Abstract
Nowadays, infectious diseases of bacterial and viral origins represent a serious medical problem worldwide. In fact, the development of antibiotic resistance is responsible for the emergence of bacterial strains that are refractory even to new classes of antibiotics. Furthermore, the recent COVID-19 pandemic suggests that new viruses can emerge and spread all over the world. The increase in infectious diseases depends on multiple factors, including malnutrition, massive migration of population from developing to industrialized areas, and alteration of the human microbiota. Alternative treatments to conventional antibiotics and antiviral drugs have intensively been explored. In this regard, plants and marine organisms represent an immense source of products, such as polyphenols, alkaloids, lanthipeptides, and terpenoids, which possess antibacterial and antiviral activities. Their main mechanisms of action involve modifications of bacterial cell membranes, with the formation of pores, the release of cellular content, and the inhibition of bacterial adherence to host cells, as well as of the efflux pump. Natural antivirals can interfere with viral replication and spreading, protecting the host with the enhanced production of interferon. Of note, these antivirals are not free of side effects, and their administration to humans needs more research in terms of safety. Preclinical research with natural antibacterial and antiviral compounds confirms their effects against bacteria and viruses, but there are still only a few clinical trials. Therefore, their full exploitation and more intensive clinical studies represent the next steps to be pursued in this area of medicine.
1. Introduction
Bacterial and viral infections still represent threatening diseases, which cause the deaths of millions of individuals per year [1,2]. With special reference to bacterial infections, the discovery of antibiotics has saved millions of people, but the overdose and misuse of antibiotics has led to the emergence of so-called multi-drug-resistant (MDR) bacteria [3,4,5,6]. Consequently, antimicrobial resistance (AMR) has developed, with the transmission from different sources of bacteria resistant to antibiotics to the general population. Moreover, the frequent use of antibiotics for livestock has greatly contributed to AMR [7]. For instance, resistant animal-borne bacteria can infect humans through direct contact with animals, saliva, and feces or bacteria, which may derive from contaminated water and food and polluted air [8]. Consequently, the dissemination of MDR bacteria in hospitals and intensive care units is extremely frequent [9,10]. Furthermore, the non-rationale use of antibiotics in humans may lead to the alteration of the gut microbiota, which, under steady-state conditions, protects from bacteria and food-borne antigens, enhancing mucosal innate and adaptive immunities or producing bacteria-derived metabolites, such as short-chain fatty acids, secondary bile acids, and tryptophan-derived metabolites that confer protection to the host [11,12,13,14,15,16]. There is evidence that microbiota depletion by antibiotics reduces the production of REG IIIγ, with the defective killing of vancomycin-resistant enterococci (VRE) [17]. In summary, the impact of antibiotics on gut microbiota may lead to a predominance of intestinal pathogenic bacteria and loss of bacterial diversity and/or certain bacterial species, increasing the risk of new infections and/or recurrence [18]. For instance, tuberculosis is again spreading around, as MDR Mycobacterium tuberculosis strains have become resistant to antibiotics [19].
Viral infections continue to endanger human life and health, with deadly viruses periodically emerging, as in the case of the human immunodeficiency virus (HIV), Ebola virus, and, mostly recently, SARS-CoV-2 [1,2,20]. Apart from vaccine-mediated preventative measures, more and more antiviral drugs have been discovered, but their uses are limited because of different factors, such as cytotoxicity to host cells and drug resistance, which is related to a high mutation rate, high replication rate, large viral load, and the genetic barrier of viruses [21,22].
Nowadays, alternative treatments to antibiotics and antivirals have been explored, mostly employing natural sources of antimicrobials derived from plants and marine organisms. These sources contain many substances with antibacterial activity, thus potentially restoring the clinical use of antibiotics, increasing their effectiveness while avoiding antibiotic resistance (AR) [23,24]. Parallelly, antiviral compounds of vegetal and marine derivations have been shown to inhibit virus survival and reproduction by targeting enzymes necessary for the replication cycle [25]. In terms of quantities, natural products are often less available, and more research is needed to increase the extraction of bioactive compounds in larger amounts [26].
In the present review, various compounds of natural origin, such as polyphenols, alkaloids, lanthipeptides, and terpenoids, will be described in terms of their antibacterial and antiviral mechanisms of action. Despite a vast arsenal of natural antimicrobials, clinical studies are still scant, while preclinical research still needs to be pursued for a full exploitation of these compounds.
2. Natural Products with Antibacterial Activity
Antibiotics still represent the optimal therapeutic approach to combat bacterial infections. However, the phenomenon of bacterial resistance is increasing with the adaptation and survival of bacteria, despite the presence of antibiotics in the environment [26]. Multiple factors contribute to bacterial resistance to antibiotics, and, among them, inappropriate use and dosage (e.g., in viral infections), bacterial carriage, and high concentrations of antibiotics in the environment are the major ones [27]. Notably, the emerging resistance of bacteria to certain antibiotics, such as carbapenems, glycopeptides, and colistin, have been reported [28,29,30,31].
Different mechanisms responsible for bacterial resistance have been documented, i.e., the inhibition of some antibiotics’ (beta-lactam antibiotics and tetracyclines) penetration into bacterial cells; prevention of fluoroquinolones and tetracyclines from reaching target cells; modification of the antibiotic target site, as in the case of resistance towards beta-lactam antibiotics, fluoroquinolones, macrolides, and glycopeptides; and the production of special enzymes, which inactivate or modify antibiotics (e.g., resistance to beta-lactam antibiotics, aminoglycosides, and chloramphenicol) [32]. Moreover, there is evidence that point mutations and the recombination of genetic material may provoke bacterial resistance through the acquisition and incorporation of free DNA fragments into the genome, the introduction of resistance genes by a bacteriophage, and the transfer of plasmid-extra-chromosomal genetic material and the transposon fragment of DNA [33]. In view of the increasing occurrence of bacterial resistance, a priority list of antibiotic-resistant bacteria has been proposed [34]. Such a list includes Pseudomonas (P.) aeruginosa and Acinetobacter (A.) baumannii being carbapenem-resistant; Enterobacterales-producing beta-lactamases being resistant to carbapenems; Mycobacterium (M.) tuberculosis complex being rifampicin-resistant; and Neisseria gonorrhoeae being resistant to all antibiotics.
On these grounds, putative alternatives to antibiotics are represented by natural products, which may exert antibacterial activity, restoring the clinical efficacy of classical antibiotics. Plant-derived substances can exert antibacterial activity through multiple mechanisms, such as the alteration of membrane function and structure and blockade of DNA/RNA synthesis and function, as well as interference with cell communication [35]. Furthermore, certain plant-derived compounds can inhibit the production of bacterial toxins. This is the case of essential oils of clove, thyme, cinnamon, and eugenol, which abrogate the production of listeriolysin by Listeria monocytogenes, as well as carvacrol, which hampers the production of Bacillus cereus and Clostridioides difficile toxins [36,37]. Furthermore, certain plant-derived compounds, such as berberine, gallic acid, and capsaicin, can inhibit efflux pumps of bacteria [38]. Figure 1 shows some representative natural products endowed with antimicrobial activity that will be described in the next paragraphs.

Figure 1.
Chemical structures of some natural molecules with antimicrobial activity.
Polyphenols like quercetin, curcumin, epigallocatechin gallate (EGCG), lanthypeptides (microbisporicin, cynnamin, and avermipeptin), and alkaloids (berberine and coptisin) are representative of natural products with antimicrobial activity.
2.1. Polyphenols
Polyphenols (flavonoids, and non-flavonoids) are largely contained in leaves, seeds, and fruits [39,40]. In general terms, polyphenols are endowed with anti-inflammatory activity, inhibiting the activation of NF-kβ and the release of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α [41]. Moreover, polyphenols in vitro and in vivo expand the T regulatory cell subset with the release of the anti-inflammatory cytokine, IL-10 [41,42]. Quite importantly, polyphenols in vitro hamper the binding of bacterial lipopolysaccharides to toll-like receptor 4 on monocytes, thus interrupting the release of pro-inflammatory cytokines [43]. All together, these activities may alleviate inflammation in infectious diseases of bacterial origin. As far as polyphenol antibacterial activity is concerned, they negatively interact with hydroxyl groups of bacterial cell membranes, thus damaging membrane phospholipids and proteins with expanded membrane permeability and leakage of cell content [41]. Flavonoids, when transformed into pro-oxidants, and phenoxyl radicals can inhibit pathogenic bacteria, causing their lysis [42]. In addition, it has been reported that flavonoids can destabilize cell membranes and cell walls, interfering with bacterial cell attachment [44]. In the next paragraphs, some representative polyphenols are discussed.
Quercetin is a flavonol present in fruits, grain products, and leafy vegetables [45]. It inhibits virulence factors, such as pyogenic proteases and pyocyanin, as well as sialic acid expression, with a decrease in the appearance of quorum-sensing genes [46,47,48]. Furthermore, quercetin has been shown to synergize with antibiotic membrane activity when combined with nanoparticles [46]. There is evidence that quercetin strongly inhibits quorum sensing, biofilm formation, and virulence factors in P. aeruginosa and Staphylococcus aureus (S. aureus) [49]. Synergistic effects have been documented using more than one flavonoid, i.e., quercetin, rutin, and more with some antibiotics, including cefradine, imipenem, ceftriaxone, and methicillin [50]. On the other hand, isoquercetin, a glycosylated flavonoid derived from quercetin, exhibits in subinhibitory concentration (MIC/8) an antagonistic effect in combination with kanamycin, amikacin, neomycin, and gentamycin, in view of mutual chelation [51]. Quercetin, only at a higher MIC, manifests antagonism with antibiotics when applied to the multi-resistant strain of E. coli. MICs/MBCs are 50 μg/mL for S. aureus, 16–256 μg/mL for S. mutans, and 16 μg/mL for MRSA [52,53,54]. Apart from antimicrobial activity, quercetin also exerts a potent anti-inflammatory function, decreasing lipopolysaccharide-mediated effects in P. gingivalis-treated human gingival fibroblasts [50]. This effect is attained by the suppression of the NF-kβ pathway and pro-inflammatory cytokine release.
Curcumin, a polyphenolic compound, is a product derived from the stem of curcuma, with a broad spectrum of antibacterial activities against both Gram-negative and Gram-positive bacteria [55,56]. Quite interestingly, curcumin possesses synergistic or additive antibacterial activity in combination with a series of antibiotics, such as polymyxin B, tetracycline, ciprofloxacin, colistin, and other natural adjuvants, i.e., berberine and epigallocatechin gallate (EGCG) [53,54]. Furthermore, curcumin has been demonstrated to inhibit biofilm formation, exerting antimicrobial effects against P. gingivalis [55,56]. At the same time, curcumin exhibits anti-inflammatory activity, reducing levels of interleukin (IL)-1β and tumor necrosis factor (TNF)-alpha while increasing the release of the anti-inflammatory cytokine, IL-10 [57].
Catechins (a species of flavan-3-ols) are the main polyphenols of green tea and encompass epicatechin, epigallocatechin (EGC), and EGCG, with EGC and EGCG exerting the most prominent antibacterial activities [58]. Among mechanisms of action, catechins reduce methicillin-resistant Staphylococcus aureus (S. aureus), inhibiting the N02A efflux pump [59].
Apart from single polyphenols, there exists a series of natural extracts enriched in polyphenols. They include wines and winery by-products (grape pomace, leaves, seeds, and skins), with quercetin, resveratrol, caffeic acid, and gallic acid as main compounds [60]. It has been reported that these extracts are active against a broad spectrum of bacteria, even including Escherichia coli, Salmonella enterica, S. aureus, Helicobacter (H.) pylori, Klebsiella (K.) pneumoniae, and oral pathogenic bacteria [61]. Antibacterial activity depends on the capacity of these compounds to form pores in the bacterial cell wall, as well as to inactivate microbial adhesion [62]. Also, olive oil by-products contain bioactive compounds, which can contribute to human health [63]. In this respect, olive mill wastewater (OMWW), produced during olive oil extraction, contains hydroxytyrosol as the main polyphenol, with lower amounts of verbascoside and oleuropein [64]. OMWW is active against both Gram-negative and Gram-positive bacteria, which are multi-resistant to antibiotics [65,66]. Of note, OMWW is endowed with anti-inflammatory activity, and its dietary supplementation prevents cell death and oxidative damage in rabbits [67].
Elderberry extracts (Sambucus nigra L.) are enriched in phenols, flavonoids, and anthocyanins [68]. Aqueous extracts of the elderflowers are active against S. aureus and S. epidermis, while ethanolic extracts in vitro exhibit antimicrobial activity against S. aureus and Bacillus cereus [69,70]. In addition, elderflower extracts are more active against Gram-negative and Gram-positive bacteria, in comparison to fruit extracts, which, instead, are more effective against respiratory infections bacteria [71,72].
Walnuts (Juglans regia L.) contain flavonoids and anthocyanins, which account for their antibacterial activities [68]. E. coli, P. aeruginosa, H. pylori, and S. aureus represent the major targets of walnuts [73,74]. The antibacterial activity of dried walnuts is enhanced by silver nanoparticles [75]. Honey is a natural supersaturated sugar solution produced by honeybees and is highly enriched in flavonoids and phenolic acids [76]. It exhibits antibacterial activity against both Gram-negative and Gram-positive bacteria, extended-spectrum beta-lactamase-producing E. coli, ciprofloxacin-resistant P. aeruginosa, and vancomycin-resistant Enterococcus (VRE) [77]. Manuka honey, for its contents of methylglyoxal and polyphenols, has been shown to prevent biofilm growth [78]. In this framework, it is worth mentioning propolis, a resin-like material made by bees, rich in polyphenols, phenols, and steroids [79]. There is evidence that flavonoids and cinnamic acid derivatives inhibit bacterial development and adhesion [80].
2.2. Essential Oils
Essential oils (EOs) contain volatile and aromatic compounds, as well as phenols in smaller amounts. EOs can modify the structure of bacterial cell membranes, interfering with enzyme and protein functions and with fatty acid metabolism [81]. Syzygium (S.) aromaticum, known as clove, belongs to the Myrtaceae family and contains eugenol, a phenyl propanoid, the most bioactive molecule [82]. Eugenol from S. aromaticum is active against both Gram-negative, and Gram-positive bacteria while synergizing with the antibiotic colistin against two resistant strains, namely A. baumannii and K. pneumoniae [83,84]. The combination S. aromaticum EOs/eugenol is very active against P. gingivalis, killing bacteria after 4 h of treatment and inhibiting the initial step of biofilm formation, while its effect on the pre-existing biofilm is negligible [85].
2.3. Alkaloids
Among alkaloids, berberine is a plant metabolite contained in leaves, stems, twigs, barks, rhizomes, and roots of many medicinal plants. It belongs to the group of isoquinoline alkaloids, and is used to synthesize several bioactive molecules [86]. Among different biological activities exerted by berberine, its antimicrobial properties have intensively been studied [87]. Berberine nanoparticles (BRBNPs) have been demonstrated to be very effective in in vitro assays against both Gram-negative, and Gram-positive bacteria. In addition, BRBNPs, when complexed with EGC, were very effective against MRSA in an in vivo murine model [88]. The above complex can affect the ability of MRSA to create a biofilm, inhibiting agrA-D gene expression [89]. In this respect, nanoparticles based on the combination of berberine with cinnamomic acid can more easily penetrate MDR bacteria, thus decreasing biofilm formation [80]. In the same direction, fusic acid, curcumin, and thymol, respectively, when combined with berberine, synergizes in the inhibition of S. aureus biofilm formation [90,91,92,93].
Berberine has been shown to be very active against K. pneumoniae strains, synergizing with certain antibiotics, i.e., norfloxacin, ciprofloxacin, and doxycycline [94]. Moreover, berberine can restore susceptibility to antibiotics (tigecycline, meropenem, ciprofloxacin, and sulbactam) against multi-drug-resistant A. baumannii [95]. Berberine can destabilize the bacterial cell membrane, intercalating and cleaving the bacterial DNA [96]. Moreover, the combination of berberine/thioridazine/ciprofloxacin can reduce the adeABC efflux pump in MDR A. baumannii [97]. As far as E. coli is concerned, berberine aqueous extract is able to synergize with the antibiotics colistin, tigecycline, and amoxicillin-clavulanate against carbapenem-resistant E. coli infections [98]. Also, berberine is an effective antimicrobial against enterotoxigenic and enteropathogenic E. coli strains in infected animals [99]. In vitro and in silico studies have demonstrated the efficacy of berberine as a potential efflux pump inhibitor against MdfA from E. coli [100].
Regarding P. aeruginosa, there is evidence that berberine synergizes with different antibiotics, such as amikacin, azithromycin, and tobramycin, against aminoglycoside-resistant P. aeruginosa strains [101,102,103]. It has been reported that berberine can act through blockage of the MexXY-OprM efflux pump, reducing biofilm formation [104,105].
2.4. Lanthipeptides
Lanthipeptides are microbial bioactive compounds mostly derived from Actinobacteria [106]. The mechanism of action of lanthipeptides is based on their ability to bind to lipid II, a highly conserved peptidoglycan structure in bacterial cytoplasmic membranes [107]. Binding to lipid II leads to pore formation in the Gram-positive bacterial cell membrane, with the release of cellular content [108].
Microbisporicin is a class I lanthipeptide produced by Microbispora sp. [109]. Microsporicin is active against a broad spectrum of bacteria, including MRSA, VRE, and penicillin-resistant S. penumoniae, as well as Nisseria meningitidis, Moraxella catarrhalis, and Haemophilus influenzae [110,111]. Microbisporicin synergistically acts in combination with the antibiotic polymyxin against Gram-negative bacteria and in murine infection models induced by drug-resistant Gram-positive bacteria. The microbicidal activity of microbisporicin is determined by an increased net charge from halogenation in the lanthipeptide structure, which leads to increased cellular permeability [112]. Microbisporicin is at preclinical stages, however, and further studies are required for its application to infections caused by multi-resistant pathogens [113].
Class II lanthipeptides encompass the cinnamycin group (duramycin, cinnamycin, mathermycin, and kyamycin) [114]. They act by binding to the phosphatidylethanolamine receptor, a major lipid component of the cellular membrane of Gram-positive bacteria [115]. Avermipeptin B belongs to class III lanthipeptides, and it is very active against S. aureus [116].
In Table 1, the main natural antibacterial products are depicted.

Table 1.
Plant-derived compounds and their antimicrobial activity.
3. Natural Product-Mediated Prevention of Biofilm Formation
Biofilm formation has been shown to play a major role in AR, and therefore, in the next paragraphs, some details on its structure and function will be provided. Biofilm formation encompasses four steps, namely, attachment, microcolony formation, maturation, and dispersion [117]. Extracellular polymeric substance (EPS) matrix formation allows bacterial adhesion, which facilitates the distribution of nutrients to resident cells [118]. EPS acts as a physical barrier, which impedes the penetration of antibiotics, with exopolysaccharides from P. aeruginosa binding to cationic antibiotics, such as aminoglycosides [119]. Quite interestingly, in the context of biofilms, there exist so-called persister cells, which are highly tolerant against antibiotics [120]. In a model of S. epidermis biofilm, the importance of persister cells in the development of tolerance to antibiotics has been documented [121].
In the previous sections, various natural products were described for their ability to prevent biofilm formation through different approaches [122]. Here, more emphasis will be placed on marine products for their capacity to target biofilms. Pontifactin, a lipopeptide produced by the marine bacterium Pontibacter korlensis, can inhibit the growth of various biofilm formations generated by some bacterial strains, e.g., B. subtilis, S. aureus, and Vibrio (V.) cholerae [123].
Lipopetides, because of their amphipathic nature, act as surfactants, reducing the adhesion properties and bacterial surface hydrophobicity [124]. Pumilacidin-like lipopetides, derived from the marine bacterium Bacillus sp. 176, inhibits the motility of the biofilm-forming pathogen V. alginolyticus 178 [125]. Such an effect is attained through the downregulation of the flagellar assembly genes flgp in V. alginolyticus 178, which are crucial for motility, flagellar stability, attachment, and colonization. Furthermore, an anthraquinone compound, emodin, isolated from the marine gorgonian coral Dichotella gemmacea-associated fungus Penicillium sp. SCSGAF 0023, is very effective against S. aureus biofilm formation [126]. Evidence has been provided that emodin can penetrate phospholipid bilayers, influencing van der Waals interactions, destabilizing membrane bilayers, and disrupting the fluidity of cell membranes [127]. Other marine peptides have been shown to target quorum sensing. This is the case of the cyclo (L-Trp-L-Pro) isolated from Rheinheimera aquinaris QS 102, DKP cyclo (L-Pro-L-Tyr) isolated from Penicillium chrysogenum DKY-1, nesfactin isolated from the marine sponge Fasciospongia cavernosa-associated bacterium Nesterenkonia sp. MSA31, and secalonic acid D, isolated from the marine fungus Penicillium sp. SCSGAF0023, respectively, which have been found to inhibit the quorum sensitivity system, preventing the formation of biofilms [128,129,130,131]. In Figure 2, the effects of natural antimicrobials on biofilm formation are summarized.
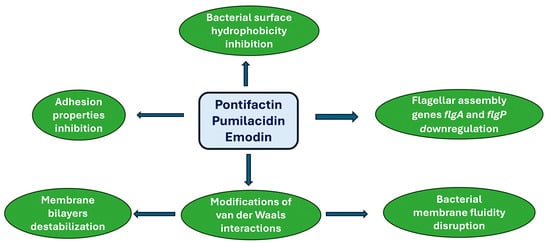
Figure 2.
Marine natural product-mediated prevention of biofilm formation.
The antimicrobial activities mediated by major lipopeptides pontifactin (class I, from Pontibacter korlensis), pumilacidin (class II, from Bacillus sp. 176), and emodin (class III, from Dichotella gemmacea) lanthipeptides are described.
5. Discussion
Bacterial and viral infections continue to pose a significant threat to human health worldwide. The ability of bacteria to adapt and develop new resistance mechanisms against antibiotics necessitates novel treatment approaches. Flavonoids, terpenes, alkaloids, and lanthipeptides derived from plant and marine sources have recently been explored as potential antibacterial agents. These natural compounds often demonstrate enhanced effectiveness when used in combination with conventional antibiotics, offering a promising strategy to combat antibiotic resistance. Various mechanisms of action are exerted by natural compounds, and among them, alteration of the bacterial membrane permeability with the formation of micropores and the release of cellular content, as well as the inhibition of the bacterial efflux pump, have been documented. Despite their potential, the clinical application of these natural products faces several challenges. The content of active metabolites in natural extracts is often low, making large-scale extraction difficult. Furthermore, the chemical structure of these compounds can be unstable, necessitating modifications that may alter their activity. More comprehensive studies are required to understand their mechanisms of action fully and to evaluate their safety and efficacy in humans.
Similar to their antibacterial counterparts, natural antiviral compounds have shown promise against a variety of viruses, including HBV, HCV, HIV, IAV, HSV, and SARS-CoV-2. Despite encouraging results obtained in in vitro and in animal models, there are some limitations for their use as antivirals in humans. For instance, the content of active metabolites is very low, and advancements in extraction and purification techniques are essential to obtain higher yields of active metabolites. Furthermore, the structure of natural products is unstable, and therefore, it is necessary to modify it, reconsidering the activity after modification has occurred. Finally, mechanisms of action of natural antivirals have not been clarified in a complete way, and comprehensive preclinical and clinical studies are needed to elucidate the mechanisms of action, optimal dosages, and potential side effects of these natural products.
Moreover, interdisciplinary research is crucial for developing novel formulations and delivery systems that maximize the therapeutic potential of natural compounds. Collaborative efforts can also facilitate the identification of new bioactive compounds from underexplored natural sources, expanding the arsenal of available antimicrobial agents.
Quite interestingly, some molecules (e.g., polyphenols) exhibit multiple biological activities in addition to their antimicrobial effects, such as antidiabetic or anticancer effects. Whether these activities may potentiate or negatively interfere with each other remains an open issue. For instance, polyphenols play an immunosuppressive role in the host, and this may be detrimental to patients with cancer.
6. Conclusions
In conclusion, natural compounds offer a vast and largely untapped resource for developing new antibacterial and antiviral therapies. While promising results have been obtained in preclinical studies, significant challenges remain in translating these findings into clinical practice. Addressing these challenges through innovative research and collaborative efforts will be key to unlocking the full potential of natural products in combating infectious diseases.
Author Contributions
Conceptualization, R.A. and E.J.; validation, A.B. and L.S.; data curation, A.B.; writing—original draft preparation, E.J.; writing—review and editing, R.A.; visualization, L.S.; supervision, A.B.; project administration, E.J.; funding acquisition, R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AMR | Antimicrobial resistance |
| BRBNPs | Berberine nanoparticles |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin gallate |
| EOs | Essential oils |
| EPS | Extracellular polymer substance |
| HADC | Histone deacetylase |
| HAT | Histone acetyl transferase |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HIV | Human immune deficiency virus |
| IAV | Influenza A virus |
| IL | Interleukin |
| MDR | Multi-drug-resistant |
| MSRA | Methicillin-resistant S. aureus |
| OMWW | Olive mill wastewater |
| RSV | Respiratory syncytial virus |
| TNF | Tumor necrosis factor |
| VRE | Vancomycin-resistant Enterococcus |
References
- Ryan, M.; Brindal, E.; Roberts, M.; Hickson, R.I. A behaviour and disease transmission model: Incorporating the Health Belief Model for human behaviour into a simple transmission model. J. R. Soc. Interface 2024, 21, 20240038. [Google Scholar] [CrossRef]
- de Lusignan, S.; Shi, T.; Fowler, T.; Andrews, N.; Todkill, D.; Gu, X.; Meza-Torres, B.; Robertson, C.; Sheikh, A. Sleeper frameworks for Pathogen X: Surveillance, risk stratification, and the effectiveness and safety of therapeutic interventions. Lancet Infect. Dis. 2024, 24, e417–e418. [Google Scholar] [CrossRef]
- Bottalico, L.; Charitos, I.A.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The war against bacteria, from the past to present and beyond. Expert Rev. Anti-Infective Ther. 2022, 20, 681–706. [Google Scholar] [CrossRef]
- Santacroce, L.; Spirito, F.; Bottalico, L.; Muzio, E.L.; Charitos, I.A.; Potenza, M.A.; Montagnani, M.; Jirillo, E. Current Issues and Perspectives in Antimicrobials use in Dental Practice. Curr. Pharm. Des. 2022, 28, 2879–2889. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 30 April 2024).
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Goldmann, D.A.; Weinstein, R.A.; Wenzel, R.P.; Tablan, O.C.; Duma, R.J.; Gaynes, R.P.; Schlosser, J.; Martone, W.J. Strategies to Prevent and Control the Emergence and Spread of Antimicrobial-Resistant Microorganisms in Hospitals. A challenge to hospital leadership. JAMA 1996, 275, 234–240. [Google Scholar] [CrossRef]
- Kollef, M.H.; Fraser, V.J. Antibiotic Resistance in the Intensive Care Unit: Strategies for Management. Ann. Intern. Med. 2001, 134, 298–314. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Leshem, A.; Liwinski, T.; Elinav, E. Immune-Microbiota Interplay and Colonization Resistance in Infection. Mol. Cell 2020, 78, 597–613. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.C.; Pandey, S.P.; Bender, M.J.; Meisel, M. Systemic Immunoregulatory Consequences of Gut Commensal Translocation. Trends Immunol. 2021, 42, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Di Domenico, M.; Montagnani, M.; Jirillo, E. Antibiotic Resistance and Microbiota Response. Curr. Pharm. Des. 2023, 29, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Brandl, K.; Plitas, G.; Mihu, C.N.; Ubeda, C.; Jia, T.; Fleisher, M.; Schnabl, B.; DeMatteo, R.P.; Pamer, E.G. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 2008, 455, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.B.; Buffie, C.G.; Carter, R.A.; Leiner, I.; Toussaint, N.C.; Miller, L.C.; Gobourne, A.; Ling, L.; Pamer, E.G. Loss of Microbiota-Mediated Colonization Resistance to Clostridium difficile Infection with Oral Vancomycin Compared with Metronidazole. J. Infect. Dis. 2015, 212, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Edlund, C.; Nord, C.E. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 2001, 1, 101–114. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Foxman, E.F.; Watkins, T.A.; Lipsitch, M. Considerations for viral co-infection studies in human populations. mBio 2024, e0065824. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Devincenzo, J.P.; Toovey, S.; Wu, J.Z.; Whitley, R.J. Comparison of antiviral resistance across acute and chronic viral infections. Antivir. Res. 2018, 158, 103–112. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Palese, L.L. The Dynamics of OXA-23 β-Lactamase from Acinetobacter baumannii. Int. J. Mol. Sci. 2023, 24, 17527. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Pacyga, K.; Pacyga, P.; Topola, E.; Viscardi, S.; Duda-Madej, A. Bioactive Compounds from Plant Origin as Natural Antimicrobial Agents for the Treatment of Wound Infections. Int. J. Mol. Sci. 2024, 25, 2100. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Damiani, E.; Scarabelli, S.; Principi, F.; Gioacchini, A.M.; Rocchi, M.B.L. EGCG and Its Antiviral Effects. Nutrients 2023, 15, 781. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Wang, Y.-W.; Yang, J.; Tong, Z.-J.; Wu, J.-Z.; Wang, Y.-B.; Wang, Q.-X.; Li, Q.-Q.; Yu, Y.-C.; Leng, X.-J.; et al. Natural Products as Potential Lead Compounds to Develop New Antiviral Drugs Over the Past Decade. Eur. J. Med. Chem. 2023, 260, 115726. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Bobate, S.; Mahalle, S.; Dafale, N.A.; Bajaj, A. Emergence of environmental antibiotic resistance: Mechanism, monitoring and management. Environ. Adv. 2023, 13, 100409. [Google Scholar] [CrossRef]
- Lepe, J.A.; Martínez-Martínez, L. Resistance mechanisms in Gram-negative bacteria. Med. Intensiv. 2022, 46, 392–402. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295, Erratum in Nat. Rev. Microbiol. 2024, 22, 255. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Kresken, M.; Klare, I.; Wichelhaus, T.A.; Wohlfarth, E.; Layer-Nicolaou, F.; Neumann, B.; Werner, G.; Study Group ‘Antimicrobial Re-sistance’ of the Paul-Ehrlich-Society for Chemotherapy. Glycopeptide resistance in Enterococcus spp. and coagulase-negative staphylococci from hospitalised patients in Germany: Occurrence, characteristics and dalbavancin susceptibility. J. Glob. Antimicrob. Resist. 2022, 28, 102–107. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Baig, M.I.R.; Kadu, P.; Bawane, P.; Nakhate, K.T.; Yele, S.; Ojha, S.; Goyal, S.N. Mechanisms of emerging resistance associated with non-antibiotic antimicrobial agents: A state-of-the-art review. J. Antibiot. 2023, 76, 629–641. [Google Scholar] [CrossRef] [PubMed]
- WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Inhibition of listeriolysin O and phosphatidylcholine-specific production in Listeria monocytogenes by subinhibitory concentrations of plant essential oils. J. Med Microbiol. 2002, 51, 567–608. [Google Scholar] [CrossRef] [PubMed]
- Mooyottu, S.; Kollanoor-Johny, A.; Flock, G.; Bouillaut, L.; Upadhyay, A.; Sonenshein, A.L.; Venkitanarayanan, K. Carvacrol and trans-cinnamaldehyde reduce Clostridium difficile toxin production and cytotoxicity in vitro. Int. J. Mol. Sci. 2014, 15, 4415–4430. [Google Scholar] [CrossRef] [PubMed]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 2020, 10, 277–290. [Google Scholar] [CrossRef]
- Santacroce, L.; Topi, S.; Charitos, I.A.; Lovero, R.; Luperto, P.; Palmirotta, R.; Jirillo, E. Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products. Pathophysiology 2024, 31, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Perz, M.; Szymanowska, D.; Janeczko, T.; Kostrzewa-Susłow, E. Antimicrobial Properties of Flavonoid Derivatives with Bromine, Chlorine, and Nitro Group Obtained by Chemical Synthesis and Biotransformation Studies. Int. J. Mol. Sci. 2024, 25, 5540. [Google Scholar] [CrossRef] [PubMed]
- Meure, C.M.; Steer, B.; Porter, J. Interrelationships between Dietary Outcomes, Readmission Rates and Length of Stay in Hospitalised Oncology Patients: A Scoping Review. Nutrients 2023, 15, 400. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E.; Magrone, M.; Russo, M.A.; Romita, P.; Massari, F.; Foti, C. Red Grape Polyphenol Oral Administration Improves Immune Response in Women Affected by Nickel-Mediated Allergic Contact Dermatitis. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 374–384. [Google Scholar] [CrossRef]
- Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Quercetin potentiates meropenem activity among pathogenic carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Appl. Microbiol. 2019, 127, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-W.; Luo, H.-Z.; Jiang, H.; Jian, T.-K.; Chen, Z.-Q.; Jia, A.-Q. Hordenine: A Novel Quorum Sensing Inhibitor and Antibiofilm Agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018, 66, 1620–1628. [Google Scholar] [CrossRef]
- Wang, D.; Xie, K.; Zou, D.; Meng, M.; Xie, M. Inhibitory effects of silybin on the efflux pump of methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2018, 18, 827–833. [Google Scholar] [CrossRef]
- Malczak, I.; Gajda, A. Interactions of naturally occurring compounds with antimicrobials. J. Pharm. Anal. 2023, 13, 1452–1470. [Google Scholar] [CrossRef] [PubMed]
- Morais-Braga, M.; Souza, T.; Santos, K.; Guedes, G.; Andrade, J.; Tintino, S.; Sobral-Souza, C.; Costa, J.; Saraiva, A.; Coutinho, H. Phenolic compounds and interaction between aminoglycosides and natural products of Lygodium venustum SW against multiresistant bacteria. Chemotherapy 2012, 58, 337–340. [Google Scholar] [CrossRef]
- Wang, J.; Song, M.; Pan, J.; Shen, X.; Liu, W.; Zhang, X.; Li, H.; Deng, X. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J. Cell. Mol. Med. 2018, 22, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Xiong, G.; Ji, W.; Wang, F.; Zhang, F.; Xue, P.; Cheng, M.; Sun, Y.; Wang, X.; Zhang, T. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-κB Signaling Pathway. BioMed Res. Int. 2019, 2019, 6282635. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Gao, P.; Xie, X.; Li, D.; Yu, D.; Yu, M. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult. Sci. 2020, 99, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Sharahi, J.Y.; Ahovan, Z.A.; Maleki, D.T.; Rad, Z.R.; Rad, Z.R.; Goudarzi, M.; Shariati, A.; Bostanghadiri, N.; Abbasi, E.; Hashemi, A. In vitro antibacterial activity of curcumin-meropenem combination against extensively drug-resistant (XDR) bacteria isolated from burn wound infections. Avicenna J. Phytomed. 2020, 10, 3–10. [Google Scholar]
- Al-Dulaimi, M.M.K.; Mutalib, S.A.; Ghani, M.A.; Zaini, N.A.M.; Ariffin, A.A. Multiple Antibiotic Resistance (MAR), Plasmid Profiles, and DNA Polymorphisms among Vibrio vulnificus Isolates. Antibiotics 2019, 8, 68. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.S.; Sivasubramanian, A.; Nagarajan, S. Simultaneous inhibition of MarR by salicylate and efflux pumps by curcumin sensitizes colistin resistant clinical isolates of Enterobacteriaceae. Microb. Pathog. 2020, 148, 104445. [Google Scholar] [CrossRef] [PubMed]
- Izui, S.; Sekine, S.; Murai, H.; Takeuchi, H.; Amano, A. Inhibitory effects of curcumin against cytotoxicity of Porphyromonas gingivalis outer membrane vesicles. Arch. Oral Biol. 2021, 124, 105058. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Guo, X.; Du, Q.; Keshavarzi, M. Curcumin and its nano-formulations combined with exercise: From molecular mechanisms to clinic. Cell Biochem. Funct. 2024, 42, e4061. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. Biomed. Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Jayaraman, A.; Mahapatra, S.K.; Vellingiri, V. Anti-virulence properties of catechin-in-cyclodextrin-in-phospholipid liposome through down-regulation of gene expression in MRSA strains. Microb. Pathog. 2022, 167, 105585. [Google Scholar] [CrossRef]
- Magrone, T.; Panaro, M.A.; Jirillo, E.; Covelli, V. Molecular effects elicited in vitro by red wine on human healthy peripheral blood mononuclear cells: Potential therapeutical application of polyphenols to diet-related chronic diseases. Curr. Pharm. Des. 2008, 14, 2758–2766. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Xu, Y.; Zhang, J.; Sui, Z.; Corke, H. Antibacterial Activity and Multi-Targeting Mechanism of Dehydrocorydaline from Corydalis turtschaninovii Bess. Against Listeria monocytogenes. Front. Microbiol. 2022, 12, 799094. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From antiquity to contemporary times: How olive oil by-products and waste water can contribute to health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef] [PubMed]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef] [PubMed]
- Sar, T.; Akbas, M.Y. Antimicrobial Activities of Olive Oil Mill Wastewater Extracts against Selected Microorganisms. Sustainability 2023, 15, 8179. [Google Scholar] [CrossRef]
- Cappelli, K.; Ferlisi, F.; Mecocci, S.; Maranesi, M.; Trabalza-Marinucci, M.; Zerani, M.; Bosco, A.D.; Acuti, G. Dietary Supplementation of Olive Mill Waste Water Polyphenols in Rabbits: Evaluation of the Potential Effects on Hepatic Apoptosis, Inflammation and Metabolism through RT-qPCR Approach. Animals 2021, 11, 2932. [Google Scholar] [CrossRef] [PubMed]
- Tăbăcariu, A.S.-B.; Ifrim, I.-L.; Patriciu, O.-I.; Ștefănescu, I.-A.; Fînaru, A.-L. Walnut By-Products and Elderberry Extracts—Sustainable Alternatives for Human and Plant Health. Molecules 2024, 29, 498. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Badim, H.; Salvador, C.; Silvestre, A.J.D.; Santos, S.A.O.; Rocha, S.M.; Sousa, A.M.; Pereira, M.O.; Wilson, C.P.; Rocha, C.M.R.; et al. Chemical Characterization of Sambucus nigra L. Flowers Aqueous Extract and Its Biological Implications. Biomolecules 2021, 11, 1222. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskiene, K.; Inkeniene, A.; Puidokaite, E.; Grigonis, A. Quality analysis of semisolid formulations with the liquid extract of elderflower (Sambucus nigra L.). Acta Pol. Pharm. Drug Res. 2019, 76, 1061–1071. [Google Scholar] [CrossRef]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plants Res. 2010, 4, 1805–1809. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Sharma, P.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia. J. Pharm. Anal. 2013, 3, 298–302. [Google Scholar] [CrossRef]
- Żurek, N.; Pycia, K.; Pawłowska, A.; Potocki, L.; Kapusta, I.T. Chemical Profiling, Bioactive Properties, and Anticancer and Antimicrobial Potential of Juglans regia L. Leaves. Molecules 2023, 28, 1989. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Panneerselvam, K. An investigation on antibacterial filler property of silver nanoparticles generated from Walnut shell powder by insitu process. Mater. Today Proc. 2021, 39, 368–372. [Google Scholar] [CrossRef]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; de Boer, L.; Ruyter-Spira, C.P.; Creemers-Molenaar, T.; Helsper, J.P.F.G.; Vandenbroucke-Grauls, C.M.J.E.; Zaat, S.A.J.; Velde, A.A.T. Medical-grade honey enriched with antimicrobial peptides has enhanced activity against antibiotic-resistant pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 251–257. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Biosci. 2019, 61, 32–36. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Propolis: Composition and Antibacterial Properties. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.N.; Casey, E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules 2021, 26, 4500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Kong, L.-C.; Liu, J.; Ma, H.-X. Synergistic effect of eugenol with Colistin against clinical isolated Colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Noghabi, S.A.; Kargar, P.G.; Bagherzade, G.; Beyzaei, H. Comparative study of antioxidant and antimicrobial activity of berberine-derived Schiff bases, nitro-berberine and amino-berberine. Heliyon 2023, 9, e22783. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, J.A.; Tereshchenkov, A.G.; Nazarov, P.A.; Lukianov, D.A.; Skvortsov, D.A.; Polshakov, V.I.; Vasilieva, B.F.; Efremenkova, O.V.; Kaiumov, M.Y.; Paleskava, A.; et al. Conjugates of Chloramphenicol Amine and Berberine as Antimicrobial Agents. Antibiotics 2022, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, S.; Popat, A.; Ziora, Z.M.; Moyle, P.M. Sortase A Inhibitor Protein Nanoparticle Formulations Demonstrate Antibacterial Synergy When Combined with Antimicrobial Peptides. Molecules 2023, 28, 2114. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ma, L.; Wang, G.; Yang, J.; Zhang, M.; Wang, X.; Su, J.; Xie, M. In vitro Antimicrobial Activity and the Mechanism of Berberine Against Methicillin-Resistant Staphylococcus aureus Isolated from Bloodstream Infection Patients. Infect. Drug Resist. 2022, 15, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, W.; Cai, L.; Yang, T. Potentiation and Mechanism of Berberine as an Antibiotic Adjuvant Against Multidrug-Resistant Bacteria. Infect. Drug Resist. 2023, 16, 7313–7326. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Pan, Q.; Fan, L.; Pan, T.; Zhu, F.; Pan, Q.; Shan, L.; Zhao, L. Berberine at sub-inhibitory concentration inhibits biofilm dispersal in Staphylococcus aureus. Microbiology 2022, 168. [Google Scholar] [CrossRef]
- Lade, H.; Chung, S.H.; Lee, Y.; Kumbhar, B.V.; Joo, H.-S.; Kim, Y.-G.; Yang, Y.-H.; Kim, J.-S. Thymol Reduces agr-Mediated Virulence Factor Phenol-Soluble Modulin Production in Staphylococcus aureus. BioMed Res. Int. 2022, 2022, 8221622. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, S. Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J. Appl. Microbiol. 2021, 130, 1154–1172. [Google Scholar] [CrossRef]
- Aksoy, C.S.; Avci, F.G.; Ugurel, O.M.; Atas, B.; Sayar, N.A.; Akbulut, B.S. Potentiating the activity of berberine for Staphylococcus aureus in a combinatorial treatment with thymol. Microb. Pathog. 2020, 149, 104542. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Ye, X.-G.; He, L.-T.; Zhang, S.-R.; Wang, R.-L.; Zhou, J.; He, Z.-S. In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumonia e. J. Antibiot. 2016, 69, 741–746. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Wang, L.; Kang, G.; Wang, P.; Yin, H.; Huang, H. A Potential Combination Therapy of Berberine Hydrochloride with Antibiotics Against Multidrug-Resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2021, 11, 660431. [Google Scholar] [CrossRef]
- Gao, W.-W.; Gopala, L.; Bheemanaboina, R.R.Y.; Zhang, G.-B.; Li, S.; Zhou, C.-H. Discovery of 2-aminothiazolyl berberine derivatives as effectively antibacterial agents toward clinically drug-resistant Gram-negative Acinetobacter baumanii. Eur. J. Med. Chem. 2018, 146, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Khalvati, B.; Eslami, S.; Mirzaii, M.; Roustaei, N.; Mazloomirad, F.; Khoramrooz, S.S. The Inhibitory Effect of Thioridazine on adeB Efflux Pump Gene Expression in Multidrug-Resistant Acinetobacter baumannii Isolates Using Real Time PCR. Avicenna J. Med Biotechnol. 2022, 14, 132–136. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal Products and Their Active Constituents Used Alone and in Combination with Antibiotics against Multidrug-Resistant Bacteria. Planta Medica 2023, 89, 168–182. [Google Scholar] [CrossRef]
- Patra, P.H.; Mahanti, A.; Mondal, D.K.; Dandapat, P.; Bandyopadhyay, S.; Samanta, I.; Lodh, C.; Bera, A.K.; Bhattacharyya, D.; Sarkar, M.; et al. Potential antibacterial activity of berberine against multi drug resistant enterovirulent Escherichia coli isolated from yaks (Poephagus grunniens) with haemorrhagic diarrhoea. Asian Pac. J. Trop. Med. 2013, 6, 315–319. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X. Role of Berberine as a Potential Efflux Pump Inhibitor against MdfA from Escherichia coli: In Vitro and In Silico Studies. Microbiol. Spectr. 2023, 11, e0332422. [Google Scholar] [CrossRef]
- Morita, Y.; Nakashima, K.-I.; Nishino, K.; Kotani, K.; Tomida, J.; Inoue, M.; Kawamura, Y. Berberine Is a Novel Type Efflux Inhibitor Which Attenuates the MexXY-Mediated Aminoglycoside Resistance in Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 1223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Li, L.; Liu, L. Synergistic Activity of Berberine with Azithromycin against Pseudomonas Aeruginosa Isolated from Patients with Cystic Fibrosis of Lung In Vitro and In Vivo. Cell. Physiol. Biochem. 2017, 42, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Guo, M.; Xu, X.; Hu, Y.; Liu, D.; Wang, C.; Liu, X.; Li, Y. In Vitro Synergistic Inhibitory Activity of Natural Alkaloid Berberine Combined with Azithromycin against Alginate Production by Pseudomonas aeruginosa PAO1. Oxidative Med. Cell. Longev. 2022, 2022, 3858500. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, L.G.; Mahoney, A.R.; Dey, D.; Wuest, W.M.; Conn, G.L. Di-berberine conjugates as chemical probes of Pseudomonas aeruginosa MexXY-OprM efflux function and inhibition. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jhanji, R.; Bhati, V.; Singh, A.; Kumar, A. Phytomolecules against bacterial biofilm and efflux pump: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2020, 38, 5500–5512. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti-Infect. Ther. 2019, 8, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, R.; Bhattarai, N.; Baral, P.; Gerstman, B.S.; Park, J.H.; Handfield, M.; Chapagain, P.P. Lipid II Binding and Transmembrane Properties of Various Antimicrobial Lanthipeptides. J. Chem. Theory Comput. 2022, 18, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rendón, D.; Guzmán-Chávez, F.; García-Ausencio, C.; Rodríguez-Sanoja, R.; Sánchez, S. The untapped potential of actinobacterial lanthipeptides as therapeutic agents. Mol. Biol. Rep. 2023, 50, 10605–10616. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Lazzarini, A.; Carrano, L.; Corti, E.; Ciciliato, I.; Gastaldo, L.; Candiani, P.; Losi, D.; Marinelli, F.; Selva, E.; et al. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem. Biol. 2008, 15, 22–31. [Google Scholar] [CrossRef]
- Münch, D.; Müller, A.; Schneider, T.; Kohl, B.; Wenzel, M.; Bandow, J.E.; Maffioli, S.; Sosio, M.; Donadio, S.; Wimmer, R.; et al. The lantibiotic NAI-107 binds to bactoprenol-bound cell wall precursors and impairs membrane functions. J. Biol. Chem. 2014, 289, 12063–12076. [Google Scholar] [CrossRef]
- Brunati, C.; Thomsen, T.T.; Gaspari, E.; Maffioli, S.; Sosio, M.; Jabes, D.; Løbner-Olesen, A.; Donadio, S. Expanding the potential of NAI-107 for treating serious ESKAPE pathogens: Synergistic combinations against Gram-negatives and bactericidal activity against non-dividing cells. J. Antimicrob. Chemother. 2018, 73, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Foulston, L.C.; Bibb, M.J. Microbisporicin gene cluster reveals unusual features of lantibiotic biosynthesis in actinomycetes. Proc. Natl. Acad. Sci. USA 2010, 107, 13461–13466. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, S.K. Perspectives on lantibiotic discovery—Where have we failed and what improvements are required? Expert Opin. Drug Discov. 2015, 10, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.C.; Mösker, E.; Faria, R.; Süssmuth, R.D.; Mendo, S.; Caetano, T. Class II two-peptide lanthipeptide proteases: Exploring LicTP for biotechnological applications. Appl. Microbiol. Biotechnol. 2023, 107, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Gao, Y.; Zhao, F.; Wang, J.; Teng, K.; Zhang, J.; Zhong, J. Dissecting the catalytic and substrate binding activity of a class II lanthipeptide synthetase BovM. Biochem. Biophys. Res. Commun. 2014, 450, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, F.; Hu, Y. Genome Mining-Mediated Discovery of a New Avermipeptin Analogue in Streptomyces actuosus ATCC 25421. ChemistryOpen 2018, 7, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends. Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudo-monas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Van den Bergh, B.; Fauvart, M.; Michiels, J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef]
- Dombach, J.L.; Quintana, J.L.J.; Detweiler, C.S. Staphylococcal Bacterial Persister Cells, Biofilms, and Intracellular Infection Are Disrupted by JD1, a Membrane-Damaging Small Molecule. mBio 2021, 12, e0180121. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Basak, A.K.; Melander, C. Natural products as inspiration for the development of bacterial antibiofilm agents. Nat. Prod. Rep. 2020, 37, 1454–1477. [Google Scholar] [CrossRef] [PubMed]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.A.; Booysen, E.; van Zyl, L.; Trindade, M. Do biosurfactants as anti-biofilm agents have a future in industrial water systems? Front. Bioeng. Biotechnol. 2023, 11, 1244595. [Google Scholar] [CrossRef] [PubMed]
- Xiu, P.; Liu, R.; Zhang, D.; Sun, C. Pumilacidin-Like Lipopeptides Derived from Marine Bacterium Bacillus sp. Strain 176 Suppress the Motility of Vibrio alginolyticus. Appl. Environ. Microbiol. 2017, 83, e00450-17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Ryu, S.Y.; Lee, J. Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.S.; Perez-Fons, L.; Estepa, A.; Micol, V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, B.; Lu, Y.; Guo, Y.; Sun, J.; Wei, B.; Zhang, H.; Wang, H. Quorum Sensing Inhibitors from Marine Microorganisms and Their Synthetic Derivatives. Mar. Drugs 2019, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, L.; Sun, S.; Chang, A.; Dai, X.; Li, H.; Wang, Y.; Zhu, H. A Cyclic Dipeptide from Marine Fungus Penicillium chrysogenum DXY-1 Exhibits Anti-quorum Sensing Activity. ACS Omega 2021, 6, 7693–7700. [Google Scholar] [CrossRef]
- Kiran, G.S.; Sajayan, A.; Priyadharshini, G.; Balakrishnan, A.; Prathiviraj, R.; Sabu, A.; Selvin, J. A novel anti-infective molecule nesfactin identified from sponge associated bacteria Nesterenkonia sp. MSA31 against multidrug resistant Pseudomonas aeruginosa. Microb. Pathog. 2021, 157, 104923. [Google Scholar] [CrossRef]
- Wang, J.; Nong, X.-H.; Zhang, X.-Y.; Xu, X.-Y.; Amin, M.; Qi, S.-H. Screening of Anti-Biofilm Compounds from Marine-Derived Fungi and the Effects of Secalonic Acid D on Staphylococcus aureus Biofilm. J. Microbiol. Biotechnol. 2017, 27, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Lorizate, M.; Kräusslich, H.-G. Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 2011, 3, a004820. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.L.; Chlanda, P. The Art of Viral Membrane Fusion and Penetration. Subcell Biochem. 2023, 106, 113–152. [Google Scholar] [CrossRef]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Bashir, S.M.; Nabi, S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytotherapy Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Wei, Z.-Q.; Zhang, Y.-H.; Ke, C.-Z.; Chen, H.-X.; Ren, P.; He, Y.-L.; Hu, P.; Ma, D.-Q.; Luo, J.; Meng, Z.-J. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J. Gastroenterol. 2017, 23, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoo, H.S.; Kim, J.C.; Park, C.S.; Choi, M.S.; Kim, M.; Choi, H.; Min, J.S.; Kim, Y.S.; Yoon, S.W.; et al. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 2009, 124, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Prusty, K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2005, 113, 951–960. [Google Scholar] [CrossRef]
- Wang, Z.; Song, X.-Q.; Xu, W.; Lei, S.; Zhang, H.; Yang, L. Stand Up to Stand Out: Natural Dietary Polyphenols Curcumin, Resveratrol, and Gossypol as Potential Therapeutic Candidates against Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Nutrients 2023, 15, 3885. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Mekky, R.Y.; El-Ekiaby, N.M.; Hamza, M.T.; Elemam, N.M.; El-Sayed, M.; Esmat, G.; Abdelaziz, A.I. Mir-194 is a hepatocyte gate keeper hindering HCV entry through targeting CD81 receptor. J. Infect. 2015, 70, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Thibault, P.A.; Wilson, J.A. MicroRNA 122 Affects both the Initiation and the Maintenance of Hepatitis C Virus Infections. J. Virol. 2022, 96, e0190321. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Song, T.; Li, M.; Chen, W.; Li, J.; Gong, S.; Zhao, Y.; Ma, L.; Yu, H.; Li, X.; et al. The medicinal value of tea drinking in the management of COVID-19. Heliyon 2023, 9, e12968. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Meyer, T. Sinecatechins (Polyphenon E) ointment for treatment of external genital warts and possible future indications. Expert Opin. Biol. Ther. 2014, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cheng, X.; Naumovski, N.; Hu, L.; Wang, K. Epigenetic regulation by quercetin: A comprehensive review focused on its biological mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 5542. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Li, L.; Chen, Y.; Jia, R.; Zou, Y.; Wan, H.; Zhao, L.; Tang, H.; Lv, C.; et al. Resveratrol Inhibits Pseudorabies Virus Replication by Targeting IE180 Protein. Front. Microbiol. 2022, 13, 891978. [Google Scholar] [CrossRef]
- Docherty, J.J.; Sweet, T.J.; Bailey, E.; Faith, S.A.; Booth, T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antivir. Res. 2006, 72, 171–177. [Google Scholar] [CrossRef]
- Xie, X.-H.; Zang, N.; Li, S.-M.; Wang, L.-J.; Deng, Y.; He, Y.; Yang, X.-Q.; Liu, E.-M. Resveratrol inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation 2012, 35, 1392–1401. [Google Scholar] [CrossRef]
- Pan, P.; Li, J.; Lin, W.; Long, G. Effects of Resveratrol on Hepatitis B Virus Replication: In vitro and in vivo Experiments. Intervirology 2022, 65, 206–214. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, P.; Gupta, G.K.; Ntie-Kang, F.; Kumar, D. Structure-Activity-Relationship and Mechanistic Insights for Anti-HIV Natural Products. Molecules 2020, 25, 2070. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Sukemori, A.; Watanabe, T.; Malla, K.J.; Yoshikawa, T.; Li, W.; Koike, K.; Chen, C.-H.; Akiyama, T.; Qian, K.; et al. Stelleralides A–C, novel potent anti-HIV daphnane-type diterpenoids from Stellera chamaejasme L. Org. Lett. 2011, 13, 2904–2907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-Y.; Chen, H.; He, H.-P.; Zhang, Y.; Li, S.-F.; Tang, G.-H.; Guo, L.-L.; Yang, W.; Zhu, F.; Zheng, Y.-T.; et al. Anti-HIV active daphnane diterpenoids from Trigonostemon thyrsoideum. Phytochemistry 2013, 96, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Guo, Z.-G.; Wang, L.; Guo, Q.-F.; Cao, F. Anti-IAV indole-diterpenoids from the marine-derived fungus Penicillium citrinum. Nat. Prod. Res. 2023, 37, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-J.; Li, Y.; Ma, S.-G.; Qu, J.; Liu, Y.-B.; Li, Y.-H.; Zhang, D.; Li, L.; Yu, S.-S. Antiviral Triterpenes from the Twigs and Leaves of Lyonia ovalifolia. J. Nat. Prod. 2016, 79, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Mair, C.E.; Grienke, U.; Wilhelm, A.; Urban, E.; Zehl, M.; Schmidtke, M.; Rollinger, J.M. Anti-Influenza Triterpene Saponins from the Bark of Burkea africana. J. Nat. Prod. 2018, 81, 515–523. [Google Scholar] [CrossRef]
- Warowicka, A.; Nawrot, R.; Goździcka-Józefiak, A. Antiviral activity of berberine. Arch. Virol. 2020, 165, 1935–1945. [Google Scholar] [CrossRef]
- Le, K.; Tran, D.; Nguyen, A.; Le, L. A Screening of Neuraminidase Inhibition Activities of Isoquinolone Alkaloids in Coptis chinensis Using Molecular Docking and Pharmacophore Analysis. ACS Omega 2020, 5, 30315–30322. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A. New trends in the practical use of isoquinoline alkaloids as potential drugs applicated in infectious and non-infectious diseases. Biomed. Pharmacother. 2023, 168, 115704. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-G.; Wang, Y.; Yang, M.-R.; Wang, C.-Y.; Meng, J.; Liu, J.; Yang, Z.; Wu, K.; Bai, L.-P.; Zhu, G.-Y.; et al. Structures, Biomimetic Synthesis, and Anti-SARS-CoV-2 Activity of Two Pairs of Enantiomeric Phenylpropanoid-Conjugated Protoberberine Alkaloids from the Rhizomes of Corydalis decumbens. Arch. Pharmacal Res. 2022, 45, 631–643. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).