Systemic Impact of Subgingival Infection Control in Periodontitis Patients with Cardiovascular Disease: A Narrative Review
Abstract
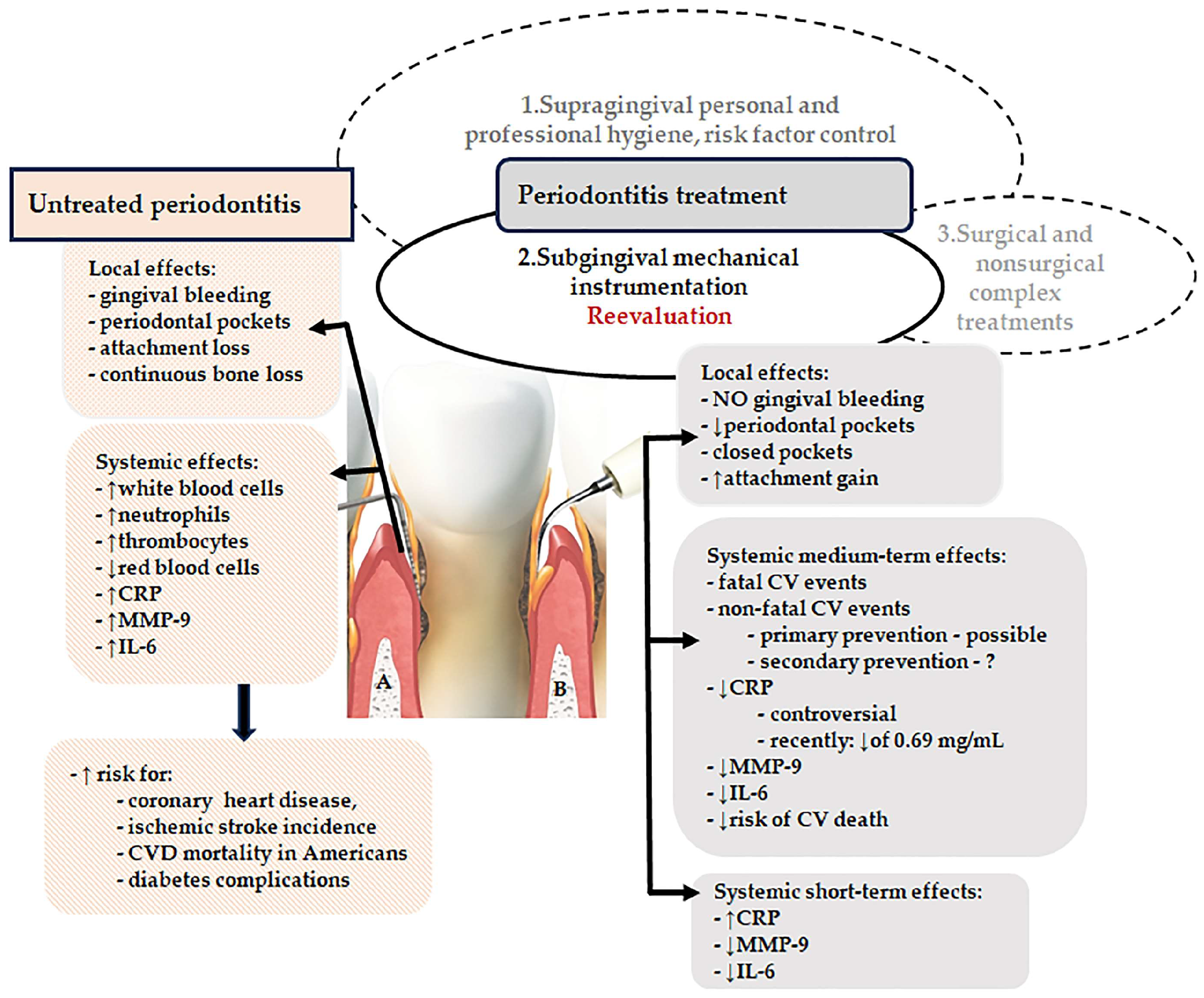
1. Introduction
2. Results
2.1. Medium-Term Effects of Subgingival Mechanical Instrumentation on Mortality- and Morbidity-Related Outcomes in Cardiovascular Disease Patients with Periodontitis
2.2. Medium-Term Effects of Subgingival Mechanical Instrumentation on Inflammatory Markers and Blood Cell Counts in Cardiovascular Disease Patients with Periodontitis
2.3. Adverse Short-Term Effects of Subgingival Mechanical Instrumentation in Periodontitis Patients
3. Discussions
4. Material and Methods
4.1. Literature Search
4.2. Inclusion Criteria
4.3. Data Collection and Synthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyle, J.; Chapple, I. Molecular Aspects of the Pathogenesis of Periodontitis. Periodontol. 2000 2015, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between Periodontal Diseases and Cardiovascular Diseases, Diabetes and Respiratory Diseases: Consensus Report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European Arm of the World Organization of Family Doctors (WONCA Europe). J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Iwasaki, M.; Yoshihara, A.; Miyazaki, H. Association between Periodontitis and Medical Expenditure in Older Adults: A 33-month Follow-up Study. Geriatr. Gerontol. Int. 2016, 16, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, Regional, and National Burden of Severe Periodontitis, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases (CVDs). Who.int. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 April 2024).
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.O.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Sharma, P.; Walter, C.; Weston, P.; Beck, J. The Epidemiological Evidence behind the Association between Periodontitis and Incident Atherosclerotic Cardiovascular Disease. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S70–S84. [Google Scholar] [CrossRef]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef]
- Xu, S.; Song, M.; Xiong, Y.; Liu, X.; He, Y.; Qin, Z. The Association between Periodontal Disease and the Risk of Myocardial Infarction: A Pooled Analysis of Observational Studies. BMC Cardiovasc. Disord. 2017, 17, 50. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal Disease and Coronary Heart Disease Incidence: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.-D.; Zeng, X.-T.; Kwong, J.S.W.; Hua, X.-P. Periodontal Disease and Risk of Coronary Heart Disease: An Updated Meta-Analysis of Prospective Cohort Studies. Int. J. Cardiol. 2015, 201, 469–472. [Google Scholar] [CrossRef]
- Zeng, X.-T.; Leng, W.-D.; Lam, Y.-Y.; Yan, B.P.; Wei, X.-M.; Weng, H.; Kwong, J.S.W. Periodontal Disease and Carotid Atherosclerosis: A Meta-Analysis of 17,330 Participants. Int. J. Cardiol. 2016, 203, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Glob. Heart 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological Effects of Ionizing Radiation: Endothelial Activation and Dysfunction. Cell. Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Bush, R.B.; Paju, S. Associations between Periodontal Disease and Risk for Nosocomial Bacterial Pneumonia and Chronic Obstructive Pulmonary Disease. A Systematic Review. Ann. Periodontol. 2003, 8, 54–69. [Google Scholar] [CrossRef]
- Janket, S.-J.; Baird, A.E.; Chuang, S.-K.; Jones, J.A. Meta-Analysis of Periodontal Disease and Risk of Coronary Heart Disease and Stroke. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 559–569. [Google Scholar] [CrossRef]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The Prevalence and Incidence of Coronary Heart Disease Is Significantly Increased in Periodontitis: A Meta-Analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Risk of Incident Cardiovascular Disease in People with Periodontal Disease: A Systematic Review and Meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 109–122. [Google Scholar] [CrossRef]
- Rydén, L.; Buhlin, K.; Ekstrand, E.; de Faire, U.; Gustafsson, A.; Holmer, J.; Kjellström, B.; Lindahl, B.; Norhammar, A.; Nygren, Å.; et al. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report from the PAROKRANK Study. Circulation 2016, 133, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Coelho, J.M.F.; Miranda, S.S.; Cruz, S.S.; Trindade, S.C.; Cerqueira, E.M.M.; Passos-Soares, J.S.; da Costa, M.C.N.; Vianna, M.I.P.; Figueiredo, A.C.M.G.; et al. Severe and Moderate Periodontitis Are Associated with Acute Myocardial Infarction. J. Periodontol. 2020, 91, 1444–1452. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, B.; Huo, N.; Cai, C.; Liu, H.; Xu, J. Association between Myocardial Infarction and Periodontis: A Meta -Analysis of Case-Control Studies. Front. Physiol. 2016, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, N.; Ahlqvist, J.; Näslund, U.; Buhlin, K.; Gustafsson, A.; Kjellström, B.; Klinge, B.; Rydén, L.; Levring Jäghagen, E. Associations among Periodontitis, Calcified Carotid Artery Atheromas, and Risk of Myocardial Infarction. J. Dent. Res. 2020, 99, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-Analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Merchant, A.T. The Association between Periodontitis and Cardiovascular Disease: An Update. Curr. Atheroscler. Rep. 2020, 22, 1–5. [Google Scholar] [CrossRef]
- Isola, G.; Santonocito, S.; Lupi, S.M.; Polizzi, A.; Sclafani, R.; Patini, R.; Marchetti, E. Periodontal Health and Disease in the Context of Systemic Diseases. Mediat. Inflamm. 2023, 2023, 9720947. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, S.; Ouyang, X.; Wang, Y.; Jiang, Y.; An, N. Crosstalk between Akt and NF-κB Pathway Mediates Inhibitory Effect of Gas6 on Monocytes-endothelial Cells Interactions Stimulated by P. Gingivalis-LPS. J. Cell. Mol. Med. 2020, 24, 7979–7990. [Google Scholar] [CrossRef]
- Deng, H.; Gong, Y.; Chen, Y.; Zhang, G.; Chen, H.; Cheng, T.; Jin, L.; Wang, Y. Porphyromonas gingivalis Lipopolysaccharide Affects the Angiogenic Function of Endothelial Progenitor Cells via Akt/FoxO1 Signaling. J. Periodontal. Res. 2022, 57, 859–868. [Google Scholar] [CrossRef]
- Vázquez-Reza, M.; Custodia, A.; López-Dequidt, I.; Aramburu-Núñez, M.; Romaus-Sanjurjo, D.; Ouro, A.; Botelho, J.; Machado, V.; Iglesias-Rey, R.; Pías-Peleteiro, J.M.; et al. Periodontal Inflammation Is Associated with Increased Circulating Levels of Endothelial Progenitor Cells: A Retrospective Cohort Study in a High Vascular Risk Population. Ther. Adv. Chronic Dis. 2023, 14, 20406223231178276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, S.; Ren, J.; Zou, H.; Liu, Y.; Chen, Y.; Qiu, Y.; Zhuang, W.; Tao, J.; Yang, J. Association of Enhanced Circulating Trimethylamine N-oxide with Vascular Endothelial Dysfunction in Periodontitis Patients. J. Periodontol. 2022, 93, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.-H.; Chen, C.-Y.; Chen, I.-C.; Huang, H.-L.; Lu, Y.-W.; Kuo, C.-S.; Chang, C.-C.; Huang, P.-H.; Chen, J.-W.; Lin, S.-J. Trimethylamine N-Oxide, Circulating Endothelial Progenitor Cells, and Endothelial Function in Patients with Stable Angina. Sci. Rep. 2019, 9, 4249. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G., III; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nemoto, H.; Nomura, R.; Inaba, H.; Yoshioka, H.; Taniguchi, K.; Amano, A.; Ooshima, T. Detection of Oral Bacteria in Cardiovascular Specimens. Oral Microbiol. Immunol. 2009, 24, 64–68. [Google Scholar] [CrossRef]
- Joshi, C.; Bapat, R.; Anderson, W.; Dawson, D.; Hijazi, K.; Cherukara, G. Detection of Periodontal Microorganisms in Coronary Atheromatous Plaque Specimens of Myocardial Infarction Patients: A Systematic Review and Meta-Analysis. Trends Cardiovasc. Med. 2021, 31, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Gaetti-Jardim, E.; Marcelino, S.L.; Feitosa, A.C.R.; Romito, G.A.; Avila-Campos, M.J. Quantitative Detection of Periodontopathic Bacteria in Atherosclerotic Plaques from Coronary Arteries. J. Med. Microbiol. 2009, 58, 1568–1575. [Google Scholar] [CrossRef]
- Pessi, T.; Karhunen, V.; Karjalainen, P.P.; Ylitalo, A.; Airaksinen, J.K.; Niemi, M.; Pietila, M.; Lounatmaa, K.; Haapaniemi, T.; Lehtimäki, T.; et al. Bacterial Signatures in Thrombus Aspirates of Patients with Myocardial Infarction. Circulation 2013, 127, 1219–1228. [Google Scholar] [CrossRef]
- Ye, Z.; Cao, Y.; Miao, C.; Liu, W.; Dong, L.; Lv, Z.; Iheozor-Ejiofor, Z.; Li, C. Periodontal Therapy for Primary or Secondary Prevention of Cardiovascular Disease in People with Periodontitis. Cochrane Libr. 2022, 10, CD009197. [Google Scholar] [CrossRef]
- Liu, W.; Cao, Y.; Dong, L.; Zhu, Y.; Wu, Y.; Lv, Z.; Iheozor-Ejiofor, Z.; Li, C. Periodontal Therapy for Primary or Secondary Prevention of Cardiovascular Disease in People with Periodontitis. Cochrane Libr. 2019, 12, CD009197. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- López, N.J.; Quintero, A.; Casanova, P.A.; Ibieta, C.I.; Baelum, V.; López, R. Effects of Periodontal Therapy on Systemic Markers of Inflammation in Patients with Metabolic Syndrome: A Controlled Clinical Trial. J. Periodontol. 2012, 83, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Couper, D.J.; Falkner, K.L.; Graham, S.P.; Grossi, S.G.; Gunsolley, J.C.; Madden, T.; Maupome, G.; Offenbacher, S.; Stewart, D.D.; et al. The Periodontitis and Vascular Events (PAVE) Pilot Study: Adverse Events. J. Periodontol. 2008, 79, 90–96. [Google Scholar] [CrossRef]
- Couper, D.J.; Beck, J.D.; Falkner, K.L.; Graham, S.P.; Grossi, S.G.; Gunsolley, J.C.; Madden, T.; Maupome, G.; Offenbacher, S.; Stewart, D.D.; et al. The Periodontitis and Vascular Events (PAVE) Pilot Study: Recruitment, Retention, and Community Care Controls. J. Periodontol. 2008, 79, 80–89. [Google Scholar] [CrossRef]
- Offenbacher, S.; Beck, J.D.; Moss, K.; Mendoza, L.; Paquette, D.W.; Barrow, D.A.; Couper, D.J.; Stewart, D.D.; Falkner, K.L.; Graham, S.P.; et al. Results from the Periodontitis and Vascular Events (PAVE) Study: A Pilot Multicentered, Randomized, Controlled Trial to Study Effects of Periodontal Therapy in a Secondary Prevention Model of Cardiovascular Disease. J. Periodontol. 2009, 80, 190–201. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Ready, D.; Tonetti, M.S. Periodontal Disease and C-reactive Protein-associated Cardiovascular Risk. J. Periodontal. Res. 2004, 39, 236–241. [Google Scholar] [CrossRef]
- Souza, D.; Okawa, A.B.; Silva, R.T.; Araújo, C.O. Short-Term Changes on C Reactive Protein (CRP) Levels after Non-Surgical Periodontal Treatment in Systemically Healthy Individuals. Clin. Oral Investig. 2017, 21, 477–484. [Google Scholar] [CrossRef]
- Subha, D.S.; Pradeep, T. Periodontal Therapy with 0.25% Lemongrass Oil Mouthwash in Reducing Risk of Cardiovascular Diseases: A 3-Arm Prospective Parallel Experimental Study. Ethiop. J. Health Sci. 2017, 27, 531–540. [Google Scholar] [CrossRef]
- Hussain Bokhari, S.A.; Khan, A.A.; Tatakis, D.N.; Azhar, M.; Hanif, M.; Izhar, M. Non-surgical Periodontal Therapy Lowers Serum Inflammatory Markers: A Pilot Study. J. Periodontol. 2009, 80, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Malekzadeh, T.; Dongari-Bagtzoglou, A. Effect of Periodontal Treatment on Serum C-reactive Protein Levels: A Systematic Review and Meta-analysis. J. Periodontol. 2006, 77, 1635–1642. [Google Scholar] [CrossRef]
- Orlandi, M.; Muñoz Aguilera, E.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the Treatment of Periodontitis on Systemic Health and Quality of Life: A Systematic Review. J. Clin. Periodontol. 2022, 49 (Suppl. 24), 314–327. [Google Scholar] [CrossRef]
- Disidoro, O.; Perrotti, V.; Hui, W.L.; Piattelli, A.; Iaculli, F.; Quaranta, A. The Impact of Non-Surgical Therapy of Periodontal Disease on Surrogate Markers for Cardiovascular Disease: A Literature Review. Am. J. Dent. 2019, 32, 191–200. [Google Scholar]
- Lam, O.L.T.; Zhang, W.; Samaranayake, L.P.; Li, L.S.W.; McGrath, C. A Systematic Review of the Effectiveness of Oral Health Promotion Activities among Patients with Cardiovascular Disease. Int. J. Cardiol. 2011, 151, 261–267. [Google Scholar] [CrossRef]
- Roca-Millan, E.; Gonzalez-Navarro, B.; Sabater-Recolons, M.M.; Mari-Roig, A.; Jane-Salas, E.; Lopez-Lopez, J. Periodontal Treatment on Patients with Cardiovascular Disease: Systematic Review and Meta-Analysis. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e681–e690. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef]
- Pitiphat, W.; Matangkasombut, O.; Merchant, A. Letter to the Editor: Re: Effect of Periodontal Treatment on Serum C-reactive Protein Levels. J. Periodontol. 2007, 78, 1184–1185. [Google Scholar] [CrossRef] [PubMed]
- Hada, D.S.; Garg, S.; Ramteke, G.B.; Ratre, M.S. Effect of Non-surgical Periodontal Treatment on Clinical and Biochemical Risk Markers of Cardiovascular Disease: A Randomized Trial. J. Periodontol. 2015, 86, 1201–1211. [Google Scholar] [CrossRef]
- Luthra, S.; Orlandi, M.; Hussain, S.B.; Leira, Y.; Botelho, J.; Machado, V.; Mendes, J.J.; Marletta, D.; Harden, S.; D’Aiuto, F. Treatment of Periodontitis and C-reactive Protein: A Systematic Review and Meta-analysis of Randomized Clinical Trials. J. Clin. Periodontol. 2023, 50, 45–60. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Mahendra, J.; Muralidharan, J.; Srinivasan, S.; Mahendra, L.; Cherian, S.M.; Fathima, L.; Prakash, P.; Namasivayam, A.; Dave, P.H.; Bedi, M.; et al. Calprotectin and Periostin Levels in Periodontitis Patients with Coronary Artery Disease. Oral Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Duan, X.Q.; Hu, R.; Ouyang, X.Y. Effect of Non-Surgical Periodontal Therapy on Serum Levels of TNF-a, IL-6 and C-Reactive Protein in Periodontitis Subjects with Stable Coronary Heart Disease. Chin. J. Dent. Res. 2013, 16, 145–151. [Google Scholar]
- Jervøe-Storm, P.-M.; Eberhard, J.; Needleman, I.; Worthington, H.V.; Jepsen, S. Full-Mouth Treatment Modalities (within 24 Hours) for Periodontitis in Adults. Cochrane Libr. 2022, 6, CD004622. [Google Scholar] [CrossRef]
- Eberhard, J.; Jepsen, S.; Jervøe-Storm, P.-M.; Needleman, I.; Worthington, H.V. Full-Mouth Treatment Modalities (within 24 Hours) for Chronic Periodontitis in Adults. Cochrane Libr. 2015, 4, CD004622. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Cei, S.; Orlandi, M.; Gennai, S.; Gabriele, M.; Filice, N.; Nisi, M.; D’Aiuto, F. Acute-phase Response Following Full-mouth versus Quadrant Non-surgical Periodontal Treatment: A Randomized Clinical Trial. J. Clin. Periodontol. 2015, 42, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Gennai, S.; Marruganti, C.; Peric, M.; Ghiadoni, L.; Marhl, U.; Petrini, M. Acute-phase Response Following One-stage Full-mouth versus Quadrant Non-surgical Periodontal Treatment in Subjects with Comorbid Type 2 Diabetes: A Randomized Clinical Trial. J. Clin. Periodontol. 2023, 50, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.C.; Cortelli, J.R.; Cortelli, S.C.; Miranda Cota, L.O.; Machado Costa, L.C.; Moreira Castro, M.V.; Oliveira Azevedo, A.M.; Costa, F.O. Clinical and Microbiologic Evaluation of Scaling and Root Planing per Quadrant and One-stage Full-mouth Disinfection Associated with Azithromycin or Chlorhexidine: A Clinical Randomized Controlled Trial. J. Periodontol. 2015, 86, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A.; Kinane, D.F. Quadrant Root Planing versus Same-day Full-mouth Root Planing: I. Clinical Findings. J. Clin. Periodontol. 2004, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Del Peloso Ribeiro, É.; Bittencourt, S.; Sallum, E.A.; Nociti, F.H., Jr.; Gonçalves, R.B.; Casati, M.Z. Periodontal Debridement as a Therapeutic Approach for Severe Chronic Periodontitis: A Clinical, Microbiological and Immunological Study. J. Clin. Periodontol. 2008, 35, 789–798. [Google Scholar] [CrossRef]
- Koshy, G.; Kawashima, Y.; Kiji, M.; Nitta, H.; Umeda, M.; Nagasawa, T.; Ishikawa, I. Effects of Single-visit Full-mouth Ultrasonic Debridement versus Quadrant-wise Ultrasonic Debridement. J. Clin. Periodontol. 2005, 32, 734–743. [Google Scholar] [CrossRef]
- Slade, G.D.; Offenbacher, S.; Beck, J.D.; Heiss, G.; Pankow, J.S. Acute-Phase Inflammatory Response to Periodontal Disease in the US Population. J. Dent. Res. 2000, 79, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Craandijk, J.; Hoek, F.J.; Dillen, P.M.E.W.; Van Der Velden, U. Elevation of Systemic Markers Related to Cardiovascular Diseases in the Peripheral Blood of Periodontitis Patients. J. Periodontol. 2000, 71, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Torrungruang, K.; Vathesatogkit, P.; Mahanonda, R.; Thienpramuk, L. Periodontitis and Hypertension Are Linked through Systemic Inflammation: A 5-year Longitudinal Study. J. Clin. Periodontol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory Processes in Cardiovascular Disease: A Route to Targeted Therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rammos, A.; Bechlioulis, A.; Kekiopoulou, A.; Kekiopoulos, P.; Katsouras, C.S.; Sioka, C. Myocardial Perfusion Imaging and C-Reactive Protein in Myocardial Ischemia: A Retrospective Single-Center Study. Life 2024, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Lapić, I.; Padoan, A.; Bozzato, D.; Plebani, M. Erythrocyte Sedimentation Rate and C-Reactive Protein in Acute Inflammation. Am. J. Clin. Pathol. 2020, 153, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Perera, C.; McNeil, H.P.; Geczy, C.L. S100 Calgranulins in Inflammatory Arthritis. Immunol. Cell Biol. 2010, 88, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Marriott, E.; Singanayagam, A.; El-Awaisi, J. Inflammation as the Nexus: Exploring the Link between Acute Myocardial Infarction and Chronic Obstructive Pulmonary Disease. Front. Cardiovasc. Med. 2024, 11, 1362564. [Google Scholar] [CrossRef] [PubMed]
- Haririan, H.; Andrukhov, O.; Pablik, E.; Neuhofer, M.; Moritz, A.; Rausch-Fan, X. Comparative Analysis of Calcium-binding Myeloid-related Protein-8/14 in Saliva and Serum of Patients with Periodontitis and Healthy Individuals. J. Periodontol. 2016, 87, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Punj, V.; Kim, J.-M.; Lee, S.; Ulmer, T.S.; Lu, W.; Rice, J.C.; An, W. MMP-9 Facilitates Selective Proteolysis of the Histone H3 Tail at Genes Necessary for Proficient Osteoclastogenesis. Genes Dev. 2016, 30, 208–219. [Google Scholar] [CrossRef]
- Kim, H.-D.; Kim, S.; Jeon, S.; Kim, S.-J.; Cho, H.-J.; Choi, Y.-N. Diagnostic and Prognostic Ability of Salivary MMP-9 and S100A8 for Periodontitis. J. Clin. Periodontol. 2020, 47, 1191–1200. [Google Scholar] [CrossRef]
- Honore, P.M.; Perriens, E.; Blackman, S. Potential Confounders in Linking Elevated S100A8/A9 to Left Ventricular Dysfunction in Septic Shock Patients. Crit. Care 2023, 27, 480. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; de Salis, I.; Hamilton, W.; Salisbury, C. ‘I’m Fishing Really—Inflammatory Marker Testing in Primary Care: A Qualitative Study. Br. J. Gen. Pract. 2016, 66, e200–e206. [Google Scholar] [CrossRef] [PubMed]
- Irwandi, R.A.; Kuswandani, S.O.; Harden, S.; Marletta, D.; D’Aiuto, F. Circulating Inflammatory Cell Profiling and Periodontitis: A Systematic Review and Meta-Analysis. J. Leukoc. Biol. 2022, 111, 1069–1096. [Google Scholar] [CrossRef]
- de França, L.F.C.; da Silva, F.R.P.; di Lenardo, D.; Alves, E.H.P.; Nascimento, H.M.S.; da Silva, I.A.T.; Vasconcelos, A.C.C.G.; Vasconcelos, D.F.P. Comparative Analysis of Blood Parameters of the Erythrocyte Lineage between Patients with Chronic Periodontitis and Healthy Patients: Results Obtained from a Meta-Analysis. Arch. Oral Biol. 2019, 97, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Darbar, U.; Rakmanee, T.; Donos, N. Anemia of Inflammation Associated with Periodontitis: Analysis of Two Clinical Studies. J. Periodontol. 2019, 90, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Angkananard, T.; Anothaisintawee, T.; McEvoy, M.; Attia, J.; Thakkinstian, A. Neutrophil Lymphocyte Ratio and Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2018, 2018, 2703518. [Google Scholar] [CrossRef]
- Do Lee, C. White Blood Cell Count and Incidence of Coronary Heart Disease and Ischemic Stroke and Mortality from Cardiovascular Disease in African-American and White Men and Women: Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2001, 154, 758–764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caloian, C.S.; Ciurea, A.; Negucioiu, M.; Roman, A.; Micu, I.C.; Picoș, A.; Soancă, A. Systemic Impact of Subgingival Infection Control in Periodontitis Patients with Cardiovascular Disease: A Narrative Review. Antibiotics 2024, 13, 359. https://doi.org/10.3390/antibiotics13040359
Caloian CS, Ciurea A, Negucioiu M, Roman A, Micu IC, Picoș A, Soancă A. Systemic Impact of Subgingival Infection Control in Periodontitis Patients with Cardiovascular Disease: A Narrative Review. Antibiotics. 2024; 13(4):359. https://doi.org/10.3390/antibiotics13040359
Chicago/Turabian StyleCaloian, Carmen Silvia, Andreea Ciurea, Marius Negucioiu, Alexandra Roman, Iulia Cristina Micu, Andrei Picoș, and Andrada Soancă. 2024. "Systemic Impact of Subgingival Infection Control in Periodontitis Patients with Cardiovascular Disease: A Narrative Review" Antibiotics 13, no. 4: 359. https://doi.org/10.3390/antibiotics13040359
APA StyleCaloian, C. S., Ciurea, A., Negucioiu, M., Roman, A., Micu, I. C., Picoș, A., & Soancă, A. (2024). Systemic Impact of Subgingival Infection Control in Periodontitis Patients with Cardiovascular Disease: A Narrative Review. Antibiotics, 13(4), 359. https://doi.org/10.3390/antibiotics13040359







