Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects
Abstract
1. Introduction
2. Natural Products with Antibacterial Activity
2.1. Polyphenols
2.2. Essential Oils
2.3. Alkaloids
2.4. Lanthipeptides
3. Natural Product-Mediated Prevention of Biofilm Formation
4. Antiviral Products of Natural Origin
4.1. Polyphenols
4.2. Terpenoids
4.3. Alkaloids
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| BRBNPs | Berberine nanoparticles |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin gallate |
| EOs | Essential oils |
| EPS | Extracellular polymer substance |
| HADC | Histone deacetylase |
| HAT | Histone acetyl transferase |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HIV | Human immune deficiency virus |
| IAV | Influenza A virus |
| IL | Interleukin |
| MDR | Multi-drug-resistant |
| MSRA | Methicillin-resistant S. aureus |
| OMWW | Olive mill wastewater |
| RSV | Respiratory syncytial virus |
| TNF | Tumor necrosis factor |
| VRE | Vancomycin-resistant Enterococcus |
References
- Ryan, M.; Brindal, E.; Roberts, M.; Hickson, R.I. A behaviour and disease transmission model: Incorporating the Health Belief Model for human behaviour into a simple transmission model. J. R. Soc. Interface 2024, 21, 20240038. [Google Scholar] [CrossRef]
- de Lusignan, S.; Shi, T.; Fowler, T.; Andrews, N.; Todkill, D.; Gu, X.; Meza-Torres, B.; Robertson, C.; Sheikh, A. Sleeper frameworks for Pathogen X: Surveillance, risk stratification, and the effectiveness and safety of therapeutic interventions. Lancet Infect. Dis. 2024, 24, e417–e418. [Google Scholar] [CrossRef]
- Bottalico, L.; Charitos, I.A.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The war against bacteria, from the past to present and beyond. Expert Rev. Anti-Infective Ther. 2022, 20, 681–706. [Google Scholar] [CrossRef]
- Santacroce, L.; Spirito, F.; Bottalico, L.; Muzio, E.L.; Charitos, I.A.; Potenza, M.A.; Montagnani, M.; Jirillo, E. Current Issues and Perspectives in Antimicrobials use in Dental Practice. Curr. Pharm. Des. 2022, 28, 2879–2889. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 30 April 2024).
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Goldmann, D.A.; Weinstein, R.A.; Wenzel, R.P.; Tablan, O.C.; Duma, R.J.; Gaynes, R.P.; Schlosser, J.; Martone, W.J. Strategies to Prevent and Control the Emergence and Spread of Antimicrobial-Resistant Microorganisms in Hospitals. A challenge to hospital leadership. JAMA 1996, 275, 234–240. [Google Scholar] [CrossRef]
- Kollef, M.H.; Fraser, V.J. Antibiotic Resistance in the Intensive Care Unit: Strategies for Management. Ann. Intern. Med. 2001, 134, 298–314. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Leshem, A.; Liwinski, T.; Elinav, E. Immune-Microbiota Interplay and Colonization Resistance in Infection. Mol. Cell 2020, 78, 597–613. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.C.; Pandey, S.P.; Bender, M.J.; Meisel, M. Systemic Immunoregulatory Consequences of Gut Commensal Translocation. Trends Immunol. 2021, 42, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Di Domenico, M.; Montagnani, M.; Jirillo, E. Antibiotic Resistance and Microbiota Response. Curr. Pharm. Des. 2023, 29, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Brandl, K.; Plitas, G.; Mihu, C.N.; Ubeda, C.; Jia, T.; Fleisher, M.; Schnabl, B.; DeMatteo, R.P.; Pamer, E.G. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 2008, 455, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.B.; Buffie, C.G.; Carter, R.A.; Leiner, I.; Toussaint, N.C.; Miller, L.C.; Gobourne, A.; Ling, L.; Pamer, E.G. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection with Oral Vancomycin Compared with Metronidazole. J. Infect. Dis. 2015, 212, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Edlund, C.; Nord, C.E. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 2001, 1, 101–114. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Foxman, E.F.; Watkins, T.A.; Lipsitch, M. Considerations for viral co-infection studies in human populations. mBio 2024, e0065824. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Devincenzo, J.P.; Toovey, S.; Wu, J.Z.; Whitley, R.J. Comparison of antiviral resistance across acute and chronic viral infections. Antivir. Res. 2018, 158, 103–112. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Palese, L.L. The Dynamics of OXA-23 β-Lactamase from Acinetobacter baumannii. Int. J. Mol. Sci. 2023, 24, 17527. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Pacyga, K.; Pacyga, P.; Topola, E.; Viscardi, S.; Duda-Madej, A. Bioactive Compounds from Plant Origin as Natural Antimicrobial Agents for the Treatment of Wound Infections. Int. J. Mol. Sci. 2024, 25, 2100. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Damiani, E.; Scarabelli, S.; Principi, F.; Gioacchini, A.M.; Rocchi, M.B.L. EGCG and Its Antiviral Effects. Nutrients 2023, 15, 781. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Wang, Y.-W.; Yang, J.; Tong, Z.-J.; Wu, J.-Z.; Wang, Y.-B.; Wang, Q.-X.; Li, Q.-Q.; Yu, Y.-C.; Leng, X.-J.; et al. Natural Products as Potential Lead Compounds to Develop New Antiviral Drugs Over the Past Decade. Eur. J. Med. Chem. 2023, 260, 115726. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Bobate, S.; Mahalle, S.; Dafale, N.A.; Bajaj, A. Emergence of environmental antibiotic resistance: Mechanism, monitoring and management. Environ. Adv. 2023, 13, 100409. [Google Scholar] [CrossRef]
- Lepe, J.A.; Martínez-Martínez, L. Resistance mechanisms in Gram-negative bacteria. Med. Intensiv. 2022, 46, 392–402. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295, Erratum in Nat. Rev. Microbiol. 2024, 22, 255. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Kresken, M.; Klare, I.; Wichelhaus, T.A.; Wohlfarth, E.; Layer-Nicolaou, F.; Neumann, B.; Werner, G.; Study Group ‘Antimicrobial Re-sistance’ of the Paul-Ehrlich-Society for Chemotherapy. Glycopeptide resistance in Enterococcus spp. and coagulase-negative staphylococci from hospitalised patients in Germany: Occurrence, characteristics and dalbavancin susceptibility. J. Glob. Antimicrob. Resist. 2022, 28, 102–107. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Baig, M.I.R.; Kadu, P.; Bawane, P.; Nakhate, K.T.; Yele, S.; Ojha, S.; Goyal, S.N. Mechanisms of emerging resistance associated with non-antibiotic antimicrobial agents: A state-of-the-art review. J. Antibiot. 2023, 76, 629–641. [Google Scholar] [CrossRef] [PubMed]
- WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Inhibition of listeriolysin O and phosphatidylcholine-specific production in Listeria monocytogenes by subinhibitory concentrations of plant essential oils. J. Med Microbiol. 2002, 51, 567–608. [Google Scholar] [CrossRef] [PubMed]
- Mooyottu, S.; Kollanoor-Johny, A.; Flock, G.; Bouillaut, L.; Upadhyay, A.; Sonenshein, A.L.; Venkitanarayanan, K. Carvacrol and trans-cinnamaldehyde reduce Clostridium difficile toxin production and cytotoxicity in vitro. Int. J. Mol. Sci. 2014, 15, 4415–4430. [Google Scholar] [CrossRef] [PubMed]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 2020, 10, 277–290. [Google Scholar] [CrossRef]
- Santacroce, L.; Topi, S.; Charitos, I.A.; Lovero, R.; Luperto, P.; Palmirotta, R.; Jirillo, E. Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products. Pathophysiology 2024, 31, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Perz, M.; Szymanowska, D.; Janeczko, T.; Kostrzewa-Susłow, E. Antimicrobial Properties of Flavonoid Derivatives with Bromine, Chlorine, and Nitro Group Obtained by Chemical Synthesis and Biotransformation Studies. Int. J. Mol. Sci. 2024, 25, 5540. [Google Scholar] [CrossRef] [PubMed]
- Meure, C.M.; Steer, B.; Porter, J. Interrelationships between Dietary Outcomes, Readmission Rates and Length of Stay in Hospitalised Oncology Patients: A Scoping Review. Nutrients 2023, 15, 400. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E.; Magrone, M.; Russo, M.A.; Romita, P.; Massari, F.; Foti, C. Red Grape Polyphenol Oral Administration Improves Immune Response in Women Affected by Nickel-Mediated Allergic Contact Dermatitis. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 374–384. [Google Scholar] [CrossRef]
- Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Quercetin potentiates meropenem activity among pathogenic carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Appl. Microbiol. 2019, 127, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-W.; Luo, H.-Z.; Jiang, H.; Jian, T.-K.; Chen, Z.-Q.; Jia, A.-Q. Hordenine: A Novel Quorum Sensing Inhibitor and Antibiofilm Agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018, 66, 1620–1628. [Google Scholar] [CrossRef]
- Wang, D.; Xie, K.; Zou, D.; Meng, M.; Xie, M. Inhibitory effects of silybin on the efflux pump of methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2018, 18, 827–833. [Google Scholar] [CrossRef]
- Malczak, I.; Gajda, A. Interactions of naturally occurring compounds with antimicrobials. J. Pharm. Anal. 2023, 13, 1452–1470. [Google Scholar] [CrossRef] [PubMed]
- Morais-Braga, M.; Souza, T.; Santos, K.; Guedes, G.; Andrade, J.; Tintino, S.; Sobral-Souza, C.; Costa, J.; Saraiva, A.; Coutinho, H. Phenolic compounds and interaction between aminoglycosides and natural products of Lygodium venustum SW against multiresistant bacteria. Chemotherapy 2012, 58, 337–340. [Google Scholar] [CrossRef]
- Wang, J.; Song, M.; Pan, J.; Shen, X.; Liu, W.; Zhang, X.; Li, H.; Deng, X. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J. Cell. Mol. Med. 2018, 22, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Xiong, G.; Ji, W.; Wang, F.; Zhang, F.; Xue, P.; Cheng, M.; Sun, Y.; Wang, X.; Zhang, T. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-κB Signaling Pathway. BioMed Res. Int. 2019, 2019, 6282635. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Gao, P.; Xie, X.; Li, D.; Yu, D.; Yu, M. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult. Sci. 2020, 99, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Sharahi, J.Y.; Ahovan, Z.A.; Maleki, D.T.; Rad, Z.R.; Rad, Z.R.; Goudarzi, M.; Shariati, A.; Bostanghadiri, N.; Abbasi, E.; Hashemi, A. In vitro antibacterial activity of curcumin-meropenem combination against extensively drug-resistant (XDR) bacteria isolated from burn wound infections. Avicenna J. Phytomed. 2020, 10, 3–10. [Google Scholar]
- Al-Dulaimi, M.M.K.; Mutalib, S.A.; Ghani, M.A.; Zaini, N.A.M.; Ariffin, A.A. Multiple Antibiotic Resistance (MAR), Plasmid Profiles, and DNA Polymorphisms among Vibrio vulnificus Isolates. Antibiotics 2019, 8, 68. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.S.; Sivasubramanian, A.; Nagarajan, S. Simultaneous inhibition of MarR by salicylate and efflux pumps by curcumin sensitizes colistin resistant clinical isolates of Enterobacteriaceae. Microb. Pathog. 2020, 148, 104445. [Google Scholar] [CrossRef] [PubMed]
- Izui, S.; Sekine, S.; Murai, H.; Takeuchi, H.; Amano, A. Inhibitory effects of curcumin against cytotoxicity of Porphyromonas gingivalis outer membrane vesicles. Arch. Oral Biol. 2021, 124, 105058. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Guo, X.; Du, Q.; Keshavarzi, M. Curcumin and its nano-formulations combined with exercise: From molecular mechanisms to clinic. Cell Biochem. Funct. 2024, 42, e4061. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. Biomed. Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Jayaraman, A.; Mahapatra, S.K.; Vellingiri, V. Anti-virulence properties of catechin-in-cyclodextrin-in-phospholipid liposome through down-regulation of gene expression in MRSA strains. Microb. Pathog. 2022, 167, 105585. [Google Scholar] [CrossRef]
- Magrone, T.; Panaro, M.A.; Jirillo, E.; Covelli, V. Molecular effects elicited in vitro by red wine on human healthy peripheral blood mononuclear cells: Potential therapeutical application of polyphenols to diet-related chronic diseases. Curr. Pharm. Des. 2008, 14, 2758–2766. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Xu, Y.; Zhang, J.; Sui, Z.; Corke, H. Antibacterial Activity and Multi-Targeting Mechanism of Dehydrocorydaline from Corydalis turtschaninovii Bess. Against Listeria monocytogenes. Front. Microbiol. 2022, 12, 799094. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From antiquity to contemporary times: How olive oil by-products and waste water can contribute to health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef] [PubMed]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef] [PubMed]
- Sar, T.; Akbas, M.Y. Antimicrobial Activities of Olive Oil Mill Wastewater Extracts against Selected Microorganisms. Sustainability 2023, 15, 8179. [Google Scholar] [CrossRef]
- Cappelli, K.; Ferlisi, F.; Mecocci, S.; Maranesi, M.; Trabalza-Marinucci, M.; Zerani, M.; Bosco, A.D.; Acuti, G. Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through RT-qPCR Approach. Animals 2021, 11, 2932. [Google Scholar] [CrossRef] [PubMed]
- Tăbăcariu, A.S.-B.; Ifrim, I.-L.; Patriciu, O.-I.; Ștefănescu, I.-A.; Fînaru, A.-L. Walnut By-Products and Elderberry Extracts—Sustainable Alternatives for Human and Plant Health. Molecules 2024, 29, 498. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Badim, H.; Salvador, C.; Silvestre, A.J.D.; Santos, S.A.O.; Rocha, S.M.; Sousa, A.M.; Pereira, M.O.; Wilson, C.P.; Rocha, C.M.R.; et al. Chemical Characterization of Sambucus nigra L. Flowers Aqueous Extract and Its Biological Implications. Biomolecules 2021, 11, 1222. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskiene, K.; Inkeniene, A.; Puidokaite, E.; Grigonis, A. Quality analysis of semisolid formulations with the liquid extract of elderflower (Sambucus nigra L.). Acta Pol. Pharm. Drug Res. 2019, 76, 1061–1071. [Google Scholar] [CrossRef]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plants Res. 2010, 4, 1805–1809. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Sharma, P.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia. J. Pharm. Anal. 2013, 3, 298–302. [Google Scholar] [CrossRef]
- Żurek, N.; Pycia, K.; Pawłowska, A.; Potocki, L.; Kapusta, I.T. Chemical Profiling, Bioactive Properties, and Anticancer and Antimicrobial Potential of Juglans regia L. Leaves. Molecules 2023, 28, 1989. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Panneerselvam, K. An investigation on antibacterial filler property of silver nanoparticles generated from Walnut shell powder by insitu process. Mater. Today Proc. 2021, 39, 368–372. [Google Scholar] [CrossRef]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; de Boer, L.; Ruyter-Spira, C.P.; Creemers-Molenaar, T.; Helsper, J.P.F.G.; Vandenbroucke-Grauls, C.M.J.E.; Zaat, S.A.J.; Velde, A.A.T. Medical-grade honey enriched with antimicrobial peptides has enhanced activity against antibiotic-resistant pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 251–257. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Propolis: Composition and Antibacterial Properties. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.N.; Casey, E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules 2021, 26, 4500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Kong, L.-C.; Liu, J.; Ma, H.-X. Synergistic effect of eugenol with Colistin against clinical isolated Colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Noghabi, S.A.; Kargar, P.G.; Bagherzade, G.; Beyzaei, H. Comparative study of antioxidant and antimicrobial activity of berberine-derived Schiff bases, nitro-berberine and amino-berberine. Heliyon 2023, 9, e22783. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, J.A.; Tereshchenkov, A.G.; Nazarov, P.A.; Lukianov, D.A.; Skvortsov, D.A.; Polshakov, V.I.; Vasilieva, B.F.; Efremenkova, O.V.; Kaiumov, M.Y.; Paleskava, A.; et al. Conjugates of Chloramphenicol Amine and Berberine as Antimicrobial Agents. Antibiotics 2022, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, S.; Popat, A.; Ziora, Z.M.; Moyle, P.M. Sortase A Inhibitor Protein Nanoparticle Formulations Demonstrate Antibacterial Synergy When Combined with Antimicrobial Peptides. Molecules 2023, 28, 2114. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ma, L.; Wang, G.; Yang, J.; Zhang, M.; Wang, X.; Su, J.; Xie, M. In vitro Antimicrobial Activity and the Mechanism of Berberine Against Methicillin-Resistant Staphylococcus aureus Isolated from Bloodstream Infection Patients. Infect. Drug Resist. 2022, 15, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, W.; Cai, L.; Yang, T. Potentiation and Mechanism of Berberine as an Antibiotic Adjuvant Against Multidrug-Resistant Bacteria. Infect. Drug Resist. 2023, 16, 7313–7326. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Pan, Q.; Fan, L.; Pan, T.; Zhu, F.; Pan, Q.; Shan, L.; Zhao, L. Berberine at sub-inhibitory concentration inhibits biofilm dispersal in Staphylococcus aureus. Microbiology 2022, 168. [Google Scholar] [CrossRef]
- Lade, H.; Chung, S.H.; Lee, Y.; Kumbhar, B.V.; Joo, H.-S.; Kim, Y.-G.; Yang, Y.-H.; Kim, J.-S. Thymol Reduces agr-Mediated Virulence Factor Phenol-Soluble Modulin Production in Staphylococcus aureus. BioMed Res. Int. 2022, 2022, 8221622. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, S. Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J. Appl. Microbiol. 2021, 130, 1154–1172. [Google Scholar] [CrossRef]
- Aksoy, C.S.; Avci, F.G.; Ugurel, O.M.; Atas, B.; Sayar, N.A.; Akbulut, B.S. Potentiating the activity of berberine for Staphylococcus aureus in a combinatorial treatment with thymol. Microb. Pathog. 2020, 149, 104542. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Ye, X.-G.; He, L.-T.; Zhang, S.-R.; Wang, R.-L.; Zhou, J.; He, Z.-S. In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumonia e. J. Antibiot. 2016, 69, 741–746. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Wang, L.; Kang, G.; Wang, P.; Yin, H.; Huang, H. A Potential Combination Therapy of Berberine Hydrochloride with Antibiotics Against Multidrug-Resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2021, 11, 660431. [Google Scholar] [CrossRef]
- Gao, W.-W.; Gopala, L.; Bheemanaboina, R.R.Y.; Zhang, G.-B.; Li, S.; Zhou, C.-H. Discovery of 2-aminothiazolyl berberine derivatives as effectively antibacterial agents toward clinically drug-resistant Gram-negative Acinetobacter baumanii. Eur. J. Med. Chem. 2018, 146, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Khalvati, B.; Eslami, S.; Mirzaii, M.; Roustaei, N.; Mazloomirad, F.; Khoramrooz, S.S. The Inhibitory Effect of Thioridazine on adeB Efflux Pump Gene Expression in Multidrug-Resistant Acinetobacter baumannii Isolates Using Real Time PCR. Avicenna J. Med Biotechnol. 2022, 14, 132–136. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal Products and Their Active Constituents Used Alone and in Combination with Antibiotics against Multidrug-Resistant Bacteria. Planta Medica 2023, 89, 168–182. [Google Scholar] [CrossRef]
- Patra, P.H.; Mahanti, A.; Mondal, D.K.; Dandapat, P.; Bandyopadhyay, S.; Samanta, I.; Lodh, C.; Bera, A.K.; Bhattacharyya, D.; Sarkar, M.; et al. Potential antibacterial activity of berberine against multi drug resistant enterovirulent Escherichia coli isolated from yaks (Poephagus grunniens) with haemorrhagic diarrhoea. Asian Pac. J. Trop. Med. 2013, 6, 315–319. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X. Role of Berberine as a Potential Efflux Pump Inhibitor against MdfA from Escherichia coli: In Vitro and In Silico Studies. Microbiol. Spectr. 2023, 11, e0332422. [Google Scholar] [CrossRef]
- Morita, Y.; Nakashima, K.-I.; Nishino, K.; Kotani, K.; Tomida, J.; Inoue, M.; Kawamura, Y. Berberine Is a Novel Type Efflux Inhibitor Which Attenuates the MexXY-Mediated Aminoglycoside Resistance in Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 1223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Li, L.; Liu, L. Synergistic Activity of Berberine with Azithromycin against Pseudomonas Aeruginosa Isolated from Patients with Cystic Fibrosis of Lung In Vitro and In Vivo. Cell. Physiol. Biochem. 2017, 42, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Guo, M.; Xu, X.; Hu, Y.; Liu, D.; Wang, C.; Liu, X.; Li, Y. In Vitro Synergistic Inhibitory Activity of Natural Alkaloid Berberine Combined with Azithromycin against Alginate Production by Pseudomonas aeruginosa PAO1. Oxidative Med. Cell. Longev. 2022, 2022, 3858500. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, L.G.; Mahoney, A.R.; Dey, D.; Wuest, W.M.; Conn, G.L. Di-berberine conjugates as chemical probes of Pseudomonas aeruginosa MexXY-OprM efflux function and inhibition. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jhanji, R.; Bhati, V.; Singh, A.; Kumar, A. Phytomolecules against bacterial biofilm and efflux pump: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2020, 38, 5500–5512. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti-Infect. Ther. 2019, 8, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, R.; Bhattarai, N.; Baral, P.; Gerstman, B.S.; Park, J.H.; Handfield, M.; Chapagain, P.P. Lipid II Binding and Transmembrane Properties of Various Antimicrobial Lanthipeptides. J. Chem. Theory Comput. 2022, 18, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rendón, D.; Guzmán-Chávez, F.; García-Ausencio, C.; Rodríguez-Sanoja, R.; Sánchez, S. The untapped potential of actinobacterial lanthipeptides as therapeutic agents. Mol. Biol. Rep. 2023, 50, 10605–10616. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Lazzarini, A.; Carrano, L.; Corti, E.; Ciciliato, I.; Gastaldo, L.; Candiani, P.; Losi, D.; Marinelli, F.; Selva, E.; et al. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem. Biol. 2008, 15, 22–31. [Google Scholar] [CrossRef]
- Münch, D.; Müller, A.; Schneider, T.; Kohl, B.; Wenzel, M.; Bandow, J.E.; Maffioli, S.; Sosio, M.; Donadio, S.; Wimmer, R.; et al. The lantibiotic NAI-107 binds to bactoprenol-bound cell wall precursors and impairs membrane functions. J. Biol. Chem. 2014, 289, 12063–12076. [Google Scholar] [CrossRef]
- Brunati, C.; Thomsen, T.T.; Gaspari, E.; Maffioli, S.; Sosio, M.; Jabes, D.; Løbner-Olesen, A.; Donadio, S. Expanding the potential of NAI-107 for treating serious ESKAPE pathogens: Synergistic combinations against Gram-negatives and bactericidal activity against non-dividing cells. J. Antimicrob. Chemother. 2018, 73, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Foulston, L.C.; Bibb, M.J. Microbisporicin gene cluster reveals unusual features of lantibiotic biosynthesis in actinomycetes. Proc. Natl. Acad. Sci. USA 2010, 107, 13461–13466. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, S.K. Perspectives on lantibiotic discovery—Where have we failed and what improvements are required? Expert Opin. Drug Discov. 2015, 10, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.C.; Mösker, E.; Faria, R.; Süssmuth, R.D.; Mendo, S.; Caetano, T. Class II two-peptide lanthipeptide proteases: Exploring LicTP for biotechnological applications. Appl. Microbiol. Biotechnol. 2023, 107, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Gao, Y.; Zhao, F.; Wang, J.; Teng, K.; Zhang, J.; Zhong, J. Dissecting the catalytic and substrate binding activity of a class II lanthipeptide synthetase BovM. Biochem. Biophys. Res. Commun. 2014, 450, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, F.; Hu, Y. Genome Mining-Mediated Discovery of a New Avermipeptin Analogue in Streptomyces actuosus ATCC 25421. ChemistryOpen 2018, 7, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends. Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudo-monas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Van den Bergh, B.; Fauvart, M.; Michiels, J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef]
- Dombach, J.L.; Quintana, J.L.J.; Detweiler, C.S. Staphylococcal Bacterial Persister Cells, Biofilms, and Intracellular Infection Are Disrupted by JD1, a Membrane-Damaging Small Molecule. mBio 2021, 12, e0180121. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Basak, A.K.; Melander, C. Natural products as inspiration for the development of bacterial antibiofilm agents. Nat. Prod. Rep. 2020, 37, 1454–1477. [Google Scholar] [CrossRef] [PubMed]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.A.; Booysen, E.; van Zyl, L.; Trindade, M. Do biosurfactants as anti-biofilm agents have a future in industrial water systems? Front. Bioeng. Biotechnol. 2023, 11, 1244595. [Google Scholar] [CrossRef] [PubMed]
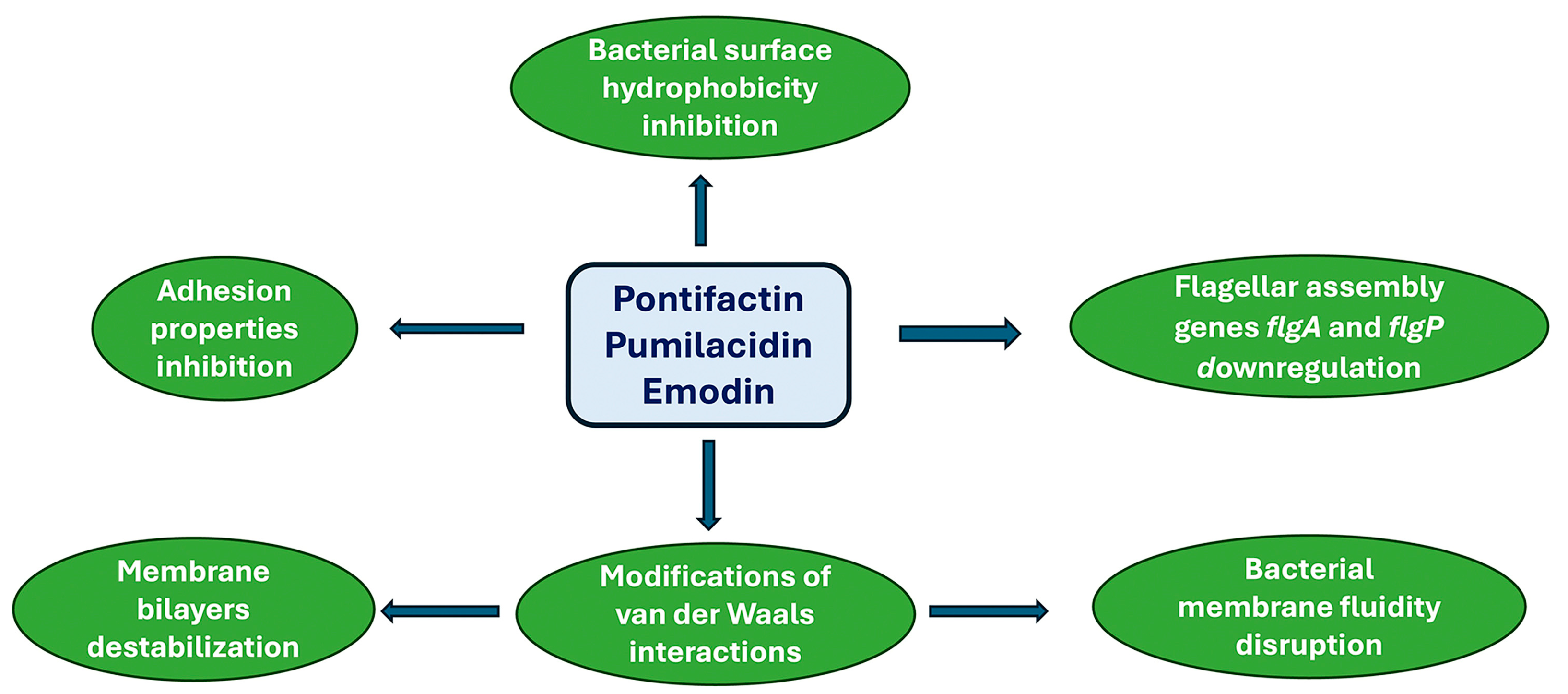
- Xiu, P.; Liu, R.; Zhang, D.; Sun, C. Pumilacidin-Like Lipopeptides Derived from Marine Bacterium Bacillus sp. Strain 176 Suppress the Motility of Vibrio alginolyticus. Appl. Environ. Microbiol. 2017, 83, e00450-17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Ryu, S.Y.; Lee, J. Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.S.; Perez-Fons, L.; Estepa, A.; Micol, V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, B.; Lu, Y.; Guo, Y.; Sun, J.; Wei, B.; Zhang, H.; Wang, H. Quorum Sensing Inhibitors from Marine Microorganisms and Their Synthetic Derivatives. Mar. Drugs 2019, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, L.; Sun, S.; Chang, A.; Dai, X.; Li, H.; Wang, Y.; Zhu, H. A Cyclic Dipeptide from Marine Fungus Penicillium chrysogenum DXY-1 Exhibits Anti-quorum Sensing Activity. ACS Omega 2021, 6, 7693–7700. [Google Scholar] [CrossRef]
- Kiran, G.S.; Sajayan, A.; Priyadharshini, G.; Balakrishnan, A.; Prathiviraj, R.; Sabu, A.; Selvin, J. A novel anti-infective molecule nesfactin identified from sponge associated bacteria Nesterenkonia sp. MSA31 against multidrug resistant Pseudomonas aeruginosa. Microb. Pathog. 2021, 157, 104923. [Google Scholar] [CrossRef]
- Wang, J.; Nong, X.-H.; Zhang, X.-Y.; Xu, X.-Y.; Amin, M.; Qi, S.-H. Screening of Anti-Biofilm Compounds from Marine-Derived Fungi and the Effects of Secalonic Acid D on Staphylococcus aureus Biofilm. J. Microbiol. Biotechnol. 2017, 27, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Lorizate, M.; Kräusslich, H.-G. Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 2011, 3, a004820. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.L.; Chlanda, P. The Art of Viral Membrane Fusion and Penetration. Subcell Biochem. 2023, 106, 113–152. [Google Scholar] [CrossRef]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Bashir, S.M.; Nabi, S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytotherapy Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Wei, Z.-Q.; Zhang, Y.-H.; Ke, C.-Z.; Chen, H.-X.; Ren, P.; He, Y.-L.; Hu, P.; Ma, D.-Q.; Luo, J.; Meng, Z.-J. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J. Gastroenterol. 2017, 23, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoo, H.S.; Kim, J.C.; Park, C.S.; Choi, M.S.; Kim, M.; Choi, H.; Min, J.S.; Kim, Y.S.; Yoon, S.W.; et al. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 2009, 124, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Prusty, K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2005, 113, 951–960. [Google Scholar] [CrossRef]
- Wang, Z.; Song, X.-Q.; Xu, W.; Lei, S.; Zhang, H.; Yang, L. Stand Up to Stand Out: Natural Dietary Polyphenols Curcumin, Resveratrol, and Gossypol as Potential Therapeutic Candidates against Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Nutrients 2023, 15, 3885. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Mekky, R.Y.; El-Ekiaby, N.M.; Hamza, M.T.; Elemam, N.M.; El-Sayed, M.; Esmat, G.; Abdelaziz, A.I. Mir-194 is a hepatocyte gate keeper hindering HCV entry through targeting CD81 receptor. J. Infect. 2015, 70, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Thibault, P.A.; Wilson, J.A. MicroRNA 122 Affects both the Initiation and the Maintenance of Hepatitis C Virus Infections. J. Virol. 2022, 96, e0190321. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Song, T.; Li, M.; Chen, W.; Li, J.; Gong, S.; Zhao, Y.; Ma, L.; Yu, H.; Li, X.; et al. The medicinal value of tea drinking in the management of COVID-19. Heliyon 2023, 9, e12968. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Meyer, T. Sinecatechins (Polyphenon E) ointment for treatment of external genital warts and possible future indications. Expert Opin. Biol. Ther. 2014, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cheng, X.; Naumovski, N.; Hu, L.; Wang, K. Epigenetic regulation by quercetin: A comprehensive review focused on its biological mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 5542. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Li, L.; Chen, Y.; Jia, R.; Zou, Y.; Wan, H.; Zhao, L.; Tang, H.; Lv, C.; et al. Resveratrol Inhibits Pseudorabies Virus Replication by Targeting IE180 Protein. Front. Microbiol. 2022, 13, 891978. [Google Scholar] [CrossRef]
- Docherty, J.J.; Sweet, T.J.; Bailey, E.; Faith, S.A.; Booth, T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antivir. Res. 2006, 72, 171–177. [Google Scholar] [CrossRef]
- Xie, X.-H.; Zang, N.; Li, S.-M.; Wang, L.-J.; Deng, Y.; He, Y.; Yang, X.-Q.; Liu, E.-M. Resveratrol inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation 2012, 35, 1392–1401. [Google Scholar] [CrossRef]
- Pan, P.; Li, J.; Lin, W.; Long, G. Effects of Resveratrol on Hepatitis B Virus Replication: In vitro and in vivo Experiments. Intervirology 2022, 65, 206–214. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, P.; Gupta, G.K.; Ntie-Kang, F.; Kumar, D. Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products. Molecules 2020, 25, 2070. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Sukemori, A.; Watanabe, T.; Malla, K.J.; Yoshikawa, T.; Li, W.; Koike, K.; Chen, C.-H.; Akiyama, T.; Qian, K.; et al. Stelleralides A–C, novel potent anti-HIV daphnane-type diterpenoids from Stellera chamaejasme L. Org. Lett. 2011, 13, 2904–2907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-Y.; Chen, H.; He, H.-P.; Zhang, Y.; Li, S.-F.; Tang, G.-H.; Guo, L.-L.; Yang, W.; Zhu, F.; Zheng, Y.-T.; et al. Anti-HIV active daphnane diterpenoids from Trigonostemon thyrsoideum. Phytochemistry 2013, 96, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Guo, Z.-G.; Wang, L.; Guo, Q.-F.; Cao, F. Anti-IAV indole-diterpenoids from the marine-derived fungus Penicillium citrinum. Nat. Prod. Res. 2023, 37, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-J.; Li, Y.; Ma, S.-G.; Qu, J.; Liu, Y.-B.; Li, Y.-H.; Zhang, D.; Li, L.; Yu, S.-S. Antiviral Triterpenes from the Twigs and Leaves of Lyonia ovalifolia. J. Nat. Prod. 2016, 79, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Mair, C.E.; Grienke, U.; Wilhelm, A.; Urban, E.; Zehl, M.; Schmidtke, M.; Rollinger, J.M. Anti-Influenza Triterpene Saponins from the Bark of Burkea africana. J. Nat. Prod. 2018, 81, 515–523. [Google Scholar] [CrossRef]
- Warowicka, A.; Nawrot, R.; Goździcka-Józefiak, A. Antiviral activity of berberine. Arch. Virol. 2020, 165, 1935–1945. [Google Scholar] [CrossRef]
- Le, K.; Tran, D.; Nguyen, A.; Le, L. A Screening of Neuraminidase Inhibition Activities of Isoquinolone Alkaloids in Coptis chinensis Using Molecular Docking and Pharmacophore Analysis. ACS Omega 2020, 5, 30315–30322. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A. New trends in the practical use of isoquinoline alkaloids as potential drugs applicated in infectious and non-infectious diseases. Biomed. Pharmacother. 2023, 168, 115704. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-G.; Wang, Y.; Yang, M.-R.; Wang, C.-Y.; Meng, J.; Liu, J.; Yang, Z.; Wu, K.; Bai, L.-P.; Zhu, G.-Y.; et al. Structures, Biomimetic Synthesis, and Anti-SARS-CoV-2 Activity of Two Pairs of Enantiomeric Phenylpropanoid-Conjugated Protoberberine Alkaloids from the Rhizomes of Corydalis decumbens. Arch. Pharmacal Res. 2022, 45, 631–643. [Google Scholar] [CrossRef] [PubMed]
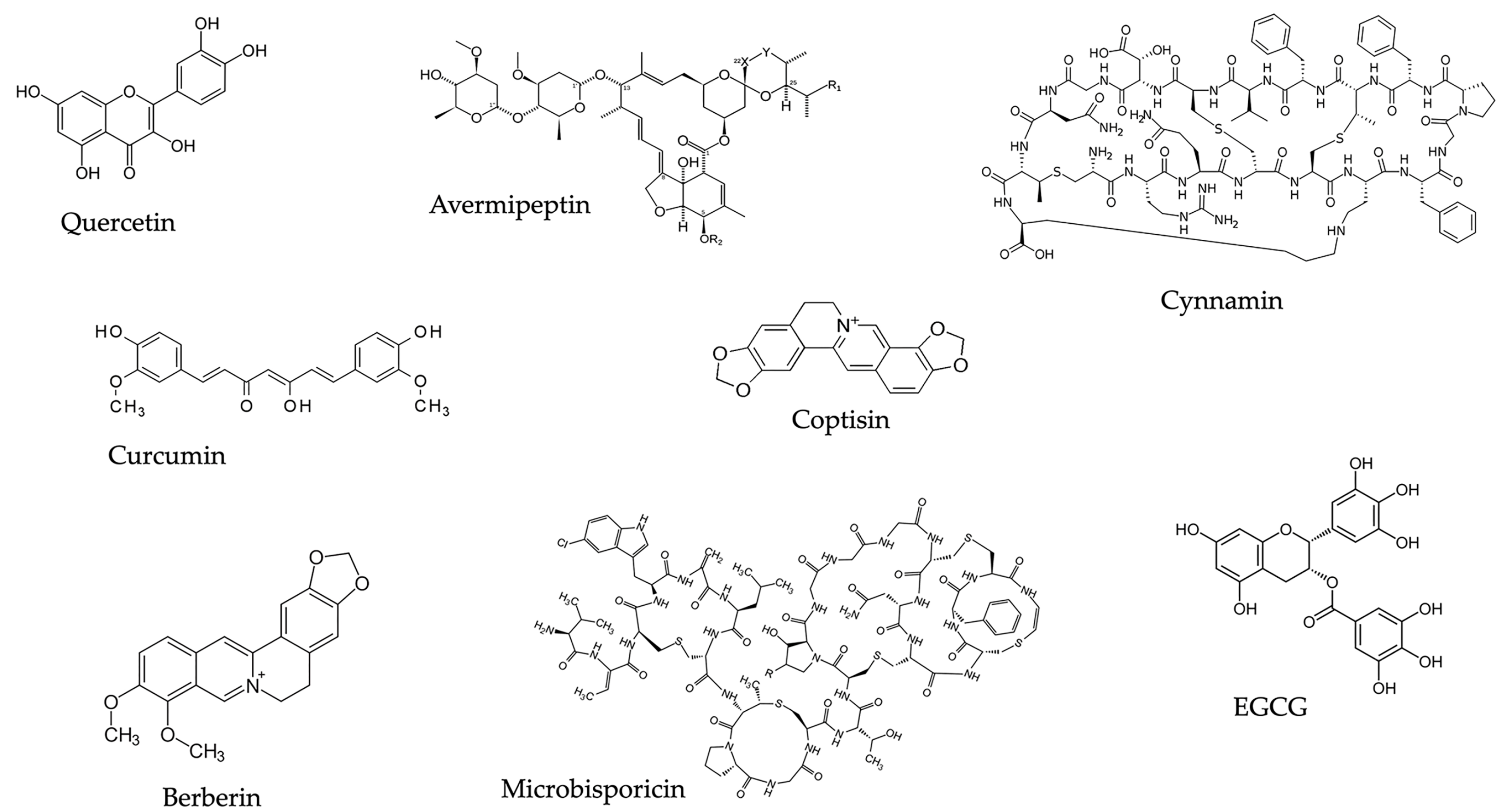
| Class | Molecule | Biological Action |
|---|---|---|
| Polyphenols | Quercetin | Inhibition of quorum-sensing genes, pyogenic proteases, pyocyanin, sialic acid expression, biofilm formation; synergism with antibiotics |
| Curcumin | Inhibition of biofilm formation; synergism with antibiotics, berberine, EGCG | |
| Catechins | Inhibition of the NorA efflux pump | |
| Essential Oils | Modification of cell membrane structure; interference with enzymes, proteins functions, and fatty acid metabolism; synergism with antibiotics | |
| Alkaloids | Berberine | Inhibition of biofilm formation; synergism with antibiotics and other natural products |
| Lanthypeptides | Class I (Microbisporin) | Pore formation and increased permeability of bacterial cell membrane |
| Class II (Cinnamycin) | Binding to the phosphatidyl ethanolamine receptor, a major lipid component of Gram-positive bacteria | |
| Class III (Avermipeptin B) | Antibacterial activity against S. aureus |
| Class | Molecule | Antiviral Activity |
|---|---|---|
| Polyphenols | Curcumin | X HDAC1, HDAC3, HDAC8, and histone acetyl transferase p300 inhibition; HBsAg and acetylation level of cccDNA-bound histones H3 and H4 inhibition; HBV X gene A p53-mediated pathway transcription inhibition; HPV-18 genes (interfering with the binding activity of activator- protein 1) transcription inhibition; enhancement of the Ac-histone H4 protein; anti-inflammatory activity in patients with COVID-19 |
| Polyphenols | EGCG | CD81 decreased expression, miR-122 inhibition, and suppression of liver HCV replication; anti-HCV activity through increased miR-548m expression |
| Polyphenols | Quercetin | Inhibition of miR-146a Abd reduced the replication of HIV; modulation of DNA methylation, histone acetylation, and SIRT1 activation |
| Polyphenols | Resveratrol | TIR domain containing adaptor molecule signaling pathway inhibition, with the induction of M2 receptor expression and decreased RSV replication; regulation of TLR3 expression, SIRT1 activation, and TNF-α release upregulation, with HBV infection inhibition |
| Terpenoids | Stelleralide A | Inhibition of HIV |
| Terpenoids | Valeransin E | Inhibition of viral HA |
| Alkaloids | Berberine | Inhibition of early gene expression; berberine-mediated inhibition of SARS-CoV-2 spike binding histone to ACE2 host cell receptors |
| Alkaloids | Coptisine | Coptisine-mediated inhibition of SARS-CoV-2 main proteases; berberine-mediated interaction with IAV neuraminidase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrigoni, R.; Ballini, A.; Jirillo, E.; Santacroce, L. Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics 2024, 13, 603. https://doi.org/10.3390/antibiotics13070603
Arrigoni R, Ballini A, Jirillo E, Santacroce L. Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics. 2024; 13(7):603. https://doi.org/10.3390/antibiotics13070603
Chicago/Turabian StyleArrigoni, Roberto, Andrea Ballini, Emilio Jirillo, and Luigi Santacroce. 2024. "Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects" Antibiotics 13, no. 7: 603. https://doi.org/10.3390/antibiotics13070603
APA StyleArrigoni, R., Ballini, A., Jirillo, E., & Santacroce, L. (2024). Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics, 13(7), 603. https://doi.org/10.3390/antibiotics13070603









