Acidic Urine pH and Clinical Outcome of Lower Urinary Tract Infection in Kidney Transplant Recipients Treated with Ciprofloxacin and Fosfomycin
Abstract
1. Introduction
2. Results
2.1. Characteristics of KTRs with E. coli and K. pneumoniae UTI Episodes
2.2. Association of Urine pH with Microbiological and Clinical Outcomes of E. coli and K. pneumoniae UTI Episodes
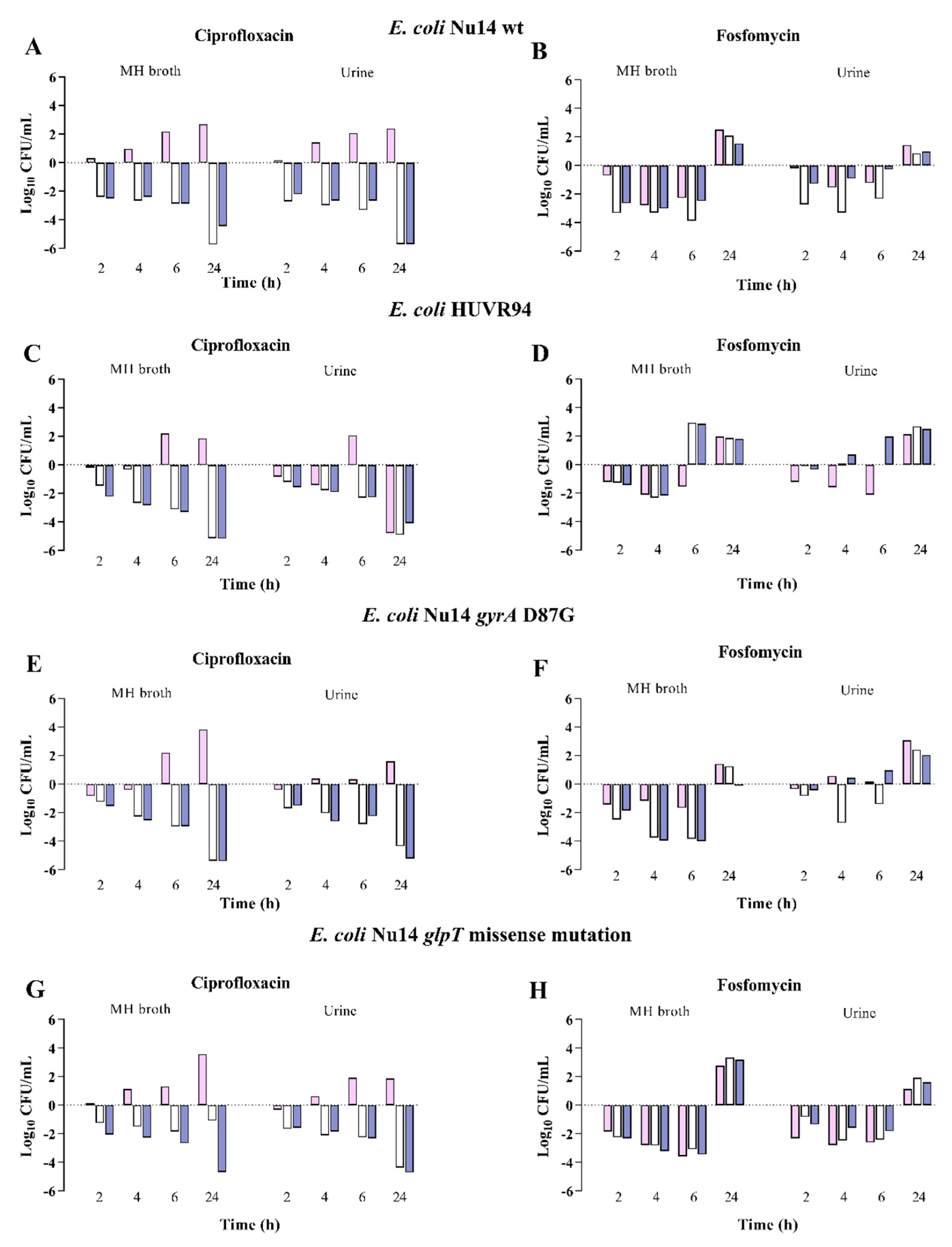
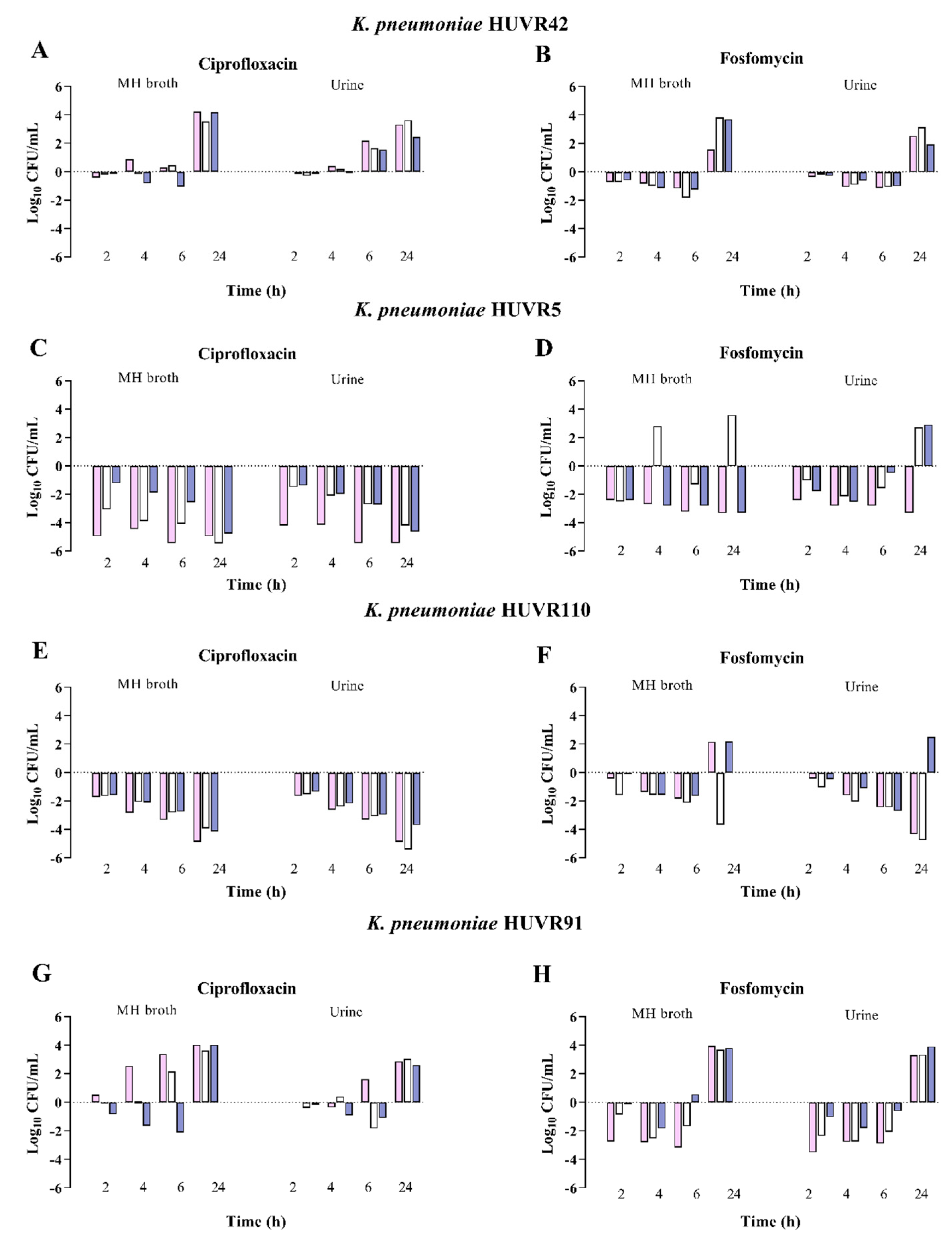
2.3. Antimicrobial and Bactericidal Activities of Ciprofloxacin and Fosfomycin against E. coli and K. pneumoniae Clinical Isolates at Neutral, Acidic, and Alkaline pH
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Antimicrobial Susceptibility of E. coli and K. pneumoniae Clinical Isolates at Different pH Conditions
4.3. Bactericidal Activity of Ciprofloxacin and Fosfomycin against E. coli and K. pneumoniae Strains at Different pH Conditions
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodro, M.; Sanclemente, G.; Lipperheide, I.; Allali, M.; Marco, F.; Bosch, J.; Cofan, F.; Ricart, M.J.; Esforzado, N.; Oppenheimer, F.; et al. Impact of urinary tract infections on short-term kidney graft outcome. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015, 21, 1104.e1–1104.e8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naik, A.S.; Dharnidharka, V.R.; Schnitzler, M.A.; Brennan, D.C.; Segev, D.L.; Axelrod, D.; Xiao, H.; Kucirka, L.; Chen, J.; Lentine, K.L. Clinical and economic consequences of first-year urinary tract infections, sepsis, and pneumonia in contemporary kidney transplantation practice. Transpl. Int. 2016, 29, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Fontserè, S.; Infante-Domínguez, C.; Suárez-Benjumea, A.; Suñer-Poblet, M.; González-Corvillo, C.; Martín-Gutiérrez, G.; Bernal, G.; Pachón, J.; Pachón-Ibáñez, M.E.; Cordero, E. Impact of Treating Asymptomatic Bacteriuria in Kidney Transplant Recipients: A Prospective Cohort Study. Antibiotics 2021, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, M.; Pezeshgi, A.; Mahdiabadi, M.Z.; Sabzghabaei, F.; Hajishah, H.; Mahdavynia, S. Prevalence and risk factors of urinary tract infection in kidney recipients: A meta-analysis study. BMC Nephrol. 2023, 24, 284. [Google Scholar] [CrossRef]
- Ny, S.; Edquist, P.; Dumpis, U.; Gröndahl-Yli-Hannuksela, K.; Hermes, J.; Kling, A.M.; Klingeberg, A.; Kozlov, R.; Källman, O.; Lis, D.O.; et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2019, 17, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, B.; Li, Y.; Zhu, S.; Xue, F.; Liu, J. Antimicrobial Susceptibility and Molecular Mechanisms of Fosfomycin Resistance in Clinical Escherichia coli Isolates in Mainland China. PLoS ONE 2015, 10, e0135269. [Google Scholar] [CrossRef]
- Seok, H.; Choi, J.Y.; Wi, Y.M.; Park, D.W.; Peck, K.R.; Ko, K.S. Fosfomycin Resistance in Escherichia coli Isolates from South Korea and in vitro Activity of Fosfomycin Alone and in Combination with Other Antibiotics. Antibiotics 2020, 9, 112. [Google Scholar] [CrossRef]
- Origüen, J.; Fernández-Ruiz, M.; López-Medrano, F.; Ruiz-Merlo, T.; González, E.; Morales, J.M.; Fiorante, S.; San-Juan, R.; Villa, J.; Orellana, M.; et al. Progressive increase of resistance in Enterobacteriaceae urinary isolates from kidney transplant recipients over the past decade: Narrowing of the therapeutic options. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2016, 18, 575–584. [Google Scholar] [CrossRef]
- López-Medrano, F.; Silva, J.T.; Fernández-Ruiz, M.; Vidal, E.; Origüen, J.; Calvo-Cano, A.; Luna-Huerta, E.; Merino, E.; Hernández, D.; Jironda-Gallegos, C.; et al. Oral fosfomycin for the treatment of lower urinary tract infections among kidney transplant recipients-Results of a Spanish multicenter cohort. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 451–462. [Google Scholar] [CrossRef]
- Coussement, J.; Kamar, N.; Matignon, M.; Weekers, L.; Scemla, A.; Giral, M.; Racapé, J.; Alamartine, É.; Mesnard, L.; Kianda, M.; et al. Antibiotics versus no therapy in kidney transplant recipients with asymptomatic bacteriuria (BiRT): A pragmatic, multicentre, randomized, controlled trial. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 398–405. [Google Scholar] [CrossRef]
- Origüen, J.; López-Medrano, F.; Fernández-Ruiz, M.; Polanco, N.; Gutiérrez, E.; González, E.; Mérida, E.; Ruiz-Merlo, T.; Morales-Cartagena, A.; Pérez-Jacoiste Asín, M.A.; et al. Should Asymptomatic Bacteriuria Be Systematically Treated in Kidney Transplant Recipients? Results From a Randomized Controlled Trial. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016, 16, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Sabé, N.; Oriol, I.; Melilli, E.; Manonelles, A.; Bestard, O.; Polo, C.; Los Arcos, I.; Perelló, M.; Garcia, D.; Riera, L.; et al. Antibiotic Treatment Versus No Treatment for Asymptomatic Bacteriuria in Kidney Transplant Recipients: A Multicenter Randomized Trial. Open Forum. Infect. Dis. 2019, 6, ofz243. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehnerand, F. EAU Guidelines on Urological Infections; EAU Guidelines Office: Arnhem, The Netherlands, 2023. [Google Scholar]
- EMA (European Medicines Agency). Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. Available online: https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-disabling-potentially-permanent-side-effects-lead_en.pdf (accessed on 8 November 2023).
- Komp Lindgren, P.; Karlsson, A.; Hughes, D. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob. Agents Chemother. 2003, 47, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Muratani, T.; Yasuda, M.; Takahashi, S.; Monden, K.; Ishikawa, K.; Kiyota, H.; Arakawa, S.; Matsumoto, T.; Shima, H.; et al. Genetic profiles of fluoroquinolone-resistant Escherichia coli isolates obtained from patients with cystitis: Phylogeny, virulence factors, PAIusp subtypes, and mutation patterns. J. Clin. Microbiol. 2009, 47, 791–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martín-Gutiérrez, G.; Rodríguez-Beltrán, J.; Rodríguez-Martínez, J.M.; Costas, C.; Aznar, J.; Pascual, Á.; Blázquez, J. Urinary Tract Physiological Conditions Promote Ciprofloxacin Resistance in Low-Level-Quinolone-Resistant Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gutiérrez, G.; Rodríguez-Martínez, J.M.; Pascual, Á.; Rodríguez-Beltrán, J.; Blázquez, J. Plasmidic qnr Genes Confer Clinical Resistance to Ciprofloxacin under Urinary Tract Physiological Conditions. Antimicrob. Agents Chemother. 2017, 61, e02615-16. [Google Scholar] [CrossRef]
- Erdogan-Yildirim, Z.; Burian, A.; Manafi, M.; Zeitlinger, M. Impact of pH on bacterial growth and activity of recent fluoroquinolones in pooled urine. Res. Microbiol. 2011, 162, 249–252. [Google Scholar] [CrossRef]
- Martín-Gutiérrez, G.; Docobo-Pérez, F.; Rodriguez-Beltrán, J.; Rodríguez-Martínez, J.M.; Aznar, J.; Pascual, A.; Blázquez, J. Urinary Tract Conditions Affect Fosfomycin Activity against Escherichia coli Strains Harboring Chromosomal Mutations Involved in Fosfomycin Uptake. Antimicrob. Agents Chemother. 2018, 62, e01899-17. [Google Scholar] [CrossRef]
- Fedrigo, N.H.; Mazucheli, J.; Albiero, J.; Shinohara, D.R.; Lodi, F.G.; Machado, A.; Sy, S.K.B.; Tognim, M.C.B. Pharmacodynamic Evaluation of Fosfomycin against Escherichia coli and Klebsiella spp. from Urinary Tract Infections and the Influence of pH on Fosfomycin Activities. Antimicrob. Agents Chemother. 2017, 61, e02498-16. [Google Scholar] [CrossRef]
- Ten Doesschate, T.; van Werkhoven, H.; Meijvis, S.; Stalenhoef, J.; van Zuilen, A.; de Vries, A.; Bonten, M. Fosfomycin-trometamol for Urinary Tract Infections in Kidney Transplant Recipients. Transplantation 2019, 103, 1272–1276. [Google Scholar] [CrossRef]
- Campos, A.; Andrade, N.L.; Couto, N.; Mutters, N.T.; de Vos, M.; Rosa, A.C.P.; Damasco, P.V.; Lo Ten Foe, J.R.; Friedrich, A.W.; Chlebowicz-Flissikowska, M.A.; et al. Characterization of fosfomycin heteroresistance among multidrug-resistant Escherichia coli isolates from hospitalized patients in Rio de Janeiro, Brazil. J. Glob. Antimicrob. Resist. 2020, 22, 584–593. [Google Scholar] [CrossRef]
- Burian, A.; Erdogan, Z.; Jandrisits, C.; Zeitlinger, M. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology 2012, 90, 281–287. [Google Scholar] [CrossRef]
- AEMPS (Agencia Española de Medicamentos y Productos Sanitarios). Fosfomicina Kern Pharma; AEMPS: Madrid, Spain, 2021.
- AEMPS (Agencia Española de Medicamentos y Productos Sanitarios). Ciprofloxacino Normon; AEMPS: Madrid, Spain, 2020.
- EUCAST (The European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 11.0; ESCMID: Base, Switzerland, 2021. [Google Scholar]
- Mehershahi, K.S.; Chen, S.L. Complete Genome Sequence of the Uropathogenic Escherichia coli Strain NU14. Genome Announc. 2017, 5, e00306-17. [Google Scholar] [CrossRef]
- Komp Lindgren, P.; Marcusson, L.L.; Sandvang, D.; Frimodt-Møller, N.; Hughes, D. Biological cost of single and multiple norfloxacin resistance mutations in Escherichia coli implicated in urinary tract infections. Antimicrob. Agents Chemother. 2005, 49, 2343–2351. [Google Scholar] [CrossRef]
- Nilsson, A.I.; Berg, O.G.; Aspevall, O.; Kahlmeter, G.; Andersson, D.I. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob. Agents Chemother. 2003, 47, 2850–2858. [Google Scholar] [CrossRef]

| Variables | E. coli 115 Episodes N (%) | K. pneumoniae 69 Episodes N (%) |
|---|---|---|
| Age (years; median [IQR]) | 58 (50–67) | 61 (50–69) |
| Female patients | 71 (61.7) | 42 (60.9) |
| Charlson Comorbidity Index (median [IQR]) | 3 (3–5) | 5 (3–5) |
| Months from transplantation (median [IQR]) | 14 (4–77) | 6 (1–77) |
| <2 months from transplantation | 21 (18.3) | 41 (59.4) |
| Previous kidney transplantation | 10 (8.7) | 8 (11.6) |
| Living donor | 11 (9.5) | 6 (8.7) |
Induction therapy within 3 previous months:
| 73 (63.5) 26 (22.6) 41 (35.7) 6 (5.2) | 28 (40.6) 12 (17.4) 14 (20.3) 2 (2.9) |
Current immunosuppression:
| 107 (93.0) 107 (93.0) 90 (78.3) 8 (6.9) 4 (3.4) | 58 (84.1) 64 (92.8) 54 (78.3) 4 (5.8) 3 (4.3) |
| Acute rejection within the previous 6 months | 11 (9.6) | 0 (0.0) |
Rejection treatment in the previous 6 months:
| 9 (7.9) 1 (0.9) 1 (0.9) | - - - |
| Creatinine (mg/dL; median [IQR]) | 1.57 (1.21–1.95) | 1.56 (1.25–1.99) |
| Bacteriuria within the previous 6 months | 57 (49.6) | 49 (71.0) |
Antibiotic use within the previous 3 months
| 48 (41.7) 11 (9.6) 6 (5.2) 14 (12.2) 15 (13.0) 2 (1.7) | 30 (43.5) 3 (4.3) 8 (11.6) 10 (14.5) 7 (10.1) 2 (2.9) |
| Cystitis | 19 (16.5) | 33 (47.8) |
| Asymptomatic bacteriuria | 96 (83.5) | 36 (52.2) |
| Urinary pH (median [IQR]) | 6 (6–6.5) | 6.5 (6–6.5) |
Baseline antibiotic resistance:
| 66 (57.4) 30 (26.1) 24 (20.9) 10 (8.7) 2 (1.7) 2 (1.7) | 42 (60.9) 20 (29.0) 15 (21.7) 8 (11.6) 21 (30.4) 20 (29.0) |
Antibiotic therapy of the UTI episodes
| 88 (76.5) 27 (23.5) | 46 (66.7) 23 (33.3) |
| Variable | Urinary pH ≤ 6 | Urinary pH > 6 | p | |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Escherichia coli UTI Episodes (N = 115) | ||||
| Microbiological cure during one-month follow-up | Total | 37/60 (61.7) | 38/55 (69.1) | 0.41 |
| Episodes treated with fosfomycin | 29/47 (61.7) | 27/41 (65.9) | 0.69 | |
| Episodes treated with ciprofloxacin | 8/13 (61.5) | 11/14 (78.6) | 0.42 | |
| Symptomatic UTI during one-month follow-up | Total | 8/60 (13.3) | 0/55 (0.0) | 0.006 |
| Episodes treated with fosfomycin | 7/47 (14.9) | 0/41 (0.0) | 0.013 | |
| Episodes treated with ciprofloxacin | 1/13 (7.7) | 0/14 (0.0) | 0.48 | |
| Symptomatic UTI during six-month follow-up | Total | 11/60 (18.3) | 9/55 (16.4) | 0.78 |
| Episodes treated with fosfomycin | 10/47 (21.3) | 9/41 (22.0) | 0.94 | |
| Episodes treated with ciprofloxacin | 1/13 (7.7) | 0/14 (0.0) | 0.48 | |
| Klebsiella pneumoniae UTI episodes (N = 69) | ||||
| Microbiological cure during one-month follow-up | Total | 10/29 (34.5) | 15/40 (37.5) | 0.69 |
| Episodes treated with fosfomycin | 4/16 (25.0) | 8/30 (26.7) | 1.00 | |
| Episodes treated with ciprofloxacin | 6/13 (46.2) | 7/10 (70.0) | 0.16 | |
| Symptomatic UTI during one-month follow-up | Total | 4/29 (13.8) | 4/40 (10.0) | 0.71 |
| Episodes treated with fosfomycin | 3/16 (18.8) | 4/30 (13.3) | 0.69 | |
| Episodes treated with ciprofloxacin | 1/13 (7.7) | 0/10 (0.0) | 1.00 | |
| Symptomatic UTI during six-month follow-up | Total | 3/29 (10.3) | 6/40 (15.0) | 0.75 |
| Episodes treated with fosfomycin | 1/16 (6.3) | 4/30 (13.3) | 1.00 | |
| Episodes treated with ciprofloxacin | 2/13 (15.4) | 2/10 (20.0) | 1.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Espejo, S.; Fontserè, S.; Infante, C.; Suárez-Benjumea, A.; Carretero-Ledesma, M.; Suñer-Poblet, M.; González-Corvillo, C.; Bernal, G.; Martín-Gutiérrez, G.; Pérez-Cáceres, J.A.; et al. Acidic Urine pH and Clinical Outcome of Lower Urinary Tract Infection in Kidney Transplant Recipients Treated with Ciprofloxacin and Fosfomycin. Antibiotics 2024, 13, 116. https://doi.org/10.3390/antibiotics13020116
Herrera-Espejo S, Fontserè S, Infante C, Suárez-Benjumea A, Carretero-Ledesma M, Suñer-Poblet M, González-Corvillo C, Bernal G, Martín-Gutiérrez G, Pérez-Cáceres JA, et al. Acidic Urine pH and Clinical Outcome of Lower Urinary Tract Infection in Kidney Transplant Recipients Treated with Ciprofloxacin and Fosfomycin. Antibiotics. 2024; 13(2):116. https://doi.org/10.3390/antibiotics13020116
Chicago/Turabian StyleHerrera-Espejo, Soraya, Sara Fontserè, Carmen Infante, Alejandro Suárez-Benjumea, Marta Carretero-Ledesma, Marta Suñer-Poblet, Carmen González-Corvillo, Gabriel Bernal, Guillermo Martín-Gutiérrez, Juan Antonio Pérez-Cáceres, and et al. 2024. "Acidic Urine pH and Clinical Outcome of Lower Urinary Tract Infection in Kidney Transplant Recipients Treated with Ciprofloxacin and Fosfomycin" Antibiotics 13, no. 2: 116. https://doi.org/10.3390/antibiotics13020116
APA StyleHerrera-Espejo, S., Fontserè, S., Infante, C., Suárez-Benjumea, A., Carretero-Ledesma, M., Suñer-Poblet, M., González-Corvillo, C., Bernal, G., Martín-Gutiérrez, G., Pérez-Cáceres, J. A., Pachón, J., Pachón-Ibáñez, M. E., & Cordero, E. (2024). Acidic Urine pH and Clinical Outcome of Lower Urinary Tract Infection in Kidney Transplant Recipients Treated with Ciprofloxacin and Fosfomycin. Antibiotics, 13(2), 116. https://doi.org/10.3390/antibiotics13020116







