Novel Antimicrobial Approaches to Combat Bacterial Biofilms Associated with Urinary Tract Infections
Abstract
1. Introduction
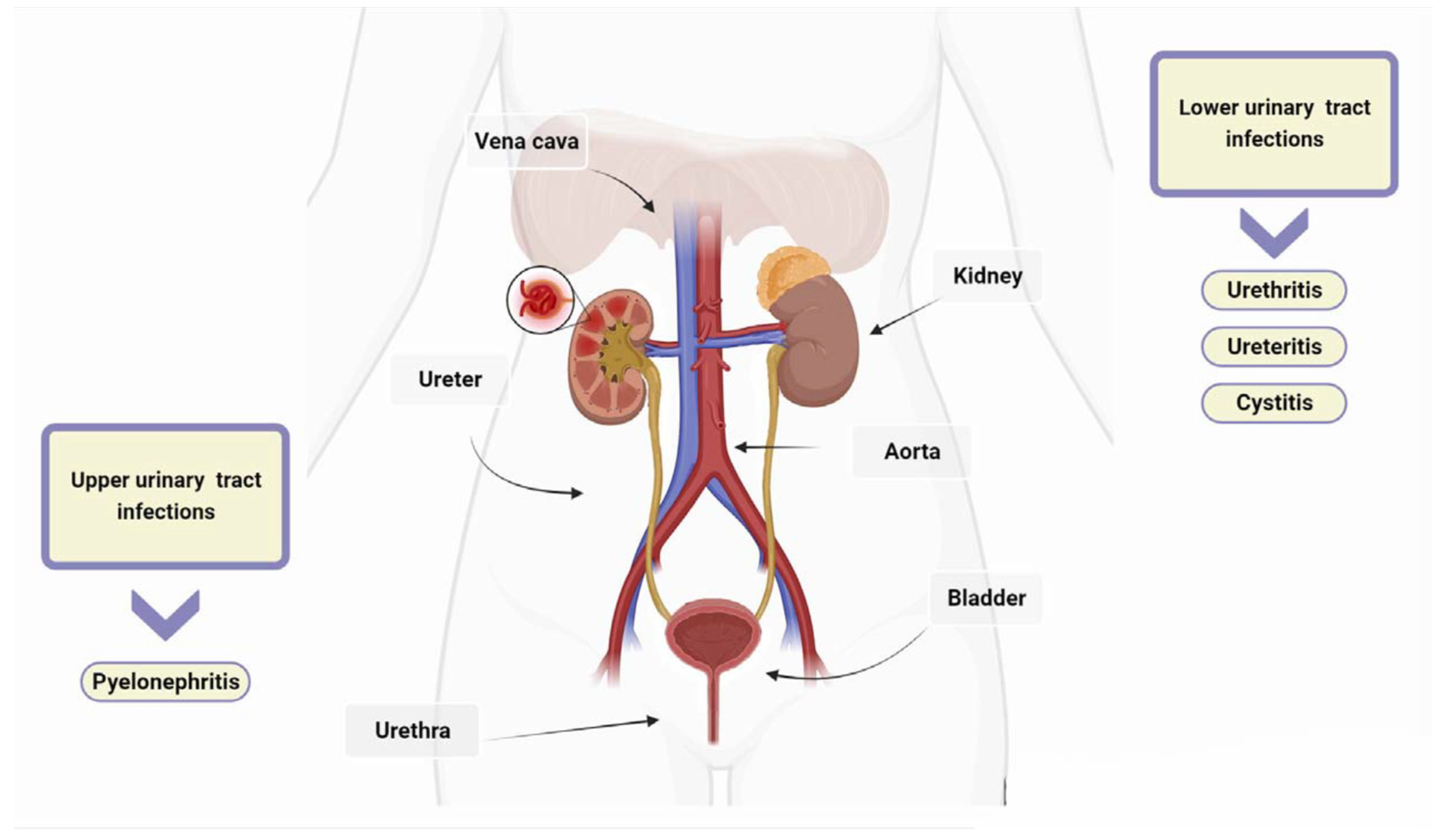
2. Classification and Pathogenesis of UTIs
2.1. Types of UTIs
2.2. Clinical Syndromes
2.3. Urinary Tract Infections Caused by Bacteria
3. Biofilm Formation
4. The Role of Biofilm in the Persistence and Recurrence of UTIs
5. Resistance of Bacteria in Biofilm
6. Strategies for Combatting Biofilm-Forming Pathogenic Microorganisms in UTIs
6.1. Effectiveness of Antimicrobial Peptides (AMPs) against Biofilm Formation
6.2. QS Inhibitors
6.3. Biofilm Inhibition by Nanoparticles
6.4. Bacteriophage Therapy for Treating UTIs
6.5. Biofilm-Dispersing Enzymes
7. Discussion and Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| AmpC | Cephalosporinase |
| AMPs | antimicrobial peptides |
| A3 | antimicrobial peptide-A3 |
| CAUTI | catheter-associated urinary tract infections |
| CDC | Centers for Disease Control and Prevention |
| CTX-M | Cefotaximase |
| EPS | Exopolysaccharides |
| GAG layer | Glycosaminoglycan |
| HGT | horizontal gene transfer |
| KPC | carbapenemase-producing Klebsiella pneumoniae |
| LL-37 | human cathelicidin antimicrobial peptide |
| MRSA | methicillin-resistant Staphylococcus aureus |
| NDM | New Delhi Metallo β-lattamasi |
| PS1 | synthetic peptides |
| OXA | oxacillinase |
| SHV | sulfhydryl variant of TEM |
| TEM | β-lactamase isolated from a patient named Temoniera in Greece |
| temporinGHa | cloned from Hylarana guentheri |
| UPEC | uropathogenic Escherichia coli |
| UTIs | urinary tract infections |
| VRE | vancomycin-resistant Enterococcus |
References
- Maddali, N.; Cantin, A.; Koshy, S.; Eiting, E.; Fedorenko, M. Antibiotic prescribing patterns for adult urinary tract infections within emergency department and urgent care settings. Am. J. Emerg. Med. 2021, 45, 464–471. [Google Scholar] [CrossRef]
- Brodie, A.; El-Taji, O.; Jour, I.; Foley, C.; Hanbury, D. A Retrospective Study of Immunotherapy Treatment with Uro-Vaxom (OM-89(R)) for Prophylaxis of Recurrent Urinary Tract Infections. Curr. Urol. 2020, 14, 130–134. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C. New Insights into the Pathogenesis and Treatment of Urinary Tract Infections. Pathogens 2023, 12, 1213. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Goebel, M.C.; Trautner, B.W.; Grigoryan, L. The Five Ds of Outpatient Antibiotic Stewardship for Urinary Tract Infections. Clin. Microbiol. Rev. 2021, 34, e0000320. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Paul, R. State of the Globe: Rising Antimicrobial Resistance of Pathogens in Urinary Tract Infection. J. Glob. Infect. Dis. 2018, 10, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Zummo, S.; Gerace, E.; Scappatura, G.; Biondo, C. Extended-spectrum beta-lactamase & carbapenemase-producing fermentative Gram-negative bacilli in clinical isolates from a University Hospital in Southern Italy. New Microbiol. 2021, 44, 227–233. [Google Scholar]
- Li, X.; Fan, H.; Zi, H.; Hu, H.; Li, B.; Huang, J.; Luo, P.; Zeng, X. Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019. J. Clin. Med. 2022, 11, 2817. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Mlugu, E.M.; Mohamedi, J.A.; Sangeda, R.Z.; Mwambete, K.D. Prevalence of urinary tract infection and antimicrobial resistance patterns of uropathogens with biofilm forming capacity among outpatients in morogoro, Tanzania: A cross-sectional study. BMC Infect. Dis. 2023, 23, 660. [Google Scholar] [CrossRef]
- Uruen, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Gronseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Rajab, A.A.H.; Abdallah, M.H.; Rizvi, S.M.D.; Moin, A.; Khafagy, E.S.; Tabrez, S.; Hegazy, W.A.H. Biofilm Lifestyle in Recurrent Urinary Tract Infections. Life 2023, 13, 148. [Google Scholar] [CrossRef]
- Hola, V.; Opazo-Capurro, A.; Scavone, P. Editorial: The Biofilm Lifestyle of Uropathogens. Front. Cell. Infect. Microbiol. 2021, 11, 763415. [Google Scholar] [CrossRef]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Y.F.; Qi, X.W.; Du, Y.; Li, C.Q. Immune-related ureteritis and cystitis induced by immune checkpoint inhibitors: Case report and literature review. Front. Immunol. 2022, 13, 1051577. [Google Scholar] [CrossRef] [PubMed]
- Mahyar, A.; Ayazi, P.; Farzadmanesh, S.; Sahmani, M.; Oveisi, S.; Chegini, V.; Esmaeily, S. The role of zinc in acute pyelonephritis. Infez. Med. 2015, 23, 238–242. [Google Scholar] [PubMed]
- Van Eyssen, S.R.; Samarkina, A.; Isbilen, O.; Zeden, M.S.; Volkan, E. FimH and Type 1 Pili Mediated Tumor Cell Cytotoxicity by Uropathogenic Escherichia coli In Vitro. Pathogens 2023, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef]
- Ingram, A.; Posid, T.; Pandit, A.; Rose, J.; Amin, S.; Bellows, F. Risk factors, demographic profiles, and management of uncomplicated recurrent urinary tract infections: A single institution study. Menopause 2021, 28, 943–948. [Google Scholar] [CrossRef]
- Zare, M.; Vehreschild, M.; Wagenlehner, F. Management of uncomplicated recurrent urinary tract infections. BJU Int. 2022, 129, 668–678. [Google Scholar] [CrossRef]
- Mekonnen, S.A.; El Husseini, N.; Turdiev, A.; Carter, J.A.; Belew, A.T.; El-Sayed, N.M.; Lee, V.T. Catheter-associated urinary tract infection by Pseudomonas aeruginosa progresses through acute and chronic phases of infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2209383119. [Google Scholar] [CrossRef]
- Guliciuc, M.; Porav-Hodade, D.; Mihailov, R.; Rebegea, L.F.; Voidazan, S.T.; Ghirca, V.M.; Maier, A.C.; Marinescu, M.; Firescu, D. Exploring the Dynamic Role of Bacterial Etiology in Complicated Urinary Tract Infections. Medicina 2023, 59, 1686. [Google Scholar] [CrossRef]
- Woodford, H.J.; George, J. Diagnosis and management of urinary infections in older people. Clin. Med. 2011, 11, 80–83. [Google Scholar] [CrossRef]
- Geerlings, S.E. Clinical Presentations and Epidemiology of Urinary Tract Infections. Microbiol. Spectr. 2016, 4, 4–5. [Google Scholar] [CrossRef]
- Ebell, M.H.; Gagyor, I. Diagnosis of Urinary Tract Infection in Women. Am. Fam. Physician 2022, 106, 335–336. [Google Scholar] [PubMed]
- Kwok, M.; McGeorge, S.; Mayer-Coverdale, J.; Graves, B.; Paterson, D.L.; Harris, P.N.A.; Esler, R.; Dowling, C.; Britton, S.; Roberts, M.J. Guideline of guidelines: Management of recurrent urinary tract infections in women. BJU Int. 2022, 130 (Suppl. S3), 11–22. [Google Scholar] [CrossRef] [PubMed]
- Caretto, M.; Giannini, A.; Russo, E.; Simoncini, T. Preventing urinary tract infections after menopause without antibiotics. Maturitas 2017, 99, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Alperin, M.; Burnett, L.; Lukacz, E.; Brubaker, L. The mysteries of menopause and urogynecologic health: Clinical and scientific gaps. Menopause 2019, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Byren, I.; Hoey, C.T. Treatment of bacterial prostatitis. Clin. Infect. Dis. 2010, 50, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Schaeffer, A.J. Chronic prostatitis. BMJ Clin. Evid. 2011, 2011, 1802. [Google Scholar] [PubMed]
- Sell, J.; Nasir, M.; Courchesne, C. Urethritis: Rapid Evidence Review. Am. Fam. Physician 2021, 103, 553–558. [Google Scholar] [PubMed]
- Sadoghi, B.; Kranke, B.; Komericki, P.; Hutterer, G. Sexually transmitted pathogens causing urethritis: A mini-review and proposal of a clinically based diagnostic and therapeutic algorithm. Front. Med. 2022, 9, 931765. [Google Scholar] [CrossRef]
- Herness, J.; Buttolph, A.; Hammer, N.C. Acute Pyelonephritis in Adults: Rapid Evidence Review. Am. Fam. Physician 2020, 102, 173–180. [Google Scholar] [PubMed]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4, 459–502. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.S.; Liden, M.; Huttner, A. The urobiome in men and women: A clinical review. Clin. Microbiol. Infect. 2023, 29, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Topi, S.; Palmirotta, R.; D’Agostino, D.; Charitos, I.A.; Lovero, R.; Santacroce, L. An Overview of the Microbiota of the Human Urinary Tract in Health and Disease: Current Issues and Perspectives. Life 2023, 13, 1486. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.K.; Kandaswamy, K. Virulence factors of uropathogens and their role in host pathogen interactions. Cell Surf. 2022, 8, 100075. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Flores-Diaz, M.; Monturiol-Gross, L.; Naylor, C.; Alape-Giron, A.; Flieger, A. Bacterial Sphingomyelinases and Phospholipases as Virulence Factors. Microbiol. Mol. Biol. Rev. 2016, 80, 597–628. [Google Scholar] [CrossRef]
- Singh, V.; Phukan, U.J. Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity. Med. Microbiol. Immunol. 2019, 208, 585–607. [Google Scholar] [CrossRef]
- Ramirez-Larrota, J.S.; Eckhard, U. An Introduction to Bacterial Biofilms and Their Proteases, and Their Roles in Host Infection and Immune Evasion. Biomolecules 2022, 12, 306. [Google Scholar] [CrossRef]
- Whelan, S.; Lucey, B.; Finn, K. Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms 2023, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M.; Amare, A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect. Drug Resist. 2022, 15, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. Adv. Biol. 2014, 2014, 13. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies against Microbial Biofilm Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Hao, Y.; Liu, Y.; Dong, Z.; Li, K. Biological and Physiochemical Methods of Biofilm Adhesion Resistance Control of Medical-Context Surface. Int. J. Biol. Sci. 2021, 17, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.M. Biofilm: Formation and Natural Products’ Approach to Control—A Review. Afr. J. Infect. Dis. 2022, 16, 59–71. [Google Scholar] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. Int. J. Mol. Sci. 2023, 24, 4030. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.G.; Sandulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Y.; Lin, S.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; et al. Strategies for Interfering with Bacterial Early Stage Biofilms. Front. Microbiol. 2021, 12, 675843. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Dhar, N.; Thacker, V.V.; Simonet, T.M.; Signorino-Gelo, F.; Knott, G.W.; McKinney, J.D. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. eLife 2021, 10, e66481. [Google Scholar] [CrossRef]
- Szabo, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. An Overview of Healthcare Associated Infections and Their Detection Methods Caused by Pathogen Bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef]
- Rubi, H.; Mudey, G.; Kunjalwar, R. Catheter-Associated Urinary Tract Infection (CAUTI). Cureus 2022, 14, e30385. [Google Scholar] [CrossRef]
- Yuan, F.; Huang, Z.; Yang, T.; Wang, G.; Li, P.; Yang, B.; Li, J. Pathogenesis of Proteus mirabilis in Catheter-Associated Urinary Tract Infections. Urol. Int. 2021, 105, 354–361. [Google Scholar] [CrossRef]
- Murray, B.O.; Flores, C.; Williams, C.; Flusberg, D.A.; Marr, E.E.; Kwiatkowska, K.M.; Charest, J.L.; Isenberg, B.C.; Rohn, J.L. Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems. Front. Cell. Infect. Microbiol. 2021, 11, 691210. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of antibiotic resistance biofilms and potential combating strategies: A review. 3 Biotech 2021, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; Yousef, A.E. Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections. Antibiotics 2023, 12, 1005. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef]
- Palusiak, A. Proteus mirabilis and Klebsiella pneumoniae as pathogens capable of causing co-infections and exhibiting similarities in their virulence factors. Front. Cell. Infect. Microbiol. 2022, 12, 991657. [Google Scholar] [CrossRef]
- Cortese, Y.J.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of Catheter-Associated Urinary Tract Infections and In Vitro Urinary Tract Models. J. Healthc. Eng. 2018, 2018, 2986742. [Google Scholar] [CrossRef]
- Kanti, S.P.Y.; Csoka, I.; Jojart-Laczkovich, O.; Adalbert, L. Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications. Biomedicines 2022, 10, 2580. [Google Scholar] [CrossRef]
- Stahlhut, S.G.; Struve, C.; Krogfelt, K.A.; Reisner, A. Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol. Med. Microbiol. 2012, 65, 350–359. [Google Scholar] [CrossRef]
- Taha, H.; Raji, S.J.; Khallaf, A.; Abu Hija, S.; Mathew, R.; Rashed, H.; Du Plessis, C.; Allie, Z.; Ellahham, S. Improving Catheter Associated Urinary Tract Infection Rates in the Medical Units. BMJ Qual. Improv. Rep. 2017, 6, u209593-w7966. [Google Scholar] [CrossRef][Green Version]
- Van Decker, S.G.; Bosch, N.; Murphy, J. Catheter-associated urinary tract infection reduction in critical care units: A bundled care model. BMJ Open Qual. 2021, 10, e001534. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Baral, R.; Khanal, B. Comparative study of antimicrobial resistance and biofilm formation among Gram-positive uropathogens isolated from community-acquired urinary tract infections and catheter-associated urinary tract infections. Infect. Drug Resist. 2019, 12, 957–963. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef] [PubMed]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hemadi, M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.S.; Hanafiah, A.; Nachimuthu, R.; Muthupandian, S.; Md Nesran, Z.N.; Patil, S. The Role of Antimicrobial Peptides as Antimicrobial and Antibiofilm Agents in Tackling the Silent Pandemic of Antimicrobial Resistance. Molecules 2022, 27, 2995. [Google Scholar] [CrossRef] [PubMed]
- Pontes, J.T.C.; Toledo Borges, A.B.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial Peptides as an Alternative for the Eradication of Bacterial Biofilms of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Zendo, T.; Sugimoto, S.; Iwase, T.; Tajima, A.; Yamada, S.; Sonomoto, K.; Mizunoe, Y. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2013, 57, 5572–5579. [Google Scholar] [CrossRef]
- Almaaytah, A.; Farajallah, A.; Abualhaijaa, A.; Al-Balas, Q. A3, a Scorpion Venom Derived Peptide Analogue with Potent Antimicrobial and Potential Antibiofilm Activity against Clinical Isolates of Multi-Drug Resistant Gram Positive Bacteria. Molecules 2018, 23, 1603. [Google Scholar] [CrossRef]
- Hwang, I.S.; Hwang, J.S.; Hwang, J.H.; Choi, H.; Lee, E.; Kim, Y.; Lee, D.G. Synergistic effect and antibiofilm activity between the antimicrobial peptide coprisin and conventional antibiotics against opportunistic bacteria. Curr. Microbiol. 2013, 66, 56–60. [Google Scholar] [CrossRef]
- Xie, Z.; Wei, H.; Meng, J.; Cheng, T.; Song, Y.; Wang, M.; Zhang, Y. The Analogs of Temporin-GHa Exhibit a Broader Spectrum of Antimicrobial Activity and a Stronger Antibiofilm Potential against Staphylococcus aureus. Molecules 2019, 24, 4173. [Google Scholar] [CrossRef]
- Park, S.C.; Lee, M.Y.; Kim, J.Y.; Kim, H.; Jung, M.; Shin, M.K.; Lee, W.K.; Cheong, G.W.; Lee, J.R.; Jang, M.K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules 2019, 24, 4560. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Nunez, C.; Cardoso, M.H.; de Souza Candido, E.; Franco, O.L.; Hancock, R.E. Synthetic antibiofilm peptides. Biochim. Biophys. Acta 2016, 1858, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Dosler, S.; Karaaslan, E.; Alev Gerceker, A. Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram-negative bacteria. J. Chemother. 2016, 28, 95–103. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Nunez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gamez, S.; Hochberg, M.E.; van Doorn, G.S. Quorum sensing as a mechanism to harness the wisdom of the crowds. Nat. Commun. 2023, 14, 3415. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhatia, S. Quorum Sensing Inhibitors: Curbing Pathogenic Infections through Inhibition of Bacterial Communication. Iran. J. Pharm. Res. 2021, 20, 486–514. [Google Scholar] [CrossRef] [PubMed]
- Duplantier, M.; Lohou, E.; Sonnet, P. Quorum Sensing Inhibitors to Quench P. aeruginosa Pathogenicity. Pharmaceuticals 2021, 14, 1262. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Imani Rad, H.; Peeri, H.; Amani, M.; Mohammadnia, A.; Ogunniyi, A.D.; Khazandi, M.; Venter, H.; Arzanlou, M. Allicin prevents the formation of Proteus-induced urinary crystals and the blockage of catheter in a bladder model in vitro. Microb. Pathog. 2019, 132, 293–301. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Warming, A.N.; Vejborg, R.M.; Moscoso, J.A.; Stegger, M.; Lorenzen, F.; Rybtke, M.; Andersen, J.B.; Petersen, R.; Andersen, P.S.; et al. A broad range quorum sensing inhibitor working through sRNA inhibition. Sci. Rep. 2017, 7, 9857. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Reddy, D.; Mohanty, S.K.; Kumavath, R.; Murali, A.; Suchiang, K.; Busi, S. Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PAO1: Insights from in vitro, in vivo and in silico studies. Microbiol. Res. 2019, 226, 19–26. [Google Scholar] [CrossRef]
- Bhargava, N.; Singh, S.P.; Sharma, A.; Sharma, P.; Capalash, N. Attenuation of quorum sensing-mediated virulence of Acinetobacter baumannii by Glycyrrhiza glabra flavonoids. Future Microbiol. 2015, 10, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, C.; Jha, N.K.; Ghosh, R.; Shetty, P.H. Anti quorum sensing and anti virulence activity of tannic acid and it’s potential to breach resistance in Salmonella enterica Typhi/Paratyphi A clinical isolates. Microb. Pathog. 2020, 138, 103813. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Dang, T.T.; Martinuzzi, R. Use of quorum sensing antagonists to deter the formation of crystalline Proteus mirabilis biofilms. Int. J. Antimicrob. Agents 2009, 34, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Crintea, A.; Carpa, R.; Mitre, A.O.; Petho, R.I.; Chelaru, V.F.; Nadasan, S.M.; Neamti, L.; Dutu, A.G. Nanotechnology Involved in Treating Urinary Tract Infections: An Overview. Nanomaterials 2023, 13, 555. [Google Scholar] [CrossRef]
- Moradi, F.; Ghaedi, A.; Fooladfar, Z.; Bazrgar, A. Recent advance on nanoparticles or nanomaterials with anti-multidrug resistant bacteria and anti-bacterial biofilm properties: A systematic review. Heliyon 2023, 9, e22105. [Google Scholar] [CrossRef]
- Garcia Vence, M.; Chantada-Vazquez, M.D.P.; Vazquez-Estevez, S.; Manuel Cameselle-Teijeiro, J.; Bravo, S.B.; Nunez, C. Potential clinical applications of the personalized, disease-specific protein corona on nanoparticles. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 501, 102–111. [Google Scholar] [CrossRef]
- Siraj, E.A.; Yayehrad, A.T.; Belete, A. How Combined Macrolide Nanomaterials are Effective against Resistant Pathogens? A Comprehensive Review of the Literature. Int. J. Nanomed. 2023, 18, 5289–5307. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D.; Kari, Z.A.; Edinur, H.A. Green Synthesis of Silver Nanoparticles Using Diospyros malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-NP). Nanomaterials 2021, 11, 1999. [Google Scholar] [CrossRef]
- Palanisamy, N.K.; Ferina, N.; Amirulhusni, A.N.; Mohd-Zain, Z.; Hussaini, J.; Ping, L.J.; Durairaj, R. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, D.; Ramanathan, S.; Arunachalam, K.; Jeyaraj, G.P.; Shunmugiah, K.P.; Arumugam, V.R. Phytosynthesized silver nanoparticles as antiquorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: An in vitro study. J. Appl. Microbiol. 2018, 124, 1425–1440. [Google Scholar] [CrossRef]
- Shunmugaperumal, T.; Ramamurthy, S. Assessment of antibiofilm activity of magnesium fluoride nanoparticles-stabilized oil-in-water nanosized emulsion. Drug Dev. Ind. Pharm. 2012, 38, 899. [Google Scholar] [CrossRef]
- Lellouche, J.; Kahana, E.; Elias, S.; Gedanken, A.; Banin, E. Antibiofilm activity of nanosized magnesium fluoride. Biomaterials 2009, 30, 5969–5978. [Google Scholar] [CrossRef] [PubMed]
- Guha-Chowdhury, N.; Clark, A.G.; Sissons, C.H. Inhibition of purified enolases from oral bacteria by fluoride. Oral Microbiol. Immunol. 1997, 12, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lellouche, J.; Friedman, A.; Gedanken, A.; Banin, E. Antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int. J. Nanomed. 2012, 7, 5611–5624. [Google Scholar] [CrossRef]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Sposito, L.; De Toledo, L.G.; Bonifacio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Baishya, D.; Sarkar, T.; Edinur, H.A.; Pati, S. Synthesis and Characterization of Diosgenin Encapsulated Poly-epsilon-Caprolactone-Pluronic Nanoparticles and Its Effect on Brain Cancer Cells. Polymers 2021, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qiao, S.; Li, L.; Qi, G.; Lin, Y.; Qiao, Z.; Wang, H.; Shao, C. Surface charge-conversion polymeric nanoparticles for photodynamic treatment of urinary tract bacterial infections. Nanotechnology 2015, 26, 495602. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkodous, M.; El-Sayyad, G.S.; Abdel Maksoud, M.I.A.; Abdelrahman, I.Y.; Mosallam, F.M.; Gobara, M.; El-Batal, A.I. Fabrication of Ultra-Pure Anisotropic Zinc Oxide Nanoparticles via Simple and Cost-Effective Route: Implications for UTI and EAC Medications. Biol. Trace Elem. Res. 2020, 196, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lutken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif. Cells Nanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef]
- Alomary, M.N.; Ansari, M.A. Proanthocyanin-Capped Biogenic TiO(2) Nanoparticles with Enhanced Penetration, Antibacterial and ROS Mediated Inhibition of Bacteria Proliferation and Biofilm Formation: A Comparative Approach. Chemistry 2021, 27, 5817–5829. [Google Scholar] [CrossRef]
- Qindeel, M.; Barani, M.; Rahdar, A.; Arshad, R.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Urinary Tract Infections. Nanomaterials 2021, 11, 546. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef]
- Agarwala, M.; Choudhury, B.; Yadav, R.N. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of Gallium and Gallium-Based Compounds as Antimicrobial Agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef]
- Polivkova, M.; Hubacek, T.; Staszek, M.; Svorcik, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piatek, B.; Piatek, R. Bacteriophages as Potential Tools for Use in Antimicrobial Therapy and Vaccine Development. Pharmaceuticals 2021, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review on the Recent Use of Phage-Based Strategies with Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef]


| Organs of Urinary Tract | Signs and Symptoms |
|---|---|
| Bladder | Dysuria *, blood in urine, frequency *, suprapubic pain |
| Urethra | Burning with urination, discharge |
| Kidneys | Nausea, vomiting, high fever, back or side pain |
| Urethritis | Dysuria *, itching, frequency * |
| AMPs | Antibiofilm Activity | Mechanism of Action | Reference |
|---|---|---|---|
| Nisin A, Mastoparan | S. aureus | Membrane depolarisation | [89] |
| A3 | E. faecalis, S. aureus | Membrane disruption | [90] |
| Coprisin | E. coli, S. aureus | Membrane disruption | [91] |
| GHaK | S. aureus | Membrane permeabilisation | [92] |
| PS1 | P. aeruginossa, S. aureus | EPS production inhibition | [93] |
| DJK 5/6 | E. coli, P. aeruginosa, K. pneumoniae | Cell signal interruption for biofilm formation | [94] |
| Melittin | E. coli, P. aeruginosa, K. pneumoniae | Membrane permeabilisation | [95] |
| LL-37 | P. aeruginosa, S. epidermidis | Preventing the transcription of specific genes necessary for quorum sensing | [96] |
| Hepcidin | S. epidermidis | Inhibition of EPS production | [97] |
| QS Inhibitors | Antibiofilm Activity | Mechanism of Action | Reference |
|---|---|---|---|
| Allicin | P. aeruginosa, P. mirabilis | QS inhibition | [103,104] |
| 5-Hydroxymethylfurfural | P. aeruginosa | Downregulation of the expression of quorum-sensing genes | [105] |
| Glycyrin and glyzarin | Acinetobacter baumannii | Inhibition of microbial-quorum-sensing-mediated virulence factors | [106] |
| Tannic acid | P. mirabilis S. typhi and S. paratyphi A | Restriction of QS-regulated virulence factors | [107,108] |
| NPs | Anti-Biofilm Activity | MIC | Mechanism of Action | Reference |
|---|---|---|---|---|
| Silver nanoparticles (AgNPs) | S. aureus, E. coli, P. aeruginosa, P. vulgaris | 0.625 mg/mL (S. aureus) | Nano-based drug delivery | [113,114,115] |
| Fluoride-based nanoparticles | E. faecalis, S. aureus | 0.1 mg/mL (S. aureus) | Inhibition of bacterial metabolism | [116,117,118,119] |
| Polymeric nanoparticles (PNs) | Gram-positive and Gram-negative bacteria | 0.340 mg/mL (S. aureus) | Controlled drug delivery | [120,121,122] |
| Zinc-based nanoparticles | E. coli, S. aureus | 0.05 mg/mL (S. aureus) | Disruption of membrane integrity | [116,123] |
| Gold nanoparticles (AuNPs) | P. aeruginossa, E. coli, S. aureus | 7.56 μg/mL (S. aureus) | Targeted drug delivery | [124,125,126] |
| Iron, aluminium oxide, copper oxide, gallium-based NPs | Gram-positive and Gram-negative bacteria | 100 μM (S. aureus) | ROS generation | [127,128,129,130,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, G.; Trinchera, M.; Midiri, A.; Zummo, S.; Vitale, G.; Biondo, C. Novel Antimicrobial Approaches to Combat Bacterial Biofilms Associated with Urinary Tract Infections. Antibiotics 2024, 13, 154. https://doi.org/10.3390/antibiotics13020154
Mancuso G, Trinchera M, Midiri A, Zummo S, Vitale G, Biondo C. Novel Antimicrobial Approaches to Combat Bacterial Biofilms Associated with Urinary Tract Infections. Antibiotics. 2024; 13(2):154. https://doi.org/10.3390/antibiotics13020154
Chicago/Turabian StyleMancuso, Giuseppe, Marilena Trinchera, Angelina Midiri, Sebastiana Zummo, Giulia Vitale, and Carmelo Biondo. 2024. "Novel Antimicrobial Approaches to Combat Bacterial Biofilms Associated with Urinary Tract Infections" Antibiotics 13, no. 2: 154. https://doi.org/10.3390/antibiotics13020154
APA StyleMancuso, G., Trinchera, M., Midiri, A., Zummo, S., Vitale, G., & Biondo, C. (2024). Novel Antimicrobial Approaches to Combat Bacterial Biofilms Associated with Urinary Tract Infections. Antibiotics, 13(2), 154. https://doi.org/10.3390/antibiotics13020154






