Abstract
Multidrug-resistant organism (MDRO) outbreaks have been steadily increasing in intensive care units (ICUs). Still, healthcare institutions and workers (HCWs) have not reached unanimity on how and when to implement infection prevention and control (IPC) strategies. We aimed to provide a pragmatic physician practice-oriented resume of strategies towards different MDRO outbreaks in ICUs. We performed a narrative review on IPC in ICUs, investigating patient-to-staff ratios; education, isolation, decolonization, screening, and hygiene practices; outbreak reporting; cost-effectiveness; reproduction numbers (R0); and future perspectives. The most effective IPC strategy remains unknown. Most studies focus on a specific pathogen or disease, making the clinician lose sight of the big picture. IPC strategies have proven their cost-effectiveness regardless of typology, country, and pathogen. A standardized, universal, pragmatic protocol for HCW education should be elaborated. Likewise, the elaboration of a rapid outbreak recognition tool (i.e., an easy-to-use mathematical model) would improve early diagnosis and prevent spreading. Further studies are needed to express views in favor or against MDRO decolonization. New promising strategies are emerging and need to be tested in the field. The lack of IPC strategy application has made and still makes ICUs major MDRO reservoirs in the community. In a not-too-distant future, genetic engineering and phage therapies could represent a plot twist in MDRO IPC strategies.
1. Introduction
Hospital-acquired infections (HAIs) are a major concern for public health and a major issue in ICUs [1]. HAIs are defined as infections acquired after hospitalization that manifest themselves 48 h after admission to the hospital. The most common HAIs include ventilator-associated pneumonia (VAP), central line-associated bloodstream infections (CLABSIs), and catheter-associated urinary tract infections (CAUTIs) [1].
According to the WHO’s Global Report on Infection Prevention and Control (IPC) of 2022, 7% of patients in high-income countries and 15% of patients in low- and middle-income countries (LMICs) acquire at least one HAI during hospitalization [2,3].
These numbers rise dramatically if we take into consideration only adult ICUs: almost one out of three (30%) hospitalized patients develop an HAI; in fact, almost half of all cases (48.7%) of sepsis with organ dysfunction treated in ICUs are hospital-acquired [2,4], resulting in death in 52.3% [4].
In particular, MDROs’ prevalence in ICUs is currently a major concern as they can account for up to 50% of all infections in this setting [5]. In the USA, more than 700,000 HAIs each year are associated with MDR bacteria in ICUs, while in Europe, a growing rate of HAIs caused by Carbapenemase-producing Enterobacteriaceae (CPE) and New Delhi Metallo-Betalactamase (NDM) producers have been observed. However, the greatest threat in ICUs is currently represented by MDR Acinetobacter baumannii, Pseudomonas, Enterobacteriaceae, and Candida auris, against which there are few weapons available [6].
These global estimates of HAI frequency are probably downgraded by several factors: the lack of HAI surveillance and outbreak reporting systems, poor adherence to standardized protocols, and poor study quality [2].
The reason for the higher percentages in ICUs is grounded only partially in its intrinsic risk factors for infection acquisition (i.e., the use of invasive devices, high severity of acute illness, predisposing underlying conditions, and being at age extremities [7]); the lack of infection control is the real killer.
According to the WHO, national infection prevention and control (IPC) programs and operational plans are not available in all countries and, when available, they may not be fully implemented. This is the case for Italy and Romania in Europe; Bolivia, Costa Rica, and Honduras in South-Central America; and India, Nepal, Bhutan, Myanmar, Tagikistan, Turkmenistan, Iraq, and Afghanistan in Asia [2]. In Africa, 15 out of 54 states find themselves in this condition [2].
These programs are a key element to put IPC into practice and prevent multidrug-resistant organisms (MDROs) from spreading in and outside of the hospital setting.
IPC is based on two different but complementary approaches: targeted and universal approaches. The targeted approach consists of screening and isolation, and the details are usually contained in a bundle.
The aim of this narrative review of the literature is to summarize and display the most successful and pragmatic strategies to achieve infection control in ICU outbreaks.
2. Methodology
This review reports the major risk factors for HAI acquisition identified in the ICU setting and proposes strategies in guidelines, the WHO’s recommendations, international institutional statements, and outbreak reports in the past 25 years, although it does not comprehensively report on all of the literature as a systematic review would. The choice of 25 years is dictated by the will to include only the most consolidated strategies and novelties in the field of IPC, including those specifically against MDROs. Every statement and statistic reported in the following section of this paper refers to adult ICU departments, unless otherwise marked.
The search was conducted on the PubMed electronic database and included only peer-reviewed articles. No language restriction was applied. Publications were firstly screened by title, abstract, and year of publishing by CF. Afterwards, CF evaluated the full articles in order to assess the eligibility for inclusion, and they were consequently reviewed by DP, LP, and PT. The quality of the data and accuracy of description of the proposed strategy, together with the novelty, were considered as the most weighted factors in the selection process.
All investigated variables in the context of IPC (patient-to-staff ratios, education, isolation, decolonization, screening and hygiene practices, outbreak reporting, cost-effectiveness, reproduction-number (R0), and future perspectives) were selected on the basis of (1) recurrency in IPC guidelines and WHO statements, (2) frequency of discussion and solution proposals in outbreak reports, and (3) real-life, daily faced IPC problematic issues authors experience as an infectologist (CF) and intensivists (DP, LP, and PT).
3. Outbreaks Genesis
An HAI outbreak could be defined as an increased number of cases of a certain HAI among patients or healthcare personnel that is superior to the expected number, which is clustered by time and place [8,9,10].
Transmission occurs differently depending on the pathogen, involving environmental, healthcare organization-dependent, laboratory, and host-dependent factors [7].
The routes of transmission for some of the most common pathogens isolated in ICUs [11] are displayed in Table 1. The hematogenous route was not listed as the routinary use of gloves for invasive procedures is commonly adopted and effectively prevents the spread of blood-borne diseases.

Table 1.
The routes of transmission for some of the most frequent difficult-to-treat pathogens in the ICU setting.
Although outbreaks involve a large number of individuals, the risk factors for HAI acquisition should be taken into consideration for both the type of patient and type of infection.
4. Risk Factors for Outbreak
Outbreaks generally depart from a non-diagnosed infected or colonized patient for a transmittable disease [7]. Therefore, the first risk factor is represented by the lack of diagnosis.
A rapid outbreak recognition tool (i.e., an easy-to-use mathematical model) should be proposed to improve early diagnosis and prevent spreading. For example, the identification of three cases in 5 days could be an outbreak triggered test, as experimented by Elliot et al. [28].
There are several factors, both generic and pathogen-specific, that interlude the outbreak genesis. All generic risk factors are listed as separate paragraphs in this review, including patients’ colonization (Box 1) [12,27,29] management, and pathogen-specific risk factors are listed as sub-paragraphs or the main focus.
Box 1. MDRO colonization.
- It is defined by the presence of an MDRO without the evidence of tissue invasion or associated symptoms:
- ○
- Regular sites: Respiratory secretions (nostrils, pharynx, and endotracheal aspirates), wounds, skin, urine, and the rectum. More than one site could be affected by the same colonization.
- ○
- Sterile sites (not interpretable as colonization): Blood, liquor, pleural, peritoneal, and synovial liquids.
- A colonized patient always represents a potential source of transmission.
- It requires contact and/or respiratory isolation beyond routine IPC procedures.
- Decolonization is not currently recommended in the ICU setting.
Among the most underrated, artificial fingernails have been associated with HAIs, such as Serratia marcescens bloodstream infections (BSIs) in patients undergoing hemodialysis [30] and ESBL-producing Klebsiella pneumoniae and Pseudomonas aeruginosa invasive infections in neonatal ICUs [31].
Notably, a body mass index (BMI) ≥30 and an elevated number of hospitalizations have also been associated with a major risk of acquiring MRSA [32,33], CRE [34], and VRE [34] colonization.
Intravenous and inhalation drug use is the important risk factor to community-acquired MRSA colonization; therefore, they have to be screened at admission [32].
5. Strategies
IPC strategies are multiple and synergic. All variables that are worth considering for the purpose of a successful infection control process are reported below. They include the patient-to-nurse ratio (PNR), patient-to-intensivist ratio (PIR), healthcare staff education, isolation types, MDRO decolonization, hand hygiene, shoe hygiene, screening, environmental cleaning, antimicrobial stewardship programs, outbreak reporting, special populations, cost-effectiveness and R0, new experiment strategies, and future perspectives. The quality of evidence and strength of these practices according to the pathogen are listed in the ESCMID’s guidelines for Infection Control 2014 [12]. To our knowledge, no further updates of these guidelines have been published.
6. Nurse-to-Patient Ratio
The connection between nurse- and intensivist-to-patient ratios and infection prevention and control (IPC) is of paramount importance. Hospital IPC is a sanitary problem involving the whole hospital structure [35]. In fact, IPC strategies are not only for first-line healthcare personnel’s utility, but also for healthcare management personnel. Isolation, decolonization, screening and hygiene practices, and outbreak reporting may not be implemented or implemented incorrectly if the healthcare staff is understaffed or not properly acquainted with IPC and healthcare-associated infections (HCAIs) [36,37]. This results in a lack of infection control, beyond a of lack infection prevention, with associated costs in terms of human lives and economic expenditure [38].
There are solid studies in the literature and strong guidelines regarding the patient-to-nurse ratio (PNR) (Table 2). This ratio should be 1:1 or 1:2 according to the kind of ICU. Several international organizations have stated that in the ICU setting, every patient must have immediate access to an ICU specialist nurse, suggesting a PNR of 1:1.

Table 2.
Studies aiming to evaluate the optimal patient-to-nurse (PNR) ratio in the ICU setting.
7. Physician/Patient Ratio
Currently, there is no clear recommendation on the patient-to-intensivist ratio (PIR) by actual guidelines (Table 3).

Table 3.
Studies evaluating the association between the intensivist-to-patient ratio and higher mortality rates in the ICU setting.
Five studies have been published on this topic before Jeremy M. Kahn et al. tried to give an answer to this question in 2023 with a multicenter cohort study on 29 ICUs in 10 hospitals in the United States of America [47]. They failed to find an association between a higher intensivist-to-patient ratio and higher mortality.
Neuraz et al., in 2015, were the first to find an association with the PIR, namely a two-fold increase in shift-specific mortality among French ICU patients cared for by doctors with > 14 vs. < 8 patients [42]. Moreover, Gershengorn HB et al. conducted a similar study in the United Kingdom in 2017, finding a positive association between the PIR (patient-to-intensivist ratio) and British ICU patients’ mortality [43]. Five years later, Georgshengorn et al. repeated the study on Australian and New Zealand ICUs, but no association with the PIR was found [45].
Furthermore, studies conducted on this topic in the USA always fail to find an association between the PIR and ICU mortality.
However, as Kerlin MP and Caruso P. stated in their paper and as Kahn et al. pointed out, all five studies that preceded theirs suffered from several methodological limitations [47,49]. For instance, they took into consideration the intensivist-to-patient ratios averaged over the length of the entire ICU stay, overlooking that the ICU census changes day by day and that it could obscure daily variations, which could influence the outcomes. Moreover, they generally extrapolated intensivist-to-patient ratios from ICU census data, neglecting that intensivists may provide care in multiple ICUs within a single day. Additionally, in all of these studies, the majority of intensivists were anesthesiologists/intensivists, but many other specialists, ranging from approximately 10% to 30%, belonged to different medical specialties.
8. Education
Beyond the nurse- and physician-to-patient ratios, HCW education on IPC is what affects infectious disease transmission and relative associated mortality the most.
Therefore, not only nurses, but all healthcare personnel (physicians, healthcare workers, medical and nursing students, and cleaning staff) [50] should undergo an ‘IPC course’ as soon as they are hired by the hospital, just before taking an active part in ward activities [50,51]. Furthermore, a ‘refresh IPC course’ should be taken periodically, established by hospital protocols, no less than once per year (or per month, according to local epidemiology). The frequency of the ‘refresh IPC course’ should be rapidly implemented in the case of an outbreak [52].
IPC courses should provide information on pathogens’ transmission, isolation and hand hygiene instructions, and a practical simulation of the procedures. An initial and final practice test should be performed in order to verify the effectiveness of the course and awareness achieved among the healthcare personnel.
The Cochrane Effective Practice and Organisation of Care (EPOC) group elaborated on a seven-item educational model to enhance the uptake of educational contents (Box 2).
Box 2. The seven educational strategies elaborated on by the Cochrane Effective Practice and Organisation of Care group [53] to promote the uptake of guidelines.
- Printed educational materials
- Educational meetings
- Educational outreach
- Local opinion leaders
- Audit and feedback
- Computerized reminders
- Tailored interventions
Currently, when IPC educational and training programs are present, they differ consistently among WHO countries [54] and are rarely provided by academic institutions, and frequently practicing IPC physicians are not specialized in infectious disease or clinical microbiology [54]. The WHO’s latest guidelines on the core components of IPC programs [50] (Box 3) suggest different and targeted training for each of the identified three categories of HCWs: IPC specialists, HCWs involved in patient care (i.e., nurses and healthcare assistants), and auxiliary personnel (cleaning, administrative, and managerial staff).
Box 3. Core components of IPC (infection prevention and control) programs at the national and acute healthcare facility levels according to the WHO’s guidelines of 2016.
- IPC programs
- IPC guidelines (both at the national level and facility level)
- IPC education and training
- Healthcare-associated infection (HCAI) surveillance
- Multimodal strategies for implementing IPC
- IPC monitoring, evaluation, and feedback
- Workload, staffing, and bed occupancy (at the facility level)
- Built environment, materials, and equipment for IPC (at the facility level)
No standardized, universal, pragmatic education protocol has been elaborated so far, so we reported some valuable examples (Table 4).

Table 4.
Education models proposed to cope with healthcare-associated infections (HCAI) in intensive care units (ICUs).
In addition to implementing proper nurse- and intensivist-to-patient ratios, an educational model on IPC should be adopted by the institution in order to assure the good quality of IPC strategy implementation.
9. Isolation
The isolation of the colonized/infected patient is a key strategy for infection control [12]. Without isolation, the other IPC approaches may not be sufficient.
According to the ESCMID’s guidelines of 2014, precautionary isolation for patients recently admitted in the ICU should always be performed in order to avoid the uprise of infection clusters among ICU patients and staff and further hospital clusters [12]. Isolation should be discontinued only after a negative result is obtained from screening procedures (see SCREENING section).
Isolation rooms should preferably be single rooms whenever possible. It is mandatory to provide a single room in the case of neutropenic patients or specific airborne diseases (measles, varicella virus, and tuberculosis) [7].
There are three kinds of isolation: contact isolation, respiratory isolation, and both.
The kind of isolation that should be adopted varies depending on where the pathogen was isolated.
9.1. Respiratory Isolation
Respiratory isolation is required every time a respiratory airway sample (rhinopharyngeal swabs, sputum, and bronchial airway liquid fluid or aspirate) has a positive result for a potential air-spreading pathogen in human beings [12]. Such pathogens are listed in Table 1.
Two kinds of respiratory isolation rooms should be available in every ICU [60]:
- A negative-pressure room for patients who were colonized or infected by potential air-spreading pathogens (i.e., coronaviruses, Mycobacterium tuberculosis, varicella zoster virus, and measles);
- A positive-pressure room for patients who are likely more susceptible to acquiring an infection, such as solid-organ transplant (SOT) recipients, hematopoietic stem cell transplant (HSCT) patients, patients with the presence of hematological disorders, and patients with chronic use of corticosteroids, calcineurin inhibitors, anti-metabolites, and other immunosuppressants.
A differential pressure is created when one space (corridor or anteroom) has a different pressure compared to an adjoining space (in this case, the isolation room). When a differential pressure is present, air is forced to flow from the high-pressure space to the lower-pressure space. The direction of the flow is called ‘negative’ when the air ventilation system generates an air flow into the room, but it does not escape from the room; it is positive when the reverse occurs (Figure 1).
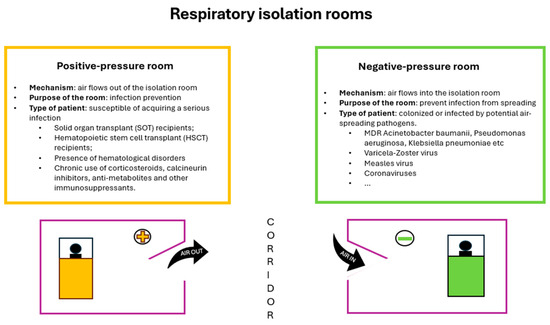
Figure 1.
Diagram representing positive-pressure and negative-pressure respiratory isolation rooms.
The duration of isolation depends on the possibility of pathogen eradication [12].
Whenever the pathogen is eradicated, the patient can leave the isolation room.
A single isolation room is mandatory in the case of some airborne pathogens (tuberculosis, measles, and varicella virus) and patients with neutropenia [7].
9.2. Contact Isolation
Contact isolation is required every time a skin or rectal sample (swabs) has a positive result for a potential pathogen transmitted through direct or indirect contact in human beings, especially MDROs (multi-drug resistant organisms) [7]. Those include all pathogens with acquirable resistance through through plasmid transmission, among others, along with ESBL (extended-spectrum beta-lactamase) resistance [7]. Such pathogens are listed in Table 1.
Contact isolation is also required in the case of the diagnosis of particular diseases known for being transmitted by contact (i.e., Ebola).
Contact isolation is mandatory for both patients who are infected and patients who are colonized by these organisms [7,12]. CRE rectal colonization could last for up to one year [61], while VRE lasts for approximately 6 months [62]. MRSA skin colonization has been reported to last for an average of 9 months [63,64,65], and an older age is associated with a longer duration of colonization for both MRSA [63] and CRE.
10. MDRO Decolonization
Although the eradication of the multidrug-resistant organism (MDRO) could possibly serve to prevent both further transmission and infection development [66], it is not currently recommended.
10.1. Gram-Negative Bacteria (GNB)
There is no recommendation in favor or against routine MDR-GNB decolonization in ICU patients by actual guidelines.
In general patients, the ESCMID-EUCIC guidelines do not recommend the routine decolonization of 3GCephRE and CRE carriers, though they do not extend this statement to immunocompromised patients (e.g., patients in the ICU, with neutropenia, or receiving a transplant) as only few studies have been conducted on this population. Its effectiveness and long-term side effects are encouraged to be assessed through appropriate RCTs (randomized controlled trials) [67].
However, several recent studies suggest an increased risk of CRE infection development in ICU patients with CRE colonization [68,69,70,71] and satisfactory rates of decolonization effectiveness [72,73].
For CRAB (carbapenem-resistant Acinetobacter baumannii), AGRE (aminoglycoside-resistant Enterobacteriaceae), CoRGNB (colistin-resistant Gram-negative organisms), CRSM (cotrimoxazole-resistant Stenotrophomonas maltophilia), FQRE (fluoroquinolone-resistant Enterobacteriaceae), PDRGNB (pan-drug-resistant Gram-negative organisms), and XDRPA (extremely drug-resistant Pseudomonas aeruginosa) carriers, evidence is still limited, and no recommendations have been proposed for ICU carriers nor for non-ICU carriers [67].
10.2. Gram Positive Bacteria (GPB)
To our knowledge, MRSA decolonization with intranasal mupirocin and chlorhexidine bathing is not explicitly recommended by any guidelines [15], except for those on an orthopedic or cardio surgery waiting list [74]. Still, there is much evidences that systemic screening followed by the decolonization of MRSA in all ICU patients (universal approach) decreases the incidence of MRSA colonization or infection by up to 52% [75]. In fact, the SHEA/IDSA/APIC guidelines highlight that active surveillance with contact precautions is inferior to universal decolonization in reducing MRSA isolation in adult ICUs [15] (REDUCE MRSA Trial) [76], and universal decolonization with daily CHG bathing plus 5 days of nasal decolonization should be performed in this setting to reduce endemic MRSA clinical cultures [15] (quality of evidence: high). Therefore, the endemic status should be assessed. Predictors of decolonization failure could be respiratory tract colonization [77], a younger age (0–17 years) [66], refugee status [66], and the presence of one or more comorbidities [66]; patients with these factors would possibly need different decolonization strategies.
However, physicians should bear in mind that MRSA colonization is associated with a 4-fold increase in the risk of MRSA infection development [78]. More than 50% of patients with MRSA colonization develop the infection in the ICU setting [79], and MRSA colonization is also associated with an increase in hospital admission, with further consequent possible transmission and outbreak development [33].
As far as we know, no guidelines have been elaborated on VRE decolonization indications or practice. This is probably due to the scarcity of studies conducted on this topic so far. Some studies on MRSA decolonization showed that chlorhexidine bathing could be effective in reducing VRE acquisition and infection development [15]. Cheng et al. obtained VRE decolonization by applying a combination of polyethylene glycol for bowel preparation, a five-day course of oral absorbable linezolid and non-absorbable daptomycin to suppress any remaining VRE, and subsequent oral Lactobacillus rhamnosus GG beyond environmental cleaning and isolation [80]. A non-antibiotic decolonization protocol consisting of a four-item bundle for both VRE and CRE was recently proposed by Choi et al.: using a glycerin enema for mechanical evacuation, daily lactobacillus ingestion for the restoration of normal gut flora, a chlorhexidine bath, and changing bed sheets and clothing every day [81]. Both proposed protocols need to be experimented with in further studies to assess their efficacy, but firstly, studies on VRE decolonization benefits should be conducted.
10.3. Candida auris
According to the Centers for Disease Control and Prevention (CDC), the efficacy of Candida auris decolonization is not known [82]. Chlorhexidine or topical antifungals have been proposed empirically, but evidence is still scarce.
Candida auris is currently the biggest emergent threat in USA and European ICUs as, contrarily to other MDROs, no antifungals, single or in combination, have shown solid efficacy. Thus, IPC measures are the best available weapon. Beyond the ECDC’s in-hospital hygiene recommendations, contact tracking, single-room contact isolation, surveillance though periodic skin swab testing of the healthcare personnel, co-hospitalized patients, and cohabitants who came into contact with the C. auris carrier could be effective in tackling the spread of C. auris.
11. Hand Hygiene
Hand hygiene (HH) is crucial for infection control. According to the WHO’s recommendations, hand hygiene should be performed when moving from one patient to another in all settings regardless of the presence of an ongoing infection or colonization.
The WHO’s recommendations on HH are based on two rules: the six movements and the five moments of hand hygiene [59].
Healthcare staff cannot exempt themselves from knowing these rules and should put them into practice as per the strong evidence these practices have shown.
It has been proven that appropriate HH is associated with a reduction in HAI incidence of up to 50% [83], including a 50% reduction in MRSA infections.
Despite the success rate in preventing HAI development and spreading declared by the WHO, a systematic review conducted by Kathryn Ann Lambe and colleagues in 2019 emphasized that the mean HH compliance was only 59.6% in adult ICUs, ranging from 64.4% in high-income countries to 9.1% in low-income countries. This percentage also varies in consideration to the type of ICU (neonatal, 67.0%; pediatric, 41.2%; and adult, 58.2%) and the type of healthcare workers (nurses, 43.4%; physicians, 32.6%; and others, 53.8%) [84].
A Brazilian study estimated that within 20 s without adhering to contact precautions, there was a 45% possibility that HCW (healthcare worker) hands became contaminated with a CRE. After shaking hands with this HCW, the possibility to become contaminated was likewise 22% [85]. If the first HCW had used a gown and gloves or would have washed their hands, the possibility rates would have been, respectively, 10% and 0% [85].
In the case of an outbreak, it would be useful to implement compliance with direct observations of the ‘five moments’ for hand hygiene performed by healthcare workers, followed by individualized verbal feedback [52].
12. Shoe and Medical Equipment Hygiene
Shoe soles represent a potential vector for pathogen transmission [86]. In addition to hand-hygiene, HCWs’ shoe bottoms can carry pathogens from one environment to another. Therefore, decontamination is needed when moving from one patient to another, especially when there is an MDRO carrier. Rashid et al. conducted a systematic review looking for an effective decontamination strategy for shoe soles in 2016 but did not succeed. This was also due to the scarcity of data present on this topic. Among mechanical strategies, the use of shoe covers or disposable boots seemed to be the most effective method in reducing the bacterial load in a sanitary setting, while adhesive mats proved to be ineffective [86]. Among chemical strategies, tanks or adhesive mats supplemented with 3–1 benzoisothiazolin or 0.2% benzylkonium were able to reduce the bacterial load [86]. Also, treating boots with a peroxygen disinfectant reduces the bacterial load by up to 1.4 log10. Boot baths with 6% sodium hypochlorite seem to prevent virus transmission [87].
On par with HCWs’ shoes, all HCW equipment, including badges, stethoscopes, oximeters, ultrasonography probes, and smartphones, should be disinfected with antiseptics such as chlorhexidine and benzalkonium, although MDR-efflux pump QAC carriers or GNB could be resistant [88].
13. Screening
The key points for MDRO screening are summarized in Box 4.
Box 4. Key points for multidrug-resistant organism (MDRO) screening.
- Use of risk-assessment scores for MDRO acquisition and infection development;
- Active screening for MDROs through weekly sample collection (skin, rectal, and/or respiratory according to the pathogen);
- Periodical environmental sample surveillance on high-touch surfaces (no standardized period has been proposed, but it would be advisable to perform this procedure at least once a week);
- Whole genome sequencing (WGS) whenever an MDRO is identified to identify putative transmission chains and to stratify patients.;
- Isolation, contact precautions, hand hygiene, and environmental cleaning should be performed in conformity with actual guidelines (see Environmental Cleaning and Hand Hygiene Sections).
- a.
- Risk-assessment scores
Risk-assessment scores (Table 5) could be applied at admission and recalculated daily in order to foresee the risk of colonization acquisition and/or infection. Hereby, HCWs can promptly put into practice the consequential IPC measures.
To our knowledge, no definitive colonization score was elaborated so far for CRAB and CRPA, although Dalben et al. identified some colonization risk factors for their acquisition in ICUs: the male sex, surgery prior to admission, the APACHE II score, and colonization pressure in the week before an outcome [89]. Tacconelli et al. identified some other risk factors for CRAB colonization and infection development, such as quinolone use [90]. Meschiari et al. identified as independent risk factors the use of permanent devices, mechanical ventilation, urinary catheters, the McCabe score, length of stay, and carbapenem use for CRAB colonization acquisition in the ICU setting [91].

Table 5.
Applicable risk-assessment scores for outbreak-related pathogen colonization and/or infection development.
Table 5.
Applicable risk-assessment scores for outbreak-related pathogen colonization and/or infection development.
| Type of MDRO | Type of Risk | Risk-Assessment Score | Description | Performance |
|---|---|---|---|---|
| Candida spp. [92] | Colonization | Candida Colonization Index [93] | Ratio of number of (non-blood) sites colonized with Candida spp. /total number of sites cultured Threshold = 0.5 | PPV = 66% NPV = 100% |
| Infection | Candida score [94] | Candida Score = TPN (1 point), surgery (1 point), severe sepsis (2 points), multifocal Candida colonization (1 point); threshold = 2.5 | Sensitivity = 81% Specificity = 74% PPV = 16% NPV = 98% | |
| Ostrosky-Zeichner Clinical Prediction Rule [95] | Mechanical ventilation ≥48 h AND systemic antibiotic AND CVP (on any day in day 1–3 period of ICU admission) plus ≥1 of the following: any major surgery (days 7–0), pancreatitis (days 7–0), use of steroids/other immunosuppressive agents (days 7–0), use of TPN (days 1–3), or dialysis (days 1–3) | Sensitivity = 50% Specificity = 83% PPV = 10% NPV = 97% | ||
| ESBL-producing Enterobacteriacae | Colonization | Tumbarello et al. [96] | Recent (≤12 months before admission) hospitalization, transfer from another healthcare facility, Charlson comorbidity score ≥ 4, recent (≤3 months before admission) β-lactam and/or fluoroquinolone treatment, recent urinary catheterization, and age ≥70 years | With cutoff score ≥ 3: Sensitivity = 94% Specificity = 41% PPV = 44% NPV = 93% |
| Infection (BSI) | ESBL Prediction Score (ESBL-PS) [97] | Outpatient procedures within 1 month, prior infections or colonization with ESBL-E within 12 months, and number of prior courses of β-lactams and/or fluoroquinolones used within 3 months of BSI | With cutoff score ≥ 1: Sensitivity = 88% Specificity = 77% PPV = 16% NPV = 99% With cutoff score ≥ 3: Sensitivity = 43% Specificity = 96% PPV = 33% NPV = 97% | |
| CPE | Colonization | Papafotiou et al. [98] | Karnofsky score, previous hospitalization, stay in a long-term care facility, history of ≥2 different interventional procedures, previous CPE colonization or infection, renal replacement therapy, and diabetes with end-organ damage | With cutoff score ≥ 27: Sensitivity = 72% Specificity = 81% PPV = 15% NPV = 98% |
| CRAB | Infection | Cogliati Dezza et al. [99] | CRAB colonization, higher CCI, multisite colonization, and the need for mechanical ventilation | Unknown |
| XDR A. baumanii | Colonization | Moghnieh et al. [100] | Urinary catheter placement >6 days, ICU contact pressure for >4 days, presence of gastrostomy tube, and previous use of carbapenems or piperacillin/tazobactam | Unknown |
| MRSA | Colonization | Torres et Sampathkumar [101] | Nursing home residence, diabetes, hospitalization in the past year, and chronic skin condition/infection | With cutoff score ≥ 8: Sensitivity = 54% Specificity = 80% |
| VRE | Colonization | PREVENT score [102] | Age of ≥60 years, hemato-oncological disease, cumulative antibiotic treatment for >4 weeks, and VRE infection | Sensitivity = 82% Specificity = 77% PPV = 57% NPV = 92% |
| MDROs | Colonization | AutoRAS- MDRO [103] | Electronic health records (EHRs) | Sensitivity = 81% Specificity = 79% PPV = 49% NPV = 94% |
BHI: brain heart infusion; CPE: carbapenemase-producing Enterobacteriaceae; CRAB: carbapenem-resistant Acinetobacter baumanii; CRE: carbapenem-resistant Enterobacteriaceae; CR-GNB: carbapenem-resistant Gram-negative bacteria; ESBL: extended-spectrum beta-lactamase; ICU: intensive care unit; IPC: infection prevention and control; HCW: healthcare workers; MDRO: multidrug-resistant organism; MRSA: meticillin-resistant Staphylococcus aureus; VRE: vancomycin-resistant Enterococci, XDR: extensively drug resistant.
- b.
- CRAB screening
Actually, there is no consensus on CRAB active screening strategies [12]. Garnacho-Montero J et al. recommend weekly rectal, pharyngeal, and tracheal swabs [104]. Valencia-Martìn et al. found a sensitivity of 96% by combining rectal and pharyngeal swabs compared to 78% when using rectal swabs only [105]. Different values but the same conclusion were drawn by Nutman et al.: a 94% sensitivity was obtained when combining buccal mucosa, skin, and rectal swabs compared to 74% when using rectal swabs only [106]. They also found that the most sensible swab was buccal mucosa swab for respiratory culture-positive patients and skin swab for respiratory culture-negative patients. Meschiari et al. found that skin samples (100%), followed by rectal samples (86%), showed the best sensitivity, but due to the waiting period to receive a screening test, they suggested adopting contact precaution measures to all ICU patients until the end of the outbreak [52].
- c.
- Rectal screening for carbapenem-resistant Gram-negative bacteria (CR-GNB)
This screening procedure should be performed at ICU admission and repeated at least once a week according to local epidemiology [12,107]. In order to promptly identify a patient with CR-GNB rectal colonization or CR-GNB infection, an active surveillance system involving the microbiology laboratory and infection control staff should be implemented [108].
Contact precautions should be adopted, including the following [12,107,108]:
- -
- Single-use gloves and gowns should be worn during assistance (worn at the moment of entering the room of the patient with CR-GNB colonization and removed at the moment of exiting the patient’s room);
- -
- Gloves and gowns should be used individually for every patient with CR-GNB colonization, since the CR-GNB could vary in species and resistance profile;
- -
- Gloves and gowns should be changed according to the WHO’s guidelines regarding the ‘Five moments’ and ‘six movements’ [59].
- d.
- Skin screening for MRSA
As for rectal screening, skin screening should be performed at admission and repeated at least weekly in the ICU [15]. Other situations in which active screening is encouraged are preoperatively, upon initiating dialysis, at admission to a particular unit, or upon identifying a potential outbreak [15]. Swab samples should be collected from the nostrils, throat, and perineum. Other sites could include the wound, sputum, or eyes [66].
- e.
- Environmental samples surveillance
Environmental samples should be collected according to the CDC Environmental Checklist for Monitoring Terminal Cleaning in order to prevent the spread of CR-GNB and other dangerous microorganisms, paying particular attention to high-touch surfaces [109] (see the Cleaning Section).
Environmental samples should be collected with a sterile BHI moistened gauze, as normal swabs revealed a low sensitivity for Acinetobacter baumanii (0 to 18%) [52,110].
- f.
- Whole genome sequencing (WGS)
The genomic characterization of CR-GNB could be useful to identify putative transmission chains [111] and to stratify patients [52]. For instance, lately, non-functional adeN was found to be associated with increased virulence and hyper invasiveness [112]. In Meschiari et al.’s study [52], only two patients who acquired a CRAB clone with the inactivation of adeN survived, probably because they had a younger age and better immune status. Their hypothesis was that the inactivation of adeN could have contributed to higher mortality rates in their outbreak, similarly to other studies [113,114,115], despite appropriate therapy with cefiderocol.
14. Environmental Cleaning
Cleaning the room and bed is essential for IPC in the ICU. For this reason, cleaning should be standardized with a hospital protocol and realized on a routine basis or when a patient is moved or discharged from a room (i.e., terminal cleaning). In the protocol, environmental service personnel training, the use of checklists, and/or monitoring ‘high-touch’ contact surfaces with healthcare workers’ hands should be provided [109].
ICU cleaning includes both surface cleaning and air cleaning.
14.1. Air Cleaning
A ventilation system, together with the appropriate use of heating and air conditioning, is fundamental in preventing the acquisition of HAIs. High-efficiency particulate air (HEPA) could be useful for the prevention of fungi infections, including Aspergillus spp [116]. Recently, air purifiers seem to be effective in reducing the microbial load in the air and on surfaces in the ICU [117], and it may be worth including them in the ICU cleaning routine.
14.2. Surface Cleaning
Cleaning, including cleaning of isolation rooms and open-space areas, should be performed with 10% sodium hypochlorite for environmental surfaces and hydrogen peroxide wipes for all medical devices. This has also proven to be effective against C. auris contamination [118,119].
It should be performed on all surfaces, with particular focus on the most ‘high-touch’ surfaces [109], defined by Kisk Huslage et al. in 2015 as sustaining more than three contacts per interaction with the patient [109]. Among the 109 ICU surfaces studied, three were identified as ‘high-touch’ surfaces, namely the bed rail, the bed surface, and the supply cart. These three surfaces accounted for 40.2% of the contacts recorded in the ICUs. Considering the medical–surgical floor, the ‘high-touch’ surfaces, defined as sustaining more than one contact per interaction, were the bed rail, the over-bed table, the intravenous pump, and the bed surface (48.6% of all contacts with medical–surgical floors). In the same study, it was found that bed rails had the highest frequency of contact in both types of healthcare settings, accounting for 7.76 contacts per interaction in the ICUs [109].
In order to write a hospital protocol, a local assessment of the ‘high-touch’ surfaces should be performed and integrated with the above-mentioned data.
Of course, the protocol must take into consideration the concentration and type of pathogens found on the specific environmental surfaces to determine the best kind of disinfection.
Several studies demonstrated that standard cleaning with self-monitoring is insufficient to control CRAB environmental spread [52,120]. This information becomes more relevant considering that environmental contamination seems to be the most frequent source of CRAB cross-transmission in the ICU [52,106,120].
Moreover, Carling PC et al. highlighted that less than 50% of standardized environmental surfaces have been cleaned during terminal room cleaning [121].
The cleaning process should not only follow the CDC’s Environmental Checklist for Monitoring Terminal Cleaning guidelines [122], but also put into practice Meschiari’s ‘cycling radical cleaning and disinfection’ from the ‘five-component bundle’ protocol [52]. Environmental contamination appeared to represent the most frequent source.
Recently, ‘no-touch’ cleaning methods have been developed, including UV cleaning and pressurized hydrogen peroxide. Although they are effective, they do not tend to be well-tolerated and they are expensive and limitedly practical, as it takes hours before the room is ready for a new patient [123,124]. This makes the cycling radical cleaning and disinfection method [52] preferable as a faster, easy-to-use, and cost-effective method.
15. Antimicrobial Stewardship Program
IPC in the ICU setting is the result of teamwork [125,126,127] and effective communication [128]. Beyond ICU personnel (doctors, nurses, and HCWs), three key roles are needed to perform antimicrobial stewardship: the infectious diseases specialist (IDS) [129], the clinical microbiologist [130], and the clinical pharmacology specialist [131].
In case no protocol has been elaborated at the facility level, the IDS should be consulted at the following times [132]:
- -
- Whenever an infectious disease is suspected;
- -
- When the patient presents fever;
- -
- Whenever a new cultural or serological positivity is released by the microbiological laboratory;
- -
- For antimicrobic therapy initiation, monitoring, and discontinuation.
Adherence to IDS recommendations by the treating doctor has been proven to be of paramount importance for disease progression and outcomes, and also in terms of mortality [133,134].
The timing of specialists’ consultation is essential, and a proactive approach compared to an event-triggered approach would be preferrable [129]. In this regard, Zwerwer et al. recently managed to develop a machine-learning model that is able to predict infection-related consultations in ICUs up to eight hours in advance based on electronic health records [129].
The IDS should perform at least the first consultation for every patient at the bedside when visiting the patient [135]. The IDS should visit the patient every time an important clinical change is present. According to the number and severity of patients suffering from bacterial, virological, or fungal infections, a minimum number of weekly visits should be planned [132].
Although many studies have witnessed the commonly inappropriate prescription of antibiotics identified as ‘reserve antibiotics’ in the WHO’s AWaRe antibiotic book [136] worldwide, no exclusivity to IDS prescribers has been established [137].
For hospitals without IDS services, Zimmermann et al. are currently conducting a trial with the purpose to identify means to comprehensively and sustainably improve the quality of care of patients with infectious diseases in those settings (trial registration: DRKS00023710) [138].
Antimicrobial stewardship (AMS) remains pivotal and complementary to IPC in fighting antimicrobial resistance.
16. Outbreak Reporting
Manuscripts on IPC are mainly conducted during outbreaks. The main limitation of this kind of study is that it is scarce, and frequently, different risk factors are taken into consideration from one study to another [7].
Another limitation is that a universal outbreak definition is lacking [7,139]. One of the most accurate definition lists for different pathogen outbreaks is the one offered by the Division of Infectious Disease Epidemiology, West Virginia, USA [8].
The ORION statement (Outbreak Reports and Intervention Studies of Nosocomial Infection statement, 2007) by Sheldon Stone and colleagues proposed a standardized way of reporting an outbreak, which could be useful in the prevention and/or management of future outbreaks, other than contributing to the current literature [140].
The statement consisted of a 22-item checklist including information on the number of colonized, infected, and deceased patients; the type of medical department; the number of beds in the ward; performance of genotyping; the study design; and data on costs.
A decade after ORION statement publication, outbreak reports globally still do not provide the basic information in the event [139]. After 2017, only a review on CRAB and CRPRA outbreaks mentioned the statement, apparently not using it for the selection of the outbreak reports, but highlighting the importance of appropriate reporting [141].
17. CRE Prevention among Special Populations
17.1. Hematological Patients
Among hematological patients with carbapenem-resistant Enterobacteriaceae (CRE) rectal colonization, in a recent retrospective study by Xia Chen et al., receiving proton pump inhibitors and admission to the ICU (p < 0.05) were identified as risk factors for subsequent CRE infection development [142]. Receiving proton pump inhibitors is also recognized to be a predisposing factor to infection by extended-spectrum β-lactamase-producing Enterobacteriaceae. Among this kind of hematological patients, gastrointestinal injury, tigecycline exposure, and the carbapenem resistance score were not associated with subsequent CRE infection, which may be responsible for subsequent CRE infection in other hematological disorders [143], as well as high-risk disease and mucositis [144].
17.2. Neutropenic Patients
According to the ESCMID-EUCIC guidelines, there is no conclusive evidence on 3GCephRE carriers’ decolonization benefits in this population. In particular, the decolonization of 3GCephRE has been associated with temporary effectiveness and an increased risk of developing ESBL-E BSI in patients with neutropenia colonization [67].
For future clinical trials on decolonization by this pathogen, it is suggested to use a combination of oral colistin sulphate (50 mg (salt) four times daily) and neomycin sulphate (250 mg (salt) four times daily) in patients with severe neutropenia [67].
17.3. Hemodialysis Patients
Patients using a temporary line for vascular access have a greater risk of colonization by MRSA [32].
18. Cost-Effectiveness and MDRO Reproduction Number (R0)
The cost-effectiveness of IPC strategy implementation, such as screening, laboratory tools, HCW personnel, and bed rotations (that require one bed off regular admissions) are to be considered.
In the WHO’s 2022 global report, the impact and cost-effectiveness of IPC measures was addressed to encourage the improvement of IPC programs [145].
Multidrug-resistant organisms and difficult-to-treat infections are associated with prolonged hospitalization with higher costs in terms of human resources, assistance, drugs, disposables, additional cleaning, length of stay, and laboratory costs. MDROs’ basic reproduction number (R0) should be kept in mind when estimating an outbreak cost (Table 6). In fact, the basic reproduction number (R0), or basic reproductive number, expresses how many successful transmission events and new infections result on average from one infection [146].

Table 6.
Estimated basic reproduction number (R0), single patient, and associated outbreak costs of the most common pathogens responsible for outbreaks in ICU based on research on the literature.
18.1. CRE
Lin et al. developed a computational model in order to predict the cost-effectiveness of CRE surveillance strategies in the ICU [152]. The cost of a single patient affected by CRE was estimated to be USD 63,948 based on a literature review. Other than reducing CRE colonization acquisition, they found out that up to USD 572,000 per year could have been saved whenever IPC strategies were implemented in Maryland, USA considering Maryland’s 2012 incidence of 4.8 CRE infections in every 100,000 persons.
The rate of CRE has risen exponentially.
A single identification of a CRE infection or colonization could be responsible for up to 11 transmissions according to a Brazilian study [85].
In this study, the authors developed a mathematical model to describe the dynamics of transmission of CRE in the ICU, and they found the CRE transmission R0 (basic reproduction number) to be 11 with routine IPC before the implementation of the experimented IPC strategies they performed. After the IPC implementations, the R0 dropped to 0.41 (range 0–2.1). To our knowledge, this is the only study that was capable of estimating the R0 of patients with CRE colonization.
Recently, many effective new antibiotics have been discovered against CRE [153], but their costs are still very high.
18.2. VRE
Mac et al. proved the same cost-effectiveness of VRE screening and isolation in a medicine ward in Canada [154]. The cost of a single VRE patient was esteemed to be USD 17,949 [155], while a VRE outbreak costed EUR 60,524 [156] based on a literature review. Similar to Lin et al., for CRE, they achieved both VRE colonization acquisition and relative mortality reduction at a cost-effectiveness threshold of 50,000 USD/QALY (quality-adjusted life years) in Toronto, Canada. According to current studies in the literature, the VRE transmission R₀ was 1.32 (range of 1.03–1.46) [157].
18.3. MRSA
Chaix et al. estimated the cost of a single MRSA infection in a French ICU to be USD 9275, while the IPC measures for MRSA would range from USD 340 to USD 1,480 per patient and USD 30,225 for the entire outbreak [158,159]. They proved that routinary screening with other IPC measures managed to reduce both costs and MRSA incidence by 14%. According to a recent review, universal decolonization would be more effective and less expensive than other IPC strategies, but the most effective would be a combination of screening, isolation, and decolonization in the ICU setting, even though it is the most expensive one [79]. Eike Steinig and colleagues conducted the first study on the community-acquired MRSA R0, resulting in a range between 0.97 and 1.60 depending on the strain [147].
In summary, IPC measures in the ICU have been proven to be cost-effective wherever MRSA colonization and infection rates are significant, although no cut-off rate has been assessed.
18.4. CRAB
The literature on carbapenem-resistant Acinetobacter baumanii (CRAB) outbreak costs is more scarce. Coyle et al. elaborated on a model estimating that a single patient with CRAB would cost up to USD 55,122 for a 13-day length of stay [160], confirmed by Young et al., who reported a real-life data cost of UAS 60,000 in a Korean ICU [38]. Considering an R0 of approximately 1.5 in Australian ICUs, the total outbreak cost would be around USD 1 million [28]. Implementing IPC measures, the threshold would be of $75,000–$93,822/QALY.
18.5. Candida auris
Taori et al. analyzed the cost a Candida auris outbreak in London, UK, which was estimated to be EUR 1,217,817. The additional length of stay accounted for half of this sum (EUR 69,645 per month) [161]. The screening cost for C. auris was estimated to be EUR 269,984 during the outbreak (EUR 51,040 per month) [161].
Considering this study, a C. auris outbreak exceeds in costs an average CRE outbreak (EUR 1.1 million) [162] and a Clostridium difficile outbreak (EUR 1,222,376) [148], taking into consideration the long-lasting contamination or the need to close the ICU for a certain period.
The cost-effectiveness of IPC measures in C. auris outbreaks still needs to be assessed. Recently, Rosa et al. managed to prove the positive economic impact of the implementation of an in-house PCR (polymerase chain reaction) to screen patients presenting risk factors for C. auris acquisition at admission in Miami hospitals in the USA [163]. The saving margin in a two-year post-intervention period was between USD 77,251,310 and USD 373,048,026 based on a deduced incidence of positivity of 3% [163].
As far as we know, no C. auris studies conducted so far identified the C. auris transmission R0.
Therefore, IPC represents a solid cost-effective solution for CRE, VRE, MRSA, and CDI outbreaks and a possibly cost-effective strategy for C. auris outbreaks, as it seems to be capable of preventing these hospitalizations with associated costs.
The reproduction numbers of other pathogens possibly responsible for outbreaks in the ICU are reported in Table 7.

Table 7.
Estimated basic reproduction numbers (R0) of other pathogens possibly responsible for outbreaks in the ICU.
19. New Experimental Strategies
Beyond HH and isolation precautions, new experimental IPC strategies have been proposed in the past 10 years. These strategies are focused on MDRO outbreaks (Table 8).

Table 8.
New experimental strategies in the past 10 years to prevent MDRO spread.
The most recent applications include the employment of artificial intelligence and machine learning, but the literature on this topic is still scarce.
One of the most relevant, easy-to-implement, and effective strategies is the five-item IPC bundle proposed by Meschiari et al. for CRAB outbreaks in the ICU (Box 5) [52]. Notably, A. baumannii stands out for its endurance, and it could survive on dry surfaces for up to 5 months [180], facilitating its spreading.
Box 5. Meschiari’s ‘five-component bundle’ of IPC57.
- Proactive reinforcement of all routine IPC practices among HCWs:
- Improving HH compliance with direct observations of the WHO’s ‘5 moments’ for HH performed by IPC nurses followed by individualized verbal feedback;
- Establishing an ‘improvement group’ with medical and nursing staff to analyze critical issues regarding HH compliance;
- Monitoring compliance with contact precautions performed by IPC nurses using two specific checklists;
- Meetings with radiology and transport personnel to reinforce compliance with IPC measures.
- Extended CRAB screening:
- For all patients with an expected ICU length of stay > 24 h in ICU;
- At admission and weekly thereafter;
- Swabs should be performed in axilla, groin, trachea, and rectum.
- Contact precaution measures:
- For all patients until discharge, independently of CRAB status;
- Single-use gloves and gowns should be worn before entering each single-patient unit, and gloves should be changed according to the WHO’s 5 moments for HH [59]).
- Environmental sampling:
- By means of pre-moistened sterile gauze pads, suggested by Corbella et al., as it showed an increased sensitivity for A. baumanii [181];
- After rubbing all ICU surfaces vigorously, moistened gauze pads should be firstly put in incubation for 24 h at 37 °C in a screw-cap container with 10 mL of brain heart infusion medium (BHI), and secondly sampled into MacConkey agar plates and incubated aerobically at 37 °C for 48 h.
- Cycling radical cleaning and disinfection:
- Performed for all rooms, common areas, and patients;
- Use of 10% sodium hypochlorite for environmental surfaces;
- Use of hydrogen peroxide in wipes for medical devices;
- Cleaning and disinfection should be performed from upper corner to opposite lower corner starting from a transitory unit, and disinfection should be checked by IPC nurses through fluorescein spray with an UV torch, with special attention to hard-to-reach areas, and if not effectively cleaned, disinfection should be repeated;
- All disinfected surfaces should dry completely before re-using them;
- After common area sanitization, the colonized patient is moved from their original unit to a transitory unit, where the patient’s disinfection is performed with 2% leave-on chlorhexidine disposable cloths while the patient’s original unit becomes disinfected, and once the bed is cleaned, the disinfected patient can come back to their original room, and the transitory room becomes cleaned thereafter;
- The whole cycle process takes around 6 h to be completed;
- The process requires the recruitment of two people dedicated to cleaning and an additional nursing shift.
Marianna Meschiari et al., when facing a CRAB outbreak in their ICU, decided to implement and systematize IPC measures, which led to the elaboration of this successful protocol (Box 4).
While previously existing items n. 2 and n. 3 were intensified and revised (multiple sites vs. rectal site for n. 2 and universal vs. CRAB carrier-only contact precaution measures for n. 3), items 4 and 5 are novelty in the field. In their study, a whole genome sequencing (WGS) analysis was performed for all CRAB isolates, environmental or clinical.
The pitfall of this new method is that it is frequently difficult to create a ‘transitory room’ due to ICU overcrowding currently affecting many ICUs all over the world [182].
Moreover, the whole process takes approximately 6 h, which implies the need for supplementary HCWs or, more realistically, healthcare assistant shifts, contributing to work overload [183]. It could be still useful to avoid ICU closure and limiting admissions due to extensive CRAB contamination. It is also applicable to open-space ICUs, the ICUs most affected by nosocomial epidemics [184]. After the introduction of cycling radical cleaning and disinfection in 2018, the Modena ICU (Italy) did not experience nosocomial ICU CRAB outbreaks anymore, but only sporadic cases [52]. Furthermore, ICU alcohol hand rub use increased by more than three times, and total antibiotic use dropped by 18.2%, while meropenem and fluoroquinolones dropped by 83.3% and 84%, respectively (the percentages were calculated based on the original article’s data).
20. Future Perspectives of IPC
It has not escaped our notice that IPC strategies could consistently change in the next few years (Table 9). Phage therapy, targeting specific virulence genes and non-antibiotic decolonization strategies, seem the most promising ones.

Table 9.
Proposed future strategies tackling MDRO spread in ICUs.
Take-home messages are displayed in Box 6.
Box 6. Take-home messages.
- The most effective IPC strategy remains unknown, as a multimodal approach does not identify the most effective strategy, given that all strategies are applied simultaneously [105].
- Any outbreak should be a reason to intensify IPC (infection prevention and control) measures [52].
- A single CRE patient could be responsible for up to 11 contagions.
- Further studies are needed to strengthen ideas in favor or against MDRO decolonization in the ICU setting.
- A standardized, universal, pragmatic protocol for HCW education should be elaborated.
- A rapid outbreak recognition tool (i.e., an easy-to-use mathematical model) should be proposed to improve early diagnosis and prevent spreading.
- Standard cleaning with self-monitoring is insufficient to control MDRO environmental spread.
- The mechanical removal of biofilm may be more relevant than the type of disinfectant [52,192].
- Weekly rectal, pharyngeal, and tracheal swabs for CR-GNB should be performed [104].
- Environmental samples should be collected with a sterile BHI moistened gauze, as normal swabs revealed a low sensitivity for some pathogens like Acinetobacter baumanii (0 to 18%) [52,110]
- IPC strategies proved their cost-effectiveness independently to the country, pathogen, or type of strategy.
- New promising strategies are emerging and need to be tested in the field.
21. Limitations
The limitations of this review include the narrative nature of this study, which led to subjectivity in article and guideline selection. This implies that we might have unconsciously overlooked other variables impacting IPC in our review. We decided to mention some of the preventive measures and risk factors among a few special populations to increase physicians’ attention towards these categories. A deep focus would require a separate dedicated, population-based review.
Considering cost-effective reporting, studies have been conducted in different countries, and the R0 should have been calculated differently in each setting; therefore, these numbers could not be equally applicable to every country or setting.
22. Conclusions
The lack of IPC strategies or its application have made and still make hospitals, and ICUs in particular, responsible for an increase in MDRO reservoir into the community [7].
Despite the great number of studies on IPC, it is still difficult to evaluate which is the most effective strategy because of intrinsic study limitations [193].
It would be surely interesting to see if Meschiari’s ‘five-bundle protocol’ for CRAB outbreaks could be applied to other difficult-to-control critical pathogen outbreaks, such as CRE and Candida auris.
A univocal, numeric, and easy-to-calculate definition of a ‘hospital outbreak’ of a certain infective disease is still lacking. This would accelerate the outbreak identification process by healthcare personnel and promptly put in place IPC strategies. Further studies based on the proposed mathematical model provided by the Brazilian group of Sao Paulo should be encouraged to assess the in-hospital-acquired pathogen R0 [85].
Hopefully, in the future, plasmid modifications by genetic engineering would represent a plot twist in CRE infection control strategies as well as phage therapies [185].
Author Contributions
D.P. suggested the topic of the manuscript and revised the paper. C.F. wrote all sections in the paper under the supervision of D.P., P.T. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authorship credit is based on individual contributions and effort to complete the task.
Conflicts of Interest
All authors have nothing to disclose and declare no conflicts of interest.
References
- Blot, S.; Ruppé, E.; Harbarth, S.; Asehnoune, K.; Poulakou, G.; Luyt, C.-E.; Rello, J.; Klompas, M.; Depuydt, P.; Eckmann, C.; et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensiv. Crit. Care Nurs. 2022, 70, 103227. [Google Scholar] [CrossRef] [PubMed]
- WHO Launches First Ever Global Report on Infection Prevention and Control. Available online: https://www.who.int/news/item/06-05-2022-who-launches-first-ever-global-report-on-infection-prevention-and-control (accessed on 1 July 2024).
- New World Bank Country Classifications by Income Level: 2022–2023. Available online: https://blogs.worldbank.org/en/opendata/new-world-bank-country-classifications-income-level-2022-2023 (accessed on 1 July 2024).
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: A systematic review and meta-analysis. Intensiv. Care Med. 2020, 46, 1536–1551. [Google Scholar] [CrossRef]
- Jiang, H.; Pu, H.; Huang, N. Risk predict model using multi-drug resistant organism infection from Neuro-ICU patients: A retrospective cohort study. Sci. Rep. 2023, 13, 1–7. [Google Scholar] [CrossRef]
- Al-Sunaidar, K.A.; Aziz, N.A.; Hassan, Y.; Jamshed, S.; Sekar, M. Association of Multidrug Resistance Bacteria and Clinical Outcomes of Adult Patients with Sepsis in the Intensive Care Unit. Trop. Med. Infect. Dis. 2022, 7, 365. [Google Scholar] [CrossRef]
- Zahar, J.-R.; Blot, S. Dilemmas in infection control in the intensive care unit. Intensiv. Crit. Care Nurs. 2018, 46, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bixler, D. Outbreak Investigation. 2014. Available online: https://oeps.wv.gov/hai/documents/lhd/hai-protocol.pdf (accessed on 1 July 2024).
- World Health Organization. World Health Organization Outbreak Communication Planning Guide; Guide de l’OMS sur la planification de la communication lors des flambées de maladies; World Health Organization: Geneva, Switzerland, 2008; Volume 30, Available online: https://www.afro.who.int/sites/default/files/2017-06/outbreak_com_plan_guide.pdf (accessed on 1 July 2024).
- Al-Dorzi, H.M.; Arabi, Y.M. Outbreaks in the adult ICUs. Curr. Opin. Infect. Dis. 2017, 30, 432–439. [Google Scholar] [CrossRef]
- Jackson, K.C.; Short, C.T.; Toman, K.R.; Mietchen, M.S.; Lofgren, E.; Program, f.t.C.M.-H. Transient dynamics of infection transmission in a simulated intensive care unit. PLoS ONE 2022, 17, e0260580. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20, 1–55. [Google Scholar] [CrossRef]
- Langford, D.; Williams, V. What Does ESBL Mean, and Why Does My Patient Require Contact Isolation? Crit. Care Nurs. Q. 2011, 34, 46–51. [Google Scholar] [CrossRef]
- Kovacic, A.; Music, M.S.; Dekic, S.; Tonkic, M.; Novak, A.; Rubic, Z.; Hrenovic, J.; Goic-Barisic, I. Transmission and survival of carbapenem-resistant Acinetobacter baumannii outside hospital setting. Int. Microbiol. 2017, 20, 165–169. [Google Scholar] [CrossRef]
- Popovich, K.J.; Aureden, K.; Ham, D.C.; Harris, A.D.; Hessels, A.J.; Huang, S.S.; Maragakis, L.L.; Milstone, A.M.; Moody, J.; Yokoe, D.; et al. SHEA/IDSA/APIC Practice Recommendation: Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute-care hospitals: 2022 Update. Infect. Control. Hosp. Epidemiol. 2023, 44, 1039–1067. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Hospital cleaning in the 21st century. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Giovagnorio, F.; Geremia, N.; Scarparo, C.; Panese, S.; Bradariolo, S.; Berti, C.; Solinas, M.; Sanguinetti, M.; Selle, V.; Cozza, A.; et al. Successful control measures to treat the transmission of Candida auris in Northern Italian Hospital. New Microbiol. 2024, 46, 395–399. [Google Scholar] [PubMed]
- Luplertlop, N. Pseudallescheria/Scedosporium complex species: From saprobic to pathogenic fungus. J. Med. Mycol. 2018, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Beam, E.L.; Schwedhelm, M.M.; Boulter, K.C.; Vasa, A.M.; Larson, L.; Cieslak, T.J.; Lowe, J.J.; Herstein, J.J.; Kratochvil, C.J.; Hewlett, A.L. Ebola Virus Disease. Nurs. Clin. North Am. 2019, 54, 169–180. [Google Scholar] [CrossRef]
- Kanamori, H.; Weber, D.J.; Rutala, W.A. Healthcare Outbreaks Associated With a Water Reservoir and Infection Prevention Strategies. Clin. Infect. Dis. 2016, 62, 1423–1435. [Google Scholar] [CrossRef]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Lackner, M.; Sprute, R.; Al-Hatmi, A.M.S.; Bassetti, M.; et al. Global guideline for the diagnosis and management of rare mould infections: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, 21, e246–e257. [Google Scholar] [CrossRef]
- Adams, C.E.; Dancer, S.J. Dynamic Transmission of Staphylococcus Aureus in the Intensive Care Unit. Int. J. Environ. Res. Public Health 2020, 17, 2109. [Google Scholar] [CrossRef]
- Demuyser, T.; De Cock, E.; Sermijn, E. Airborne Aspergillus fumigatus contamination in an intensive care unit: Detection, management and control. J. Infect. Public Health 2019, 12, 904–906. [Google Scholar] [CrossRef]
- Fiore, V.; De Vito, A.; Fanelli, C.; Geremia, N.; Princic, E.; Nivoli, A.; Maida, I.; Lorettu, L.; Madeddu, G.; Babudieri, S. Mood Reactive Disorders among COVID-19 Inpatients: Experience from a Monocentric Cohort. Med Princ. Pr. 2021, 30, 535–541. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Moschopoulos, C.D.; Karofylakis, E.; Kelesidis, T.; Tsiodras, S. Update in Viral Infections in the Intensive Care Unit. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Shariff, M.; Aditi, A.; Beri, K. Corynebacterium striatum: An emerging respiratory pathogen. J. Infect. Dev. Ctries 2018, 12, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, H.; Chen, D.; Du, P.; Lan, R.; Qiu, X.; Hou, X.; Liu, Z.; Sun, L.; Xu, S.; et al. Whole-Genome Sequencing Reveals a Prolonged and Persistent Intrahospital Transmission of Corynebacterium striatum, an Emerging Multidrug-Resistant Pathogen. J. Clin. Microbiol. 2019, 57, e00683-19. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.M.; Harris, P.N.; Roberts, L.W.; Doidge, M.; Hurst, T.; Hajkowicz, K.; Forde, B.; Paterson, D.L.; Gordon, L.G. Cost-effectiveness analysis of whole-genome sequencing during an outbreak of carbapenem-resistant Acinetobacter baumannii. Antimicrob. Steward. Health Epidemiol. 2021, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; Mutters, N.T.; Guirao, X.; del Toro, M.D.; Eckmann, C.; Friedrich, A.W.; Giannella, M.; Kluytmans, J.; Presterl, E.; Christaki, E.; et al. ESCMID/EUCIC clinical practice guidelines on perioperative antibiotic prophylaxis in patients colonized by multidrug-resistant Gram-negative bacteria before surgery. Clin. Microbiol. Infect. 2022, 29, 463–479. [Google Scholar] [CrossRef]
- Gordin, F.M.; Schultz, M.E.; Huber, R.; Zubairi, S.; Stock, F.; Kariyil, J. A Cluster of Hemodialysis-Related Bacteremia Linked to Artificial Fingernails. Infect. Control. Hosp. Epidemiol. 2007, 28, 743–744. [Google Scholar] [CrossRef]
- Moolenaar, R.L.; Crutcher, J.M.; Joaquin, V.H.S.; Sewell, L.V.; Hutwagner, L.C.; Carson, L.A.; Robison, D.A.; Smithee, L.M.; Jarvis, W.R. A Prolonged Outbreak ofPseudomonas Aeruginosain a Neonatal Intensive Care Unit Did Staff Fingernails Play a Role in Disease Transmission? Infect. Control. Hosp. Epidemiol. 2000, 21, 80–85. [Google Scholar] [CrossRef]
- Price, A.M.; Sarween, N.; Gupta, I.; Baharani, J. Risk factors and Short-term Outcomes for Methicillin-resistant Staphylococcus aureus and Methicillin-sensitive Staphylococcus aureus Colonization among Hemodialysis Patients. Saudi J. Kidney Dis. Transplant. 2019, 30, 1351–1363. [Google Scholar] [CrossRef]
- Olsen, K.; Danielsen, K.; Wilsgaard, T.; Sangvik, M.; Sollid, J.U.E.; Thune, I.; Eggen, A.E.; Simonsen, G.S.; Furberg, A.-S. Obesity and Staphylococcus aureus Nasal Colonization among Women and Men in a General Population. PLoS ONE 2013, 8, e63716. [Google Scholar] [CrossRef]
- Dossett, L.A.; Dageforde, L.A.; Swenson, B.R.; Metzger, R.; Bonatti, H.; Sawyer, R.G.; May, A.K. Obesity and Site-Specific Nosocomial Infection Risk in the Intensive Care Unit. Surg. Infect. 2009, 10, 137–142. [Google Scholar] [CrossRef]
- WHO 2022 IPC.pdf. Available online: https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/ipc/ipc-global-report/who_ipc_global-report_executive-summary.pdf (accessed on 1 July 2024).
- West, E.; Barron, D.N.; Harrison, D.; Rafferty, A.M.; Rowan, K.; Sanderson, C. Nurse staffing, medical staffing and mortality in Intensive Care: An observational study. Int. J. Nurs. Stud. 2014, 51, 781–794. [Google Scholar] [CrossRef]
- Mogyoródi, B.; Skultéti, D.; Mezőcsáti, M.; Dunai, E.; Magyar, P.; Hermann, C.; Gál, J.; Hauser, B.; Iványi, Z.D. Effect of an educational intervention on compliance with care bundle items to prevent ventilator-associated pneumonia. Intensiv. Crit. Care Nurs. 2023, 75, 103342. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Sabel, A.L.; Price, C.S. Epidemiologic, Clinical, and Economic Evaluation of an Outbreak of Clonal Multidrug-ResistantAcinetobacter baumanniiInfection in a Surgical Intensive Care Unit. Infect. Control. Hosp. Epidemiol. 2007, 28, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Bray, K.; Wren, I.; Baldwin, A.; Ledger, U.S.; Gibson, V.; Goodman, S.; Walsh, D. Standards for nurse staffing in critical care units determined by: The British Association of Critical Care Nurses, The Critical Care Networks National Nurse Leads, Royal College of Nursing Critical Care and In-flight Forum. Nurs. Crit. Care 2010, 15, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Rani, R. Nurse-to-patient ratio and nurse staffing norms for hospitals in India: A critical analysis of national benchmarks. J. Fam. Med. Prim. Care 2020, 9, 2631–2637. [Google Scholar] [CrossRef]
- Falk, A. Nurse staffing levels in critical care: The impact of patient characteristics. Nurs. Crit. Care 2022, 28, 281–287. [Google Scholar] [CrossRef]
- Neuraz, A.; Guérin, C.; Payet, C.; Polazzi, S.; Aubrun, F.; Dailler, F.; Lehot, J.-J.; Piriou, V.; Neidecker, J.; Rimmelé, T.; et al. Patient Mortality Is Associated With Staff Resources and Workload in the ICU. Crit. Care Med. 2015, 43, 1587–1594. [Google Scholar] [CrossRef]
- Gershengorn, H.B.; Harrison, D.A.; Garland, A.; Wilcox, M.E.; Rowan, K.M.; Wunsch, H. Association of Intensive Care Unit Patient-to-Intensivist Ratios with Hospital Mortality. JAMA Intern. Med. 2017, 177, 388–396. [Google Scholar] [CrossRef]
- I Dara, S.; Afessa, B. Intensivist-to-Bed Ratio. Chest 2005, 128, 567–572. [Google Scholar] [CrossRef]
- Gershengorn, H.B.; Pilcher, D.V.; Litton, E.; Anstey, M.; Garland, A.; Wunsch, H. Association of patient-to-intensivist ratio with hospital mortality in Australia and New Zealand. Intensiv. Care Med. 2021, 48, 179–189. [Google Scholar] [CrossRef]
- Agarwal, A.; Chen, J.-T.; Coopersmith, C.M.; Denson, J.L.; Dickert, N.W.; Ferrante, L.E.; Gershengorn, H.B.; Gosine, A.D.; Hayward, B.J.; Kaur, N.; et al. SWEAT ICU—An Observational Study of Physician Workload and the Association of Physician Outcomes in Academic ICUs. Crit. Care Explor. 2022, 4, e0774. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.M.; Yabes, J.G.; Bukowski, L.A.; Davis, B.S. Intensivist physician-to-patient ratios and mortality in the intensive care unit. Intensiv. Care Med. 2023, 49, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Estenssoro, E.; Alegría, L.; Murias, G.; Friedman, G.; Castro, R.; Nin Vaeza, N.; Loudet, C.; Bruhn, A.; Jibaja, M.; Ospina-Tascon, G.; et al. Organizational Issues, Structure, and Processes of Care in 257 ICUs in Latin America: A Study From the Latin America Intensive Care Network. Crit. Care Med. 2017, 45, 1325–1336. [Google Scholar] [CrossRef]
- Kerlin, M.P.; Caruso, P. Towards evidence-based staffing: The promise and pitfalls of patient-to-intensivist ratios. Intensiv. Care Med. 2022, 48, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Guidelines on Core Components of Infection Prevent.pdf. Available online: https://iris.who.int/bitstream/10665/251730/1/9789241549929-eng.pdf (accessed on 1 July 2024).
- Menegueti, M.G.; Ciol, M.A.; Bellissimo-Rodrigues, F.; Auxiliadora-Martins, M.; Gaspar, G.G.; Canini, S.R.M.d.S.; Basile-Filho, A.; Laus, A.M. Long-term prevention of catheter-associated urinary tract infections among critically ill patients through the implementation of an educational program and a daily checklist for maintenance of indwelling urinary catheters. Medicine 2019, 98, e14417. [Google Scholar] [CrossRef]
- Meschiari, M.; Lòpez-Lozano, J.-M.; Di Pilato, V.; Gimenez-Esparza, C.; Vecchi, E.; Bacca, E.; Orlando, G.; Franceschini, E.; Sarti, M.; Pecorari, M.; et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob. Resist. Infect. Control. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Flodgren, G.; Hall, A.M.; Goulding, L.; Eccles, M.P.; Grimshaw, J.M.; Leng, G.C.; Shepperd, S. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst. Rev. 2016, 2016, CD010669. [Google Scholar] [CrossRef]
- Moghnieh, R.; Al-Maani, A.S.; Berro, J.; Ibrahim, N.; Attieh, R.; Abdallah, D.; Al-Ajmi, J.; Hamdani, D.; Abdulrazzaq, N.; Omar, A.; et al. Mapping of infection prevention and control education and training in some countries of the World Health Organization’s Eastern Mediterranean Region: Current situation and future needs. Antimicrob. Resist. Infect. Control. 2023, 12, 1–12. [Google Scholar] [CrossRef]
- McNett, M.P.; O’mathúna, D.B.; Tucker, S.P.; Roberts, H.B.; Mion, L.C.P.; Balas, M.C.P. A Scoping Review of Implementation Science in Adult Critical Care Settings. Crit. Care Explor. 2020, 2, e0301. [Google Scholar] [CrossRef]
- Barbash, I.J.; Pike, F.; Gunn, S.R.; Seymour, C.W.; Kahn, J.M. Effects of Physician-targeted Pay for Performance on Use of Spontaneous Breathing Trials in Mechanically Ventilated Patients. Am. J. Respir. Crit. Care Med. 2017, 196, 56–63. [Google Scholar] [CrossRef]
- Phan, H.T.; Tran, H.T.T.; Dinh, A.P.P.; Ngo, H.T.; Theorell-Haglow, J.; Gordon, C.J. An educational intervention to improve hand hygiene compliance in Vietnam. BMC Infect. Dis. 2018, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- your-5-moments-for-hand-hygiene-poster.pdf. Available online: https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/infection-prevention-and-control/your-5-moments-for-hand-hygiene-poster.pdf?sfvrsn=83e2fb0e_21 (accessed on 1 July 2024).
- World Health Organization & WHO Patient Safety. WHO Guidelines on Hand Hygiene in Health Care; World Health Organization: Geneva, Switzerland, 2009; Volume 262. [Google Scholar]
- Verderber, S.; Gray, S.; Suresh-Kumar, S.; Kercz, D.; Parshuram, C. Intensive Care Unit Built Environments: A Comprehensive Literature Review (2005–2020). HERD: Health Environ. Res. Des. J. 2021, 14, 368–415. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Hernandez-Koutoucheva, A.; Musicha, P.; Bertrand, D.; Lye, D.; Ng, O.T.; Fenlon, S.N.; Chen, S.L.; Ling, M.L.; Tang, W.Y.; et al. Duration of Carbapenemase-Producing Enterobacteriaceae Carriage in Hospital Patients. Emerg. Infect. Dis. 2020, 26, 2182–2185. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, E.S.; Paras, M.L.; Noubary, F.; Walensky, R.P.; Hooper, D.C. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus(VRE): A systematic review. BMC Infect. Dis. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Scanvic, A.; Denic, L.; Gaillon, S.; Giry, P.; Andremont, A.; Lucet, J.-C. Duration of Colonization by Methicillin-Resistant Staphylococcus aureus after Hospital Discharge and Risk Factors for Prolonged Carriage. Clin. Infect. Dis. 2001, 32, 1393–1398. [Google Scholar] [CrossRef][Green Version]
- Sanford, M.D.; Widmer, A.F.; Bale, M.J.; Jones, R.N.; Wenzel, R.P. Efficient Detection and Long-Term Persistence of the Carriage of Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 1994, 19, 1123–1128. [Google Scholar] [CrossRef]
- Larsson, A.-K.; Gustafsson, E.; Nilsson, A.C.; Odenholt, I.; Ringberg, H.; Melander, E. Duration of methicillin-resistant Staphylococcus aureus colonization after diagnosis: A four-year experience from southern Sweden. Scand. J. Infect. Dis. 2011, 43, 456–462. [Google Scholar] [CrossRef]
- Yiek, W.-K.; Tromp, M.; Strik-Albers, R.; van Aerde, K.; van der Geest-Blankert, N.; Wertheim, H.F.L.; Meijer, C.; Tostmann, A.; Bleeker-Rovers, C.P. Success rates of MRSA decolonization and factors associated with failure. Antimicrob. Resist. Infect. Control. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Tacconelli, E.; Mazzaferri, F.; de Smet, A.M.; Bragantini, D.; Eggimann, P.; Huttner, B.D.; Kuijper, E.J.; Lucet, J.-C.; Mutters, N.T.; Sanguinetti, M.; et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 2019, 25, 807–817. [Google Scholar] [CrossRef]
- Debby, B.D.; Ganor, O.; Yasmin, M.; David, L.; Nathan, K.; Ilana, T.; Dalit, S.; Smollan, G.; Galia, R. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1811–1817. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Marangos, M.; Fligou, F.; Christofidou, M.; Sklavou, C.; Vamvakopoulou, S.; Anastassiou, E.D.; Filos, K.S. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: The significance of risk factors for its development and its impact on mortality. Diagn. Microbiol. Infect. Dis. 2013, 77, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Pisney, L.M.; Barron, M.A.; Kassner, E.; Havens, D.; Madinger, N.E. Carbapenem-Resistant Enterobacteriaceae Rectal Screening during an Outbreak of New Delhi Metallo-β-Lactamase–Producing Klebsiella pneumoniae at an Acute Care Hospital. Infect. Control. Hosp. Epidemiol. 2014, 35, 434–436. [Google Scholar] [CrossRef]
- Dickstein, Y.; Edelman, R.; Dror, T.; Hussein, K.; Bar-Lavie, Y.; Paul, M. Carbapenem-resistant Enterobacteriaceae colonization and infection in critically ill patients: A retrospective matched cohort comparison with non-carriers. J. Hosp. Infect. 2016, 94, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Saidel-Odes, L.; Polachek, H.; Peled, N.; Riesenberg, K.; Schlaeffer, F.; Trabelsi, Y.; Eskira, S.; Yousef, B.; Smolykov, R.; Codish, S.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Selective Digestive Decontamination Using Oral Gentamicin and Oral Polymyxin E for Eradication of Carbapenem-Resistant Klebsiella pneumoniae Carriage. Infect. Control. Hosp. Epidemiol. 2012, 33, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Oren, I.; Sprecher, H.; Finkelstein, R.; Hadad, S.; Neuberger, A.; Hussein, K.; Raz-Pasteur, A.; Lavi, N.; Saad, E.; Henig, I.; et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: A prospective controlled trial. Am. J. Infect. Control. 2013, 41, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Herwaldt, L. Nasal decolonization: What antimicrobials and antiseptics are most effective before surgery and in the ICU. Am. J. Infect. Control. 2023, 51, A64–A71. [Google Scholar] [CrossRef]
- Ridenour, G.; Lampen, R.; Federspiel, J.; Kritchevsky, S.; Wong, E.; Climo, M. Selective Use of Intranasal Mupirocin and Chlorhexidine Bathing and the Incidence of Methicillin-Resistant Staphylococcus aureus Colonization and Infection Among Intensive Care Unit Patients. Infect. Control. Hosp. Epidemiol. 2007, 28, 1155–1161. [Google Scholar] [CrossRef]
- Huang, S.S.; Septimus, E.; Kleinman, K.; Moody, J.; Hickok, J.; Avery, T.R.; Lankiewicz, J.; Gombosev, A.; Terpstra, L.; Hartford, F.; et al. Targeted versus Universal Decolonization to Prevent ICU Infection. N. Engl. J. Med. 2013, 368, 2255–2265. [Google Scholar] [CrossRef]
- Kohler, P.; Bregenzer-Witteck, A.; Rettenmund, G.; Otterbech, S.; Schlegel, M. MRSA decolonization: Success rate, risk factors for failure and optimal duration of follow-up. Infection 2012, 41, 33–40. [Google Scholar] [CrossRef]
- Safdar, N.; Bradley, E.A. The Risk of Infection after Nasal Colonization with Staphylococcus Aureus. Am. J. Med. 2008, 121, 310–315. [Google Scholar] [CrossRef]
- Samuel, P.; Kumar, Y.S.; Suthakar, B.J.; Karawita, J.; Kumar, D.S.; Vedha, V.; Shah, H.; Thakkar, K. Methicillin-Resistant Staphylococcus aureus Colonization in Intensive Care and Burn Units: A Narrative Review. Cureus 2023, 15, e47139. [Google Scholar] [CrossRef]
- Cheng, V.C.; Chen, J.H.; Tai, J.W.; Wong, S.C.; Poon, R.W.; Hung, I.F.; To, K.K.; Chan, J.F.; Ho, P.-L.; Lo, C.-M.; et al. Decolonization of gastrointestinal carriage of vancomycin-resistant Enterococcus faecium: Case series and review of literature. BMC Infect. Dis. 2014, 14, 514. [Google Scholar] [CrossRef]
- Choi, E.; Lee, S.J.; Lee, S.; Yi, J.; Lee, Y.S.; Chang, S.-Y.; Jeong, H.Y.; Joo, Y. Comprehensive, multisystem, mechanical decolonization of Vancomycin-Resistant Enterococcus and Carbapenem-Resistant Enterobacteriacease without the use of antibiotics. Medicine 2021, 100, e23686. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Professionals FAQ|Candida auris | Fungal Diseases|CDC. 2023. Available online: https://www.cdc.gov/candida-auris/hcp/clinical-overview/index.html (accessed on 4 July 2024).
- Key Facts and Figures. Available online: https://www.who.int/campaigns/world-hand-hygiene-day/2021/key-facts-and-figures (accessed on 4 July 2024).
- Lambe, K.A.; Lydon, S.; Madden, C.; Vellinga, A.; Hehir, A.; Walsh, M.; O’connor, P. Hand Hygiene Compliance in the ICU: A Systematic Review. Crit. Care Med. 2019, 47, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- DalBen, M.d.F.; Mendes, E.T.; Moura, M.L.; Rahman, D.A.; Peixoto, D.; dos Santos, S.A.; de Figueiredo, W.B.; Mendes, P.V.; Taniguchi, L.U.; Coutinho, F.A.B.; et al. A Model-Based Strategy to Control the Spread of Carbapenem-Resistant Enterobacteriaceae: Simulate and Implement. Infect. Control. Hosp. Epidemiol. 2016, 37, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.; VonVille, H.; Hasan, I.; Garey, K.W. Shoe soles as a potential vector for pathogen transmission: A systematic review. J. Appl. Microbiol. 2016, 121, 1223–1231. [Google Scholar] [CrossRef]
- Dee, S.; Deen, J.; Pijoan, C. Evaluation of 4 intervention strategies to prevent the mechanical transmission of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2004, 68, 19–26. [Google Scholar] [PubMed]
- Funk, C.R.; Ravindranathan, S.; Matelski, A.; Zhang, H.; Taylor, C.; Chandrasekaran, S.; Arellano, M.; Langston, A.A.; Joseph, N.; Waller, E.K. Passenger pathogens on physicians. Am. J. Infect. Control. 2022, 51, 807–811. [Google Scholar] [CrossRef]
- DalBen, M.F.; Basso, M.; Garcia, C.P.; Costa, S.F.; Toscano, C.M.; Jarvis, W.R.; Lobo, R.D.; Oliveira, M.S.; Levin, A.S. Colonization pressure as a risk factor for colonization by multiresistant Acinetobacter spp and carbapenem-resistant Pseudomonas aeruginosa in an intensive care unit. Clinics 2013, 68, 1128–1133. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; De Pascale, G.; Manno, D.; Spanu, T.; Cambieri, A.; Antonelli, M.; Sanguinetti, M.; Fadda, G.; Cauda, R. Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J. Antimicrob. Chemother. 2008, 62, 1130–1137. [Google Scholar] [CrossRef]
- Meschiari, M.; Kaleci, S.; Orlando, G.; Selmi, S.; Santoro, A.; Bacca, E.; Menozzi, M.; Franceschini, E.; Puzzolante, C.; Bedini, A.; et al. Risk factors for nosocomial rectal colonization with carbapenem-resistant Acinetobacter baumannii in hospital: A matched case–control study. Antimicrob. Resist. Infect. Control. 2021, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensiv. Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef]
- Pittet, D.; Monod, M.; Suter, P.M.; Frenk, E.; Auckenthaler, R. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 1994, 220, 751–758. [Google Scholar] [CrossRef]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Almirante, B.; Nolla-Salas, J.; Álvarez-Lerma, F.; Garnacho-Montero, J.; León, M.A.; EPCAN Study Group. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit. Care Med. 2006, 34, 730–737. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Pappas, P.G.; Shoham, S.; Reboli, A.; Barron, M.A.; Sims, C.; Wood, C.; Sobel, J.D. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses 2010, 54, 46–51. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; Bassetti, M.; De Rosa, F.G.; Spanu, T.; Di Meco, E.; Losito, A.R.; Parisini, A.; Pagani, N.; Cauda, R. Identifying Patients Harboring Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae on Hospital Admission: Derivation and Validation of a Scoring System. Antimicrob. Agents Chemother. 2011, 55, 3485–3490. [Google Scholar] [CrossRef] [PubMed]
- Augustine, M.R.; Testerman, T.L.; Justo, J.A.; Bookstaver, P.B.; Kohn, J.; Albrecht, H.; Al-Hasan, M.N. Clinical Risk Score for Prediction of Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae in Bloodstream Isolates. Infect. Control. Hosp. Epidemiol. 2016, 38, 266–272. [Google Scholar] [CrossRef]
- Papafotiou, C.; Roussos, S.; Sypsa, V.; Bampali, S.; Spyridopoulou, K.; Karapanou, A.; Moussouli, A.; Samarkos, M.; Daikos, G.L.; Psichogiou, M. Predictive score for patients with carbapenemase-producing enterobacterales colonization upon admission in a tertiary care hospital in an endemic area. J. Antimicrob. Chemother. 2022, 77, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Dezza, F.C.; Covino, S.; Petrucci, F.; Sacco, F.; Viscido, A.; Gavaruzzi, F.; Ceccarelli, G.; Raponi, G.; Borrazzo, C.; Alessandri, F.; et al. Risk factors for carbapenem-resistant Acinetobacter baumannii (CRAB) bloodstream infections and related mortality in critically ill patients with CRAB colonization. JAC-Antimicrobial Resist. 2023, 5, dlad096. [Google Scholar] [CrossRef]
- Moghnieh, R.; Siblani, L.; Ghadban, D.; El Mchad, H.; Zeineddine, R.; Abdallah, D.; Ziade, F.; Sinno, L.; Kiwan, O.; Kerbaj, F.; et al. Extensively drug-resistant Acinetobacter baumannii in a Lebanese intensive care unit: Risk factors for acquisition and determination of a colonization score. J. Hosp. Infect. 2016, 92, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Torres, K.; Sampathkumar, P. Predictors of methicillin-resistant Staphylococcus aureus colonization at hospital admission. Am. J. Infect. Control. 2013, 41, 1043–1047. [Google Scholar] [CrossRef]
- Boeing, C.; Correa-Martinez, C.L.; Schuler, F.; Mellmann, A.; Karch, A.; Kampmeier, S. Development and Validation of a Tool for the Prediction of Vancomycin-Resistant Enterococci Colonization Persistence—The PREVENT Score. Microbiol. Spectr. 2021, 9, e0035621. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.Y.; Jin, Y.J.; Jin, T.X.; Lee, S.M. Development and evaluation of the automated risk assessment system for multidrug-resistant organisms (autoRAS-MDRO). J. Hosp. Infect. 2018, 98, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Dimopoulos, G.; Poulakou, G.; Akova, M.; Cisneros, J.M.; De Waele, J.; Petrosillo, N.; Seifert, H.; Timsit, J.F.; Vila, J.; et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensiv. Care Med. 2015, 41, 2057–2075. [Google Scholar] [CrossRef]
- Valencia-Martín, R.; Program, I.R.O.A.B.E.; Gonzalez-Galan, V.; Alvarez-Marín, R.; Cazalla-Foncueva, A.M.; Aldabó, T.; Gil-Navarro, M.V.; Alonso-Araujo, I.; Martin, C.; Gordon, R.; et al. A multimodal intervention program to control a long-term Acinetobacter baumannii endemic in a tertiary care hospital. Antimicrob. Resist. Infect. Control. 2019, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nutman, A.; Lerner, A.; Schwartz, D.; Carmeli, Y. Evaluation of carriage and environmental contamination by carbapenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2016, 22, 949.e5–949.e7. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Magiorakos, A.P.; Burns, K.; Baño, J.R.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control. 2017, 6, 113. [Google Scholar] [CrossRef]
- Huslage, K.; Rutala, W.A.; Sickbert-Bennett, E.; Weber, D.J. A quantitative approach to defining ‘high-touch’ surfaces in hospitals. Infect. Control Hosp. Epidemiol. 2010, 31, 850–853. [Google Scholar] [CrossRef]
- Enfield, K.B.; Huq, N.N.; Gosseling, M.F.; Low, D.J.; Hazen, K.C.; Toney, D.M.; Slitt, G.; Zapata, H.J.; Cox, H.L.; Lewis, J.D.; et al. Control of Simultaneous Outbreaks of Carbapenemase-Producing Enterobacteriaceae and Extensively Drug-Resistant Acinetobacter baumannii Infection in an Intensive Care Unit Using Interventions Promoted in the Centers for Disease Control and Prevention 2012 Carbapenemase-Resistant Enterobacteriaceae Toolkit. Infect. Control. Hosp. Epidemiol. 2014, 35, 810–817. [Google Scholar] [CrossRef]
- Mangioni, D.; Fox, V.; Chatenoud, L.; Bolis, M.; Bottino, N.; Cariani, L.; Gentiloni Silverj, F.; Matinato, C.; Monti, G.; Muscatello, A.; et al. Genomic Characterization of Carbapenem-Resistant Acinetobacter baumannii (CRAB) in Mechanically Ventilated COVID-19 Patients and Impact of Infection Control Measures on Reducing CRAB Circulation during the Second Wave of the SARS-CoV-2 Pandemic in Milan, Italy. Microbiol. Spectr. 2023, 11, e0020923. [Google Scholar]
- Bogaty, C.; Mataseje, L.; Gray, A.; Lefebvre, B.; Lévesque, S.; Mulvey, M.; Longtin, Y. Investigation of a Carbapenemase-producing Acinetobacter baumannii outbreak using whole genome sequencing versus a standard epidemiologic investigation. Antimicrob. Resist. Infect. Control. 2018, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Gikas, A.; Astrinaki, E.; Kritsotakis, E.I. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J. Hosp. Infect. 2020, 106, 447–453. [Google Scholar] [CrossRef]
- Dickstein, Y.; Lellouche, J.; Amar, M.B.D.; Schwartz, D.; Nutman, A.; Daitch, V.; Yahav, D.; Leibovici, L.; Skiada, A.; Antoniadou, A.; et al. Treatment Outcomes of Colistin- and Carbapenem-resistant Acinetobacter baumannii Infections: An Exploratory Subgroup Analysis of a Randomized Clinical Trial. Clin. Infect. Dis. 2018, 69, 769–776. [Google Scholar] [CrossRef]
- Jones, C.L.; Clancy, M.; Honnold, C.; Singh, S.; Snesrud, E.; Onmus-Leone, F.; McGann, P.; Ong, A.C.; Kwak, Y.; Waterman, P.; et al. Fatal Outbreak of an Emerging Clone of Extensively Drug-Resistant Acinetobacter baumannii with Enhanced Virulence. Clin. Infect. Dis. 2015, 61, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Eckmanns, T.; Rüden, H.; Gastmeier, P. The Influence of High-Efficiency Particulate Air Filtration on Mortality and Fungal Infection among Highly Immunosuppressed Patients: A Systematic Review. J. Infect. Dis. 2006, 193, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Arıkan, I.; Genç, Ö.; Uyar, C.; Tokur, M.E.; Balcı, C.; Renders, D.P. Effectiveness of air purifiers in intensive care units: An intervention study. J. Hosp. Infect. 2021, 120, 14–22. [Google Scholar] [CrossRef]
- Cadnum, J.L.; Shaikh, A.A.; Piedrahita, C.T.; Sankar, T.; Jencson, A.L.; Larkin, E.L.; Ghannoum, M.A.; Donskey, C.J. Effectiveness of Disinfectants Against Candida auris and Other Candida Species. Infect. Control. Hosp. Epidemiol. 2017, 38, 1240–1243. [Google Scholar] [CrossRef]
- Rapti, V.; Iliopoulou, K.; Poulakou, G. The Gordian Knot of C. auris: If You Cannot Cut It, Prevent It. Pathogens 2023, 12, 1444. [Google Scholar] [CrossRef]
- Lerner, A.O.; Abu-Hanna, J.; Carmeli, Y.; Schechner, V. Environmental contamination by carbapenem-resistant Acinetobacter baumannii: The effects of room type and cleaning methods. Infect. Control. Hosp. Epidemiol. 2019, 41, 1–6. [Google Scholar] [CrossRef]
- Carling, P.C.; Parry, M.M.; Rupp, M.E.; Po, J.L.; Dick, B.; Von Beheren, S. Healthcare Environmental Hygiene Study Group Improving Cleaning of the Environment Surrounding Patients in 36 Acute Care Hospitals. Infect. Control. Hosp. Epidemiol. 2008, 29, 1035–1041. [Google Scholar] [CrossRef]
- CDC Environmental Checklist for Monitoring Terminal Cleaning. Available online: https://www.cdc.gov/infection-control/media/pdfs/Guidelines-Environmental-Cleaning-Checklist-2010-P.pdf (accessed on 1 July 2024).
- Molter, G.; Seifert, H.; Mandraka, F.; Kasper, G.; Weidmann, B.; Hornei, B.; Öhler, M.; Schwimmbeck, P.G.; Kröschel, P.; Higgins, P.; et al. Outbreak of carbapenem-resistant Acinetobacter baumannii in the intensive care unit: A multi-level strategic management approach. J. Hosp. Infect. 2016, 92, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Allard, R.; Paré, R.; Tannenbaum, T.; Lefebvre, B.; Lévesque, S.; Mulvey, M.; Maalouf, L.; Perna, S.; Longtin, Y. Management of a hospital outbreak of extensively drug-resistant Acinetobacter baumannii using a multimodal intervention including daily chlorhexidine baths. J. Hosp. Infect. 2016, 93, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Cornistein, W.; Santonato, D.; Novau, P.A.; Fabbro, L.G.; Jorge, M.F.; Malvicini, M.A.; Vilches, V.; Iudica, F.M. Synergy between infection control and antimicrobial stewardship programs to control carbapenem-resistant Enterobacterales. Antimicrob. Steward. Heal. Epidemiol. 2023, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.P.; Johansson, A.C.; Schonberg, M.A.; Howell, M.D. Elements of a High-Quality Inpatient Consultation in the Intensive Care Unit. A Qualitative Study. Ann. Am. Thorac. Soc. 2013, 10, 220–227. [Google Scholar] [CrossRef]
- Guillamet, M.C.V.; Burnham, J.P.; Pérez, M.; Kollef, M.H.; Manthous, C.A.; Jeffe, D.B. Antimicrobial stewardship for sepsis in the intensive care unit: Survey of critical care and infectious diseases physicians. Infect. Control. Hosp. Epidemiol. 2022, 43, 1368–1374. [Google Scholar] [CrossRef]
- Bonaconsa, C.; Mbamalu, O.; Surendran, S.; George, A.; Mendelson, M.; Charani, E. Optimizing infection control and antimicrobial stewardship bedside discussion: A scoping review of existing evidence on effective healthcare communication in hospitals. Clin. Microbiol. Infect. 2024, 30, 336–352. [Google Scholar] [CrossRef]
- Zwerwer, L.R.; Luz, C.F.; Soudis, D.; Giudice, N.; Nijsten, M.W.N.; Glasner, C.; Renes, M.H.; Sinha, B. Identifying the need for infection-related consultations in intensive care patients using machine learning models. Sci. Rep. 2024, 14, 1–13. [Google Scholar] [CrossRef]
- Kampmeier, S.; Correa-Martinez, C.; Peters, G.; Mellmann, A.; Kahl, B. Personal microbiological consultations improve the therapeutic management of Staphylococcus aureus bacteremia. J. Infect. 2018, 77, 349–356. [Google Scholar] [CrossRef]
- Lazure, P.; Augustyniak, M.; A Goff, D.; Villegas, M.V.; Apisarnthanarak, A.; Péloquin, S. Gaps and barriers in the implementation and functioning of antimicrobial stewardship programmes: Results from an educational and behavioural mixed methods needs assessment in France, the United States, Mexico and India. JAC-Antimicrobial Resist. 2022, 4, dlac094. [Google Scholar] [CrossRef]
- Raineri, E.; Pan, A.; Mondello, P.; Acquarolo, A.; Candiani, A.; Crema, L. Role of the infectious diseases specialist consultant on the appropriateness of antimicrobial therapy prescription in an intensive care unit. Am. J. Infect. Control. 2008, 36, 283–290. [Google Scholar] [CrossRef]
- Sellier, E.; Pavese, P.; Gennai, S.; Stahl, J.-P.; Labarère, J.; François, P. Factors and outcomes associated with physicians’ adherence to recommendations of infectious disease consultations for inpatients. J. Antimicrob. Chemother. 2009, 65, 156–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Botelho-Nevers, E.; Thuny, F.; Casalta, J.P.; Richet, H.; Gouriet, F.; Collart, F.; Riberi, A.; Habib, G.; Raoult, D. Dramatic Reduction in Infective Endocarditis–Related Mortality With a Management-Based Approach. Arch. Intern. Med. 2009, 169, 1290–1298. [Google Scholar] [CrossRef]
- Forsblom, E.; Ruotsalainen, E.; Ollgren, J.; Järvinen, A. Telephone Consultation Cannot Replace Bedside Infectious Disease Consultation in the Management of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2012, 56, 527–535. [Google Scholar] [CrossRef] [PubMed]
- WHO AWaRe antibiotic book.pdf. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 1 July 2024).
- Seah, V.X.F.; Ong, R.Y.L.; Lim, A.S.Y.; Chong, C.Y.; Tan, N.W.H.; Thoon, K.C. Impact of a Carbapenem Antimicrobial Stewardship Program on Patient Outcomes. Antimicrob. Agents Chemother. 2017, 61, e00736-17. [Google Scholar] [CrossRef]
- Zimmermann, N.; Allen, R.; Fink, G.; Först, G.; Kern, W.V.; Farin-Glattacker, E.; Rieg, S.; Solzbach, U.; Friedrich, H.; van Uden, C.; et al. Antimicrobial Stewardship with and without Infectious Diseases Specialist Services to Improve Quality-of-Care in Secondary and Tertiary Care Hospitals in Germany: Study Protocol of the ID ROLL OUT Study. Infect. Dis. Ther. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wieland, K.; Chhatwal, P.; Vonberg, R.-P. Outbreak reporting a decade after ORION: Where do we stand? Lancet Infect. Dis. 2017, 17, 476. [Google Scholar] [CrossRef][Green Version]
- Stone, S.P.; Cooper, B.S.; Kibbler, C.C.; Cookson, B.D.; A Roberts, J.; Medley, G.F.; Duckworth, G.; Lai, R.; Ebrahim, S.; Brown, E.M.; et al. The ORION statement: Guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect. Dis. 2007, 7, 282–288. [Google Scholar] [CrossRef]
- Wieland, K.; Chhatwal, P.; Vonberg, R.-P. Nosocomial outbreaks caused by Acinetobacter baumannii and Pseudomonas aeruginosa: Results of a systematic review. Am. J. Infect. Control. 2018, 46, 643–648. [Google Scholar] [CrossRef]
- Chen, X.; Wen, X.; Jiang, Z.; Yan, Q. Prevalence and factors associated with carbapenem-resistant Enterobacterales (CRE) infection among hematological malignancies patients with CRE intestinal colonization. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 1–10. [Google Scholar] [CrossRef]
- Ferreira, A.; Moreira, F.; Guimaraes, T.; Spadão, F.; Ramos, J.; Batista, M.; Filho, J.; Costa, S.; Rocha, V. Epidemiology, risk factors and outcomes of multi-drug-resistant bloodstream infections in haematopoietic stem cell transplant recipients: Importance of previous gut colonization. J. Hosp. Infect. 2018, 100, 83–91. [Google Scholar] [CrossRef]
- Zhang, L.; Zhai, W.; Lin, Q.; Zhu, X.; Xiao, Z.; Yang, R.; Zheng, Y.; Zhang, F.; Li, S.; Wang, C.; et al. Carbapenem-resistant Enterobacteriaceae in hematological patients: Outcome of patients with Carbapenem-resistant Enterobacteriaceae infection and risk factors for progression to infection after rectal colonization. Int. J. Antimicrob. Agents 2019, 54, 527–529. [Google Scholar] [CrossRef] [PubMed]
- WHO Launches First ever Global Report on infection.pdf. Available online: https://www.who.int/publications/i/item/9789240051164 (accessed on 1 July 2024).
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (R 0 ) of measles: A systematic review. Lancet Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef] [PubMed]
- Steinig, E.; Aglua, I.; Duchene, S.; Meehan, M.T.; Yoannes, M.; Firth, C.; Jaworski, J.; Drekore, J.; Urakoko, B.; Poka, H.; et al. Phylodynamic signatures in the emergence of community-associated MRSA. Proc. Natl. Acad. Sci. USA 2022, 119. [Google Scholar] [CrossRef] [PubMed]
- van Beurden, Y.; Bomers, M.; van der Werff, S.; Pompe, E.; Spiering, S.; Vandenbroucke-Grauls, C.; Mulder, C. Cost analysis of an outbreak of Clostridium difficile infection ribotype 027 in a Dutch tertiary care centre. J. Hosp. Infect. 2017, 95, 421–425. [Google Scholar] [CrossRef]
- Zhang, S.; Palazuelos-Munoz, S.; Balsells, E.M.; Nair, H.; Chit, A.; Kyaw, M.H. Cost of hospital management of Clostridium difficile infection in United States—A meta-analysis and modelling study. BMC Infect. Dis. 2016, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Otete, E.H.; Ahankari, A.S.; Jones, H.; Bolton, K.J.; Jordan, C.W.; Boswell, T.C.; Wilcox, M.H.; Ferguson, N.M.; Beck, C.R.; Puleston, R.L. Parameters for the Mathematical Modelling of Clostridium difficile Acquisition and Transmission: A Systematic Review. PLoS ONE 2013, 8, e84224. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Gong, C.L.; Hitchcock, M.M.; Holubar, M.; Deresinski, S.; Hay, J.W. Cost-effectiveness of bezlotoxumab and fidaxomicin for initial Clostridioides difficile infection. Clin. Microbiol. Infect. 2021, 27, 1448–1454. [Google Scholar] [CrossRef]
- Lin, G.; Tseng, K.K.; Gatalo, O.; Martinez, D.A.; Hinson, J.S.; Milstone, A.M.; Levin, S.; Klein, E.; Program, F.T.C.M.I.D.I.H. Cost-effectiveness of carbapenem-resistant Enterobacteriaceae (CRE) surveillance in Maryland. Infect. Control. Hosp. Epidemiol. 2021, 43, 1162–1170. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Marelli, C.; Cattardico, G.; Fanelli, C.; Signori, A.; Di Meco, G.; Di Pilato, V.; Mikulska, M.; Mazzitelli, M.; Cattelan, A.M.; et al. Mortality in KPC-producing Klebsiella pneumoniae bloodstream infections: A changing landscape. J. Antimicrob. Chemother. 2023, 78, 2505–2514. [Google Scholar] [CrossRef]
- Mac, S.; Fitzpatrick, T.; Johnstone, J.; Sander, B. Vancomycin-resistant enterococci (VRE) screening and isolation in the general medicine ward: A cost-effectiveness analysis. Antimicrob. Resist. Infect. Control. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Lloyd-Smith, P.; Younger, J.; Lloyd-Smith, E.; Green, H.; Leung, V.; Romney, M. Economic analysis of vancomycin-resistant enterococci at a Canadian hospital: Assessing attributable cost and length of stay. J. Hosp. Infect. 2013, 85, 54–59. [Google Scholar] [CrossRef]
- Vancomycin-Resistant Enterococci Isolation and Screening Strategies: Clinical Evidence and Cost-Effectiveness. Available online: https://www.cadth.ca/sites/default/files/pdf/htis/mar-2014/RA0662%20VRE%20Screening%20final.pdf (accessed on 1 July 2024).
- Satilmis, L.; Vanhems, P.; Bénet, T. Outbreaks of Vancomycin-Resistant Enterococci in Hospital Settings: A Systematic Review and Calculation of the Basic Reproductive Number. Infect. Control. Hosp. Epidemiol. 2015, 37, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, A.S.; A Fappiano, S.; A Lipsett, P. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad. Med. J. 2002, 78, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Chaix, C.; Durand-Zaleski, I.; Alberti, C.; Brun-Buisson, C. Control of endemic methicillin-resistant Staphylococcus aureus: A cost-benefit analysis in an intensive care unit. JAMA 1999, 282, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.R.; Kaye, K.S.; Taylor, T.; Tansek, R.; Campbell, M.; Hayakawa, K.; Marchaim, D. Effectiveness and cost of implementing an active surveillance screening policy for Acinetobacter baumannii: A Monte Carlo simulation model. Am. J. Infect. Control. 2014, 42, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Taori, S.K.; Khonyongwa, K.; Hayden, I.; Athukorala, G.D.A.; Letters, A.; Fife, A.; Desai, N.; Borman, A.M. Candida auris outbreak: Mortality, interventions and cost of sustaining control. J. Infect. 2019, 79, 601–611. [Google Scholar] [CrossRef]
- Otter, J.; Burgess, P.; Davies, F.; Mookerjee, S.; Singleton, J.; Gilchrist, M.; Parsons, D.; Brannigan, E.; Robotham, J.; Holmes, A. Counting the cost of an outbreak of carbapenemase-producing Enterobacteriaceae: An economic evaluation from a hospital perspective. Clin. Microbiol. Infect. 2017, 23, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Jimenez, A.; Andrews, D.; Dinh, H.; Parra, K.; Martinez, O.; Abbo, L.M. Impact of In-house Candida auris Polymerase Chain Reaction Screening on Admission on the Incidence Rates of Surveillance and Blood Cultures With C. auris and Associated Cost Savings. Open Forum Infect. Dis. 2023, 10, ofad567. [Google Scholar] [CrossRef]
- Elsaid, M.; Nasef, M.A.; Huy, N.T. R 0 of COVID-19 and its impact on vaccination coverage: Compared with previous outbreaks. Hum. Vaccines Immunother. 2021, 17, 3850–3854. [Google Scholar] [CrossRef]
- Merler, S.; Ajelli, M.; Roe, J.H.; Morreale, S.J.; Paladino, F.V.; Shillinger, G.L.; Benson, S.R.; Eckert, S.A.; Bailey, H.; Tomillo, P.S.; et al. Deciphering the relative weights of demographic transition and vaccination in the decrease of measles incidence in Italy. Proc. R. Soc. B: Biol. Sci. 2014, 281, 20132676. [Google Scholar] [CrossRef]
- Fisman, D.; Rivers, C.; Lofgren, E.; Majumder, M.S. Estimation of MERS-Coronavirus Reproductive Number and Case Fatality Rate for the Spring 2014 Saudi Arabia Outbreak: Insights from Publicly Available Data. PLoS Curr. 2014, 6. [Google Scholar] [CrossRef]
- Liu, W.; Tang, S.; Xiao, Y. Model Selection and Evaluation Based on Emerging Infectious Disease Data Sets including A/H1N1 and Ebola. Comput. Math. Methods Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Menardo, F.; Biology, M. Switzerland Understanding drivers of phylogenetic clustering and terminal branch lengths distribution in epidemics of Mycobacterium tuberculosis. eLife 2022, 11. [Google Scholar] [CrossRef]
- Shrestha, S.; Winglee, K.; Hill, A.N.; Shaw, T.; Smith, J.P.; Kammerer, J.S.; Silk, B.J.; Marks, S.M.; Dowdy, D. Model-based Analysis of Tuberculosis Genotype Clusters in the United States Reveals High Degree of Heterogeneity in Transmission and State-level Differences Across California, Florida, New York, and Texas. Clin. Infect. Dis. 2022, 75, 1433–1441. [Google Scholar] [CrossRef]
- Mathis, A.D.; Clemmons, N.S.; Redd, S.B.; Pham, H.; Leung, J.; Wharton, A.K.; Anderson, R.; McNall, R.J.; Rausch-Phung, E.; Rosen, J.B.; et al. Maintenance of Measles Elimination Status in the United States for 20 Years Despite Increasing Challenges. Clin. Infect. Dis. 2021, 75, 416–424. [Google Scholar] [CrossRef]
- Villela, D.A.M.; Bastos, L.S.; DE Carvalho, L.M.; Cruz, O.G.; Gomes, M.F.C.; Durovni, B.; Lemos, M.C.; Saraceni, V.; Coelho, F.C.; Codeço, C.T. Zika in Rio de Janeiro: Assessment of basic reproduction number and comparison with dengue outbreaks. Epidemiol. Infect. 2017, 145, 1649–1657. [Google Scholar] [CrossRef]
- Chowell, G.; Fuentes, R.; Olea, A.; Aguilera, X.; Nesse, H.; Hyman, J.M. The basic reproduction number R0 and effectiveness of reactive interventions during dengue epidemics: The 2002 dengue outbreak in Easter Island, Chile. Math. Biosci. Eng. 2013, 10, 1455–1474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, M.A. Fatmawati Dengue infection modeling and its optimal control analysis in East Java, Indonesia. Heliyon 2021, 7, e06023. [Google Scholar] [CrossRef]
- Valencia, V.A.N.; Díaz, Y.; Pascale, J.M.; Boni, M.F.; Sanchez-Galan, J.E. Using compartmental models and Particle Swarm Optimization to assess Dengue basic reproduction number R0 for the Republic of Panama in the 1999-2022 period. Heliyon 2023, 9, e15424. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Kitahara, K.; Mitra, D.; Ntontolo, N.P.; Ngatu, N.R.; Ohno, A.; Khatiwada, J.; Dutta, S.; Miyoshi, S.-I. The basic reproduction number (R0) of ebola virus disease: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2024, 57, 102685. [Google Scholar] [CrossRef]
- Nsubuga, R.N.; White, R.G.; Mayanja, B.N.; Shafer, L.A. Estimation of the HIV Basic Reproduction Number in Rural South West Uganda: 1991–2008. PLoS ONE 2014, 9, e83778. [Google Scholar] [CrossRef] [PubMed]
- Stachel, A.; Pinto, G.; Stelling, J.; Fulmer, Y.; Shopsin, B.; Inglima, K.; Phillips, M. Implementation and evaluation of an automated surveillance system to detect hospital outbreak. Am. J. Infect. Control. 2017, 45, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.; Doherty, A.; Lacey, G. Using Artificial Intelligence in Infection Prevention. Curr. Treat Options Infect. Dis. 2020, 12, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Piaggio, D.; Zarro, M.; Pagliara, S.; Andellini, M.; Almuhini, A.; Maccaro, A.; Pecchia, L. The use of smart environments and robots for infection prevention control: A systematic literature review. Am. J. Infect. Control. 2023, 51, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Corbella, X.; Pujol, M.; Argerich, M.J.; Ayats, J.; Sendra, M.; Peña, C.; Ariza, J. Environmental Sampling of Acinetobacter baumannii: Moistened Swabs Versus Moistened Sterile Gauze Pads. Infect. Control. Hosp. Epidemiol. 1999, 20, 458–460. [Google Scholar] [CrossRef][Green Version]
- Schwab, F.; Meyer, E.; Geffers, C.; Gastmeier, P. Understaffing, overcrowding, inappropriate nurse:ventilated patient ratio and nosocomial infections: Which parameter is the best reflection of deficits? J. Hosp. Infect. 2012, 80, 133–139. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Garcia, P.C.; Nogueira, L.d.S. Nursing workload and occurrence of adverse events in intensive care: A systematic review. Rev. Esc. Enferm. USP 2016, 50, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Bracco, D.; Dubois, M.-J.; Bouali, R.; Eggimann, P. Single rooms may help to prevent nosocomial bloodstream infection and cross-transmission of methicillin-resistant Staphylococcus aureus in intensive care units. Intensiv. Care Med. 2007, 33, 836–840. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Pei, J.; Yi, W.; Fan, L.; Wang, C.; Xiao, X. Isolation and identification of a novel phage targeting clinical multidrug-resistant Corynebacterium striatum isolates. Front. Cell. Infect. Microbiol. 2024, 14, 1361045. [Google Scholar] [CrossRef] [PubMed]
- Skurnik, D.; Roux, D.; Pons, S.; Guillard, T.; Lu, X.; Cywes-Bentley, C.; Pier, G.B. Extended-spectrum antibodies protective against carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2016, 71, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Kalfopoulou, E.; Huebner, J. Advances and Prospects in Vaccine Development against Enterococci. Cells 2020, 9, 2397. [Google Scholar] [CrossRef]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2019, 44, 123–153. [Google Scholar] [CrossRef]
- Bae, J.Y.; Yun, I.; Jun, K.I.; Kim, C.-J.; Lee, M.; Choi, H.J. Association between Pneumonia Development and Virulence Gene Expression in Carbapenem-Resistant Acinetobacter baumannii Isolated from Clinical Specimens. Can. J. Infect. Dis. Med Microbiol. 2023, 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; de la Fuente-Nunez, C.; Collins, J.J. Leveraging artificial intelligence in the fight against infectious diseases. Science 2023, 381, 164–170. [Google Scholar] [CrossRef]
- Ben-Chetrit, E.; Wiener-Well, Y.; Lesho, E.; Kopuit, P.; Broyer, C.; Bier, L.; Assous, M.V.; Benenson, S.; Cohen, M.J.; McGann, P.T.; et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: Long-term impact for the ICU and hospital. Crit. Care 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Landelle, C.; Pagani, L.; Harbarth, S. Is patient isolation the single most important measure to prevent the spread of multidrug-resistant pathogens? Virulence 2013, 4, 163–171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).