A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health
Abstract
1. Introduction
2. Overview of Results
2.1. Epidemiology of Streptococcal Infections
2.1.1. Diseases Caused by S. pneumoniae
| Country | Target Population | (Study Period) Total n | Antimicrobial Resistance | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Europe | Pen | Cro | Ery | Cli | Tet | Levo | ||||
| Bulgaria | Non-IPD, children up to 9 years | (2019–2021) n = 147 | 38.1% | 16.3% | 58.5% | 46.9% | 39.5% | - | [16] | |
| Bulgaria | IPD and non-IPD, all age groups | (2011–2016) n = 198 | 46.5% | 19.7% | 43.9% | 36.4% | 37.4% | 1.0% | [26] | |
| Serbia | Respiratory and IPD isolates from children up to 18 years | (2004–2009) n = 5293 | - | - | 44.9% (2009) | - | - | - | [27] | |
| Croatia | IPD, adults | (2005–2019) n = 1108 | 19.6% IE +R | ≤2% IE | 23.0% | - | - | 0.4% | [28] | |
| Turkey | Healthy children 0–6 years | (2015) n = 150 | 14.3% | - | 47.7% | 52.4% | - | - | [29] | |
| Poland | Children at 2–5 years with recurrent acute pharyngotonsillitis | (2011) n = 57 | 45.1% | - | 52.9% | 51.0% | 43.1% | - | [21] | |
| Spain | IPD, all age groups | (1979–2008) n = 19 266 | 22.3% (in 2008) | 5.9% (in 2005) | 26.6% (in children) 20.7% (in adults) | - | - | - | [30] | |
| USA and Latin America | USA | Non-IPD and IPD, all age groups | (2009–2017) n = 7254 | 13.9–3.8% in period (2009–2017) | 11.6–2.8% | 37.5–45.2% | 19.3–17.0% | 22.4–20.8% | - | [31] |
| Argentina | Children ≤ 6 years with IPD | 2019 (n = 115) | 39.7% | 2.6% | - | - | - | - | [32] | |
| Brazil | Retrospective study for patients with IPD | (2007–2012) n = 328 | 42.8% | 18.6% | - | 7.9% | - | 0.3% | [33] | |
| Asia | Japan | Non-encapsulated SPN | (2011–2019) n = 71 | PISP: 33.8%, PRSP: 33.8% | - | 94.3% | - | - | - | [34] |
| Japan | Pediatric population | (2001, 2004, 2007, 2010, 2012) | 64.6%, 67.0%, 56.2%, 76.9% 49.5%, | - | - | - | - | - | [25] | |
| China | Meta analysis among children with IPD | (2006–2013) | 32% (total n = 1345 | 14.7% (1216) | 94.4% (1396) | 92.3% (1204) | 83.7% (1335) | - | [35] | |
| Africa | Tunisia | Ery-R respiratory and non-respiratory SPN | (2010–2016) n = 86 | 81.4% PNSP 1.2% high R | 64.0% | 39.5% | 2.32% | [36] | ||
| Ethiopia | Hospital-based prospective study, all age groups | (2018–2019) n = 57 | 17.5% | 1.8% | 59.6% | 17.5% | 38.6% | [37] | ||
2.1.2. Infections Caused by Beta-Hemolytic Streptococci
S. pyogenes (GAS) Infections
| Country | (Study Period) Total n | Antimicrobial Resistance | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Europe | Macrolide | Lincosamide | Tetracycline | Qionolone | |||
| Bulgaria | (2013–2016) n = 329 | 23–40% | - | - | - | [14] | |
| Greece | (2018–2023) n = 52 | 20.4% | 18.7% | 40.8% | 2% | [42] | |
| Spain | (2007–2020) n = 1983 | 8.7% | 3.9% | 12.0% | - | [43] | |
| Hungary | (2008–2017) n = 1104 | 10.5% | 9.2% | - | 13.5% | [44] | |
| Russia | (2014–2017) n = 792 | 12.1–17.2% | 2.4% | - | 0.3–0.8% | [45] | |
| North and South America | USA | (2016–2017) n = 3873 | 16–23% | - | 22.6% | 1.4% | [41] |
| Brazil | (2008–2012) n = 92 | 14.3% | 15.4% | 20.9% | 0% | [46] | |
| Asia and Australia | China | (2020–2021) n = 114 | 94.74% | 92.98% | 87.72% | - | [47] |
| China | (2009–2016) n = 140 | 93.5% | 94.2% | 86.4% | - | [48] | |
| Japan | (2007–2008; 2012; 2018) n = 634 | 34.9–60% | - | - | 11.5–14.3% | [49] | |
| Taiwan | (2000–2019) n = 320 | 18.1–58.4% | 6–58.4% | - | - | [6] | |
| Australia | (2007–2021) n = 318 | 6% | - | 10% | 0% | [50] | |
| Africa | Northwest Ethiopia | (2020) n = 14 | 21.4% | 50% | 14.3% | 7.2% | [51] |
| Southwest Ethiopia | (2013) n = 355 | 0% | 0% | 52.5% | - | [52] | |
| Middle East and North Africa region | Cyprus, Saudi Arabia, Egypt, etc. | (1995–2015) review | Ranged from 1.1%–12–70% | - | - | - | [15] |
| Country | (Study Period) Total n | Antimicrobial Resistance | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Europe | Macrolide | Lincosamide | Tetracycline | Qionolone | |||
| Bulgaria | (2018–2019) n = 107 | 58.88% | 15.89% | 94.62% | 10.28% | [17] | |
| Denmark | (2005–2018) n = 1875 | 8.1% (2007) 23.8% (2010) | 6.5% (2006) 20.4% (2009) | - | - | [4] | |
| Denmark | (2018–2019) n = 101 | 21.0% | 26.0% | - | - | [53] | |
| France | (2007–2019) n = 1262 | 21.0% | - | 91.0% | - | [54] | |
| France | (2007–2014) n = 8757 | 36.2% | 26.3% | 86.5% | 0.8% | [55] | |
| Germany | (2009–2010) n = 978 | 22.4% | 14.1% | - | - | [56] | |
| Iceland | (1976–2015) n = 118 | 9.0% | 1.0% | 81.6% | 0% | [57] | |
| Portugal | (2005–2015) n = 218 | 16.1% | 14.2% | 85.8% | - | [58] | |
| Portugal | (2009–2015) n = 555 | 35.1% | 33.9% | - | 0.5% | [59] | |
| Serbia | (2009–2016) n = 432 | 23.1% | - | 86.0% | 0% | [12] | |
| Serbia | (2015–2020) n = 1071 | 26.7% | 22.1% | 85.2% | 0% | [60] | |
| Spain | (2010–2016) n = 242 | 21.5% | 17.6% | - | - | [61] | |
| North and South America | USA | (2008–2016) n = 21,250 | 54.8% | 43.2% | 83.9% | 2.3% | [62] |
| Nicaragua | (2019–2020) n = 85 | 37.6% | 31.7% | - | 0% | [7] | |
| Asia | Iran | (2017) n = 27 | 44.4% | 29.6% | - | 11.1% | [63] |
| China | (2008–2015) n = 193 | 74.1% | 64.2% | 68.9% | - | [64] | |
| China | (2015–2017) n = 304 | 78.3% | 68.2% | 80.1% | - | [65] | |
| Taiwan | (2006–2015) n = 225 | 48.9% | 51.4% | - | - | [66] | |
| Taiwan | (2003–2017) n = 182 | 68.1% | 65.9% | - | - | [67] | |
| Africa | 21 countries | (1989–2019) n = 4564 | 20.82% | 19.63% | 82.6% | 24.56% | [68] |
S. agalactiae (GBS) Infections
2.1.3. Infections Due to Viridans Streptococci
2.2. Evolution of Antibiotic Resistance in Streptococcus genus
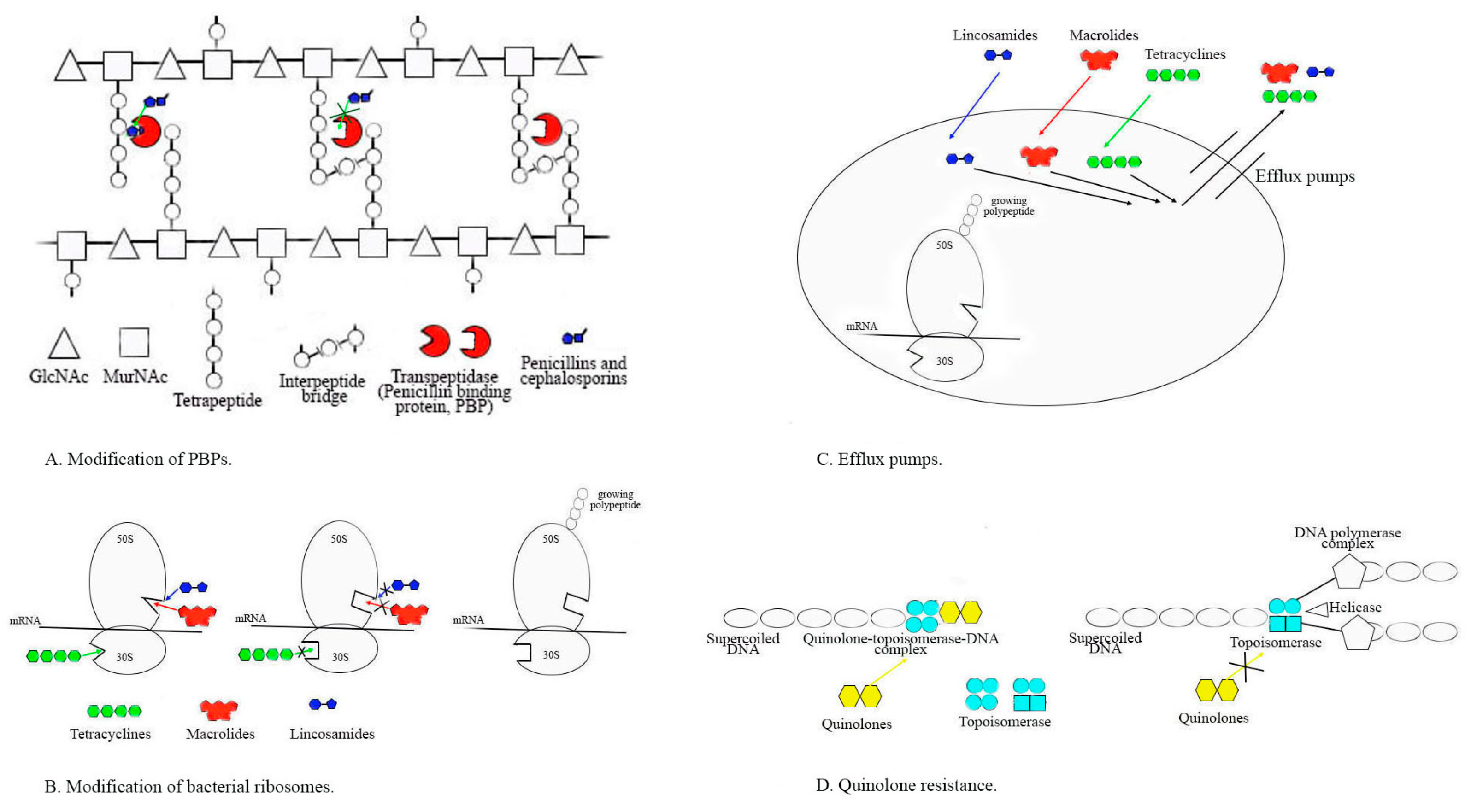
2.2.1. Alterations in PBPs and Susceptibility to Penicillin and Other Beta-Lactams
2.2.2. Resistance to MLSB Antibiotics
2.2.3. Resistance to Tetracyclines
2.2.4. Resistance to Fluoroquinolones
3. Methods
3.1. Search Strategy and Inclusion and Exclusion Criteria
3.2. Quality Assessment and Data Extraction
3.3. Characteristics of Eligible Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creti, R. Have group A and B streptococcal infections become neglected diseases in Europe? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1063–1064. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Duan, N.; Chen, W.; Zhao, X.; Wang, L.; Du, P.; Guo, J. Genomic Epidemiology of Streptococcus pneumoniae Isolated in a Tertiary Hospital in Beijing, China, from 2018 to 2022. Pathogens 2023, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Slotved, H.C.; Hoffmann, S. The Epidemiology of Invasive Group B Streptococcus in Denmark from 2005 to 2018. Front. Public Health 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E. Streptococcus pyogenes Impetigo, Erysipelas, and Cellulitis. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet], 2nd ed.; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, USA, 2022; Chapter 23. Available online: https://www.ncbi.nlm.nih.gov/books/NBK587091/ (accessed on 1 March 2024).
- Tsai, W.C.; Shen, C.F.; Lin, Y.L.; Shen, F.C.; Tsai, P.J.; Wang, S.Y.; Lin, Y.S.; Wu, J.J.; Chi, C.Y.; Liu, C.C. Emergence of macrolide-resistant Streptococcus pyogenes emm12 in southern Taiwan from 2000 to 2019. J. Microbiol. Immunol. Infect. 2021, 54, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Alemán, T.; Vielot, N.A.; Herrera, R.; Velasquez, R.; Berrios, T.; Toval-Ruíz, C.; Téllez, E.; Herrera, A.; Aguilar, S.; Becker-Dreps, S.; et al. Rectovaginal Colonization with Serotypes of Group B Streptococci with Reduced Penicillin Susceptibility among Pregnant Women in León, Nicaragua. Pathogens 2022, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Beres, S.B.; Zhu, L.; Pruitt, L.; Olsen, R.J.; Faili, A.; Kayal, S.; Musser, J.M. Integrative Reverse Genetic Analysis Identifies Polymorphisms Contributing to Decreased Antimicrobial Agent Susceptibility in Streptococcus pyogenes. mBio 2022, 13, e0361821. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zheng, Y.; Yang, Y. Is There Emergence of β-Lactam Antibiotic-Resistant Streptococcus pyogenes in China? Infect. Drug Resist. 2020, 13, 2323–2327. [Google Scholar] [CrossRef]
- Jin, Z.; Li, J.; Zhou, H.; Wang, Z.; Yi, L.; Liu, N.; Du, J.; Chang, C.Y.; Ji, W. Serotype Distribution, Virulence Determinants and Antimicrobial Susceptibility of Streptococcus agalactiae Isolated from Young Infants. Pathogens 2022, 11, 1355. [Google Scholar] [CrossRef]
- Gajic, I.; Mijac, V.; Opavski, N.; Stanojevic, M.; Lazarevic, I.; Åmitran, A.; Hadnadjev, M.; Ranin, L. Distribution of macrolide-resistant genes among isolates of macrolide-resistant Streptoccocus pyogenes and Streptococcus pneumoniae in Serbia. Arch. Biol. Sci. 2014, 66, 93–98. [Google Scholar] [CrossRef]
- Gajic, I.; Plainvert, C.; Kekic, D.; Dmytruk, N.; Mijac, V.; Tazi, A.; Glaser, P.; Ranin, L.; Poyart, C.; Opavski, N. Molecular epidemiology of invasive and non-invasive group B Streptococcus circulating in Serbia. Int. J. Med. Microbiol. 2019, 309, 19–25. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, S.; Pérez-Abeledo, M.; Ramos, B.; García, L.; Arce, A.; Martínez-Arce, R.; Yuste, J.; Sanz, J.C. Distribution of Multidrug-Resistant Invasive Serotypes of Streptococcus pneumoniae during the Period 2007–2021 in Madrid, Spain. Antibiotics 2023, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Muhtarova, A.; Gergova, R.; Mitov, I. Distribution of macrolide resistance mechanisms in Bulgarian clinical isolates of Streptococcus pyogenes during the years of 2013-2016. J. Glob. Antimicrob. Resist. 2017, 10, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Hawli, M.; Osman, M.; Dabboussi, F.; Hamze, M. Distribution of emm types and macrolide resistance determinants among group A streptococci in the Middle East and North Africa region. J. Glob. Antimicrob. Resist. 2020, 22, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, A.; Pencheva, D.; Setchanova, L.; Gergova, R. Association of pili with widespread multidrug-resistant genetic lineages of non-invasive pediatric Streptococcus pneumoniae isolates. Acta Microbiol. Immunol. Hung. 2022, 69, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gergova, R.; Muhtarova, A.; Tsitou, V.M.; Mitov, I. Emergence of multidrug-resistant and-hypervirulent Streptococcus agalactiae in Bulgarian patients. Balkan Med. J. 2021, 38, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.N.; Chang, L.L.; Lai, C.H.; Huang, Y.H.; Chen, W.F.; Yang, C.H.; Hsu, J.; Lin, H.H.; Chen, Y.H. High prevalence of fluoroquinolone-nonsusceptible Streptococcus pyogenes emm12 in Taiwan. Diagn. Microbiol. Infect. Dis. 2015, 83, 187–192. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Report about Antibiotic Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 2 April 2024).
- Gergova, R.; Petrova, G.; Gergov, S.; Minchev, P.; Mitov, I.; Strateva, T. Microbiological features of the upper respiratory tract infections in Bulgarian children for the period 1998-2014 our university’s experience. Balk. Med. J. 2016, 33, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Niedzielski, A.; Korona-Glowniak, I.; Malm, A. High prevalence of Streptococcus pneumoniae in adenoids and nasopharynx in preschool children with recurrent upper respiratory tract infections in Poland--distribution of serotypes and drug resistance patterns. Med. Sci. Monit. 2013, 19, 54–60. [Google Scholar] [CrossRef]
- Brealey, J.C.; Chappell, K.J.; Galbraith, S.; Fantino, E.; Gaydon, J.; Tozer, S.; Young, P.R.; Holt, P.G.; Sly, P.D. Streptococcus pneumoniae colonization of the nasopharynx is associated with increased severity during respiratory syncytial virus infection in young children. Respirology 2017, 23, 220–227. [Google Scholar] [CrossRef]
- Andam, C.P.; Hanage, W.P. Mechanisms of genome evolution of Streptococcus. Infect. Genet. Evol. 2015, 33, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Danino, D.; Weinberger, D.M. The Pneumococcus-Respiratory Virus Connection—Unexpected Lessons from the COVID-19 Pandemic. JAMA Netw. Open 2022, 5, e2218966. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Sato, Y.; Toyonaga, Y.; Hanaki, H.; Sunakawa, K. Nationwide survey of Streptococcus pneumoniae drug resistance in the pediatric field in Japan. Pediatr. Int. 2016, 58, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Setchanova, L.; Murdjeva, M.; Stancheva, I.; Alexandrova, A.; Sredkova, M.; Stoeva, T.; Yoneva, M.; Kurchatova, A.; Mitov, I. Serotype changes and antimicrobial nonsusceptibility rates of invasive and non-invasive Streptococcus pneumoniae isolates after implementation of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Bulgaria. Braz. J. Infect. Dis. 2017, 21, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Berbel, D.; González-Díaz, A.; López de Egea, G.; Càmara, J.; Ardanuy, C. An Overview of Macrolide Resistance in Streptococci: Prevalence, Mobile Elements and Dynamics. Microorganisms 2022, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Butić, I.; Gužvinec, M.; Jelić, M.; Groš, I.; Lucić, S.; Bošnjak, M.; Tambić, A.A. Serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates among Croatian adults during a fifteen-year period (2005–2019). Croat. Med. J. 2022, 63, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Arvas, A.; Çokuğraş, H.; Gür, E.; Gönüllü, N.; Taner, Z.; Tokman, H.B. Pneumococcal nasopharyngeal carriage in young healthy children after pneumococcal conjugate vaccine in Turkey. Balkan Med. J. 2017, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Sempere, J.; González-Camacho, F.; Domenech, M.; Llamosí, M.; Del Río, I.; López-Ruiz, B.; Gimeno, M.; Coronel, P.; Yuste, J. A national longitudinal study evaluating the activity of cefditoren and other antibiotics against non-susceptible Streptococcus pneumoniae strains during the period 2004-20 in Spain. J. Antimicrob. Chemother. 2022, 77, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Suaya, J.; Mendes, R.; Sings, H.; Arguedas, A.; Reinert, R.; Jodar, L.; Isturiz, R.E.; Gessner, B.D. Streptococcus pneumoniae serotype distribution and antimicrobial non susceptibility trends among adults with pneumonia in the United States, 2009–2017. J. Infect. 2020, 81, 557–566. [Google Scholar] [CrossRef]
- von Specht, M.; García Gabarrot, G.; Mollerach, M.; Bonofiglio, L.; Gagetti, P.; Kaufman, S.; Vigliarolo, L.; Toresani, I.; Lopardo, H.A. Resistance to β-lactams in Streptococcus pneumoniae. Rev. Argent. Microbiol. 2021, 53, 266–271. [Google Scholar] [CrossRef]
- Caierão, J.; Hawkins, P.; Sant’anna, F.H.; da Cunha, G.R.; d’Azevedo, P.A.; McGee, L.; Dias, C. Serotypes and genotypes of invasive Streptococcus pneumoniae before and after PCV10 implementation in southern Brazil. PLoS ONE 2014, 9, e111129. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Kudo, K.; Ito, M.; Sumi, A.; Kobayashi, N. Clonal lineages and antimicrobial resistance of nonencapsulated Streptococcus pneumoniae in the post-pneumococcal conjugate vaccine era in Japan. Int. J. Infect. Dis. 2021, 105, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yi, R.; Jiang, Y.; Xu, S.; Qin, P.; Liang, Z.; Chen, J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae causing invasive diseases in China: A meta-analysis. BMC Pediatr. 2019, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.B.; Mehiri, E.; Draoui, H.; Ghariani, A.; Essalah, L.; Raoult, D.; Fournier, P.E.; Slim-Saidi, L.N. Phenotypic and molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae isolated in Tunisia. J. Med. Microbiol. 2020, 69, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Mijac, V.; Opavski, N.; Markovic, M.; Gajic, I.; Vasiljevic, Z.; Sipetic, T.; Bajcetic, M. Trends in macrolide resistance of respiratory tract pathogens in the paediatric population in Serbia from 2004 to 2009. Epidemiol. Infect. 2015, 143, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Gergova, R.; Muhtarova, A.; Mitov, I.; Setchanova, L.; Mihova, K.; Kaneva, R.; Markovska, R. Relation between emm types and virulence gene profiles among Bulgarian Streptococcus pyogenes clinical isolates. Infect. Dis. 2019, 51, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, T.; Tominaga, K.; Shima, T.; Okuno, R.; Kubota, H.; Ogata, K.; Chiba, K.; Katsukawa, C.; Ohya, H.; Tada, Y. Increased prevalence of group A streptococcus isolates in streptococcal toxic shock syndrome cases in Japan from 2010 to 2012. Epidemiol. Infect. 2015, 143, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Katsuragi, S.; Hasegawa, J.; Tanaka, K.; Osato, K.; Nakata, M.; Murakoshi, T.; Sekizawa, A.; Kanayama, N.; Ishiwata, I.; et al. The most common causative bacteria in maternal sepsis-related deaths in Japan were group A Streptococcus: A nationwide survey. J. Infect. Chemother. 2019, 25, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rivers, J.; Mathis, S.; Li, Z.; Velusamy, S.; Nanduri, S.A.; Van Beneden, C.A.; Snippes-Vagnone, P.; McGee, L.; Chochua, s.; et al. Genomic Surveillance of Streptococcus pyogenes Strains Causing Invasive Disease, United States, 2016-2017. Front. Microbiol. 2020, 11, 1547. [Google Scholar] [CrossRef]
- Arêas, G.P.; Schuab, R.B.; Neves, F.P.; Barros, R.R. Antimicrobial susceptibility patterns, emm type distribution and genetic diversity of Streptococcus pyogenes recovered in Brazil. Mem. Inst. Oswaldo Cruz 2014, 109, 935–939. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Beta-Haemolytic Group A, C and G Streptococcal Infections in Southern Hungary: A 10-Year Population-Based Retrospective Survey (2008-2017) and a Review of the Literature. Infect. Drug. Resist. 2020, 13, 4739–4749. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G.; Soulopoulos Ketikidis, A.L.; Floropoulou, N.; Tychala, A.; Kagkalou, G.; Vasilaki, O.; Mantzana, P.; Skoura, L.; Protonotariou, E. Antimicrobial resistance rates of Streptococcus pyogenes in a Greek tertiary care hospital: 6-year data and literature review. New Microbiol. 2023, 46, 37–42. [Google Scholar] [PubMed]
- Butler, T.A.J.; Story, C.; Green, E.; Williamson, K.M.; Newton, P.; Jenkins, F.; Varadhan, H.; van Hal, S. Insights gained from sequencing Australian non-invasive and invasive Streptococcus pyogenes isolates. Microb. Genom. 2024, 10, 001152. [Google Scholar] [CrossRef] [PubMed]
- Sharew, B.; Moges, F.; Yismaw, G.; Abebe, W.; Fentaw, S.; Vestrheim, D.; Tessema, B. Antimicrobial resistance profile and multidrug resistance patterns of Streptococcus pneumoniae isolates from patients suspected of pneumococcal infections in Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Moeineddini, L.; Ghandehari, F.; Khorasani, M.R.; Shoaei, P.; Ebrahimi, N. Macrolide-resistance, capsular genotyping and associated factors of group B Streptococci colonized pregnant women in Isfahan, Iran. Iran J. Microbiol. 2021, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tesfaw, G.; Kibru, G.; Mekonnen, D.; Abdissa, A. Prevalence of group A β-haemolytic Streptococcus among children with pharyngitis in Jimma town, Southwest Ethiopia. Egypt. Soc. Ear Nose Throat Allied Sci. 2015, 16, 35–40. [Google Scholar] [CrossRef]
- Lu, B.; Fang, Y.; Fan, Y.; Chen, X.; Wang, J.; Zeng, J.; Li, Y.; Zhang, Z.; Huang, L.; Li, H.; et al. High Prevalence of Macrolide-resistance and Molecular Characterization of Streptococcus pyogenes Isolates Circulating in China from 2009 to 2016. Front. Microbiol. 2017, 8, 1052. [Google Scholar] [CrossRef] [PubMed]
- Muhtarova, A.; Mihova, K.; Markovska, R.; Mitov, I.; Kaneva, R.; Gergova, R. Molecular emm typing of Bulgarian macrolide-resistant Streptococcus pyogenes isolates. Acta Microbiol. Immunol. Hung. 2019, 67, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Kebede, D.; Admas, A.; Mekonnen, D. Prevalence and antibiotics susceptibility profiles of Streptococcus pyogenes among pediatric patients with acute pharyngitis at Felege Hiwot Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Microbiol. 2021, 21, 135. [Google Scholar] [CrossRef]
- Rafei, R.; Al Iaali, R.; Osman, M.; Dabboussi, F.; Hamze, M. A global snapshot on the prevalent macrolide-resistant emm types of Group A Streptococcus worldwide, their phenotypes and their resistance marker genotypes during the last two decades: A systematic review. Infect. Genet. Evol. 2022, 99, 105258. [Google Scholar] [CrossRef]
- Francois Watkins, L.K.; McGee, L.; Schrag, S.J.; Beall, B.; Jain, J.H.; Pondo, T.; Farley, M.M.; Harrison, L.H.; Zansky, S.M.; Baumbach, J.; et al. Epidemiology of Invasive Group B Streptococcal Infections Among Nonpregnant Adults in the United States, 2008-2016. JAMA Intern. Med. 2019, 179, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kekic, D.; Gajic, I.; Opavski, N.; Kojic, M.; Vukotic, G.; Smitran, A.; Boskovic, L.; Stojkovic, M.; Ranin, L. Trends in molecular characteristics and antimicrobial resistance of group B Streptococci: A multicenter study in Serbia, 2015–2020. Sci. Rep. 2021, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Plainvert, C.; Hays, C.; Touak, G.; Joubrel-Guyot, C.; Dmytruk, N.; Frigo, A.; Poyart, C.; Tazi, A. Multidrug-Resistant Hypervirulent Group B Streptococcus in Neonatal Invasive Infections, France, 2007–2019. Emerg. Infect. Dis. 2020, 26, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Hays, C.; Louis, M.; Plainvert, C.; Dmytruk, N.; Touak, G.; Trieu-Cuot, P.; Poyart, C.; Tazi, A. Changing Epidemiology of Group B Streptococcus Susceptibility to Fluoroquinolones and Aminoglycosides in France. Antimicrob. Agents Chemother. 2016, 60, 7424–7430. [Google Scholar] [CrossRef] [PubMed]
- López, Y.; Parra, E.; Cepas, V.; Sanfeliú, I.; Juncosa, T.; Andreu, A.; Xercavins, M.; Pérez, J.; Sanz, S.; Vergara, A.; et al. Serotype, virulence profile, antimicrobial resistance and macrolide-resistance determinants in Streptococcus agalactiae isolates in pregnant women and neonates in Catalonia, Spain. Enferm. Infect. Microbiol. Clin. 2018, 36, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Slotved, H.-C.; Jens, K.M.; Mohammad, R.K.; Stine, Y.N. The serotype distribution of Streptococcus agalactiae (GBS) carriage isolates among pregnant women having risk factors for early-onset GBS disease: A comparative study with GBS causing invasive infections during the same period in Denmark. BMC Infect. Dis. 2021, 21, 1129. [Google Scholar] [CrossRef]
- Martins, E.R.; Pedroso-Roussado, C.; Melo-Cristino, J.; Ramirez, M. Portuguese Group for the Study of Streptococcal Infections. Streptococcus agalactiae Causing Neonatal Infections in Portugal (2005–2015): Diversification and Emergence of a CC17/PI-2b Multidrug Resistant Sublineage. Front. Microbiol. 2017, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.; Fernandes, T.; Machado, M.P.; Carriço, J.A.; Melo-Cristino, J.; Ramirez, M.; Martins, E.R. Portuguese Group for the Study of Streptococcal Infections. Increasing macrolide resistance among Streptococcus agalactiae causing invasive disease in non-pregnant adults was driven by a single capsular-transformed lineage, Portugal, 2009 to 2015. Euro. Surveill. 2018, 23, 1700473. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, F.; Berg, A.; Wicker, E.; Imm, A.; Krause, G.; Zürn, K.; Berner, R.; Hufnagel, M.; Lander, F. Prevalence of capsular serotype, pilus island distribution, and antibiotic resistance in pediatric and adult invasive group B Streptococcus isolates: Data from a nationwide prospective surveillance study in Germany. Pediatr. Infect. Dis. J. 2021, 40, 76–82. [Google Scholar] [CrossRef]
- Ivanchik, N.V.; Sukhorukova, M.V.; Chagaryan, A.N.; Dekhnich, A.V.; Kozlov, R.S.; Andreev, V.A.; Bekker, G.G.; Varganova, A.N.; Gudkova, L.V.; Ershova, M.G.; et al. Antimicrobial resistance of clinical Streptococcus pyogenes isolates in Russia: The results of multicenter epidemiological study. PEHASus 2014–2017. Antimicrob. Agents Chemother. 2020, 22, 40–45. [Google Scholar] [CrossRef]
- Boscarino, G.; Romano, R.; Iotti, C.; Tegoni, F.; Perrone, S.; Esposito, S. An Overview of Antibiotic Therapy for Early- and Late-Onset Neonatal Sepsis: Current Strategies and Future Prospects. Antibiotics 2024, 13, 250. [Google Scholar] [CrossRef]
- Ji, W.; Liu, H.; Madhi, S.A.; Cunnington, M.; Zhang, Z.; Dangor, Z.; Zhou, H.; Mu, X.; Jin, Z.; Wang, A.; et al. Clinical and Molecular Epidemiology of Invasive Group B Streptococcus Disease among Infants, China. Emerg. Infect. Dis. 2019, 25, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Björnsdóttir, E.S.; Martins, E.R.; Erlendsdóttir, H.; Haraldsson, G.; Melo-Cristino, J.; Ramirez, M.; Kristinsson, K.G. Group B Streptococcal Neonatal and Early Infancy Infections in Iceland, 1976-2015. Pediatr. Infect. Dis. J. 2019, 38, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Chen, X.; Wang, J.; Wang, D.; Zeng, J.; Li, Y.; Li, D.; Zhu, F.; Cui, Y.; Huang, L. Molecular characteristics and antimicrobial resistance in invasive and noninvasive Group B Streptococcus between 2008 and 2015 in China. Diagn. Microbiol. Infect. Dis. 2016, 86, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, J.F.; Lai, M.Y.; Lin, L.C.; Chu, S.M.; Huang, H.R.; Chiang, M.C.; Fu, R.H.; Lu, J.J. Molecular Characteristics and Antimicrobial Resistance of Group B Streptococcus Strains Causing Invasive Disease in Neonates and Adults. Front. Microbiol. 2019, 10, 264. [Google Scholar] [CrossRef]
- Kao, Y.; Tsai, M.H.; Lai, M.Y.; Chu, S.M.; Huang, H.R.; Chiang, M.C.; Fu, R.H.; Lu, J.J.; Hsu, J.F. Emerging serotype III sequence type 17 group B streptococcus invasive infection in infants: The clinical characteristics and impacts on outcomes. BMC Infect. Dis. 2019, 19, 538. [Google Scholar] [CrossRef] [PubMed]
- Gizachew, M.; Tiruneh, M.; Moges, F.; Adefris, M.; Tigabu, Z.; Tessema, B. Streptococcus agalactiae from Ethiopian pregnant women; prevalence, associated factors and antimicrobial resistance: Alarming for prophylaxis. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Halperin, S.A.; Devlieger, R.; Baker, S.; Forte, P.; Wittke, F.; Slobod, K.S.; Dull, P.M. Maternal Immunization with an Investigational Trivalent Group B Streptococcal Vaccine: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Rench, M.A.; Rinaudo, C.D.; Fabbrini, M.; Tuscano, G.; Buffi, G.; Bartolini, E.; Bonacci, S.; Baker, C.J.; Margarit, I. Immune Responses to Invasive Group B Streptococcal Disease in Adults. Emerg. Infect. Dis. 2016, 22, 1877–1883. [Google Scholar] [CrossRef]
- Ahirwar, S.S.; Gupta, M.K.; Snehi, S.K. Dental caries and lactobacillus: Role and ecology in the oral cavity. Int. J. Pharm. Sci. & Res. 2019, 10, 4818–4829. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Bueno, P.C.P.; Cavalheiro, A.J.; Klein, M.I. Effect of Extracts, Fractions, and Isolated Molecules of Casearia sylvestris to Control Streptococcus mutans Cariogenic Biofilm. Antibiotics. 2023, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Setchanova, L.; Alexandrova, A.; Pencheva, D.; Sirakov, I.; Mihova, K.; Kaneva, R.; Mitov, I. Rise of multidrug-resistant Streptococcus pneumoniae clones expressing non-vaccine serotypes among children following introduction of the 10-valent pneumococcal conjugate vaccine in Bulgaria. J. Glob. Antimicrob. Resist. 2018, 15, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Nakano, K.; Masuda, K.; Wada, K.; Ardin, A.C.; Nomura, R.; Ooshima, T. Distribution of oral streptococci highly resistant to amoxicillin in dental plaque specimens from Japanese children and adolescents. J. Med. Microbiol. 2011, 60, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Nemoto, H.; Nakano, K.; Naka, S.; Nomura, R.; Ooshima, T. Amoxicillin-resistant oral streptococci identified in dental plaque specimens from healthy Japanese adults. J. Cardiol. 2012, 59, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V. Mechanisms of Antibiotic Resistance. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books (accessed on 2 April 2024).
- Yu, D.; Guo, D.; Zheng, Y.; Yang, Y. A review of penicillin binding protein and group A Streptococcus with reduced-β-lactam susceptibility. Front. Cell. Infect. Microbiol. 2023, 13, 1117160. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1117160 (accessed on 2 April 2024). [CrossRef] [PubMed]
- Hayes, K.; O’Halloran, F.; Cotter, L. A review of antibiotic resistance in Group B Streptococcus: The story so far. Crit. Rev. Microbiol. 2020, 46, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kimura, K.; Reid, M.; Miyazaki, A.; Banno, H.; Jin, W.; Wachino, J.; Yamada, K.; Arakawa, Y. High isolation rate of MDR group B Streptococci with reduced penicillin susceptibility in Japan. J. Antimicrob. Chemother. 2015, 70, 2725–2728. [Google Scholar] [CrossRef] [PubMed]
- Djuikoue, C.I.; Djoulako, P.D.D.; Wouambo, R.K.; Foutsa, R.Y.; Ngatcheu, D.E.; Apalata, T. Frequency and Antibiotic Susceptibility Patterns of Streptococcus agalactiae Strains Isolated from Women in Yaounde, Cameroon. Microbiol. Res. 2022, 13, 954–962. [Google Scholar] [CrossRef]
- Park, C.; Nichols, M.; Schrag, S.J. Two cases of invasive vancomycin-resistant group B streptococcus infection. N. Engl. J. Med. 2014, 370, 885–886. [Google Scholar] [CrossRef]
- Li, H.; Zhou, L.; Zhao, Y.; Ma, L.; Zhang, H.; Liu, Y.; Liu, X.; Hu, J. Epidemiological analysis of Group A streptococcus infection diseases among children in Beijing, China under COVID-19 pandemic. BMC Pediatr. 2023, 23, 76. [Google Scholar] [CrossRef]
- Ruiz-Garbajosa, P.; Cantón, R. COVID-19: Impact on prescribing and antimicrobial resistance. Rev. Esp. Quimioter. 2021, 34, 63–68. [Google Scholar] [CrossRef]
- Villalón, P.; Bárcena, M.; Medina-Pascual, M.J.; Garrido, N.; Pino-Rosa, S.; Carrasco, G.; Valdezate, S. National Surveillance of Tetracycline, Erythromycin, and Clindamycin Resistance in Invasive Streptococcus pyogenes: A Retrospective Study of the Situation in Spain, 2007–2020. Antibiotics 2023, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, K.; Wajima, T.; Morozumi, M.; Sakuma, M.; Tajima, T.; Matsubara, K.; Itahashi, K.; Iwata, S. Changes in epidemiologic characteristics and antimicrobial resistance of Streptococcus pyogenes isolated over 10 years from Japanese children with pharyngotonsillitis. J. Med. Microbiol. 2020, 69, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Xu, M.; Zhou, Y.; Xing, X.; Shen, A.; Wang, B. A multicomponent vaccine provides immunity against local and systemic infections by Group A Streptococcus across serotypes. mBio 2019, 10, e02600-19. [Google Scholar] [CrossRef] [PubMed]
- Gizachew, M.; Tiruneh, M.; Moges, F.; Tessema, B. Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: A meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Santoro, F.; Santagati, M.; Docquier, J.-D.; Lazzeri, E.; Pastore, G.; Cassone, M.; Oggioni, M.R.; Rossolini, G.M.; Stefani, S.; et al. Type M Resistance to Macrolides Is Due to a Two-GeneEfflux Transport System of the ATP-Binding Cassette (ABC) Superfamily. Front. Microbiol. 2018, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, X.; Li, J.; Ji, W.; Zhou, H.; Gong, X.; Miao, B.; Meng, S.; Duan, L.; Shi, Q.; et al. Molecular characteristics and antibiotic resistance mechanisms of clindamycin-resistant Streptococcus agalactiae isolates in China. Front. Microbiol. 2023, 14, 1138039. [Google Scholar] [CrossRef] [PubMed]
- Mudzana, R.; Mavenyengwa, R.T.; Gudza-Mugabe, M. Analysis of virulence factors and antibiotic resistance genes in group B streptococcus from clinical samples. BMC Infect. Dis. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Ousmane, S.; Diallo, B.A.; Ouedraogo, R. Genetic determinants of tetracycline resistance in clinical Streptococcus pneumoniae serotype 1 isolates from Niger. Antibiotics 2018, 7, 19. [Google Scholar] [CrossRef]
- Jespersen, M.G.; Lacey, J.A.; Tong, S.Y.C.; Davies, M.R. Global genomic epidemiology of Streptococcus pyogenes. Infect. Genet. Evol. 2020, 86, 104609. [Google Scholar] [CrossRef]
- Van Heirstraeten, L.; Leten, G.; Lammens, C.; Goossens, H.; Malhotra-Kumar, S. Increase in fluoroquinolone non-susceptibility among clinical Streptococcus pyogenes in Belgium during 2007–10. J. Antimicrob. Chemother. 2012, 67, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Lin, M.; Bao, J.; Wang, G.; Dong, R.; Zou, P.; Chen, Y.; Li, N.; Zhang, T.; et al. Maternal colonization with group B Streptococcus and antibiotic resistance in China: Systematic review and meta-analyses. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 5. [Google Scholar] [CrossRef]
- Kargar, M.; Moein Jahromi, F.; Doosti, A.; Handali, S. Molecular Investigation of Quinolone Resistance of Quinolone Resistance-Determining Region in Streptococcus pneumoniae Strains Isolated from Iran Using Polymerase Chain Reaction-Restriction Fragment Length Polymorphism Method. Osong Public Health Res. Perspect. 2014, 5, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics 2024, 13, 360. https://doi.org/10.3390/antibiotics13040360
Gergova R, Boyanov V, Muhtarova A, Alexandrova A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics. 2024; 13(4):360. https://doi.org/10.3390/antibiotics13040360
Chicago/Turabian StyleGergova, Raina, Vasil Boyanov, Adile Muhtarova, and Alexandra Alexandrova. 2024. "A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health" Antibiotics 13, no. 4: 360. https://doi.org/10.3390/antibiotics13040360
APA StyleGergova, R., Boyanov, V., Muhtarova, A., & Alexandrova, A. (2024). A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics, 13(4), 360. https://doi.org/10.3390/antibiotics13040360







