.10. Cellular Structure of E. coli O157:H7
4.10.1. SEM Analysis
4.10.2. Cell Membrane Damage Assays
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,20-azinobis-(3-ethylbenzothiazolin-6-sulfonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazil |
| FRAP | ferric reducing antioxidant power |
| GAE | gallic acid equivalent |
| HPLC | high-performance liquid chromatography |
| LC-MS | liquid chromatography–mass spectrometry |
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| QE | quercetin equivalent |
| TEAC | trolox equivalent antioxidant capacity |
References
- Getie, M.; Abebe, W.; Tessema, B. Prevalence of Enteric Bacteria and Their Antimicrobial Susceptibility Patterns among Food Handlers in Gondar Town, Northwest Ethiopia. Antimicrob. Resist. Infect. Control 2019, 8, 111. [Google Scholar] [CrossRef]
- Wallace, M.J.; Fishbein, S.R.S.; Dantas, G. Antimicrobial Resistance in Enteric Bacteria: Current State and next-Generation Solutions. Gut Microbes 2020, 12, 1799654. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.R.; Rana, S.S.; Idrishi, R.; Sharma, V.; Ghosh, P. A Review on Nutritional, Bioactive, Toxicological Properties and Preservation of Edible Flowers. Future Foods 2021, 4, 100078. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Al-Shammari, E.; Elkhalifa, A.O.; Adnan, M.; Siddiqui, A.J.; Mahmood, D.; Azad, Z.R.A.A.; Patel, M.; Mehmood, K.; Danciu, C.; et al. Anti-Adhesion and Antibiofilm Activity of Eruca Sativa Miller Extract Targeting Cell Adhesion Proteins of Food-Borne Bacteria as a Potential Mechanism: Combined in Vitro-in Silico Approach. Plants 2022, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Cui, L.; Zhou, H.; Liu, Y.; Meng, L.; Chen, S.; Xi, X.; Zhang, Y.; Kang, W. Flowers: Precious Food and Medicine Resources. Food Sci. Hum. Wellness 2023, 12, 1020–1052. [Google Scholar] [CrossRef]
- Książkiewicz, M.; Karczewska, M.; Nawrot, F.; Korybalska, K.; Studzińska-Sroka, E. Traditionally Used Edible Flowers as a Source of Neuroactive, Antioxidant, and Anti-Inflammatory Extracts and Bioactive Compounds: A Narrative Review. Molecules 2025, 30, 677. [Google Scholar] [CrossRef]
- Kuerban, M.; Ma, F.; Shan, L.; Wang, Y.; Zhou, G. Comparative Discriminant Analysis of Mesua Ferrea L. and Its Adulterants. Heliyon 2024, 10, e28459. [Google Scholar] [CrossRef]
- Chahar, M.K.; Kumar, S.D.S.; Geetha, L.; Lokesh, T.; Manohara, K.P. Mesua Ferrea L.: A Review of the Medical Evidence for Its Phytochemistry and Pharmacological Actions. Afr. J. Pharm. Pharmacol. 2013, 7, 211–219. [Google Scholar] [CrossRef]
- Luo, F.; Manse, Y.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Morikawa, T. Structures of Mammeasins P and Q, Coumarin-Related Polysubstituted Benzofurans, from the Thai Medicinal Plant Mammea Siamensis (Miq.) T. Anders.: Anti-Proliferative Activity of Coumarin Constituents against Human Prostate Carcinoma Cell Line LNCaP. Pharmaceuticals 2023, 16, 231. [Google Scholar] [CrossRef]
- Sitthisuk, P.; Poorahong, W.; Innajak, S.; Krajarng, A.; Samosorn, S.; Watanapokasin, R. Mammea Siamensis Flower Extract-Induced Cell Death Apoptosis in HCT116 Colon Cancer Cells via Vacuolar-Type H+-ATPase Inhibition Associated with GSK-3β/β-Catenin, PI3K/Akt/NF-κB, and MAPK Signaling Pathway. Pharmaceuticals 2025, 18, 441. [Google Scholar] [CrossRef]
- Escher, G.B.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; da Silva, M.C.; Genovese, M.I.; Wen, M.; Zhang, L.; et al. Clitoria Ternatea L. Petal Bioactive Compounds Display Antioxidant, Antihemolytic and Antihypertensive Effects, Inhibit α-Amylase and α-Glucosidase Activities and Reduce Human LDL Cholesterol and DNA Induced Oxidation. Food Res. Int. 2020, 128, 108763. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Antioxidant, Cytotoxic, and Antibacterial Activities of Clitoria Ternatea Flower Extracts and Anthocyanin-Rich Fraction. Sci. Rep. 2022, 12, 14890. [Google Scholar] [CrossRef] [PubMed]
- Sulastri, E.; Zubair, M.; Anas, N.; Abidin, S.; Hardani, R.; Yulianti, R.; Yulianti, R. Total Phenolic, Total Flavonoid, Quercetin Content and Antioxidant Activity of Standardized Extract of Moringa Oleifera Leaf from Regions with Different Elevation. Phcog J. 2018, 10, s104–s108. [Google Scholar] [CrossRef]
- Sulaiman, M.; Ebehairy, L.; Nissapatorn, V.; Rahmatullah, M.; Villegas, J.; Dupa, H.J.; Verzosa, R.C.; Dolma, K.G.; Shabaz, M.; Lanting, S.; et al. Antibacterial Phenolic Compounds from the Flowering Plants of Asia and the Pacific: Coming to the Light. Pharm. Biol. 2024, 62, 713–766. [Google Scholar] [CrossRef] [PubMed]
- Plekratoke, K.; Boonyarat, C.; Monthakantirat, O.; Nualkaew, N.; Wangboonskul, J.; Awale, S.; Chulikhit, Y.; Daodee, S.; Khamphukdee, C.; Chaiwiwatrakul, S.; et al. The Effect of Ethanol Extract from Mesua Ferrea Linn Flower on Alzheimer’s Disease and Its Underlying Mechanism. Curr. Issues Mol. Biol. 2023, 45, 4063–4079. [Google Scholar] [CrossRef]
- Srichaikul, B. Ultrasonication extraction, bioactivity, antioxidant activity, total flavonoid, total phenolic and antioxidant of Clitoria ternatea Linn flower extract for anti-aging drinks. Pharmacogn. Mag. 2018, 14, 56. [Google Scholar] [CrossRef]
- Dong, M.; Llanas, A. The Essence of Modern HPLC: Advantages, Limitations, Fundamentals, and Opportunities. LCGC Asia Pac. 2013, 31, 472. [Google Scholar]
- Oldoni, T.L.C.; Da Silva, R.C.; Carpes, S.T.; Massarioli, A.P.; Alencar, S.M.D. Antioxidant Activity and Development of One Chromatographic Method to Determine the Phenolic Compounds from Agroindustrial Pomace. Ann. Acad. Bras. Ciênc. 2020, 92, e20181068. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Kar, A.; Mukherjee, S.K.; Barik, S.; Hossain, S.T. Antimicrobial Activity of Trigonelline Hydrochloride Against Pseudomonas Aeruginosa and Its Quorum-Sensing Regulated Molecular Mechanisms on Biofilm Formation and Virulence. ACS Infect. Dis. 2024, 10, 746–762. [Google Scholar] [CrossRef]
- Sekhar, M.G.; Ramudu Shanmugam, K.; Chakrapani, I.S. Trigonelline, a Fenugreek Bioactive Protects Heart Tissue against Alcohol Intoxication: An in-Vivo Study Focusing on Antioxidant Perspective. J. Ayurveda Integr. Med. 2024, 15, 100963. [Google Scholar] [CrossRef]
- Tatsimo, S.J.N.; Tamokou, J.d.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and Antioxidant Activity of Kaempferol Rhamnoside Derivatives from Bryophyllum Pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Q.; Zhang, J.; Zhang, Y.-T.; Sun, J.-Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.-Y.; Liu, C. The Link between the Phenolic Composition and the Antioxidant Activity in Different Small Berries: A Metabolomic Approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [PubMed]
- Batm, A.S.; Chaiyawatthanananthn, P.; Itharat, A. Antibacterial Activity of Extracts from a Thai Traditional Remedy Called Prasachandaeng and Its Plant Components. J. Med. Assoc. Thai 2017, 100, S58–S66. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute, Ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015; ISBN 978-1-56238-987-1. [Google Scholar]
- Hossain, T.J. Methods for Screening and Evaluation of Antimicrobial Activity: A Review of Protocols, Advantages, and Limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Saharan, B.S.; Beniwal, N.; Duhan, J.S. From Formulation to Function: A Detailed Review of Microbial Biofilms and Their Polymer-Based Extracellular Substances. Microbe 2024, 5, 100194. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. npj Antimicrob. Resist. 2024, 2, 1–10. [Google Scholar] [CrossRef]
- Vani, S.; Vadakkan, K.; Mani, B. A Narrative Review on Bacterial Biofilm: Its Formation, Clinical Aspects and Inhibition Strategies. Future J. Pharm. Sci. 2023, 9, 50. [Google Scholar] [CrossRef]
- Banerjee, A.; Chowdhury, P.; Bauri, K.; Saha, B.; De, P. Inhibition and Eradication of Bacterial Biofilm Using Polymeric Materials. Biomater. Sci. 2023, 11, 11–36. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.A.G.; Afonina, I.; Kline, K.A. Eradicating Biofilm Infections: An Update on Current and Prospective Approaches. Curr. Opin. Microbiol. 2021, 63, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Mahmud, R. Chemical Analysis, Inhibition of Biofilm Formation and Biofilm Eradication Potential of Euphorbia Hirta L. against Clinical Isolates and Standard Strains. BMC Complement. Altern. Med. 2013, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The in Vitro Antibiofilm Activity of Selected Culinary Herbs and Medicinal Plants against Listeria Monocytogenes. Lett. Appl. Microbiol. 2010, 50, 30–35. [Google Scholar] [CrossRef]
- Kshirsagar, P.R.; Patil, S.M. Phytochemistry and Pharmacology of Mesua Ferrea L. In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 223–256. ISBN 978-3-030-30182-8. [Google Scholar]
- Morikawa, T.; Sueyoshi, M.; Chaipech, S.; Matsuda, H.; Nomura, Y.; Yabe, M.; Matsumoto, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; et al. Suppressive Effects of Coumarins from Mammea Siamensis on Inducible Nitric Oxide Synthase Expression in RAW264.7 Cells. Bioorg Med. Chem. 2012, 20, 4968–4977. [Google Scholar] [CrossRef]
- Chaniad, P.; Chukaew, A.; Payaka, A.; Phuwajaroanpong, A.; Techarang, T.; Plirat, W.; Punsawad, C. Antimalarial Potential of Compounds Isolated from Mammea Siamensis T. Anders. Flowers: In Vitro and Molecular Docking Studies. BMC Complement. Med. Ther. 2022, 22, 266. [Google Scholar] [CrossRef]
- Tung, N.H.; Uto, T.; Sakamoto, A.; Hayashida, Y.; Hidaka, Y.; Morinaga, O.; Lhieochaiphant, S.; Shoyama, Y. Antiproliferative and Apoptotic Effects of Compounds from the Flower of Mammea Siamensis (Miq.) T. Anders. on Human Cancer Cell Lines. Bioorg Med. Chem. Lett. 2013, 23, 158–162. [Google Scholar] [CrossRef]
- Multisona, R.R.; Shirodkar, S.; Arnold, M.; Gramza-Michalowska, A. Clitoria Ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods. Appl. Sci. 2023, 13, 2134. [Google Scholar] [CrossRef]
- Shamim, A.; Ali, A.; Iqbal, Z.; Mirza, M.A.; Aqil, M.; Kawish, S.M.; Siddiqui, A.; Kumar, V.; Naseef, P.P.; Alshadidi, A.A.F.; et al. Natural Medicine a Promising Candidate in Combating Microbial Biofilm. Antibiotics 2023, 12, 299. [Google Scholar] [CrossRef]
- Summer, K.; Browne, J.; Hollanders, M.; Benkendorff, K. Out of Control: The Need for Standardised Solvent Approaches and Data Reporting in Antibiofilm Assays Incorporating Dimethyl-Sulfoxide (DMSO). Biofilm 2022, 4, 100081. [Google Scholar] [CrossRef]
- Bonetti, A.; Toschi, A.; Tugnoli, B.; Piva, A.; Grilli, E. A Blend of Selected Botanicals Maintains Intestinal Epithelial Integrity and Reduces Susceptibility to Escherichia Coli F4 Infection by Modulating Acute and Chronic Inflammation in Vitro. Front. Vet. Sci. 2023, 10, 1275802. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial Activity and Mode of Action of Acetone Crude Leaf Extracts of Under-Investigated Syzygium and Eugenia (Myrtaceae) Species on Multidrug Resistant Porcine Diarrhoeagenic Escherichia Coli. BMC Vet. Res. 2019, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, R.T.; Velsko, I.; Sampaio, S.C.F.; Elias, W.P.; Robins-Browne, R.M.; Gomes, T.A.T.; Girón, J.A. Fimbrial Adhesins Produced by Atypical Enteropathogenic Escherichia Coli Strains. Appl. Environ. Microbiol. 2011, 77, 8391–8399. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical Screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Borges, A.; Saavedra, M.J.; Simões, M. The Activity of Ferulic and Gallic Acids in Biofilm Prevention and Control of Pathogenic Bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria—Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Klančnik, A.; Šimunović, K.; Sterniša, M.; Ramić, D.; Smole Možina, S.; Bucar, F. Anti-Adhesion Activity of Phytochemicals to Prevent Campylobacter Jejuni Biofilm Formation on Abiotic Surfaces. Phytochem. Rev. 2021, 20, 55–84. [Google Scholar] [CrossRef]
- Lewis, A.J.; Richards, A.C.; Mendez, A.A.; Dhakal, B.K.; Jones, T.A.; Sundsbak, J.L.; Eto, D.S.; Rousek, A.A.; Mulvey, M.A. Plant Phenolics Inhibit Focal Adhesion Kinase and Suppress Host Cell Invasion by Uropathogenic Escherichia Coli. Infect. Immun. 2024, 92, e00080-24. [Google Scholar] [CrossRef]
- Haile, A.F.; Alonso, S.; Berhe, N.; Atoma, T.B.; Boyaka, P.N.; Grace, D. Prevalence, Antibiogram, and Multidrug-Resistant Profile of E. Coli O157: H7 in Retail Raw Beef in Addis Ababa, Ethiopia. Front. Vet. Sci. 2022, 9, 734896. [Google Scholar] [CrossRef]
- Zulkamal, L.M.; Zelan, M.A.H.A.; Aris, F.; Zakaria, N.A.; Yusof, F.Z.M.; Ibrahim, D.; Jalil, M.T.M.J. Aqueous Extract of Clitoria Ternatea Attenuates the Growth of Streptococcus Mutans. J. Pure Appl. Microbiol. 2023, 17, 1047–1055. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.; Bilal, M.; Wei, L.; Tang, S.; Luo, H.; Zhao, Y.; Wang, Z.; Duan, X. Toxicity and Inhibition Mechanism of Gallic Acid on Physiology and Fermentation Performance of Escherichia Coli. Bioresour. Bioprocess. 2022, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Aruldass, C.A.; Marimuthu, M.M.; Ramanathan, S.; Mansor, S.M.; Murugaiyah, V. Effects of Mesua Ferrea Leaf and Fruit Extracts on Growth and Morphology of Staphylococcus Aureus. Microsc. Microanal. 2013, 19, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, J.; Zhang, L.; Zhang, R.; Zhang, S.; Ye, S.; Zhao, Z.; Yang, D. Exploring the Antibacterial Mechanism of Essential Oils by Membrane Permeability, Apoptosis and Biofilm Formation Combination with Proteomics Analysis against Methicillin-Resistant Staphylococcus Aureus. Int. J. Med. Microbiol. 2020, 310, 151435. [Google Scholar] [CrossRef]
- Sulistyani, N.; Mahfudh, N.; Mawardi, R.H.; Zakaria, Z.A. Cell Leakage Mechanism and Time Kill Studies on Staphylococcus Aureus after Exposure to Ethanol Leaf Extract of Muntingia Calabura L. Trop. J. Pharm. Res. 2023, 22, 355–362. [Google Scholar] [CrossRef]
- He, M.; Wu, T.; Pan, S.; Xu, X. Antimicrobial Mechanism of Flavonoids against Escherichia Coli ATCC 25922 by Model Membrane Study. Appl. Surf. Sci. 2014, 305, 515–521. [Google Scholar] [CrossRef]
- Hossain, M.A.; Park, H.-C.; Lee, K.-J.; Park, S.-W.; Park, S.-C.; Kang, J. In Vitro Synergistic Potentials of Novel Antibacterial Combination Therapies against Salmonella Enterica Serovar Typhimurium. BMC Microbiol. 2020, 20, 118. [Google Scholar] [CrossRef]
- Yilmaz, H.; Gultekin Subasi, B.; Celebioglu, H.U.; Ozdal, T.; Capanoglu, E. Chemistry of Protein-Phenolic Interactions toward the Microbiota and Microbial Infections. Front. Nutr. 2022, 9, 914118. [Google Scholar] [CrossRef]
- Palamae, S.; Mittal, A.; Buatong, J.; Zhang, B.; Hong, H.; Benjakul, S. Chitooligosaccharide-Catechin Conjugate: Antimicrobial Mechanisms toward Vibrio Parahaemolyticus and Its Use in Shucked Asian Green Mussel. Food Control 2023, 151, 109794. [Google Scholar] [CrossRef]
- Tagrida, M.; Palamae, S.; Saetang, J.; Ma, L.; Hong, H.; Benjakul, S. Comparative Study of Quercetin and Hyperoside: Antimicrobial Potential towards Food Spoilage Bacteria, Mode of Action and Molecular Docking. Foods 2023, 12, 4051. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Mansour, F.R.; Abdallah, I.A.; Bedair, A.; Hamed, M. Analytical Methods for the Determination of Quercetin and Quercetin Glycosides in Pharmaceuticals and Biological Samples. Crit. Rev. Anal. Chem. 2025, 55, 187–212. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 107–115. ISBN 978-1-119-13538-8. [Google Scholar]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A Modification of the ABTS• Decolorization Method and an Insight into Its Mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 77–106. ISBN 978-1-119-13538-8. [Google Scholar]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Silver Nanoparticles against Staphylococcus Aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Qureshi, K.A.; Imtiaz, M.; Parvez, A.; Rai, P.K.; Jaremko, M.; Emwas, A.-H.; Bholay, A.D.; Fatmi, M.Q. In Vitro and in Silico Approaches for the Evaluation of Antimicrobial Activity, Time-Kill Kinetics, and Anti-Biofilm Potential of Thymoquinone (2-Methyl-5-Propan-2-Ylcyclohexa-2, 5-Diene-1, 4-Dione) against Selected Human Pathogens. Antibiotics 2022, 11, 79. [Google Scholar] [CrossRef]
- Prusokiene, A.; Hawkins, M.; Nieduszynski, C.A.; Retkute, R. Effectiveness of Glass Beads for Plating Cell Cultures. Phys. Rev. E 2021, 103, 052410. [Google Scholar] [CrossRef]
- Gascón, E.; Merino, N.; Pagán, E.; Berdejo, D.; Pagán, R.; García-Gonzalo, D. Assessment of in Vitro Biofilms by Plate Count and Crystal Violet Staining: Is One Technique Enough? In Detection and Enumeration of Bacteria, Yeast, Viruses, and Protozoan in Foods and Freshwater; Magnani, M., Ed.; Springer: New York, NY, USA, 2021; pp. 53–63. ISBN 978-1-0716-1932-2. [Google Scholar]
- Molina Bertrán, S.d.C.; Monzote, L.; Cappoen, D.; Escalona Arranz, J.C.; Gordillo Pérez, M.J.; Rodríguez-Ferreiro, A.O.; Chill Nuñez, I.; Novo, C.P.; Méndez, D.; Cos, P.; et al. Inhibition of Bacterial Adhesion and Biofilm Formation by Seed-Derived Ethanol Extracts from Persea Americana Mill. Molecules 2022, 27, 5009. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, M.; Janczura, A.; Nowicka, J.; Kamysz, W. Methods Used for the Eradication of Staphylococcal Biofilms. Antibiotics 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Wanes, D.; Naim, H.Y.; Dengler, F. Proliferation and Differentiation of Intestinal Caco-2 Cells Are Maintained in Culture with Human Platelet Lysate Instead of Fetal Calf Serum. Cells 2021, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zakłos-Szyda, M.; Żyżelewicz, D.; Koszucka, A.; Motyl, I. Acrylamide Decreases Cell Viability, and Provides Oxidative Stress, DNA Damage, and Apoptosis in Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 368. [Google Scholar] [CrossRef]
- Minuti, A.E.; Labusca, L.; Herea, D.-D.; Stoian, G.; Chiriac, H.; Lupu, N. A Simple Protocol for Sample Preparation for Scanning Electron Microscopic Imaging Allows Quick Screening of Nanomaterials Adhering to Cell Surface. Int. J. Mol. Sci. 2023, 24, 430. [Google Scholar] [CrossRef]
- SEM Preparation. Available online: https://electron-microscopy.hms.harvard.edu/sem-preparation (accessed on 27 March 2025).
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Preparation of Slides and Coverslips for Microscopy. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4988. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial Activity and Mechanism of Clove Essential Oil against Foodborne Pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]





| Flower Extracts | Yield (%) |
|---|---|
| Mesua ferrea | 18.91 |
| Mammea siamensis | 16.03 |
| Clitoria ternatea | 17.01 |
| Flower Extracts | Gallic Acid (mg/g Extract) | Quercetin (mg/g Extract) |
|---|---|---|
| Mesua ferrea | 16.956 ± 0.059 b | 0.260 ± 0.027 a |
| Mammea siamensis | 0.921 ± 0.015 a | 0.678 ± 0.025 b |
| Clitoria ternatea | ND | ND |
| Flower Extracts | Total Phenolic Content (mg GAE/g Extract) | Total Flavonoid Content (mg QE/g Extract) |
|---|---|---|
| Mesua ferrea | 50.09 ± 1.01 c | 12.48 ± 0.48 a |
| Mammea siamensis | 26.02 ± 0.62 b | 16.51 ± 0.01 b |
| Clitoria ternatea | 10.66 ± 0.85 a | 19.37 ± 0.91 c |
| Flower Extracts | DPPH | ABTS | FRAP | ||
|---|---|---|---|---|---|
| IC50 (mg/mL) | Antioxidant Activity (mg GAE/g Extract) | IC50 (mg/mL) | Antioxidant Activity (mg TEAC/g Extract) | Antioxidant Activity (mg FeSO4/g Extract) | |
| Mesua ferrea | 2.09 ± 0.06 c | 2.31 ± 0.14 a | 10.96 ± 1.04 c | 15.78 ± 1.59 a | 186.49 ± 9.36 c |
| Mammea siamensis | 0.47 ± 0.02 b | 10.20 ± 0.25 b | 2.87 ± 0.28 b | 60.29 ± 5.81 b | 81.03 ± 4.02 b |
| Clitoria ternatea | 0.30 ± 0.03 a | 16.48 ± 2.00 c | 1.00 ± 0.14 a | 159.46 ± 3.21 c | 37.45 ± 3.11 a |
| Flower Extracts (500 mg/mL) | Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|---|
| E. coli | E. coli O157:H7 | S. Typhi | S. dysenteriae | V. cholerae | |
| Mesua ferrea | 13.00 ± 0.87 cd | 13.33 ± 1.15 cd | 15.00 ± 0.00 d | 14.00 ± 2.65 d | 14.83 ± 1.04 d |
| Mammea siamensis | 10.00 ± 0.00 b | 0.00 a | 11.00 ± 1.73 bc | 10.67 ± 1.53 bc | 0.00 a |
| Clitoria ternatea | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
| Gentamicin (1 mg/mL) | 26.67 ± 0.58 e | 29.33 ± 0.58 f | 26.67 ± 0.58 e | 28.33 ± 0.58 ef | 26.67 ± 0.58 e |
| Flower Extracts | Concentration of Flower Extracts (mg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | E. coli O157:H7 | S. Typhi | S. dysenteriae | V. cholerae | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Mesua ferrea | 62.5 | 62.5 | 62.5 | 62.5 | 31.25 | 31.25 | 31.25 | 31.25 | 31.25 | 31.25 |
| Mammea siamensis | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 31.25 | 31.25 | 62.5 | 62.5 |
| Clitoria ternatea | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| Gentamicin | 0.0078 | 0.0078 | 0.0078 | 0.0078 | 0.0078 | 0.0078 | 0.0078 | 0.0078 | 0.0078 | 0.0078 |
| Flower Extracts | Inhibition of Bacterial Adhesion (%) | ||||
|---|---|---|---|---|---|
| E. coli | E. coli O157:H7 | S. Typhi | S. dysenteriae | V. cholerae | |
| Mesua ferrea | 20.49 ± 1.78 de | 24.08 ± 0.36 e | 3.14 ± 1.22 a | 5.13 ± 0.52 ab | 12.43 ± 1.41 bc |
| Mammea siamensis | 16.06 ± 0.95 cd | 24.78 ± 3.61 e | 57.41 ± 0.21 g | 6.41 ± 0.26 ab | 24.29 ± 6.06 e |
| Clitoria ternatea | 16.11 ± 3.53 cd | 38.61 ± 0.39 f | 55.18 ± 0.54 g | 4.76 ± 2.07 ab | 17.86 ± 1.01 cde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriyaprom, S.; Cheepchirasuk, N.; Ngamsaard, P.; Intachaisri, V.; Inta, A.; Tragoolpua, Y. Thai Medicinal Flowers as Natural Antioxidants and Antibacterial Agents Against Pathogenic Enteric Bacteria: A Comparative Study of Mesua ferrea, Mammea siamensis, and Clitoria ternatea. Antibiotics 2025, 14, 1038. https://doi.org/10.3390/antibiotics14101038
Suriyaprom S, Cheepchirasuk N, Ngamsaard P, Intachaisri V, Inta A, Tragoolpua Y. Thai Medicinal Flowers as Natural Antioxidants and Antibacterial Agents Against Pathogenic Enteric Bacteria: A Comparative Study of Mesua ferrea, Mammea siamensis, and Clitoria ternatea. Antibiotics. 2025; 14(10):1038. https://doi.org/10.3390/antibiotics14101038
Chicago/Turabian StyleSuriyaprom, Sureeporn, Nitsanat Cheepchirasuk, Pornpimon Ngamsaard, Varachaya Intachaisri, Angkhana Inta, and Yingmanee Tragoolpua. 2025. "Thai Medicinal Flowers as Natural Antioxidants and Antibacterial Agents Against Pathogenic Enteric Bacteria: A Comparative Study of Mesua ferrea, Mammea siamensis, and Clitoria ternatea" Antibiotics 14, no. 10: 1038. https://doi.org/10.3390/antibiotics14101038
APA StyleSuriyaprom, S., Cheepchirasuk, N., Ngamsaard, P., Intachaisri, V., Inta, A., & Tragoolpua, Y. (2025). Thai Medicinal Flowers as Natural Antioxidants and Antibacterial Agents Against Pathogenic Enteric Bacteria: A Comparative Study of Mesua ferrea, Mammea siamensis, and Clitoria ternatea. Antibiotics, 14(10), 1038. https://doi.org/10.3390/antibiotics14101038









