Detection of Salmonella Pathogenicity Islands and Antimicrobial-Resistant Genes in Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Broiler Chickens
Abstract
1. Introduction
2. Results
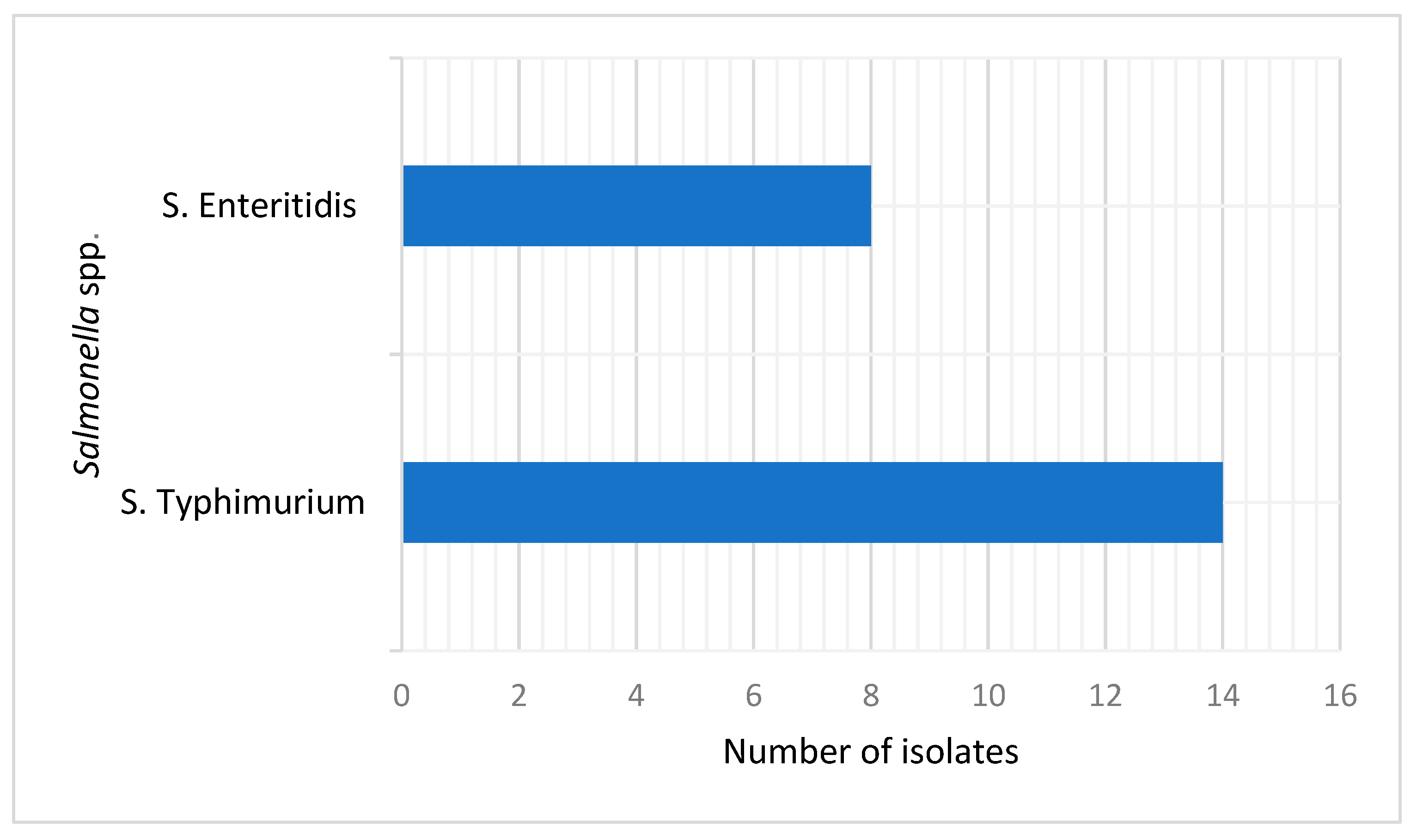
2.1. Prevalence of S. Typhimurium and S. Enteritidis Serovars
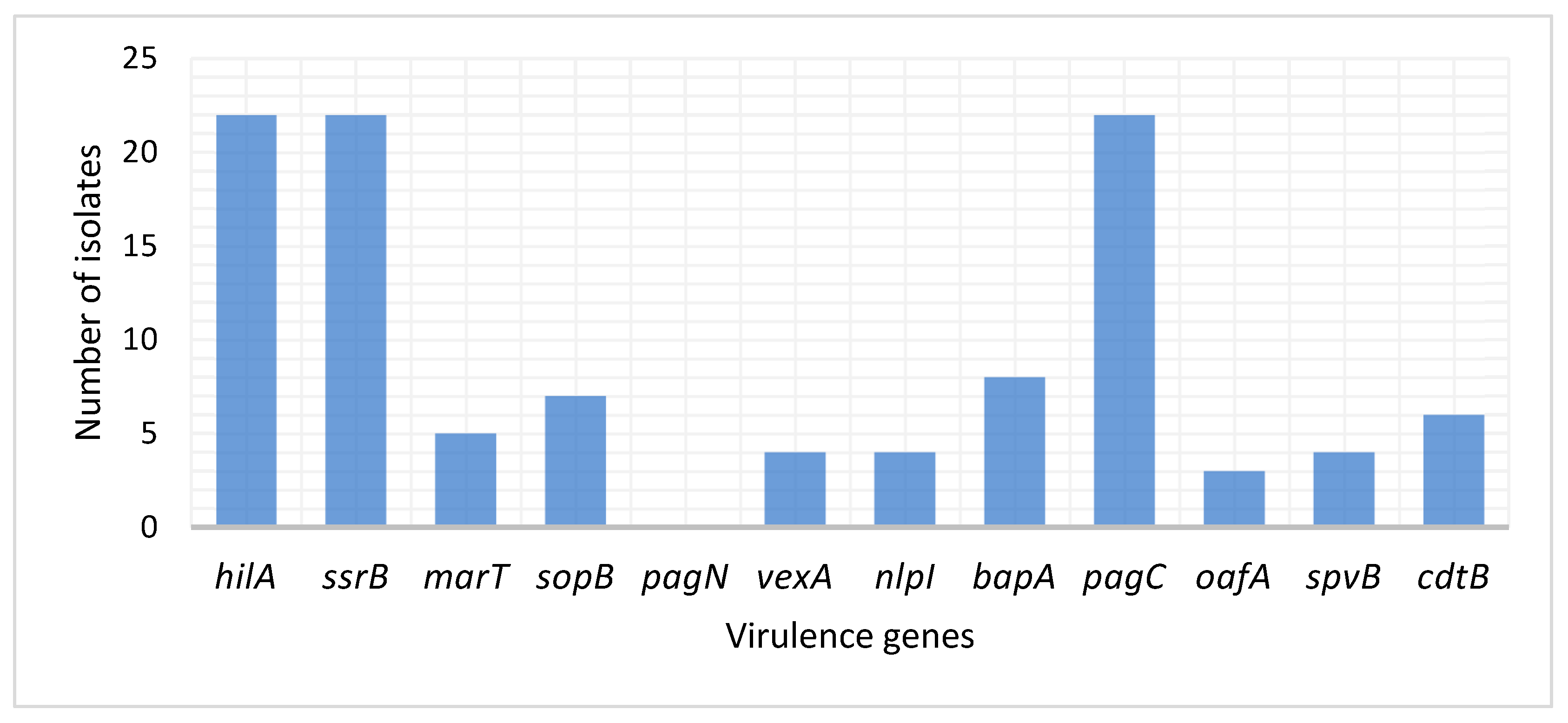
2.2. Detection of Virulence Genes in S. Typhimurium and S. Enteritidis
2.3. Phenotypic Detection of Antibiotic Resistance Profiles
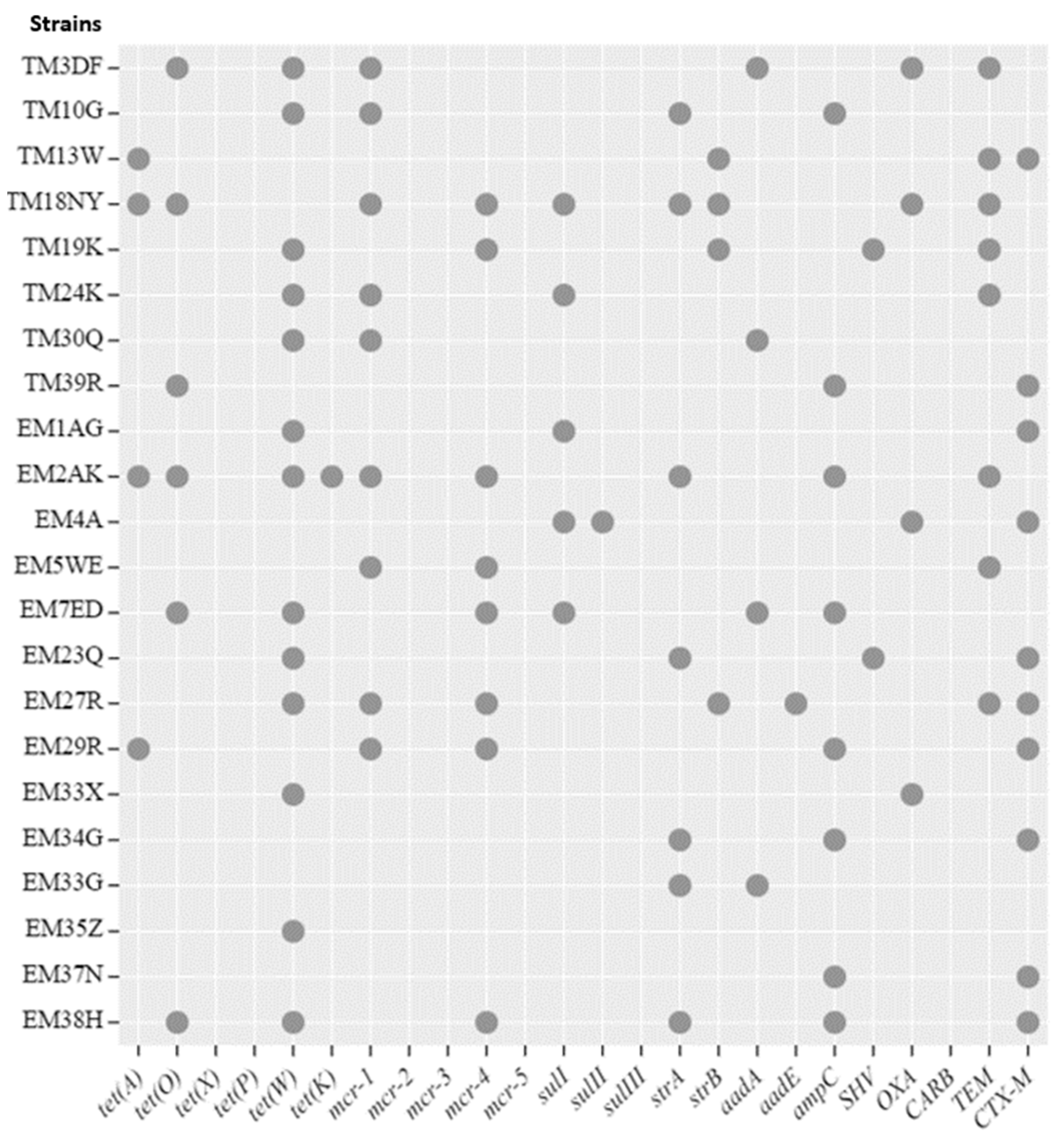
2.4. Genotypic Detection of Antibiotic Resistance Profiles
2.5. Coexistence of Phenotypic and Genotypic Antibiotic Resistance Traits
3. Discussion
4. Materials and Methods
4.1. Sampling
4.2. Microbiological Analysis
4.3. Genomic DNA Extraction
4.4. Molecular Identification of Salmonella Serovars
4.5. Identification of Salmonella Serovars Using 16S rRNA
4.6. Detection of Virulence Genes
4.7. Phenotypic Antimicrobial Susceptibility Test
4.8. Genotypic Detection of Antibiotic Resistance Genes
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alghoribi, M.F.; Doumith, M.; Alrodayyan, M.; Al Zayer, M.; Köster, W.L.; Muhanna, A.; Aljohani, S.M.; Balkhy, H.H.; Desin, T.S. S. Enteritidis and S. Typhimurium harboring SPI-1 and SPI-2 are the predominant serotypes associated with human salmonellosis in Saudi Arabia. Front. Cell. Infect. Microbiol. 2019, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.E.; Smith, T. Report on Swine Plague. US Bureau of Animal Industries, Second Annual Report; US Gov’t. Printing Office: Washington, DC, USA, 1885; p. 184. [Google Scholar]
- Nazari Moghadam, M.; Rahimi, E.; Shakerian, A.; Momtaz, H. Prevalence of Salmonella Typhimurium and Salmonella Enteritidis isolated from poultry meat: Virulence and antimicrobial-resistant genes. BMC Microbiol. 2023, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.; Hassan, M.; Shaltout, F.; El-Hosseny, S. Detection of E. coli and Salmonella organisms in cattle and camel meat. BVMJ 2013, 25, 198–204. [Google Scholar]
- de Freitas, C.G.; Santana, Â.P.; da Silva, P.H.; Gonçalves, V.S.; Barros, M.D.; Torres, F.A.; Murata, L.S.; Perecmanis, S. PCR multiplex for detection of Salmonella Enteritidis, Typhi and Typhimurium and occurrence in poultry meat. Int. J. Food Microbiol. 2010, 139, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Olobatoke, R.Y.; Mulugeta, S.D. Incidence of non-typhoidal Salmonella in poultry products in the North West Province, South Africa. S. Afr. J. Sci. 2015, 111, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Ibuki, M.; Yokomizo, F.; Mine, Y. Effect of dietary β 1–4 mannobiose in the prevention of Salmonella Enteritidis infection in broilers. Br. Poult. Sci. 2007, 48, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ramatla, T.A.; Mphuthi, N.; Ramaili, T.; Taioe, M.O.; Thekisoe, O.M.; Syakalima, M. Molecular detection of virulence genes in Salmonella spp. isolated from chicken faeces in Mafikeng, South Africa. J. S. Afr. Vet. Assoc. 2020, 91, 1994. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Prasanna Kumar, V. Studies on Virulence, Antimicrobial Resistance and Genetic Diversity of Salmonella Typhimurium Isolates from North India; GB Pant University of Agriculture and Technology: Pantnagar, Uttarakhand, 2016. [Google Scholar]
- De la Cruz, M.A.; Pérez-Morales, D.; Palacios, I.J.; Fernández-Mora, M.; Calva, E.; Bustamante, V.H. The two-component system CpxR/A represses the expression of Salmonella virulence genes by affecting the stability of the transcriptional regulator HilD. Front. Microbiol. 2015, 6, 807. [Google Scholar] [CrossRef]
- Haneda, T.; Ishii, Y.; Shimizu, H.; Ohshima, K.; Iida, N.; Danbara, H.; Okada, N. Salmonella type III effector SpvC, a phosphothreonine lyase, contributes to reduction in inflammatory response during intestinal phase of infection. Cell. Microbiol. 2012, 14, 485–499. [Google Scholar] [CrossRef]
- Card, R.; Vaughan, K.; Bagnall, M.; Spiropoulos, J.; Cooley, W.; Strickland, T.; Davies, R.; Anjum, M.F. Virulence characterisation of Salmonella enterica isolates of differing antimicrobial resistance recovered from UK livestock and imported meat samples. Front. Microbiol. 2016, 7, 640. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.; Fatima, M.; Zaheer, C.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2023, 13, 1067284. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The complex relationship between virulence and antibiotic resistance. Genes 2017, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Ramatla, T.; Tutubala, M.; Motlhaping, T.; de Wet, L.; Mokgokong, P.; Thekisoe, O.; Lekota, K. Molecular detection of Shiga toxin and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolates from sheep and goats. Mol. Biol. Rep. 2024, 51, 57. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.F.; Wong, W.C.; Chai, L.C.; Tunung, R.; Jeyaletchumi, P.; Noor Hidayah, M.S.; Ubong, A.; Farinazleen, M.G.; Cheah, Y.K.; Son, R. Salmonella: A foodborne pathogen. Int. Food Res. J. 2011, 18, 465–473. [Google Scholar]
- Ramatla, T.; Tawana, M.; Onyiche, T.E.; Lekota, K.E.; Thekisoe, O. Prevalence of antibiotic resistance in Salmonella serotypes concurrently isolated from the environment, animals, and humans in South Africa: A systematic review and meta-analysis. Antibiotics 2021, 10, 1435. [Google Scholar] [CrossRef]
- Mossoro-Kpinde, C.D.; Manirakiza, A.; Mbecko, J.R.; Misatou, P.; Le Faou, A.; Frank, T. Antimicrobial resistance of enteric Salmonella in Bangui, central African republic. J. Trop. Med. 2015, 2015, 483974. [Google Scholar] [CrossRef] [PubMed]
- Parry, C.M.; Threlfall, E.J. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 2008, 21, 531–538. [Google Scholar] [CrossRef]
- Zishiri, O.T.; Mkhize, N.; Mukaratirwa, S. Prevalence of virulence and antimicrobial resistance genes in Salmonella spp. isolated from commercial chickens and human clinical isolates from South Africa and Brazil. Onderstepoort J. Vet. Res. 2016, 83, a1067. [Google Scholar] [CrossRef]
- Ramatla, T.; Taioe, M.O.; Thekisoe, O.M.; Syakalima, M. Confirmation of antimicrobial resistance by using resistance genes of isolated Salmonella spp. in chicken houses of North West, South Africa. World’s Vet. J. 2019, 9, 158–165. [Google Scholar] [CrossRef]
- Akinola, S.A.; Mwanza, M.; Ateba, C.N. Occurrence, genetic diversities and antibiotic resistance profiles of Salmonella serovars isolated from chickens. Infect. Drug Resist. 2019, 12, 3327–3342. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.; Tahoun, A.; El-Gohary, A.E.; El-Abasy, M.; El-Khayat, F.; Gillespie, T.; Kitade, Y.; Hafez, H.M.; Neubauer, H.; El-Adawy, H. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Ateba, C.N.; Mochaiwa, B. Use of invA gene specific PCR analysis for the detection of virulent Salmonella species in beef products in the north west province, South Africa. J. Food Nutr. Res. 2014, 2, 294–300. [Google Scholar] [CrossRef][Green Version]
- Lou, L.; Zhang, P.; Piao, R.; Wang, Y. Salmonella pathogenicity island 1 (SPI-1) and its complex regulatory network. Front. Cell. Infect. Microbiol. 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.T. Identification and bioinformatic analysis of invA gene of Salmonella in free range chicken. Braz. J. Biol. 2022, 84, e263363. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh, Z.; Khaki, P. Detection of invA, sivH, and agfA virulence genes in Salmonella spp. isolated from broiler breeder farms in Alborz Province, Iran. Arch. Razi Inst. 2022, 77, 607. [Google Scholar] [CrossRef] [PubMed]
- Refai, M.; Hatem, M.E.; Elhariri, M.; Salem, G.H.; El-Said, A.H. Using of molecular biology techniques compared with conventional detection methods for detection of Salmonella in cattle in Egypt. J. Am. Sci. 2017, 13, 46–50. [Google Scholar] [CrossRef]
- Fekry, E.; Ammar, A.M.; Hussien, A.E. Molecular detection of InvA, OmpA and Stn genes in Salmonella serovars from broilers in Egypt. Alex. J. Vet. Sci. 2018, 68, 188–195. [Google Scholar] [CrossRef]
- Dishan, A.; Hizlisoy, H.; Onmaz, N.E.; Yildirim, Y.; Gonulalan, Z.; Al, S. Comprehensive analysis of Salmonella in poultry meat and products in Türkiye: Prevalence, antibiotic susceptibility and genomic characterisation. Int. J. Food Sci. Technol. 2024, 59, 3412–3422. [Google Scholar] [CrossRef]
- Dowidar, M.S.; Homouda, S.N.; Abd El Tawab, A.A. Antibiogram Pattern and Molecular Characterization of Multiple drug resistant Salmonella Isolated from Different Food Products in Egypt. Benha Vet. Med. J. 2024, 46, 119–124. [Google Scholar] [CrossRef]
- Tarabees, R.; Elsayed, M.S.; Shawish, R.; Basiouni, S.; Shehata, A.A. Isolation and characterization of Salmonella Enteritidis and Salmonella Typhimurium from chicken meat in Egypt. J. Infect. Dev. Ctries. 2017, 11, 314–319. [Google Scholar] [CrossRef]
- Myeni, S.K.; Wang, L.; Zhou, D. SipB-SipC complex is essential for translocon formation. PLoS ONE 2013, 8, e60499. [Google Scholar] [CrossRef] [PubMed]
- Eichelberg, K.; Galán, J.E. Differential regulation of Salmonella Typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect. Immun. 1999, 67, 4099–4105. [Google Scholar] [CrossRef]
- Walthers, D.; Carroll, R.K.; Navarre, W.W.; Libby, S.J.; Fang, F.C.; Kenney, L.J. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol. Microbiol. 2007, 65, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Walthers, D.; Oropeza, R.; Kenney, L.J. The response regulator SsrB activates transcription and binds to a region overlapping OmpR binding sites at Salmonella pathogenicity island 2. Mol. Microbiol. 2004, 54, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, B.; Tsai, C.N.; Coombes, B.K. Evolution of Salmonella-host cell interactions through a dynamic bacterial genome. Front. Cell. Infect. Microbiol. 2017, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Nešić, D.; Hsu, Y.; Stebbins, C.E. Assembly and function of a bacterial genotoxin. Nature 2004, 429, 429–433. [Google Scholar] [CrossRef]
- Mezal, E.H.; Bae, D.; Khan, A.A. Detection and functionality of the CdtB, PltA, and PltB from Salmonella enterica serovar Javiana. Pathog. Dis. 2014, 72, 95–103. [Google Scholar] [CrossRef]
- Haghjoo, E.; Galán, J.E. Identification of a transcriptional regulator that controls intracellular gene expression in Salmonella Typhi. Mol. Microbiol. 2007, 64, 1549–1561. [Google Scholar] [CrossRef]
- Libby, S.J.; Lesnick, M.; Hasegawa, P.; Kurth, M.; Belcher, C.; Fierer, J.; Guiney, D.G. Characterization of the spv locus in Salmonella enterica serovar Arizona. Infect. Immun. 2002, 70, 3290–3294. [Google Scholar] [CrossRef]
- Derakhshandeh, A.; Firouzi, R.; Khoshbakht, R. Association of three plasmid-encoded spv genes among different Salmonella serotypes isolated from different origins. Indian. J. Microbiol. 2013, 53, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wang, T.; Gao, S.; Xu, G.M.; Niu, H.; Huang, R.; Wu, S.Y. Salmonella plasmid virulence gene spvB enhances bacterial virulence by inhibiting autophagy in a zebrafish infection model. Fish. Shellfish. Immunol. 2016, 49, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Lesnick, M.L.; Reiner, N.E.; Fierer, J.; Guiney, D.G. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol. Microbiol. 2001, 39, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Passaris, I.; Cambré, A.; Govers, S.K.; Aertsen, A. Bimodal expression of the Salmonella Typhimurium spv operon. Genetics 2018, 210, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Sodagari, H.R.; Mashak, Z.; Ghadimianazar, A. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from retail chicken meat and giblets in Iran. J. Infect. Dev. Ctries. 2015, 9, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ramatla, T.; Mileng, K.; Ndou, R.; Mphuti, N.; Syakalima, M.; Lekota, K.E.; Thekisoe, O.M. Molecular detection of integrons, colistin and β-lactamase resistant genes in Salmonella enterica serovars Enteritidis and Typhimurium isolated from chickens and rats inhabiting poultry farms. Microorganisms 2022, 10, 313. [Google Scholar] [CrossRef]
- Ezzatpanah, A.; Moradi, B.S.; Khaki, P.; Ghaderi, R.; Seyedan Jasbi, S. Isolation, determination of serotype and pattern of antibiotic resistance of isolated Salmonella from poultry in Arak. Iran. Vet. J. 2013, 9, 88–96. [Google Scholar]
- Matchawe, C.; Machuka, E.M.; Kyallo, M.; Bonny, P.; Nkeunen, G.; Njaci, I.; Esemu, S.N.; Githae, D.; Juma, J.; Nfor, B.M.; et al. Detection of antimicrobial resistance, pathogenicity, and virulence potentials of non-typhoidal Salmonella isolates at the yaounde abattoir using whole-genome sequencing technique. Pathogens 2022, 11, 502. [Google Scholar] [CrossRef]
- Mohammed Jajere, S.; Hassan, L.; Zakaria, Z.; Abu, J.; Abdul Aziz, S. Antibiogram profiles and risk factors for multidrug resistance of salmonella enterica recovered from village chickens (gallus gallus domesticus linnaeus) and other environmental sources in the central and southern peninsular malaysia. Antibiotics 2020, 9, 701. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.; Xiong, Z.; Ma, Y.; Wei, Y.; Qu, X.; Zhang, H.; Zhang, J.; Liao, M. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong, China. Front. Microbiol 2018, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.S.; Hasan, M.M.; Ali, M.Y.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Prevalence and multidrug resistance pattern of Salmonella carrying extended-spectrum β-lactamase in frozen chicken meat in Bangladesh. J. Food Prot. 2020, 83, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Adesiji, Y.O.; Deekshit, V.K.; Karunasagar, I. Antimicrobial-resistant genes associated with Salmonella spp. isolated from human, poultry, and seafood sources. Food Sci. Nutr. 2014, 2, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Mokgophi, T.M.; Gcebe, N.; Fasina, F.; Adesiyun, A.A. Antimicrobial resistance profiles of Salmonella isolates on chickens processed and retailed at outlets of the informal market in Gauteng Province, South Africa. Pathogens 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Frye, J.G.; Chang, C.W.; Liu, J.I.; Cerniglia, C.E.; Nayak, R.A. Microarray analysis of antimicrobial resistance genes in Salmonella enterica from preharvest poultry environment. J. Appl. Microbiol. 2009, 107, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Glenn, L.M.; Lindsey, R.L.; Frank, J.F.; Meinersmann, R.J.; Englen, M.D.; Fedorka-Cray, P.J.; Frye, J.G. Analysis of antimicrobial resistance genes detected in multidrug-resistant Salmonella enterica serovar Typhimurium isolated from food animals. Microb. Drug Resist. 2011, 17, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Mąka, Ł.; Maćkiw, E.; Ścieżyńska, H.; Modzelewska, M.; Popowska, M. Resistance to sulfonamides and dissemination of sul genes among Salmonella spp. isolated from food in Poland. Foodborne Pathog. Dis. 2015, 12 (Suppl. S5), 383–389. [Google Scholar] [CrossRef] [PubMed]
- Bertelloni, F.; Cagnoli, G.; Turchi, B.; Ebani, V.V. Low Level of Colistin Resistance and mcr Genes Presence in Salmonella spp.: Evaluation of Isolates Collected between 2000 and 2020 from Animals and Environment. Antibiotics 2022, 11, 272. [Google Scholar] [CrossRef]
- Mei, C.Y.; Jiang, Y.; Ma, Q.C.; Lu, M.J.; Wu, H.; Wang, Z.Y.; Jiao, X.; Wang, J. Chromosomally and Plasmid-Located mcr in Salmonella from Animals and Food Products in China. Microbiol. Spectr. 2022, 10, e02773-22. [Google Scholar] [CrossRef]
- Raeisi, E.; Ghiamirad, M. Survey on prevalence of Salmonella serogroups and antibiotics susceptibility pattern in chicken meat in Ardabil, Iran. J. Ardabil Univ. Med. Sci. 2015, 15, 320–329. [Google Scholar]
- Hasman, H.; Mevius, D.; Veldman, K.; Olesen, I.; Aarestrup, F.M. β-Lactamases among extended-spectrum β-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 2005, 56, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Zhou, X.; Cui, Y.; Shi, C.; Shi, X. Prevalence and characterization of antimicrobial resistance in Salmonella enterica isolates from retail foods in Shanghai, China. Foodborne Pathog. Dis. 2020, 17, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Checkley, S.; Avery, B.; Chalmers, G.; Bohaychuk, V.; Gensler, G.; Reid-Smith, R.; Boerlin, P. Phenotypic and genetic characterization of antimicrobial resistance in Salmonella serovars isolated from retail meats in Alberta, Canada. Food Microbiol. 2012, 32, 110–117. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.M.; Wu, G.J.; Zhao, H.Y.; He, T.; Cao, X.Y.; Dai, L.; Xia, L.N.; Qin, S.S.; Shen, J.Z. Prevalence of antimicrobial resistance among Salmonella isolates from chicken in China. Foodborne Pathog. Dis. 2011, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Siddiky, N.A.; Sarker, S.; Khan, S.R.; Rahman, T.; Kafi, A.; Samad, M.A. Virulence and antimicrobial resistance profile of non-typhoidal Salmonella enterica serovars recovered from poultry processing environments at wet markets in Dhaka, Bangladesh. PLoS ONE 2022, 17, e0254465. [Google Scholar] [CrossRef]
- Eguale, T.; Birungi, J.; Asrat, D.; Njahira, M.N.; Njuguna, J.; Gebreyes, W.A.; Gunn, J.S.; Djikeng, A.; Engidawork, E. Genetic markers associated with resistance to beta-lactam and quinolone antimicrobials in non-typhoidal Salmonella isolates from humans and animals in central Ethiopia. Antimicrob. Resist. Infect. Control 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, J.D.; Ferreira, H.M. “Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in cattle production–a threat around the world. Heliyon 2020, 6, e03206. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. “The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Shin, S.K.; Lee, Y.; Kwon, H.; Rhee, J.S.; Kim, J.K. Validation of Direct Boiling Method for Simple and Efficient Genomic DNA Extraction and PCR-based Macroalgal Species Determination. J. Phycol. 2021, 57, 1368–1372. [Google Scholar] [CrossRef]
- Thung, T.Y.; Radu, S.; Mahyudin, N.A.; Rukayadi, Y.; Zakaria, Z.; Mazlan, N.; Tan, B.H.; Lee, E.; Yeoh, S.L.; Chin, Y.Z.; et al. Prevalence, virulence genes and antimicrobial resistance profiles of Salmonella serovars from retail beef in Selangor, Malaysia. Front. Microbiol. 2018, 8, 2697. [Google Scholar] [CrossRef] [PubMed]
- Mlangeni, L.N.; Ramatla, T.; Lekota, K.E.; Price, C.; Thekisoe, O.; Weldon, C. Occurrence, Antimicrobial Resistance, and Virulence Profiles of Salmonella Serovars Isolated from Wild Reptiles in South Africa. Int. J. Microbiol. 2024, 2024, 5213895. [Google Scholar] [CrossRef]
- Khasapane, N.G.; Koos, M.; Nkhebenyane, S.J.; Khumalo, Z.T.; Ramatla, T.; Thekisoe, O. Detection of staphylococcus isolates and their antimicrobial resistance profiles and virulence genes from subclinical mastitis cattle milk using MALDI-TOF MS, PCR and sequencing in free state province, South Africa. Animals 2024, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Li, X.; Liu, D.; Hu, X. Serotypes, antibiotic resistance, and virulence genes of Salmonella in children with diarrhea. J. Clin. Lab. Anal. 2020, 34, e23525. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 2018, 16, e05182. [Google Scholar]
- Sun, W.; Qian, X.; Gu, J.; Wang, X.-J.; Duan, M.-L. Mechanism and Effect of Temperature on Variations in Antibiotic Resistance Genes during Anaerobic Digestion of Dairy Manure. Sci. Rep. 2016, 6, 30237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, X.; Xu, T.; Zhang, H.; Sheng, D.; Yin, D. Prevalence of antibiotic resistance genes and their relationship with antibiotics in the Huangpu River and the drinking water sources, Shanghai, China. Sci. Total. Environ. 2013, 458-460, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Ba, Y.; Niu, L.; Lou, F.; Zhang, Z.; Liu, H.; Pan, Y.; Zhao, Y. A Comprehensive Research on Antibiotic Resistance Genes in Microbiota of Aquatic Animals. Front. Microbiol. 2018, 9, 1617. [Google Scholar] [CrossRef]
- Gundran, R.S.; Cardenio, P.A.; Villanueva, M.A.; Sison, F.B.; Benigno, C.C.; Kreausukon, K.; Pichpol, D.; Punyapornwithaya, V. Prevalence and distribution of blaTX-M, blaSHV, blaTEM genes in extended-spectrum β-lactamase-producing E. coli isolates from broiler farms in the Philippines. BMC Vet. Res. 2019, 15, 227. [Google Scholar] [CrossRef]
- Liu, G.; Ding, L.; Han, B.; Piepers, S.; Naqvi, S.A.; Barkema, H.W.; Ali, T.; De Vliegher, S.; Xu, S.; Gao, J. Characteristics of Escherichia coli Isolated from Bovine Mastitis Exposed to Subminimum Inhibitory Concentrations of Cefalotin or Ceftazidime. BioMed Res. Int. 2018, 2018, 4301628. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef] [PubMed]

| Gene | Location | Primer Name | Primer Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temp (°C) |
|---|---|---|---|---|---|
| hilA | SPI-1 | hilA-F hilA-R | GACAGAGCTGGACCACAATAAGACA GAGCGTAATTCATCGCCTAAAC | 312 | 55 °C |
| ssrB | SPI-2 | ssrB-F ssrB-R | CTCATTCTTCGGGCACAGTTA CCTTATTACCCTGGCCTCATTT | 558 | 55 °C |
| marT | SPI-3 | marT- F marT-R | CGTCGTCTCACAACAAACATTC CTGACAAATCAATGCCGTAACC | 556 | 55 °C |
| sopB | SPI-5 | sopB-F sopB-R | TCACTAAAAACCCAGGAGGCTTTT CGCCATCTTTATTGCGGATTTTTA | 1000 | 65 °C |
| pagN | SPI-6 | pagN-F pagN-R | TTCCAGCTTCCAGTACGTTTAG GCCTTTGTGTCTGCATCATAAG | 440 | 55 °C |
| vexA | SPI-7 | vexA-F vexA-R | AAACTAAGCGCTCCCGATAC CAGTCGCGCAGTGAAATAATG | 504 | 55 °C |
| nlpI | SPI-8 | nlpI-F nlpI-R | AGTCTTGGTTTGAGGGCATTAG TTCTTTCGCCTGCTTCTCATTA | 333 | 55 °C |
| bapA | SPI-9 | bapA-F bapA-R | TAAGCGTCGGACTTGGAATG CGTTCTTCAGCGTGTAGGTATAG | 543 | 55 °C |
| pagC | SPI-11 | pagC-F pagC-R | CGCCTTTTCCGTGGGGTATGC GAAGCCGTTTATTTTTGTAGAGGAGATGTT | 454 | 66.5 °C |
| oafA | SPI-12 | oafA-F oafA-R | CGAGTGACTGGAACCAAAGA CAAGCATAGAGCCAGAGTAGAG | 510 | 55 °C |
| spvB | Plasmid | spvB-F spvB-R | CTATCAGCCCCGCACGGAGAGCAGTTTTTA GGAGGAGGCGGTGGCGGTGGCATCATA | 717 | 66.5 °C |
| cdtB | Genome | cdtB-F cdtB-R | ACAACTGTCGCATCTCGCCCCGTCATT CAATTTGCGTGGGTTCTGTAGGTGCGAGT | 268 | 66.5 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramatla, T.; Khasapane, N.G.; Mlangeni, L.N.; Mokgokong, P.; Ramaili, T.; Ndou, R.; Nkhebenyane, J.S.; Lekota, K.; Thekisoe, O. Detection of Salmonella Pathogenicity Islands and Antimicrobial-Resistant Genes in Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Broiler Chickens. Antibiotics 2024, 13, 458. https://doi.org/10.3390/antibiotics13050458
Ramatla T, Khasapane NG, Mlangeni LN, Mokgokong P, Ramaili T, Ndou R, Nkhebenyane JS, Lekota K, Thekisoe O. Detection of Salmonella Pathogenicity Islands and Antimicrobial-Resistant Genes in Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Broiler Chickens. Antibiotics. 2024; 13(5):458. https://doi.org/10.3390/antibiotics13050458
Chicago/Turabian StyleRamatla, Tsepo, Ntelekwane G. Khasapane, Lungile N. Mlangeni, Prudent Mokgokong, Taole Ramaili, Rendani Ndou, Jane S. Nkhebenyane, Kgaugelo Lekota, and Oriel Thekisoe. 2024. "Detection of Salmonella Pathogenicity Islands and Antimicrobial-Resistant Genes in Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Broiler Chickens" Antibiotics 13, no. 5: 458. https://doi.org/10.3390/antibiotics13050458
APA StyleRamatla, T., Khasapane, N. G., Mlangeni, L. N., Mokgokong, P., Ramaili, T., Ndou, R., Nkhebenyane, J. S., Lekota, K., & Thekisoe, O. (2024). Detection of Salmonella Pathogenicity Islands and Antimicrobial-Resistant Genes in Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Broiler Chickens. Antibiotics, 13(5), 458. https://doi.org/10.3390/antibiotics13050458






