Abstract
Pseudomonas aeruginosa bacteremia is associated with a high mortality rate, and meropenem (MEPM) is commonly used to treat it. However, the relationship between the time above the minimum inhibitory concentration (fT>MIC) of MEPM and its therapeutic efficacy in P. aeruginosa bacteremia has not been explored. This study aimed to investigate this relationship by defining the target % fT>MIC of MEPM as 75%. The retrospective study spanned 14 years and included hospitalized patients treated with MEPM for P. aeruginosa bacteremia. Monte Carlo simulation was used to calculate the probability of target attainment (PTA) for each patient, and the threshold for a PTA of 75% fT>MIC associated with in-hospital survival was determined using receiver operating characteristic (ROC) curves. The ROC curve-derived PTA associated with improved in-hospital survival was 65.0%, a significant finding in multivariate logistic regression analysis adjusted for patient background factors (odds ratio: 20.49, 95% confidence interval: 3.02–245.23, p = 0.005). This result suggests a dosing regimen that achieves a PTA of at least 65% when the target fT>MIC of MEPM for treating P. aeruginosa bacteremia is defined as 75%.
1. Introduction
Gram-negative bacteria have been reported in over 40% of all bloodstream infections [1], with Pseudomonas aeruginosa accounting for 3–7% of all bacteremia and 23–26% of Gram-negative bacteria-induced bacteremia [2,3,4]. Furthermore, mortality rates for P. aeruginosa-induced bacteremia range from 18% to 46% [2,4,5,6,7,8]. Risk factors for P. aeruginosa bacteremia, categorized as a glucose nonfermenting Gram-negative rod, include hematologic malignancies like leukemia, chronic kidney disease, organ transplantation, and other immunosuppressive conditions [9,10]. Common sources of bacteremia include pulmonary, central line-associated, urinary tract, pancreaticobiliary, and unknown origins [10,11,12,13,14]. While antimicrobial susceptibility is crucial for treating infections, P. aeruginosa is intrinsically resistant to some commonly used antibiotics and can develop resistance to multiple antibiotic classes [15,16,17,18]. Therefore, the limited availability of antibiotics for P. aeruginosa infections complicates treatment, emphasizing the importance of appropriate antimicrobial therapy for P. aeruginosa bacteremia [6,11].
Meropenem (MEPM), a carbapenem, possesses a broad antibacterial spectrum and finds widespread use in antimicrobial therapy for various bacterial infections, including P. aeruginosa bacteremia [19,20]. Similar to other β-lactams, MEPM demonstrates time-dependent bactericidal activity. Its microbial efficacy is assessed based on the duration of time in percentage between doses at which the unbound drug concentration remains above the minimum inhibitory concentration (MIC), i.e., the time above the MIC (% fT>MIC) [21,22,23]. Furthermore, achieving fT>MIC of 20–30% and 40–50% for MEPM is necessary for its bacteriostatic and bactericidal activities, respectively [24,25]. Few studies have reported a connection between the pharmacokinetics (PK)/pharmacodynamics (PD) parameter and clinical outcomes [26,27]. In critically ill patients, a higher survival rate has been reported in those with 75–100% fT>MIC for MEPM compared to those with 40% fT>MIC [26]. Among patients with febrile neutropenia, a higher clinical response rate was observed in those with >75% fT>MIC for MEPM than in those with 50–75% fT>MIC [27]. These findings indicate that achieving >75% fT>MIC for MEPM is essential for enhancing therapeutic efficacy in severe infections.
Although it is necessary to know the drug concentration profiles at the target site while calculating the detailed PK/PD parameters, MEPM concentration measurements are still at the spreading stage. Therefore, it is crucial to predict the PK/PD parameters of antimicrobials for each patient based on available information and design appropriate dosage regimens. Monte Carlo simulation, a stochastic method utilizing random numbers, serves as an evaluation technique. In antimicrobial treatment, the probability of target attainment (PTA) of PK/PD parameters for various dosage regimens, including dosage and infusion time, can be determined based on population PK parameters and susceptibility results of organisms [26,28,29,30,31]. If the target value for efficacy, akin to achieving 75% fT>MIC in the treatment of P. aeruginosa bacteremia with MEPM, is considered, the Monte Carlo simulation’s target achievement rate for patients on MEPM becomes a crucial efficacy indicator.
In this study, we utilized the reported population PK parameters for MEPM to estimate individual PK, using patient information with P. aeruginosa bacteremia. We then calculated PTA for each patient, targeting a value of 75% fT>MIC. Furthermore, we explored the correlation between the calculated PTA threshold and the outcomes of patients with P. aeruginosa bacteremia.
2. Results
2.1. Patient Background
In total, 111 patients with P. aeruginosa bacteremia were screened for this retrospective study from January 2009 to December 2022, and 41 fulfilled the inclusion criteria, excluding 70 patients who met the exclusion criteria (Figure 1).
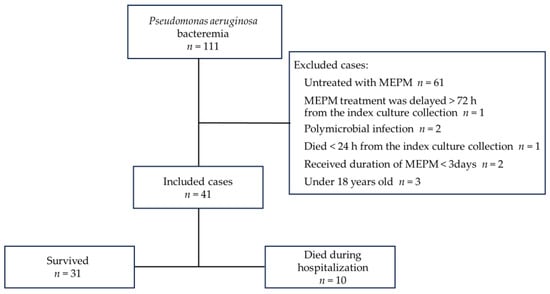
Figure 1.
Study flow chart.
The patient background is described in Table 1. The median (interquartile range (IQR)) age of the patients was 77 (70–84), 70.7% were male, and the median (IQR) modified acute physiology and chronic health evaluation II (APACHE II) score at the time of collection of blood for the index culture was 20 (13–22). The median (IQR) creatinine clearance (CLcr) was 45 (20.9–75.5) mL/min, and 16 patients (39.0%) had acute kidney injury (AKI). Comorbidities included hypertension, chronic kidney disease (CKD), and hematologic malignancies in approximately 40% of the patients. Urinary tract infection (UTI) and unknown causes were the most common sources of bacteremia (34.1% [n = 14/41]).

Table 1.
Characteristics of 41 patients with P. aeruginosa bacteremia.
The primary outcome, in-hospital survival, occurred in 31 (75.6%) of eligible patients. The duration of MEPM treatment did not differ significantly between survivors and non-survivors (median (IQR) of 8 (5.5–13) days and 10 (9–16) days, respectively; p = 0.171). Similarly, there was no significant distinction between the two groups regarding the concomitant use of other antimicrobials active against P. aeruginosa (12.9% [n = 4/31], 30% [n = 3/10]; p = 0.332). Conversely, patients who survived exhibited significantly lower modified APACHE II scores than patients who died (median (IQR) of 18 (12–22) and 22 (21–27), respectively; p = 0.005).
The MIC distribution of MEPM for P. aeruginosa is described in Figure 2. In the isolated strains, 90.3% (n = 28/31) of the survivor strains and 70.0% (n = 7/10) of the non-survivor strains had MIC ≤ 1 μg/mL. The overall MIC50 and MIC90 values were 0.5 μg/mL and 2 μg/mL, respectively.
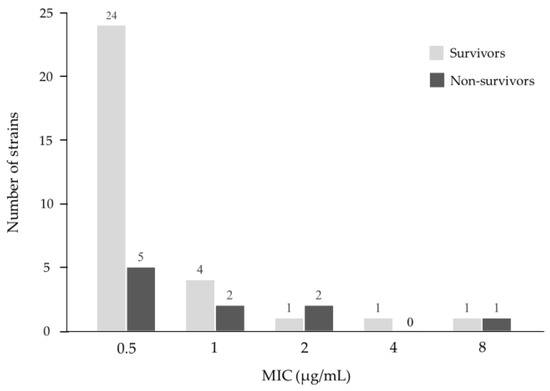
Figure 2.
Minimum inhibitory concentration (MIC) distribution of meropenem for P. aeruginosa in this study.
2.2. Probability of Target Attainment
The median (IQR) PTA for 75% fT>MIC was 90.2% (72.0–97.4) and 57.6% (24.6–73.1) for survivors and non-survivors, respectively, which were significantly different (Figure 3). The PTA threshold for 75% fT>MIC to determine the likelihood of survival was identified by the receiver operating characteristic (ROC) curve (Figure 4). The area under the curve was 0.78 (95% confidence interval [CI]: 0.584–0.968), and the optimal threshold was 65.0%, with sensitivity and specificity of 83.9% and 70.0%, respectively. From the abovementioned data, achieving a PTA of >65%, the threshold for 75% fT>MIC derived from the ROC curve was assessed as a candidate factor that may improve patient survival.
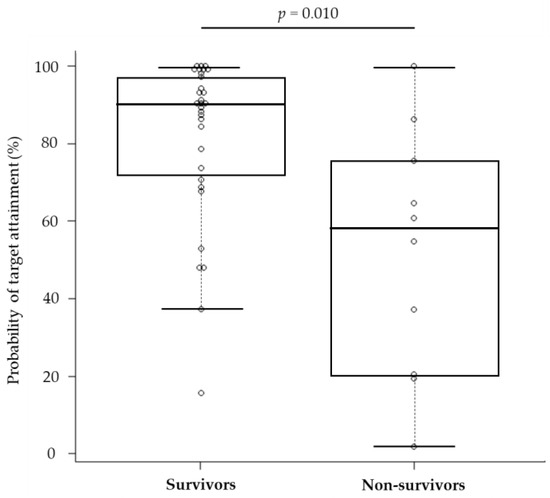
Figure 3.
Comparison of the probability of target attainment between survivors and non-survivors. The complot shows the maximum value, 75% tile value, median value, 25% tile value, and minimum value in order from the top. The white circle symbol indicates the individual value.
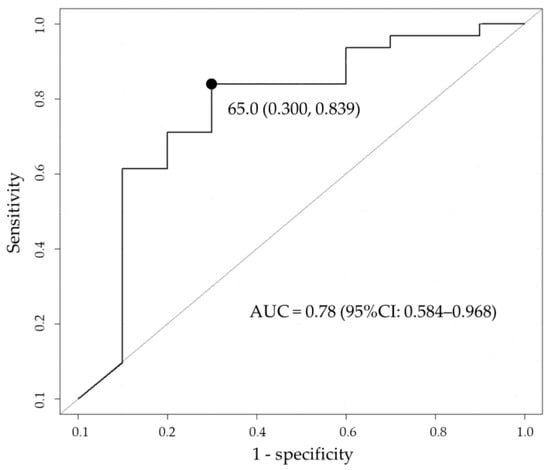
Figure 4.
Cutoff value of probability of target attainment (PTA) that influences in-hospital survival based on receiver operating characteristic (ROC) analysis. The black circle indicates the optimal threshold. AUC, area under the curve; CI, confidence interval.
2.3. Investigating the Influence of the Probability of Target Attainment on In-Hospital Survival by Adjusting Patient Background Factors
The multivariate logistic regression analysis model included the modified APACHE II score, comorbid hematologic malignancies, and UTI as the cause of bacteremia, with p ≦ 0.15 in univariate analysis between survivors and non-survivors. The ROC curve-derived PTA threshold for 75% fT>MIC was included as an independent variable. A PTA threshold of >65% was introduced into the final model as a categorical variable. The results indicated improved in-hospital survival for those with a PTA threshold of >65% (odds ratio [OR]: 20.49, 95% CI: 3.02–245.23, p = 0.005) and a modified APACHE II score (OR: 0.83, 95% CI: 0.69–0.96, p = 0.024) after controlling for clinical covariates (Table 2).

Table 2.
Logistic regression model of in-hospital survival.
3. Discussion
The key finding of this study is that the ROC curve-derived threshold of PTA of >65%, with the target % fT>MIC of MEPM defined as 75%, influences the improvement in in-hospital survival in P. aeruginosa bacteremia.
In total, 10 patients died in our study; all were receiving antimicrobial therapy since blood collection for the index culture till death, suggesting that all deaths were affected by infection. Among the 10 patients who died, 3 were in the intensive care unit (ICU) during the index blood culture collection. For the other patients, three out of seven were moved to the ICU a few days later for treatment, and four were treated in the regular ward because their condition was considered too futile to warrant ICU admission. Conversely, 2 out of the 27 survivors who were not initially admitted to the ICU were later transferred to the ICU. In 25 cases, the attending physicians decided to treat the patients in the regular ward due to their relatively stable vital signs.
In a study on the relationship between % fT>MIC and the clinical efficacy of MEPM, Boonpeng et al. reported that, in the ICU, the number of patients with sepsis treated with MEPM achieving 40% fT>MIC, 75% fT>MIC, and 100% fT>MIC was 92.9%, 71.4%, and 71.4%, respectively, in the survivor group and 83.3%, 66.7%, and 16.7%, respectively, in the non-survivor group [26]. Despite reporting more resistant organisms in the non-survivor group, these results suggest that an increase in % fT>MIC of MEPM in critically ill patients may be associated with improved survival. The median (IQR) APACHE II score of the patient population in their study was 20 (14–23). In the present study, 17.1% of the patients were admitted to the ICU, but the median (IQR) modified APACHE II score of 20 (13–22) was similar in severity to that of the Boonpeng et al. group. Ariano et al. reported that, in febrile neutropenia patients with bacteremia, the mean % fT>MIC of 42 patients in the clinical responders was 83%, whereas the mean % fT>MIC of 18 patients in the non-responders was 59% (p = 0.04). In the same report, they also stated that a rate of % fT>MIC for MEPM exceeding 75% was associated with an improved clinical response [27]. Although their report did not adjust for patient severity or comorbidities, the neutropenic status of their patients suggests susceptibility to infection. In the present study, 39.0% of the patients also had comorbid hematologic malignancies, suggesting that their background included patients with the same trends as those reported by Ariano et al. Hence, it was considered appropriate to set the target % fT>MIC for MEPM at 75% in this study.
Sjövall et al. employed a Monte Carlo simulation to examine MEPM dosing in septic shock patients with potential augmented renal clearance [32]. They found that achieving a sufficient 100% fT>MIC against P. aeruginosa requires either a prolonged infusion of 1 g every 8 h with a 3 h infusion time or a continuous infusion of 3 g/day over 24 h. Fukumoto et al. noted that in sepsis patients, with an MIC of MEPM set at 4 μg/mL, 1 g per dose (infusion time: 3–8 h), three times a day is necessary to achieve a 50% fT>MIC in patients with CLcr >85 mL/min [33]. Roberts et al. used Monte Carlo simulation based on the PK parameters of MEPM in septic patients without renal dysfunction to compare the achievement rates of 50% fT>MIC among continuous, prolonged, and intermittent bolus infusions [34]. Reportedly, for MIC > 4 μg/mL, continuous infusion had the highest rate of achieving the target, followed by prolonged infusion; however, intermittent bolus infusion revealed the lowest achievement rate. Based on these findings, patients with conserved renal function and predicted higher MEPM clearance and/or organisms with MIC > 4 μg/mL may benefit from prolonged infusion times or continuous infusion to increase % fT>MIC. However, no studies explored the correlation between % fT>MIC and clinical efficacy. As the Clinical and Laboratory Standards Institute (CLSI) criteria set the breakpoint for MEPM against P. aeruginosa at 2 μg/mL [35], clinical assessment becomes crucial when the MIC surpasses this level in the clinical setting. In this study, the MIC50 and MIC90 of MEPM against P. aeruginosa were 0.5 μg/mL and 2 μg/mL, respectively, indicating overall high susceptibility to MEPM. Furthermore, participants in this study had overall impaired renal function, with a median (IQR) CLcr of 45 (20.9–75.5) mL/min, suggesting that these factors may have significantly impacted the elevation of the patients’ PTA in this study.
For treating infections, drug concentrations in the infection site are also an important factor affecting clinical efficacy, and there are several reports on blood concentrations of MEPM and its penetration into various organs [36,37,38,39,40]. In a study of patients with severe pneumonia, the concentration of MEPM in epithelial lining fluid (ELF) was reported to be much lower than that in plasma, with a penetration rate to ELF of approximately 30% of that in blood [36,37]. Concurrently, it has been reported that the usual dosage of MEPM (1 g–2 g per dose every 8 h) does not achieve 40–50% fT>MIC in the ELF for pathogens with MIC ≤ 2 μg/mL. Conversely, MEPM is mainly eliminated from the kidney, with the urinary excretion rate of unchanged MEPM in healthy adults being approximately 65% [41,42]. Therefore, higher drug concentrations can be obtained in tissues with urinary tract infections compared with infections in other organs. Based on these findings, it is possible that differences in the penetration of MEPM into the infected organ that caused the bacteremia in this study also affected the outcome of the treatment. In fact, higher survival rates were observed in the group of patients in whom urinary tract infection was considered the cause of bacteremia.
This study has several limitations. First, it is a single-center, retrospective study, making it challenging to adjust for all confounding factors affecting clinical outcomes, compounded by the small number of included patients. The limited sample size may have impacted the estimation of the adjusted odds ratio for PTA > 65% on in-hospital survival, as indicated by large confidence intervals in some results. Second, the study did not measure the actual blood levels of MEPM in patients; thus, it is not possible to consider the effect of the changes in individual patients’ pathophysiology on the PK of MEPM. Critical illness, such as septic shock, may cause changes in vascular hyperpermeability and decreased peripheral vascular resistance with subsequent tissue edema, leading to an increase in capillary-to-cell diffusion distances [43]. Additionally, aggressive fluid resuscitation in patients with sepsis will lead to significant changes in the volume of distribution of antibiotics, potentially resulting in low trough serum and tissue concentrations. Therefore, the population’s PK parameters used in this study did not accurately reflect the PK parameters of MEPM in critically ill patients [44]. Next, critically ill patients are likely to have changes in the clearance of drugs excreted by the kidney due to the concomitant augmented renal clearance or AKI [44,45]. Therefore, it is difficult to exactly assess the clearance of MEPM—which changes daily—by estimating the Ccr level using a mathematical model. Herein, 16 patients (39.0%) had AKI complications, and of these, 5 were admitted to the ICU. Especially in this patient group, the PK of MEPM changed during the bacteremia treatment period, which may have affected the PTA results. Despite these constraints, this is the first report examining the relationship between the target % fT>MIC achievement rate of MEPM and in-hospital survival in P. aeruginosa bacteremia. We believe our findings will contribute to the appropriate dosage design for MEPM. The study suggests that defining the target % fT>MIC of MEPM as 75% and achieving PTA of >65% may enhance in-hospital survival in patients with P. aeruginosa bacteremia. As we have shown, PTA cannot be predicted using data on dose alone. Most of the P. aeruginosa bacteria isolated in this study were susceptible to MEPM with MIC ≦ 1 μg/mL, but investigating the dosing methods, such as continuous or prolonged dosing, may be required for more resistant pathogens in future studies. Furthermore, the population’s PK parameter applied for calculating PTA using the Monte Carlo simulation method are quite important, and for critical diseases such as sepsis, it may be necessary to use the population’s PK parameters constructed using PK data for individual diseases. We hope that the results obtained in the present study will serve as the foundation for further investigations.
4. Conclusions
When MEPM concentration monitoring is still in the spreading stage, defining a target % fT>MIC and calculating PTA using an appropriate PK model based on patient information and pathogen’s MIC could enable the prediction of a dosing regimen that will result in a higher clinical efficacy. It is recommended to design MEPM dosing for patients with P. aeruginosa bacteremia to achieve a PTA of >65%, defined as a target 75% fT>MIC.
5. Materials and Methods
5.1. Study Population
This retrospective study included patients with positive blood cultures for P. aeruginosa admitted to Japan Community Health Care Organization Kyushu Hospital from January 2009 to December 2022. Patients < 18 years of age, who were untreated with MEPM, subjected to delayed treatment beyond 72 h from the index blood culture collection, treated with MEPM for less than 3 days, and those with polymicrobial blood culture findings were excluded. Patients who died within 24 h of blood culture collection were also excluded.
5.2. Clinical Background
Patient characteristics and clinical outcomes were retrospectively retrieved from the electronic medical records. Information on P. aeruginosa-positive blood cultures, including the MIC for MEPM, was obtained from the microbiology laboratory database. The extracted data encompassed age, sex, body weight, admission to ICU versus non-ICU, modified APACHE II score [46], serum creatinine (Scr), presence of AKI, infection source, duration of hospitalization until the collection of the index blood culture, duration of MEPM therapy, receipt of active combination therapy with MEPM, and comorbidities. CLcr was calculated using the Cockcroft–Gault equation [47]. The source of infection referred to the attending physician’s written diagnosis. Patient comorbidities were considered present if documented in the admission history and physical examination. In-hospital survival was investigated as the outcome.
5.3. Definition
The day of positive blood culture collection was considered the first day of onset of bacteremia. In the presence of AKI, we adopted class 1 of the Acute Kidney Injury Network classification [48], defined as an increase in Scr level of > 0.3 mg/dL or >50% in at least two consecutive measurements during MEPM treatment. Combination antimicrobial therapy was defined as the administration of any other antimicrobial agent active against P. aeruginosa, following the CLSI criteria, concurrently with MEPM within the same 24 h period. MEPM dose intensities were categorized as 1 g every 8 h, 1 g every 12 h, 500 mg every 8 h, 500 mg every 12 h, or 500 mg every 24 h. MEPM doses, prescribed per the attending physician’s decision, were administered intravenously over 30 min to 1 h using a venous catheter. In-hospital survival was defined as the successful completion of antimicrobial therapy for bacteremia during hospitalization.
5.4. PK/PD Simulation of Individual Patients
Population PK parameters based on the two-compartment model reported by Ikawa et al. were used to determine PK parameters for individual patients, with a plasma protein binding rate of 2.43% [49]. The population PK parameters used for the calculation are shown below. The residual variability was disregarded.
CL (L/h) = 0.0905 × CLcr + 2.03 (IIV: 41.1%)
Vc (L) = 0.199 × BW (IIV: 39.8%)
Q (L/h) = 4.02 (IIV: 32.8%)
Vp (L) = 4.55 (IIV: 29.9%)
PBR (%) = 2.43
CL, clearance; Vc, central volume of distribution; Q, intercompartmental clearance; Vp, peripheral volume of distribution; PBR, blood plasma protein binding ratio; CLcr, creatinine clearance (L/h); BW, body weight (kg); and IIV, interindividual variability.
The PTA for individual patients was calculated using Monte Carlo simulation (n = 10,000) with a target value of 75% fT>MIC, which was reported to be highly effective in critically ill and febrile neutropenia patients [26,27]. The PK/PD simulation software BMs-Pod version 8.06 was used for Monte Carlo simulation [50].
5.5. Statistical Analysis
The threshold of PTA for 75% fT>MIC linked to improved survival was determined through a ROC curve. To assess the correlation between the PTA threshold for 75% fT>MIC derived from the ROC curves and the survival outcome in patients with P. aeruginosa bacteremia, a multivariate logistic regression analysis was conducted after adjusting for significant patient background factors. For the selection of patient background factors in the comparison between survivors and non-survivors, the Mann–Whitney U test was employed for continuous variables, and Fisher’s exact probability test was used for categorical variables. Candidate variables with a univariate significance level of p ≤ 0.15 were assessed as the potential predictors of in-hospital survival, and the stepwise method was applied for variable imputation in the multivariate logistic regression analysis. Statistical analysis was carried out using R version 4.3.1 (https://cran.r-project.org/, accessed on 3 May 2023).
Author Contributions
Conceptualization, H.N., M.M., T.K. and O.I.; methodology, H.N. and K.O.; software, H.N. and K.O.; validation, H.N., M.M. and T.K.; formal analysis, H.N.; investigation, H.N., T.K. and Y.H.; resources, Y.H.; data curation, H.N., M.M. and Y.H.; writing—original draft preparation, H.N.; writing—review and editing, H.N., M.M., T.K., K.O. and O.I.; visualization, H.N.; supervision, O.I.; project administration, O.I.; funding acquisition, O.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by Japan Community Health Care Organization Kyushu Hospital (approval number: 909, 20 June 2023).
Informed Consent Statement
Patient consent was waived due to the retrospective design of this study. In this study, we used the opt-out method. The disclosure document for this clinical study was provided through the website of Japan Community Health Care Organization Kyushu Hospital.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Hidemi Ogura for assistance in conducting the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Muñoz, P.; Cruz, A.F.; Rodríguez-Créixems, M.; Bouza, E. Gram-negative bloodstream infections. Int. J. Antimicrob. Agents 2008, 32, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Mensa, J.; Almela, M.; Martínez, J.A.; Marco, F.; Casals, C.; Gatell, J.M.; Soriano, E.; Jimenez de Anta, M.T. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch. Intern. Med. 1996, 156, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Bisbe, J.; Gatell, J.M.; Puig, J.; Mallolas, J.; Martinez, J.A.; Jimenez de Anta, M.T.; Soriano, E. Pseudomonas aeruginosa bacteremia: Univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev. Infect. Dis. 1988, 10, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.I.; Kim, S.H.; Kim, H.B.; Park, S.W.; Choe, Y.J.; Oh, M.D.; Kim, E.C.; Choe, K.W. Pseudomonas aeruginosa bacteremia: Risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 2003, 37, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.S.; Kang, C.I.; Wi, Y.M.; Ko, K.S.; Chung, D.R.; Lee, N.Y.; Song, J.H.; Peck, K.R. Inappropriate initial antimicrobial therapy as a risk factor for mortality in patients with community-onset Pseudomonas aeruginosa bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Lloyd, A.E.; Ritchie, D.J.; Reichley, R.M.; Fraser, V.J.; Kollef, M.H. Pseudomonas aeruginosa bloodstream infection: Importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 2005, 49, 1306–1311. [Google Scholar] [CrossRef]
- Gomez, J.; Alcantara, M.; Simarro, E.; Martinez, B.; Ruiz, J.; Guerra, B.; Valdés, M. Pseudomonas aeruginosa bacteremia: Clinical and epidemiological patterns and treatment. Rev. Esp. Quimioter. 2002, 15, 360–365. [Google Scholar]
- Lodise, T.P., Jr.; Patel, N.; Kwa, A.; Graves, J.; Furuno, J.P.; Graffunder, E.; Lomaestro, B.; McGregor, J.C. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: Impact of delayed appropriate antibiotic selection. Antimicrob. Agents Chemother. 2007, 51, 3510–3515. [Google Scholar] [CrossRef]
- Iversen, B.G.; Brantsaeter, A.B.; Aavitsland, P. Nationwide study of invasive Pseudomonas aeruginosa infection in Norway: Importance of underlying disease. J. Infect. 2008, 57, 139–146. [Google Scholar] [CrossRef]
- Parkins, M.D.; Gregson, D.B.; Pitout, J.D.; Ross, T.; Laupland, K.B. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection 2010, 38, 25–32. [Google Scholar] [CrossRef]
- Kang, C.I.; Kim, S.H.; Park, W.B.; Lee, K.D.; Kim, H.B.; Kim, E.C.; Oh, M.D.; Choe, K.W. Clinical features and outcome of patients with community-acquired Pseudomonas aeruginosa bacteraemia. Clin. Microbiol. Infect. 2005, 11, 415–418. [Google Scholar] [CrossRef]
- Choi, Y.; Paik, J.H.; Kim, J.H.; Han, S.B.; Durey, A. Clinical Predictors of Pseudomonas aeruginosa Bacteremia in Emergency Department. Emerg. Med. Int. 2018, 2018, 7581036. [Google Scholar] [CrossRef]
- Fabre, V.; Amoah, J.; Cosgrove, S.E.; Tamma, P.D. Antibiotic Therapy for Pseudomonas aeruginosa Bloodstream Infections: How Long Is Long Enough? Clin. Infect. Dis. 2019, 169, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Fattah, M.A.; Haquin, J.; et al. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteremia: A retrospective multicentre study. Int. J. Antimicrob. Agents 2020, 55, 105847. [Google Scholar] [CrossRef]
- Steinberg, J.P.; Burd, E.M. Other gram-negative and gram-variable bacilli. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier/Saunders: Philadelphia, PA, USA, 2015; Volume 3023, pp. 2667–2683. [Google Scholar]
- Quinn, J.P. Clinical problems posed by multiresistant nonfermenting Gram-negative pathogens. Clin. Infect. Dis. 1998, 27, S117–S124. [Google Scholar] [CrossRef]
- Livermore, D.M. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare? Clin. Infect. Dis. 2002, 34, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.A.; Szabo, D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 43, S49–S56. [Google Scholar] [CrossRef]
- Baldwin, C.M.; Lyseng-Williamson, K.A.; Keam, S.J. Meropenem: A review of its use in the treatment of serious bacterial infections. Drugs 2008, 68, 803–838. [Google Scholar] [CrossRef] [PubMed]
- Kwee, F.; Walker, S.A.; Elligsen, M.; Palmay, L.; Simor, A.; Daneman, N. Outcomes in Documented Pseudomonas aeruginosa Bacteremia Treated with Intermittent IV Infusion of Ceftazidime, Meropenem, or Piperacillin-Tazobactam: A Retrospective Study. Can. J. Hosp. Pharm. 2015, 68, 386–394. [Google Scholar] [CrossRef]
- Vogelman, B.; Gudmundsson, S.; Leggett, J.; Turnidge, J.; Ebert, S.; Craig, W.A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 1988, 158, 831–847. [Google Scholar] [CrossRef]
- Nicolau, D.P. Optimizing outcomes with antimicrobial therapy through pharmacodynamic profiling. J. Infect. Chemother. 2003, 9, 292–296. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin. Infect. Dis. 2008, 47, S32–S40. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boonpeng, A.; Jaruratanasirikul, S.; Jullangkoon, M.; Samaeng, M.; Wattanavijitkul, T.; Bhurayanontachai, R.; Pattharachayakul, S. Population Pharmacokinetics/Pharmacodynamics and Clinical Outcomes of Meropenem in Critically Ill Patients. Antimicrob. Agents Chemother. 2022, 66, e0084522. [Google Scholar] [CrossRef]
- Ariano, R.E.; Nyhlén, A.; Donnelly, J.P.; Sitar, D.S.; Harding, G.K.; Zelenitsky, S.A. Pharmacokinetics and Pharmacodynamics of Meropenem in Febrile Neutropenic Patients with Bacteremia. Ann. Pharmacother. 2005, 39, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kuti, J.L.; Dandekar, P.K.; Nightingale, C.H.; Nicolau, D.P. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J. Clin. Pharmacol. 2003, 43, 1116–1123, Erratum in J. Clin. Pharmacol. 2005, 45, 357. [Google Scholar] [CrossRef]
- Ellis, J.M.; Kuti, J.L.; Nicolau, D.P. Pharmacodynamic evaluation of meropenem and cefotaxime for pediatric meningitis: A report from the OPTAMA program. Paediatr. Drugs 2006, 8, 131–138. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.H.; Kim, J.S.; Jung, W.B.; Heo, W.; Kim, Y.K.; Kim, S.H.; No, T.H.; Jo, K.M.; Ko, J.; et al. Pharmacokinetics and Monte Carlo Simulation of Meropenem in Critically Ill Adult Patients Receiving Extracorporeal Membrane Oxygenation. Front. Pharmacol. 2021, 12, 768912. [Google Scholar] [CrossRef]
- Yamada, T.; Minami, K.; Oda, K.; Suzuki, K.; Nishihara, M.; Uchiyama, K.; Ukimura, A. Probability of target attainment of oral antimicrobials for Escherichia coli and Klebsiella pneumoniae based on Monte Carlo simulations. Diagn. Microbiol. Infect. Dis. 2022, 103, 115662. [Google Scholar] [CrossRef]
- Sjövall, F.; Alobaid, A.S.; Wallis, S.C.; Perner, A.; Lipman, J.; Roberts, J.A. Maximally effective dosing regimens of meropenem in patients with septic shock. J. Antimicrob. Chemother. 2018, 73, 191–198. [Google Scholar] [CrossRef]
- Fukumoto, S.; Ohbayashi, M.; Okada, A.; Kohyama, N.; Tamatsukuri, T.; Inoue, H.; Kato, A.; Kotani, T.; Sagara, H.; Dohi, K.; et al. Population Pharmacokinetic Model and Dosing Simulation of Meropenem Using Measured Creatinine Clearance for Patients with Sepsis. Ther. Drug Monit. 2023, 45, 392–399. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kirkpatrick, C.M.; Roberts, M.S.; Robertson, T.A.; Dalley, A.J.; Lipman, J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: Intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J. Antimicrob. Chemother. 2009, 64, 142–150. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th Edition. CLSI supplement M100. Clinical and Laboratory Standards Institute: Wayne, NY, USA, 2020, 42–45.
- Frippiat, F.; Musuamba, F.T.; Seidel, L.; Albert, A.; Denooz, R.; Charlier, C.; Van Bambeke, F.; Wallemacq, P.; Descy, J.; Lambermont, B.; et al. Modelled target attainment after meropenem infusion in patients with severe nosocomial pneumonia: The PROMESSE study. J. Antimicrob. Chemother. 2015, 70, 207–216. [Google Scholar] [CrossRef]
- Lodise, T.P.; Sorgel, F.; Melnick, D.; Mason, B.; Kinzig, M.; Drusano, G.L. Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia. Antimicrob. Agents Chemother. 2011, 55, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Blassmann, U.; Roehr, A.C.; Frey, O.R.; Vetter-Kerkhoff, C.; Thon, N.; Hope, W.; Briegel, J.; Huge, V. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: A prospective observational study. Crit. Care 2016, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Hamanoue, S.; Suwabe, T.; Ubara, Y.; Kikuchi, K.; Hazue, R.; Mise, K.; Ueno, T.; Takaichi, K.; Matsumoto, K.; Morita, K. Cyst infection in autosomal dominant polycystic kidney disease: Penetration of meropenem into infected cysts. BMC Nephrol. 2018, 19, 272. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, R.A.; Downey, P.; Soto, D.; Wong, K.Y.; Leung, Y.C.; So, L.Y.; Andresen, M. Plasma and Renal Cortex Meropenem Concentrations in Patients Undergoing Percutaneous Renal Biopsy. Biomed Res. Int. 2019, 2019, 1368397. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Chung, K.C.; Gill, M.A. Pharmacokinetics of meropenem in animals, healthy volunteers, and patients. Clin. Infect. Dis. 1997, 24, S249–S255. [Google Scholar] [CrossRef]
- Harrison, M.P.; Moss, S.R.; Featherstone, A.; Fowkes, A.G.; Sanders, A.M.; Case, D.E. The disposition and metabolism of meropenem in laboratory animals and man. J. Antimicrob. Chemother. 1989, 24, 265–277. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Udy, A.A.; Baptista, J.P.; Lim, N.L.; Joynt, G.M.; Jarrett, P.; Wockner, L.; Boots, R.J.; Lipman, J. Augmented renal clearance in the ICU: Results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit. Care Med. 2014, 42, 520–527. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Sunenshine, R.H.; Wright, M.O.; Maragakis, L.L.; Harris, A.D.; Song, X.; Hebden, J.; Cosgrove, S.E.; Anderson, A.; Carnell, J.; Jernigan, D.B.; et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg. Infect. Dis. 2007, 13, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, K.; Morikawa, N.; Ohge, H.; Ikeda, K.; Sueda, T.; Taniwaki, M.; Kurisu, K. Pharmacokinetic–pharmacodynamic target attainment analysis of meropenem in Japanese adult patients. J. Infect. Chemother. 2010, 16, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Oda, K. Development of Software for Antimicrobial PK/PD Simulation incorporating Montecarlo Simulation Based on Microsoft® Office Excel. Iryo Yakugaku 2011, 37, 335–344. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).