A New Convenient Method to Assess Antibiotic Resistance and Antimicrobial Efficacy against Pathogenic Clostridioides difficile Biofilms
Abstract
1. Introduction
2. Results and Discussion
2.1. Weight Loss Analysis
2.2. C. difficile 630∆erm Treatment with Vancomycin
2.2.1. Planktonic and Sessile Cell Enumeration
2.2.2. Confocal Laser Scanning Microscopy (CLSM) Biofilm Imaging
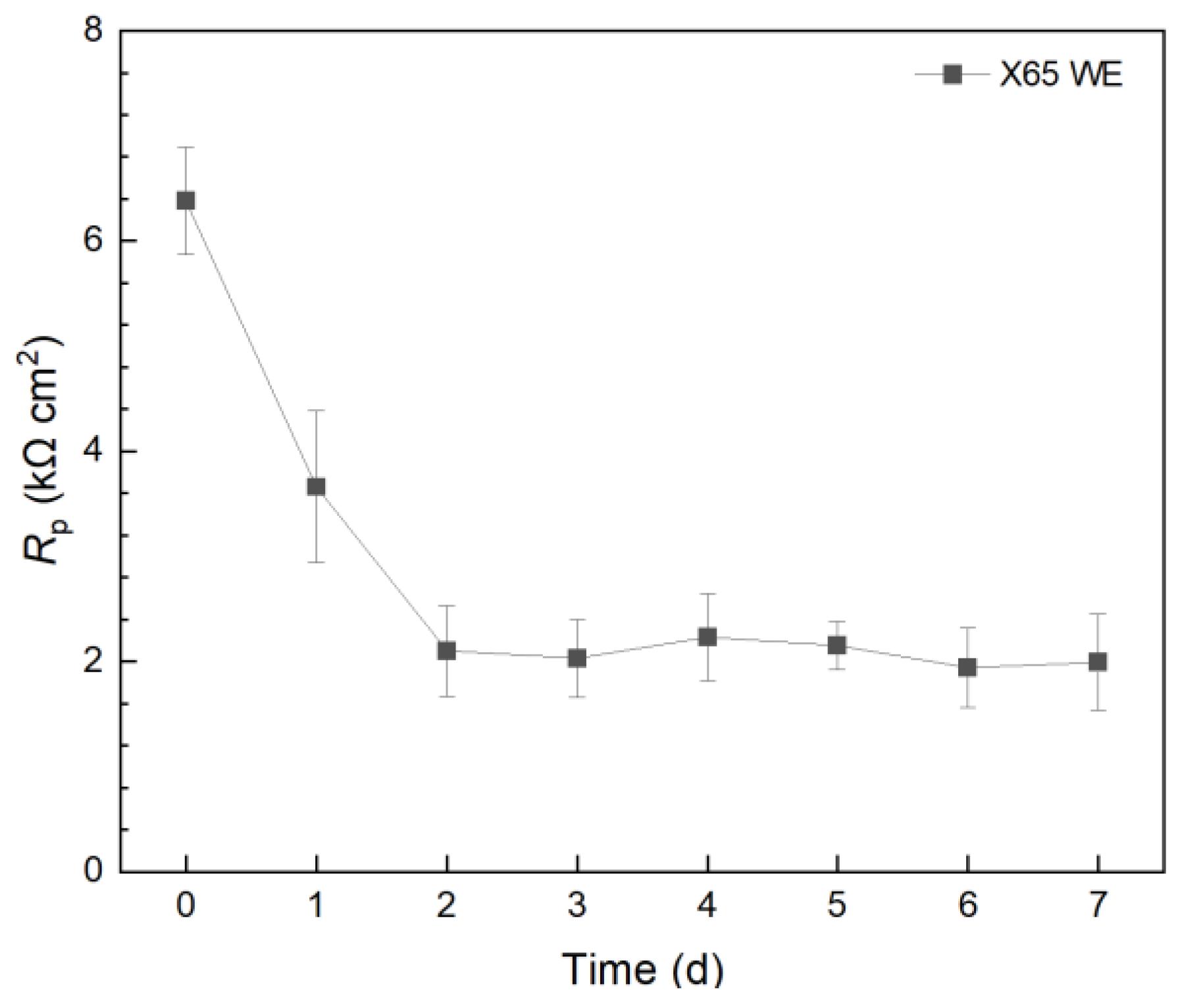
2.2.3. Electrochemical Biofilm Test Kit Measurements
2.3. Investigating C. difficile 630∆erm Adp-4 Antibiotic Resistance
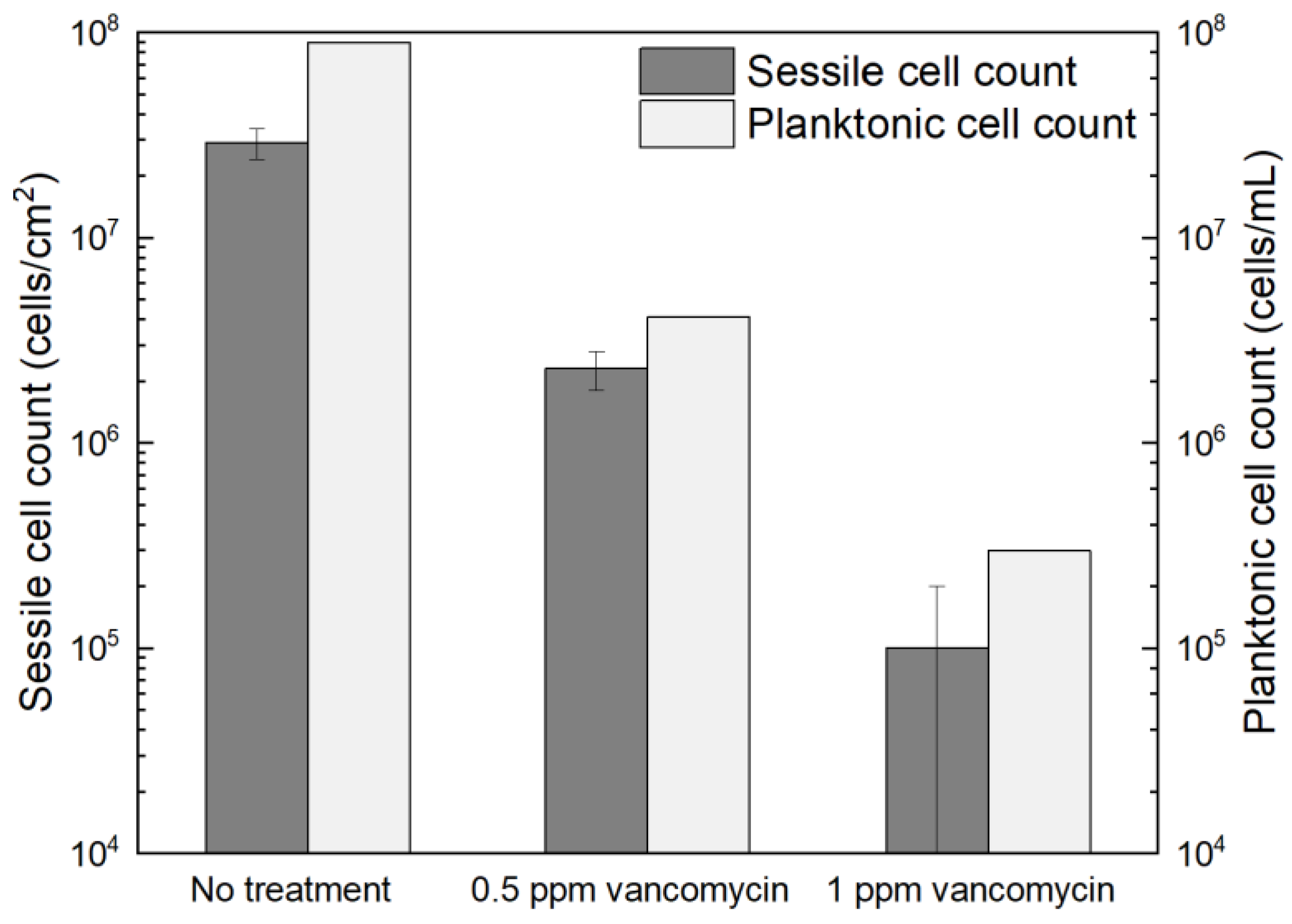
2.3.1. Planktonic and Sessile Cell Enumeration with Vancomycin Treatment
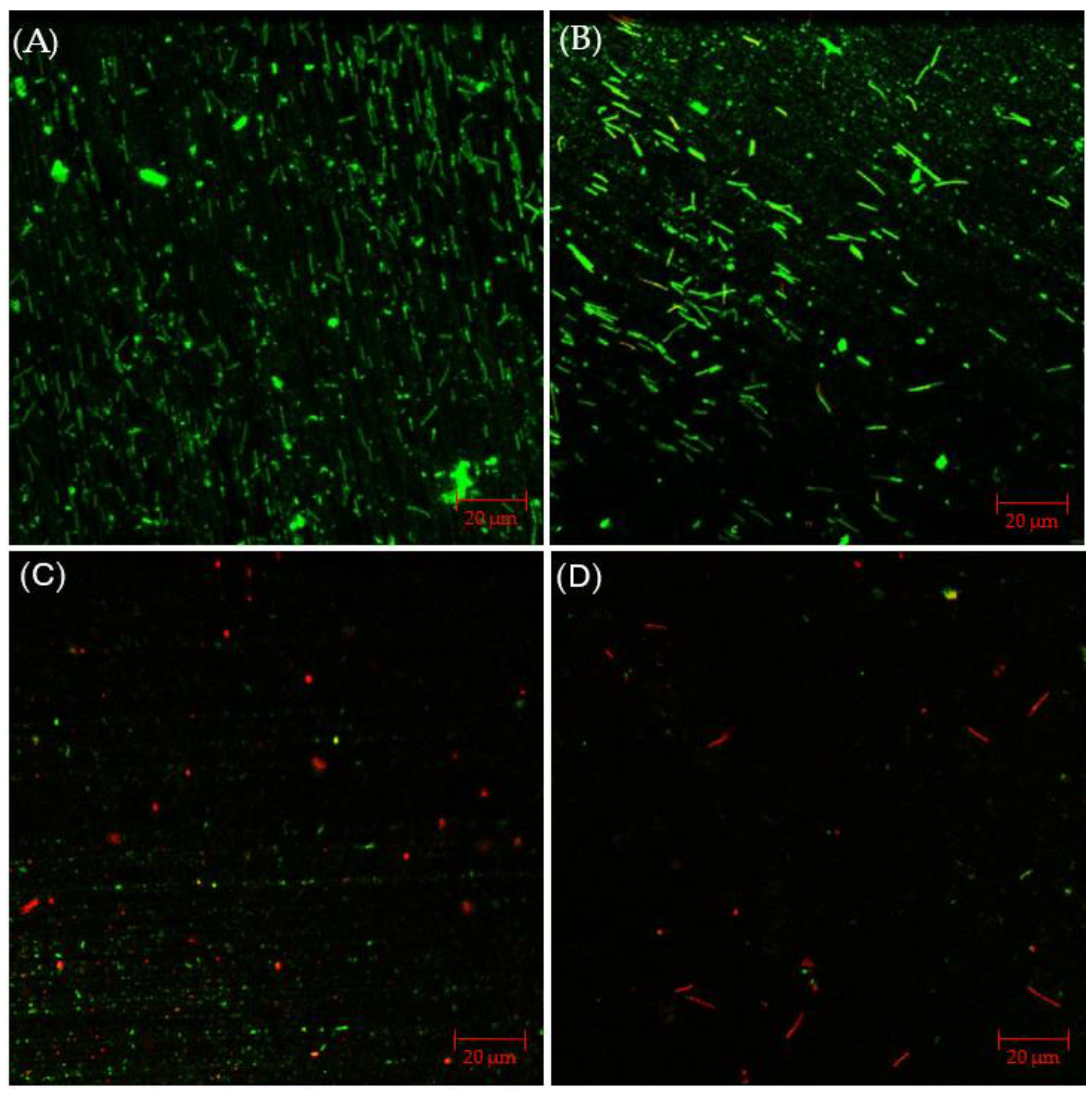
2.3.2. CLSM Biofilm Images with Vancomycin Treatment
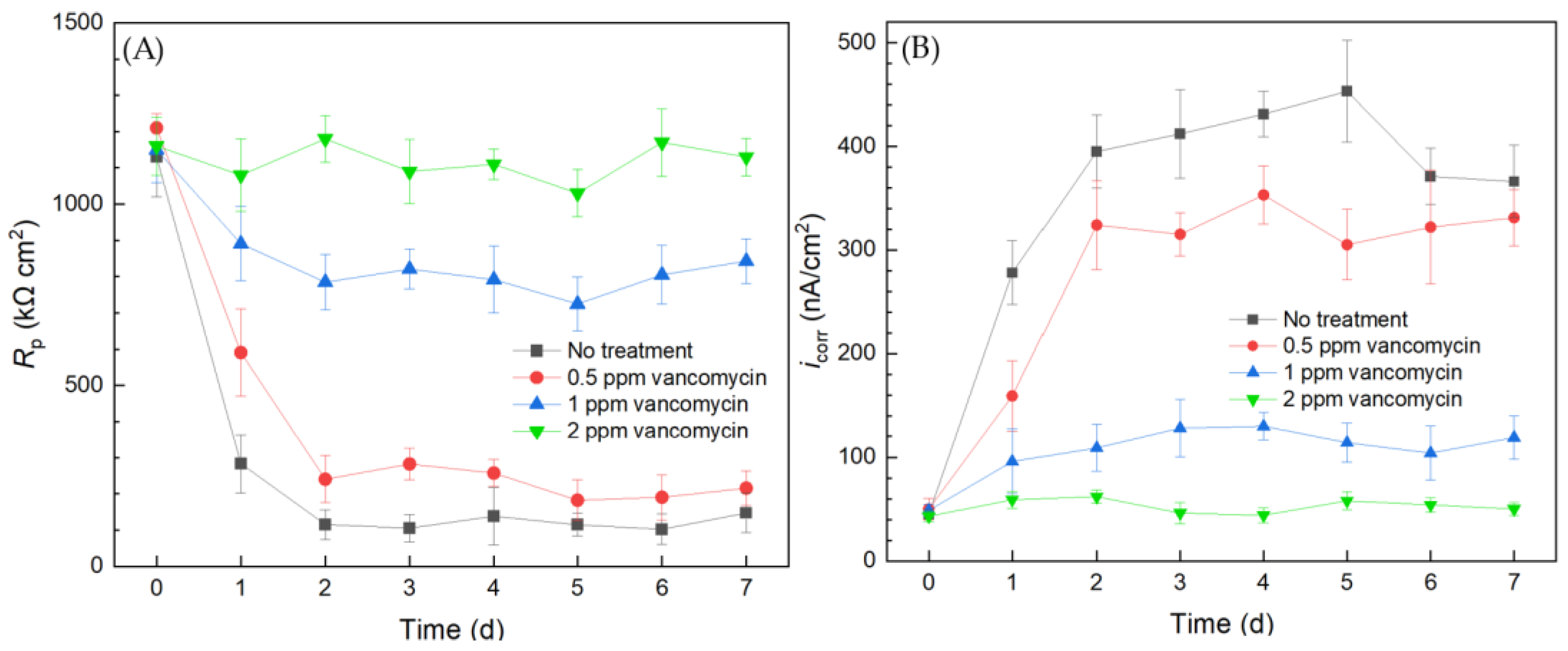
2.3.3. Antibiotic Resistance Investigation Using the Biofilm Test Kit
2.4. C. difficile 630∆erm with Disinfectant Treatment for Sanitation
2.4.1. Planktonic and Sessile Cell Enumerations with Disinfectant Treatment
2.4.2. CLSM Biofilm Images with Disinfectant Treatment
2.4.3. Assessing Biocide Prevention of C. difficile Biofilm Using a 10 mL Biofilm Test Kit
2.5. Injection Tests to Kill Pre-Established C. difficile 630∆erm Biofilm Using 10 mL Biofilm Test Kit
3. Materials and Methods
3.1. Metals, Bacteria, and Chemicals
3.2. Weight Loss Measurement
3.3. Planktonic and Sessile Cell Counts
3.4. CLSM Biofilm Observation
3.5. Electrochemical Tests in Biofilm Test Kit (10 mL Electrochemical Cell)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiersnowska, Z.M.; Lemiech-Mirowska, E.; Michałkiewicz, M.; Sierocka, A.; Marczak, M. Detection and Analysis of Clostridioides difficile Spores in a Hospital Environment. Int. J. Environ. Res. Public Health 2022, 19, 15670. [Google Scholar] [CrossRef] [PubMed]
- Moudgal, V.; Sobel, J.D. Clostridium difficile Colitis: A Review. Hosp. Pract. 2012, 40, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, S.; Klena, J.; Huang, H. Clinical Characteristics of Clostridium difficile Infection in Hospitalized Patients with Antibiotic-Associated Diarrhea in a University Hospital in China. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1773–1779. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grinspan, A.; Boutros, M. Organ-Preserving Strategies in the Management of Fulminant Clostridium difficile Colitis. In The SAGES Manual of Colorectal Surgery; Springer: Cham, Switzerland, 2019; pp. 563–576. [Google Scholar]
- Bhorgir, K.; Dhumal, K.; Palve, R. Clostridium difficile Infection: In Human Diagnosis and Management. Am. J. Pharm. Health Res. 2018, 6, 63–74. [Google Scholar] [CrossRef]
- Krouss, M.; Israilov, S.; Alaiev, D.; Tsega, S.; Talledo, J.; Chandra, K.; Zaurova, M.; Manchego, P.A.; Cho, H.J. SEE the DIFFerence: Reducing Unnecessary C. difficile Orders through Clinical Decision Support in a Large, Urban Safety-Net System. Am. J. Infect. Control 2023, 51, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Asempa, T.E.; Nicolau, D.P. Clostridium difficile Infection in the Elderly: An Update on Management. Clin. Interv. Aging 2017, 12, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- DePestel, D.D.; Aronoff, D.M. Epidemiology of Clostridium difficile Infection. J. Pharm. Pract. 2013, 26, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Garey, K.; Sethi, S.; Yadav, Y.; DuPont, H. Meta-Analysis to Assess Risk Factors for Recurrent Clostridium difficile Infection. J. Hosp. Infect. 2008, 70, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Balsells, E.; Shi, T.; Leese, C.; Lyell, I.; Burrows, J.; Wiuff, C.; Campbell, H.; Kyaw, M.H.; Nair, H. Global Burden of Clostridium difficile Infections: A Systematic Review and Meta-Analysis. J. Glob. Health 2019, 9, 010407. [Google Scholar] [CrossRef]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M. Polymer Alternative for CDI Treatment (PACT) investigators Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results from Two Multinational, Randomized, Controlled Trials. Clin. Infect. Dis. 2014, 59, 345–354. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Off. J. Am. Coll. Gastroenterol. ACG 2013, 108, 478–498. [Google Scholar] [CrossRef]
- Banawas, S.S. Clostridium difficile Infections: A Global Overview of Drug Sensitivity and Resistance Mechanisms. BioMed Res. Int. 2018, 2018, 8414257. [Google Scholar] [CrossRef] [PubMed]
- Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Renaissance of Vancomycin: Approaches for Breaking Antibiotic Resistance in Multidrug-Resistant Bacteria. Can. J. Microbiol. 2020, 66, 11–16. [Google Scholar] [CrossRef]
- Sjöberg, M.; Eriksson, M.; Andersson, J.; Norén, T. Transmission of Clostridium difficile Spores in Isolation Room Environments and through Hospital Beds. Apmis 2014, 122, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Barra-Carrasco, J.; Paredes-Sabja, D. Clostridium difficile Spores: A Major Threat to the Hospital Environment. Future Microbiol. 2014, 9, 475–486. [Google Scholar] [CrossRef]
- Lemiech-Mirowska, E.; Michałkiewicz, M.; Sierocka, A.; Gaszyńska, E.; Marczak, M. The Hospital Environment as a Potential Source for Clostridioides difficile Transmission Based on Spore Detection Surveys Conducted at Paediatric Oncology and Gastroenterology Units. Int. J. Environ. Res. Public Health 2023, 20, 1590. [Google Scholar] [CrossRef]
- Terveer, E.M.; Crobach, M.J.; Sanders, I.M.; Vos, M.C.; Verduin, C.M.; Kuijper, E.J. Detection of Clostridium difficile in Feces of Asymptomatic Patients Admitted to the Hospital. J. Clin. Microbiol. 2017, 55, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, A.; Sartini, M.; Battistella, A.; Casini, B.; Pinto, G.L.; Schinca, E.; Cristina, M.; Galliera, H.I.C.O.G. A Clostridium difficile Outbreak in an Italian Hospital: The Efficacy of the Multi-Disciplinary and Multifaceted Approach. J. Prev. Med. Hyg. 2018, 59, E132. [Google Scholar]
- Thanh, N.H.; Lan, D.T.N.; Ha, P.T.T.; An, V.T.T.; Khanh, C.C. High Performance Liquid Chromatography Analytical Method for Glutaraldehyde Determination in Disinfectants. Vietnam J. Food Control 2022, 5, 160–169. [Google Scholar]
- Rideout, K.; Teschke, K.; Dimich-Ward, H.; Kennedy, S. Considering Risks to Healthcare Workers from Glutaraldehyde Alternatives in High-Level Disinfection. J. Hosp. Infect. 2005, 59, 4–11. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, K.; Gu, T.; Raad, I. Chelators Enhanced Biocide Inhibition of Planktonic Sulfate-Reducing Bacterial Growth. World J. Microbiol. Biotechnol. 2010, 26, 1053–1057. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Phukoetphim, N.; Laopaiboon, P. Effect of Glutaraldehyde Biocide on Laboratory-Scale Rotating Biological Contactors and Biocide Efficacy. Electron. J. Biotechnol. 2006, 9, 358–369. [Google Scholar] [CrossRef]
- GLUTARALDEHYDE|Occupational Safety and Health Administration. Available online: https://www.osha.gov/chemicaldata/123 (accessed on 29 June 2024).
- Conlette, O. Impacts of Tetrakis-Hydroxymethyl Phosphonium Sulfate (THPS) Based Biocides on the Functional Group Activities of Some Oil Field Microorganisms Associated with Corrosion and Souring. Br. Microbiol. Res. J. 2014, 4, 1463–1475. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Gu, T. d-Methionine as a Biofilm Dispersal Signaling Molecule Enhanced Tetrakis Hydroxymethyl Phosphonium Sulfate Mitigation of Desulfovibrio vulgaris Biofilm and Biocorrosion Pitting. Mater. Corros. 2014, 65, 837–845. [Google Scholar] [CrossRef]
- Marrs, T.C.; Ballantyne, B. Pesticide Toxicology and International Regulation; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Xu, L.; Guan, F.; Ma, Y.; Zhang, R.; Zhang, Y.; Zhai, X.; Dong, X.; Wang, Y.; Duan, J.; Hou, B. Inadequate Dosing of THPS Treatment Increases Microbially Influenced Corrosion of Pipeline Steel by Inducing Biofilm Growth of Desulfovibrio hontreensis SY-21. Bioelectrochemistry 2022, 145, 108048. [Google Scholar] [CrossRef] [PubMed]
- Gaines, R.H. Bacterial Activity as a Corrosive Influence in the Soil. J. Ind. Eng. Chem. 1910, 2, 128–130. [Google Scholar] [CrossRef]
- Xu, D.; Gu, T.; Lovley, D.R. Microbially Mediated Metal Corrosion. Nat. Rev. Microbiol. 2023, 21, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Jurawan, I.; Frenzel, M.; Price, A.C. The Identification and Mechanism of a Scenedesmus Spp. Causing Bio-Fouling of an Oil Field Produced Water Treatment Plant. Int. Biodeterior. Biodegrad. 2016, 108, 207–213. [Google Scholar] [CrossRef]
- Ramírez, G.A.; Hoffman, C.L.; Lee, M.D.; Lesniewski, R.A.; Barco, R.A.; Garber, A.; Toner, B.M.; Wheat, C.G.; Edwards, K.J.; Orcutt, B.N. Assessing Marine Microbial Induced Corrosion at Santa Catalina Island, California. Front. Microbiol. 2016, 7, 1679. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, P.; Yuan, Z.; Guo, J. Aerobic Condition Enhances Bacteriostatic Effects of Silver Nanoparticles in Aquatic Environment: An Antimicrobial Study on Pseudomonas aeruginosa. Sci. Rep. 2017, 7, 7398. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Yawata, Y.; Toyofuku, M.; Uchiyama, H.; Nomura, N. Interspecies Interaction between Pseudomonas aeruginosa and Other Microorganisms. Microbes Environ. 2013, 28, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa Infection: Lessons from a Versatile Opportunist. Microbes Infect. 2000, 2, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Choong, A.M.; Pehkonen, S.O. The Influence of the Marine Aerobic Pseudomonas Strain on the Corrosion of 70/30 Cu–Ni Alloy. Corros. Sci. 2007, 49, 4352–4385. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Microbially Influenced Corrosion of Steels by Pseudomonas aeruginosa. Corros. Rev. 2014, 32, 129–141. [Google Scholar] [CrossRef]
- Xu, L.; Ivanova, S.A.; Gu, T. Mitigation of Galvanized Steel Biocorrosion by Pseudomonas aeruginosa Biofilm Using a Biocide Enhanced by Trehalase. Bioelectrochemistry 2023, 154, 108508. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Pu, Y.; Chen, S.; Lv, G.; Wang, W.; Li, W. Mitigation Effects of Ammonium on Microbiologically Influenced Corrosion of 90/10 Copper-Nickel Alloy Caused by Pseudomonas aeruginosa. Int. Biodeterior. Biodegrad. 2024, 189, 105762. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Mitigation of a Nitrate Reducing Pseudomonas aeruginosa Biofilm and Anaerobic Biocorrosion Using Ciprofloxacin Enhanced by D-Tyrosine. Sci. Rep. 2017, 7, 6946. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, R.; Zhou, E.; Zhao, Y.; Dou, W.; Xu, D.; Yang, K.; Gu, T. Antimicrobial Cu-Bearing 2205 Duplex Stainless Steel against MIC by Nitrate Reducing Pseudomonas aeruginosa Biofilm. Int. Biodeterior. Biodegrad. 2018, 132, 132–138. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Xu, J.; Xu, D.; Gu, T. Microbiologically Influenced Corrosion of C1018 Carbon Steel by Nitrate Reducing Pseudomonas aeruginosa Biofilm under Organic Carbon Starvation. Corros. Sci. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Jabbar, K.A.; Mackey, H.R.; Mahmoud, K.A. Recent Advancements of Nanomaterials as Coatings and Biocides for the Inhibition of Sulfate Reducing Bacteria Induced Corrosion. Curr. Opin. Chem. Eng. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Silva, P.; Oliveira, S.H.; Vinhas, G.M.; Carvalho, L.J.; Barauna, O.S.; Urtiga Filho, S.L.; Lima, M.A.G. Tetrakis Hydroxymethyl Phosphonium Sulfate (THPS) with Biopolymer as Strategy for the Control of Microbiologically Influenced Corrosion in a Dynamic System. Chem. Eng. Process.-Process Intensif. 2021, 160, 108272. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Al-Mahamedh, H.H.; Gu, T. Electrochemical Testing of Biocide Enhancement by a Mixture of d-Amino Acids for the Prevention of a Corrosive Biofilm Consortium on Carbon Steel. Ind. Eng. Chem. Res. 2017, 56, 7640–7649. [Google Scholar] [CrossRef]
- Gómez, N.C.; Abriouel, H.; Grande, M.J.; Pulido, R.P.; Gálvez, A. Combined Treatments of Enterocin AS-48 with Biocides to Improve the Inactivation of Methicillin-Sensitive and Methicillin-Resistant Staphylococcus aureus Planktonic and Sessile Cells. Int. J. Food Microbiol. 2013, 163, 96–100. [Google Scholar] [CrossRef]
- Ibusquiza, P.S.; Herrera, J.; Cabo, M. Resistance to Benzalkonium Chloride, Peracetic Acid and Nisin during Formation of Mature Biofilms by Listeria monocytogenes. Food Microbiol. 2011, 28, 418–425. [Google Scholar] [CrossRef]
- Fajardo, S.; Garcia-Galvan, F.R.; Barranco, V.; Galvan, J.C.; Feliu, S., Jr. A critical review of the application of electrochemical techniques for studying corrosion of Mg and Mg-alloys; opportunities and challenges. In Magnesium Alloys—Selected Issue; IntechOpen: London, UK, 2018; pp. 694–738. [Google Scholar]
- Unsal, T.; Xu, L.; Jia, R.; Kijkla, P.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.; Saleh, M.A.; Gu, T. Microbiologically Influenced Corrosion of Titanium by Desulfovibrio vulgaris Biofilm under Organic Carbon Starvation. Bioelectrochemistry 2023, 149, 108307. [Google Scholar] [CrossRef]
- Dong, Y.; Lekbach, Y.; Li, Z.; Xu, D.; El Abed, S.; Koraichi, S.I.; Wang, F. Microbiologically Influenced Corrosion of 304L Stainless Steel Caused by an Alga Associated Bacterium Halomonas titanicae. J. Mater. Sci. Technol. 2020, 37, 200–206. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Yang, K.; Liu, H.; Cheng, Y.F. Corrosion of Antibacterial Cu-Bearing 316L Stainless Steels in the Presence of Sulfate Reducing Bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Sharma, M.; Liu, H.; Chen, S.; Cheng, F.; Voordouw, G.; Gieg, L. Effect of Selected Biocides on Microbiologically Influenced Corrosion Caused by Desulfovibrio ferrophilus IS5. Sci. Rep. 2018, 8, 16620. [Google Scholar] [CrossRef]
- Raman, V.; Tamilselvi, S.; Rajendran, N. Evaluation of Effective Biocides for SRB to Control Microbiologically Influenced Corrosion. Mater. Corros. 2008, 59, 329–334. [Google Scholar] [CrossRef]
- Pal, M.K.; Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J. Bio-Tribo-Corros. 2022, 8, 76. [Google Scholar] [CrossRef]
- Xu, L.; Khan, A.; Wang, S.; Kijkla, P.; Kumseranee, S.; Punpruk, S.; Gu, T. Preliminary Investigations of Microbiologically Influenced Corrosion of 304 Stainless Steel by Anaerobic Clostridioides difficile Biofilm. Int. Biodeterior. Biodegrad. 2024, 194, 105871. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Kijkla, P.; Kumseranee, S.; Punpruk, S.; El-Said Mohamed, M.; Saleh, M.A.; Gu, T. Comparison of 304 SS, 2205 SS, and 410 SS Corrosion by Sulfate-Reducing Desulfovibrio ferrophilus. J. Chem. 2021, 2021, 3268404. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Kampf, G.; Kampf, G. Glutaraldehyde. In Antiseptic Stewardship: Biocide Resistance and Clinical Implications; Springer: Cham, Switzerland, 2018; pp. 131–160. [Google Scholar]
- Liu, L.; Fu, Q.; Peng, C.; Wei, B.; Qin, Q.; Gao, L.; Bai, Y.; Xu, J.; Sun, C. Effect of Glutaraldehyde as a Biocide against the Microbiologically Influenced Corrosion of X80 Steel Pipeline. J. Pipeline Syst. Eng. Pract. 2022, 13, 04022014. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Mohamed, M.E.-S.; Saleh, M.A.; Gu, T. Mitigation of Sulfate Reducing Desulfovibrio ferrophilus Microbiologically Influenced Corrosion of X80 Using THPS Biocide Enhanced by Peptide A. J. Mater. Sci. Technol. 2022, 107, 43–51. [Google Scholar] [CrossRef]
- Jolibois, B.; Guerbet, M.; Vassal, S. Glutaraldehyde in Hospital Wastewater. Arch. Environ. Contam. Toxicol. 2002, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Yang, D.; Dou, W.; Liu, J.; Zlotkin, A.; Kumseranee, S.; Punpruk, S.; Li, X.; Gu, T. A Sea Anemone-Inspired Small Synthetic Peptide at Sub-Ppm Concentrations Enhanced Biofilm Mitigation. Int. Biodeterior. Biodegrad. 2019, 139, 78–85. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Al-Mahamedh, H.H.; Gu, T. Enhanced Biocide Treatments with D-Amino Acid Mixtures against a Biofilm Consortium from a Water Cooling Tower. Front. Microbiol. 2017, 8, 1538. [Google Scholar] [CrossRef]
- Astuti, D.; Purwasena, I.A.; Putri, F.Z. Potential of Biosurfactant as an Alternative Biocide to Control Biofilm Associated Biocorrosion. J. Environ. Sci. Technol. 2018, 11, 104–111. [Google Scholar] [CrossRef]
- Bardouniotis, E.; Ceri, H.; Olson, M.E. Biofilm Formation and Biocide Susceptibility Testing of Mycobacterium Fortuitum and Mycobacterium Marinum. Curr. Microbiol. 2003, 46, 0028–0032. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.A.; Mergulhão, F.; Melo, L.; Simões, M. The Ability of an Antimicrobial Agent to Penetrate a Biofilm Is Not Correlated with Its Killing or Removal Efficiency. Biofouling 2014, 30, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Russell, A. Glutaraldehyde: Current Status and Uses. Infect. Control Hosp. Epidemiol. 1994, 15, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Verran, J.; Whitehead, K. Factors Affecting Microbial Adhesion to Stainless Steel and Other Materials Used in Medical Devices. Int. J. Artif. Organs 2005, 28, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Schlisselberg, D.B.; Yaron, S. The Effects of Stainless Steel Finish on Salmonella Typhimurium Attachment, Biofilm Formation and Sensitivity to Chlorine. Food Microbiol. 2013, 35, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hong, W.; Dong, S.; Zhang, Z.-T.; Zhang, J.; Wang, L.; Wang, Y. Genome Engineering of Clostridium difficile Using the CRISPR-Cas9 System. Clin. Microbiol. Infect. 2018, 24, 1095–1099. [Google Scholar] [PubMed]
- ASTM G1-03; Standard Practice for Preparing, Cleaning and Evaluating Corrosion Test Specimens. ASTM international: West Conshohocken, PA, USA, 2011.
- Xu, L.; Kijkla, P.; Kumseranee, S.; Punpruk, S.; Gu, T. “Corrosion-Resistant” Chromium Steels for Oil and Gas Pipelines Can Suffer from Very Severe Pitting Corrosion by a Sulfate-Reducing Bacterium. J. Mater. Sci. Technol. 2024, 174, 23–29. [Google Scholar] [CrossRef]
- Wang, D.; Kijkla, P.; Saleh, M.A.; Kumseranee, S.; Punpruk, S.; Gu, T. Tafel Scan Schemes for Microbiologically Influenced Corrosion of Carbon Steel and Stainless Steel. J. Mater. Sci. Technol. 2022, 130, 193–197. [Google Scholar] [CrossRef]










| Metal | C | P | S | N | Si | Mn | Cr | Ni | Cu | Mo | V | Nb | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 304 SS | 0.063 | 0.031 | 0.002 | 0.045 | 0.312 | 1.42 | 18.3 | 8.12 | 0.316 | 0.297 | |||
| X65 | 0.16 | 0.02 | 0.01 | 0.45 | 1.65 | 0.09 | 0.05 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Gurung, B.; Gu, C.; Wang, S.; Gu, T. A New Convenient Method to Assess Antibiotic Resistance and Antimicrobial Efficacy against Pathogenic Clostridioides difficile Biofilms. Antibiotics 2024, 13, 728. https://doi.org/10.3390/antibiotics13080728
Xu L, Gurung B, Gu C, Wang S, Gu T. A New Convenient Method to Assess Antibiotic Resistance and Antimicrobial Efficacy against Pathogenic Clostridioides difficile Biofilms. Antibiotics. 2024; 13(8):728. https://doi.org/10.3390/antibiotics13080728
Chicago/Turabian StyleXu, Lingjun, Bijay Gurung, Chris Gu, Shaohua Wang, and Tingyue Gu. 2024. "A New Convenient Method to Assess Antibiotic Resistance and Antimicrobial Efficacy against Pathogenic Clostridioides difficile Biofilms" Antibiotics 13, no. 8: 728. https://doi.org/10.3390/antibiotics13080728
APA StyleXu, L., Gurung, B., Gu, C., Wang, S., & Gu, T. (2024). A New Convenient Method to Assess Antibiotic Resistance and Antimicrobial Efficacy against Pathogenic Clostridioides difficile Biofilms. Antibiotics, 13(8), 728. https://doi.org/10.3390/antibiotics13080728






