Intrapulmonary and Systemic Pharmacokinetics of Colistin Following Nebulization of Low-Dose Colistimethate Sodium in Patients with Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii
Abstract
1. Introduction
2. Results
2.1. Study Population
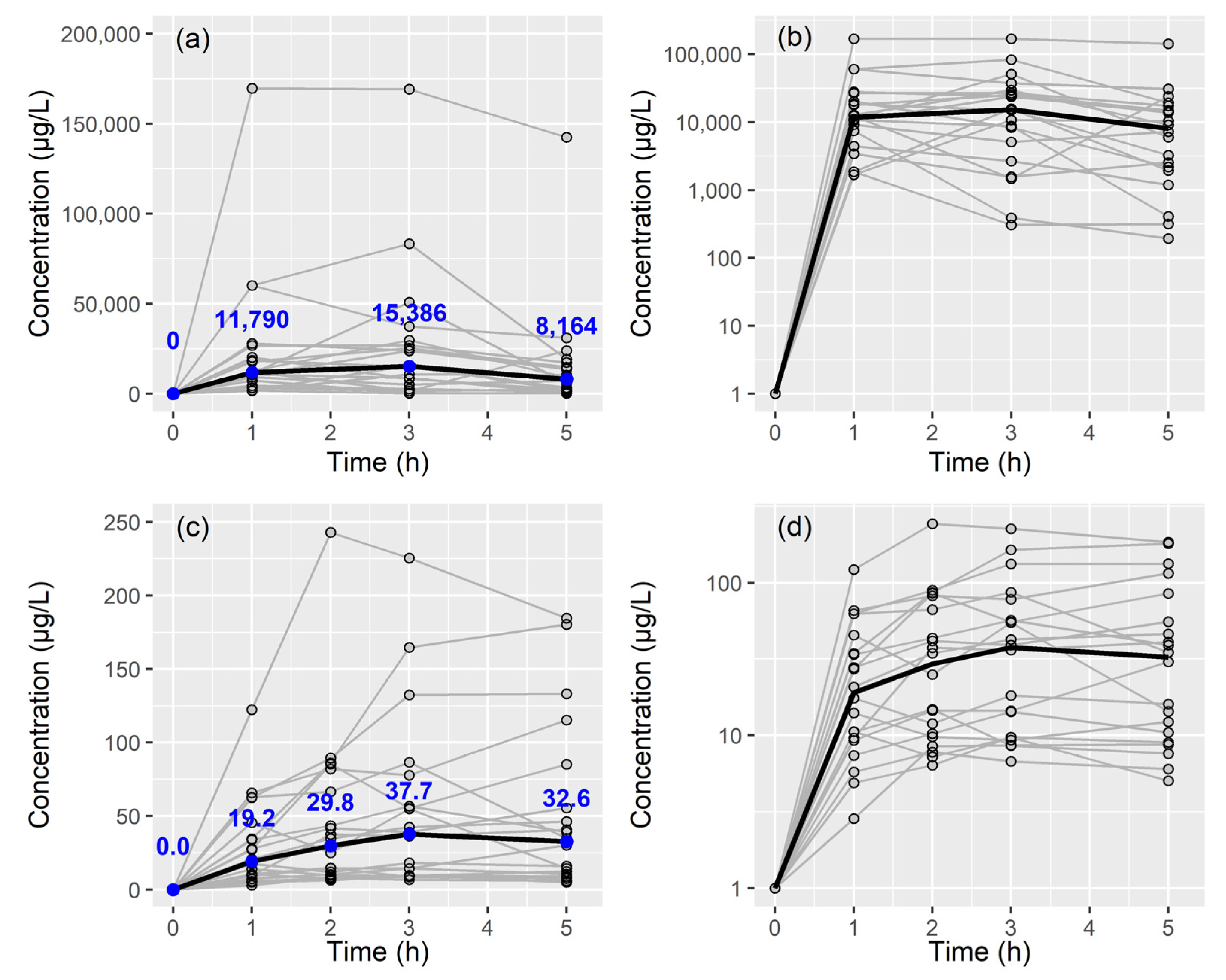
2.2. Colistin A and B Assay in BAL Fluid and Plasma
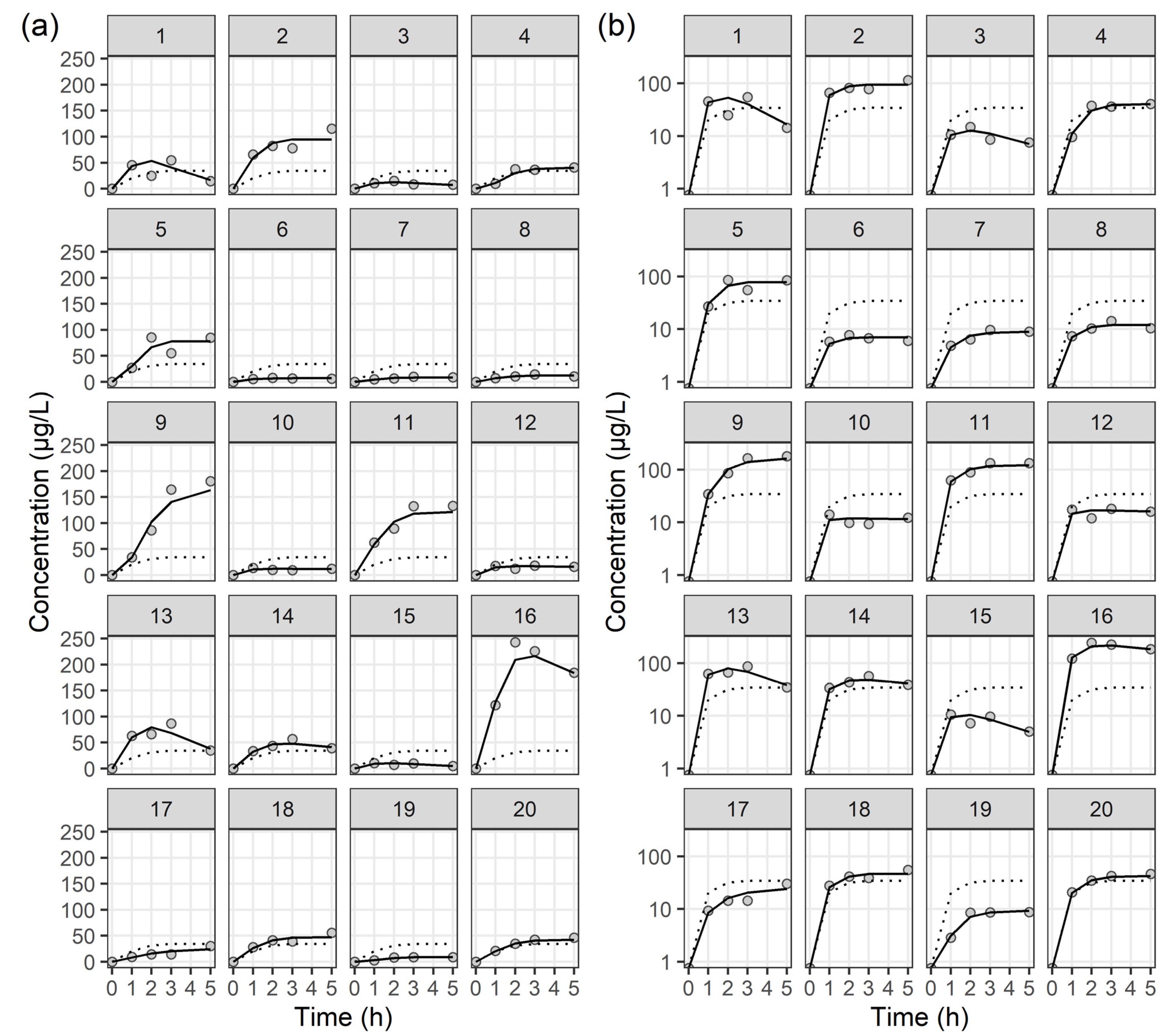
2.3. Population PK Analysis
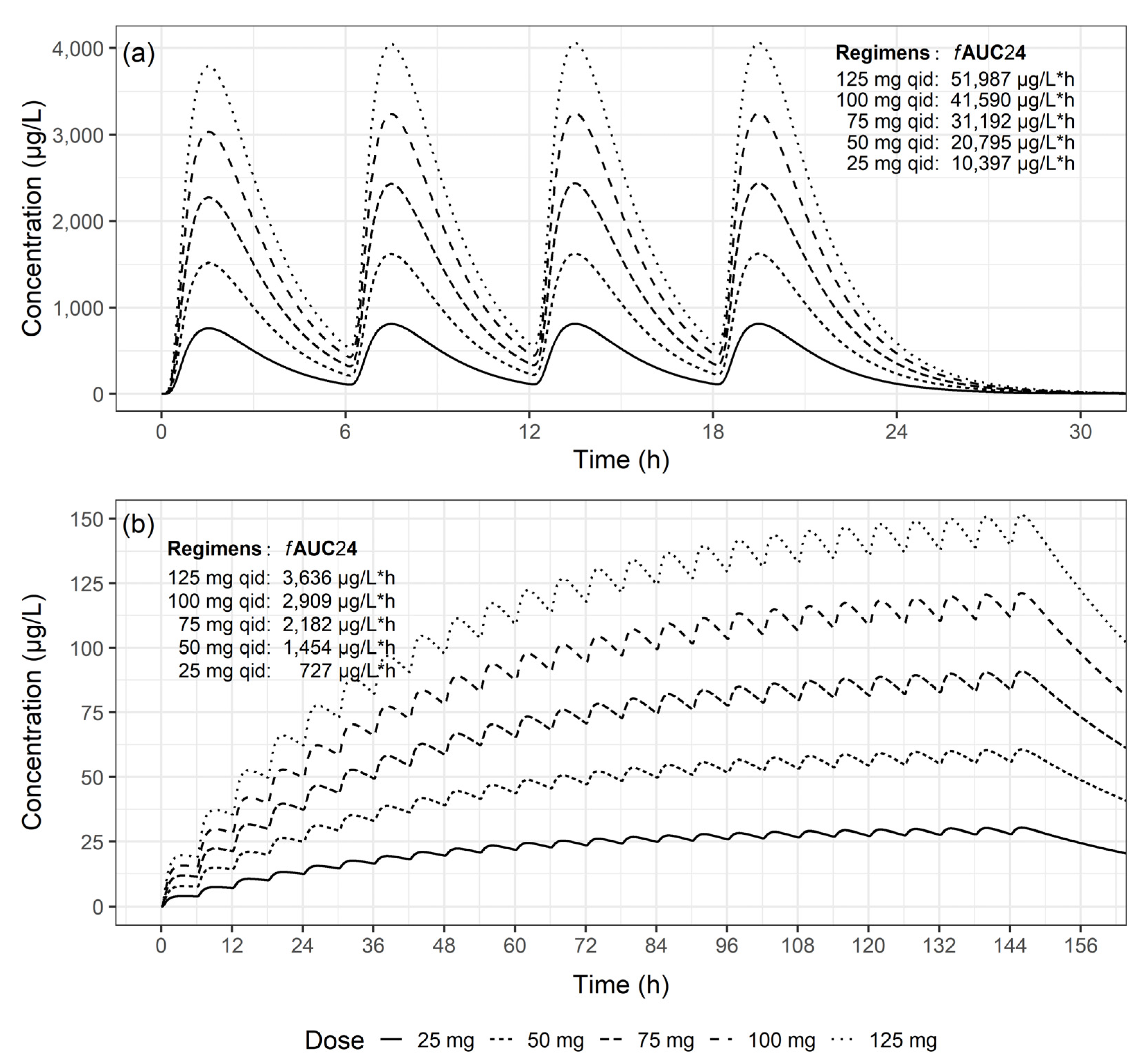
2.4. PK/PD Properties
3. Discussion
4. Materials and Methods
4.1. Study Population
- Adults aged 18 years and older, who had undergone endotracheal intubation or initiation of mechanical ventilation, and were diagnosed with VAP.
- Pneumonia was defined as the presence of new or worsening radiographic opacities on chest X-rays, accompanied by two or more of the following criteria: fever exceeding 38 °C, purulent tracheal secretions, and an elevated (>11,000/µL) or reduced white blood cell count (<4000/µL).
- VAP was defined according to the 2016 clinical practice guideline by the Infectious Diseases Society of America and the American Thoracic Society [14] as pneumonia in patients receiving mechanical ventilation that occurred at least 48 h after endotracheal intubation.
- Detection of CRAB bacteria exclusively in respiratory samples obtained through BAL or endobronchial aspiration, based on the specified criteria (growth thresholds considered significant at 103 colony forming unit (CFU)/mL for endotracheal bronchial aspiration and 104 CFU/mL for BAL fluid).
- The quality and suitability of the samples was judged according to Murray and Washington’s grading system [30], and only samples belonging to Grade 5 (epithelial cell count < 10/LPF, white blood cell count > 25/LPF).
- Patients were not eligible to participate if their legal representative did not provide consent or if the infecting pathogen was not susceptible to colistin.
4.2. Colistin Administration and Sampling Procedures
4.3. Colistin Assay in BAL Fluid and Plasma
4.4. Population PK Analysis
4.5. PK/PD Properties
4.6. Definition of Clinical Course Assessment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Ollendorf, D.A.; Oster, G.; Vera-Llonch, M.; Bellm, L.; Redman, R.; Kollef, M.H.; VAP Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002, 122, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Kollef, K.E.; Schramm, G.E.; Wills, A.R.; Reichley, R.M.; Micek, S.T.; Kollef, M.H. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 2008, 134, 281–287. [Google Scholar] [CrossRef]
- Siempos, I.I.; Vardakas, K.Z.; Kyriakopoulos, C.E.; Ntaidou, T.K.; Falagas, M.E. Predictors of mortality in adult patients with ventilator-associated pneumonia: A meta-analysis. Shock 2010, 33, 590–601. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. S1), S81–S87. [Google Scholar] [CrossRef] [PubMed]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S.; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Shorr, A.F. Review of studies of the impact on Gram-negative bacterial resistance on outcomes in the intensive care unit. Crit. Care Med. 2009, 37, 1463–1469. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, J.Y.; Lee, C.H.; Jang, S.J.; Lee, H.; Yong, D.; Jeong, S.H.; Lee, K. Increasing Resistance to Extended-Spectrum Cephalosporins, Fluoroquinolone, and Carbapenem in Gram-Negative Bacilli and the Emergence of Carbapenem Non-Susceptibility in Klebsiella pneumoniae: Analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) Data From 2013 to 2015. Ann. Lab. Med. 2017, 37, 231–239. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Imberti, R.; Cusato, M.; Villani, P.; Carnevale, L.; Iotti, G.A.; Langer, M.; Regazzi, M. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 2010, 138, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Monsel, A.; Roberts, J.A.; Pontikis, K.; Mimoz, O.; Rello, J.; Qu, J.; Rouby, J.J.; European Investigators Network for Nebulized Antibiotics in Ventilator-Associated Pneumonia. Nebulized Colistin in Ventilator-Associated Pneumonia and Tracheobronchitis: Historical Background, Pharmacokinetics and Perspectives. Microorganisms 2021, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Kovanda, L.; Kowalski, D.; Lu, Q.; Townsend, R.; Bonate, P.L. Population Pharmacokinetics of Isavuconazole from Phase 1 and Phase 3 (SECURE) Trials in Adults and Target Attainment in Patients with Invasive Infections Due to Aspergillus and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2016, 60, 5483–5491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.; Fang, Y.; Wang, X.; Chen, Y.; Qi, Q.; Huang, F.; Xiao, X. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci. Rep. 2015, 5, 17091. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Lucenteforte, E.; Pea, F.; Soriano, A.; Tavoschi, L.; Steele, V.R.; Henriksen, A.S.; Longshaw, C.; Manissero, D.; Pecini, R.; et al. Systematic review on estimated rates of nephrotoxicity and neurotoxicity in patients treated with polymyxins. Clin. Microbiol. Infect. 2021, 27, 671–686. [Google Scholar] [CrossRef]
- Oliota, A.F.; Penteado, S.T.; Tonin, F.S.; Fernandez-Llimos, F.; Sanches, A.C. Nephrotoxicity prevalence in patients treated with polymyxins: A systematic review with meta-analysis of observational studies. Diagn. Microbiol. Infect. Dis. 2019, 94, 41–49. [Google Scholar] [CrossRef]
- Rattanaumpawan, P.; Lorsutthitham, J.; Ungprasert, P.; Angkasekwinai, N.; Thamlikitkul, V. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 2645–2649. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Pontikis, K.; Valsami, G.; Avgeropoulou, S.; Neroutsos, E.; Christodoulou, E.; Moraitou, E.; Markantonis, S.L.; Dokoumetzidis, A.; Rello, J.; et al. Pharmacokinetic Characteristics of Nebulized Colistimethate Sodium Using Two Different Types of Nebulizers in Critically Ill Patients with Ventilator-Associated Respiratory Infections. Antibiotics 2022, 11, 1528. [Google Scholar] [CrossRef]
- Rouby, J.J.; Sole-Lleonart, C.; Rello, J.; European Investigators Network for Nebulized Antibiotics in Ventilator-Associated Pneumonia. Ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: Understanding nebulization of aminoglycosides and colistin. Intensive Care Med. 2020, 46, 766–770. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Samonis, G.; Falagas, M.E. Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2018, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boisson, M.; Gregoire, N.; Cormier, M.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Pharmacokinetics of nebulized colistin methanesulfonate in critically ill patients. J. Antimicrob. Chemother. 2017, 72, 2607–2612. [Google Scholar] [CrossRef]
- Boisson, M.; Jacobs, M.; Gregoire, N.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 7331–7339. [Google Scholar] [CrossRef]
- Gkoufa, A.; Sou, T.; Karaiskos, I.; Routsi, C.; Lin, Y.W.; Psichogiou, M.; Zakynthinos, S.; Giamarellou, H.; Li, J.; Friberg, L.E. Pulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and formed colistin following nebulisation of CMS among patients with ventilator-associated pneumonia. Int. J. Antimicrob. Agents 2022, 59, 106588. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.F.; Karaiskos, I.; Plachouras, D.; Karvanen, M.; Pontikis, K.; Jansson, B.; Papadomichelakis, E.; Antoniadou, A.; Giamarellou, H.; Armaganidis, A.; et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: Population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob. Agents Chemother. 2012, 56, 4241–4249. [Google Scholar] [CrossRef] [PubMed]
- Dudhani, R.V.; Turnidge, J.D.; Nation, R.L.; Li, J. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J. Antimicrob. Chemother. 2010, 65, 1984–1990. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC Distribution; European Society of Clinical Microbiology and Infectious Diseases: Basel, Switzerland, 2023. [Google Scholar]
- Rouby, J.J.; Monsel, A. Nebulized Antibiotics: Epithelial Lining Fluid Concentrations Overestimate Lung Tissue Concentrations. Anesthesiology 2019, 131, 229–232. [Google Scholar] [CrossRef]
- Murray, P.R.; Washington, J.A. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin. Proc. 1975, 50, 339–344. [Google Scholar]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology, C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Kofteridis, D.P.; Alexopoulou, C.; Valachis, A.; Maraki, S.; Dimopoulou, D.; Georgopoulos, D.; Samonis, G. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: A matched case-control study. Clin. Infect. Dis. 2010, 51, 1238–1244. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Mean (SD) † or No. ‡ or Median (IQR) § |
|---|---|
| Demographic characteristics | |
| Sex | Male 13/Female 7 ‡ |
| Age, years | 68.9 (12.6) † |
| Weight, kg | 64.9 (15.6) † |
| Height, cm | 167.3 (7.35) † |
| Clinical characteristics | |
| Hypertension | Yes 13/No 7 ‡ |
| Diabetes | Yes 6/No 14 ‡ |
| ICU duration, days | 16.5 (12.5–23) § |
| MV duration, days | 14 (11.75–19.25) § |
| Admission duration, days | 44 (32.5–88) § |
| CMS nebulization duration, days | 16 (14–20.25) |
| ECMO | Yes 4/No 16 ‡ |
| CRRT | Yes 2/No 18 ‡ |
| Shock | Yes 3/No 17 ‡ |
| Clinical outcomes | Cured 16/Failed 4 ‡ |
| Microbiologic outcomes | Eradication 8/Persistence 10/Indeterminate 2 ‡ |
| Survival | Yes 5/No 15 ‡ |
| Laboratory characteristics | |
| C-reactive protein, mg/dL | 8.69 (5.52) † |
| Albumin, mg/dL | 2.75 (0.333) † |
| Procalcitonin, ng/dL | 0.31 (0.2125–1.585) § |
| Platelets, no. | 205 (111) † |
| Creatinine, mg/dL | 0.635 (0.405–1.115) § |
| ABGA lactate, mmol/L | 1.5 (1.15–2.575) § |
| Parameter | Epithelial Lining Fluid | Plasma | ||||
|---|---|---|---|---|---|---|
| Estimates | RSE | Bootstrap Median | Estimates | RSE | Bootstrap Median | |
| (%) | (95% CI) | (%) | (95% CI) | |||
| Structural model | ||||||
| CL/fm (mL/min) | 0.541 | 23.2 | 0.529 (0.352–0.77) | 26.3 | 27.3 | 26.1 (6.66–67) |
| V/fm (mL or L) | 23.0 | 28.9 | 24.3 (11.0–46.7) | 67.4 | 22.2 | 67.2 (46.6–93) |
| KAMAX | 0.531 | 16.1 | 0.533 (0.335–0.973) | 1.20 | 17.2 | 1.20 (0.912–1.59) |
| RA | 2.16 * | 2.70 * | ||||
| GAM | 2.92 * | 1.79 * | ||||
| Interindividual variability | ||||||
| CL/fm (%) | 124 | 18.6 | 124 (96.3–156) | 196 | 16.2 | 190 (134–303) |
| V/fm (%) | 59.7 | 25.8 | 58.2 (0.000–106) | 111 | 9.8 | 110 (94.6–124) |
| KAMAX (%) | 84.3 | 15.4 | 84.9 (37.1–109) | 64.3 | 23.3 | 62.8 (0.0734–86.5) |
| RA | 83.0 * | 83.0 * | ||||
| GAM | 0 * | 0 * | ||||
| Residual variability | ||||||
| Proportional error (%) | 27.1 | 18.6 | 26.7 (19.0–33.3) | 18.5 | 11.3 | 18.4 (15.8–21.2) |
| Outcome | No. | Epithelial Lining Fluid | Plasma |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Clinical outcomes | |||
| Cured/Improved | 16 | 36.7 (18.2–62.9) | 1.40 (0.752–3.74) |
| Failed | 4 | 31.7 (15.4–47.8) | 2.00 (1.87–2.38) |
| p-value = 0.6366 ‖ | p-value = 0.7055 ‖ | ||
| Microbiological outcomes | |||
| Eradicated | 8 | 61.2 (12.5–111) | 2.36 (0.645–4.11) |
| Persistent | 10 | 28.5 (17.7–50.5) | 1.56 (1.00–2.05) |
| Indeterminate | 2 | 33.9 (30.0–37.9) | 5.96 (5.00–6.91) |
| p-value = 0.5977 ¶ | p-value = 0.1163 ¶ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Kim, S.-Y.; Kim, Y.-K.; Jung, S.-Y.; Jang, J.-H.; Jang, H.-J.; Lee, J.-H. Intrapulmonary and Systemic Pharmacokinetics of Colistin Following Nebulization of Low-Dose Colistimethate Sodium in Patients with Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii. Antibiotics 2024, 13, 258. https://doi.org/10.3390/antibiotics13030258
Lee D-H, Kim S-Y, Kim Y-K, Jung S-Y, Jang J-H, Jang H-J, Lee J-H. Intrapulmonary and Systemic Pharmacokinetics of Colistin Following Nebulization of Low-Dose Colistimethate Sodium in Patients with Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii. Antibiotics. 2024; 13(3):258. https://doi.org/10.3390/antibiotics13030258
Chicago/Turabian StyleLee, Dong-Hwan, Shin-Young Kim, Yong-Kyun Kim, So-Young Jung, Ji-Hoon Jang, Hang-Jea Jang, and Jae-Ha Lee. 2024. "Intrapulmonary and Systemic Pharmacokinetics of Colistin Following Nebulization of Low-Dose Colistimethate Sodium in Patients with Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii" Antibiotics 13, no. 3: 258. https://doi.org/10.3390/antibiotics13030258
APA StyleLee, D.-H., Kim, S.-Y., Kim, Y.-K., Jung, S.-Y., Jang, J.-H., Jang, H.-J., & Lee, J.-H. (2024). Intrapulmonary and Systemic Pharmacokinetics of Colistin Following Nebulization of Low-Dose Colistimethate Sodium in Patients with Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii. Antibiotics, 13(3), 258. https://doi.org/10.3390/antibiotics13030258






