A Surveillance Study of Culturable and Antimicrobial-Resistant Bacteria in Two Urban WWTPs in Northern Spain
Abstract
1. Introduction
2. Results
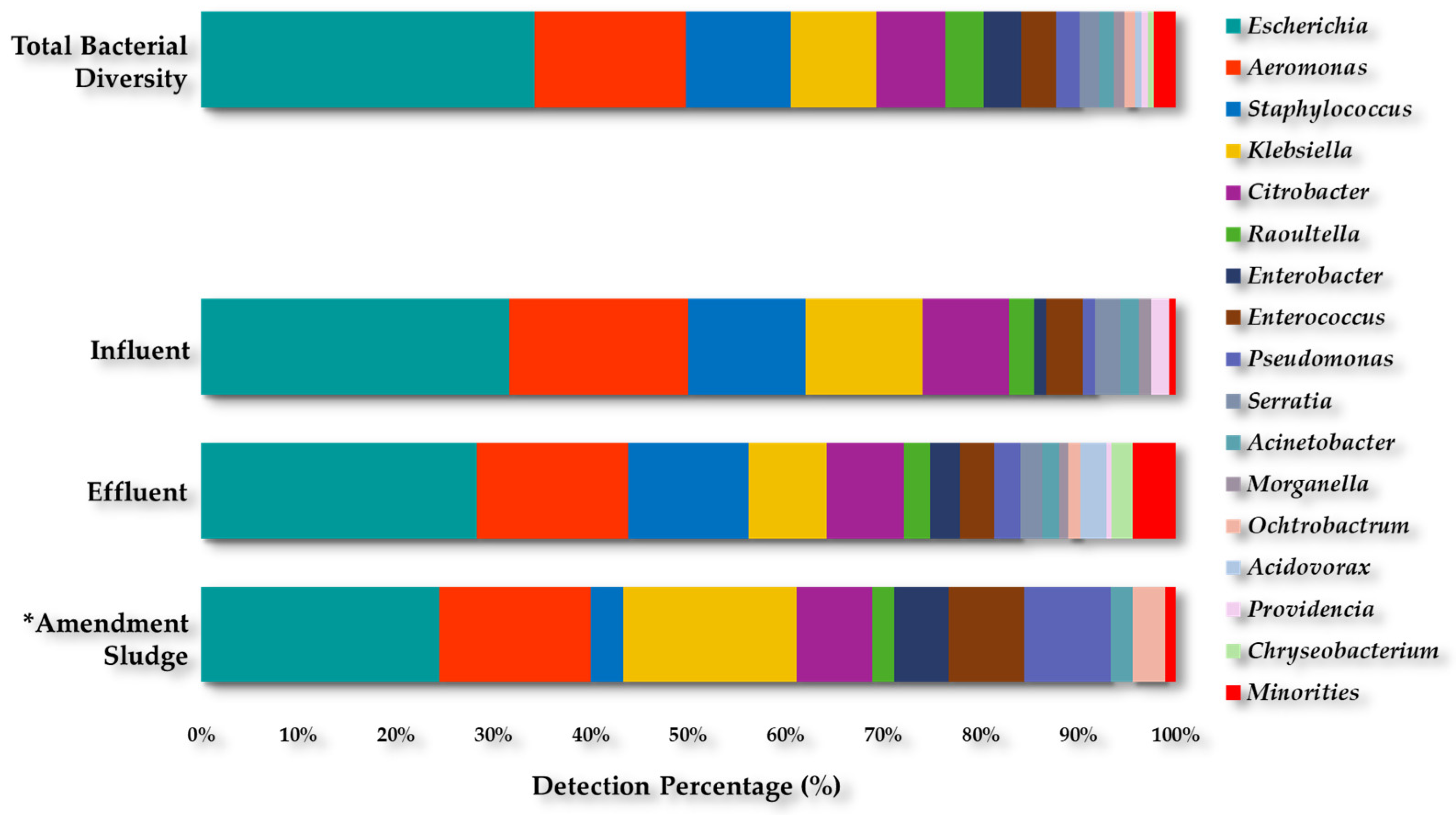
2.1. Culturable Bacterial Diversity
2.1.1. Bacterial Collection Recovered from Both WWTPs
2.1.2. Diversity of Aeromonas spp. and Pseudomonas spp.
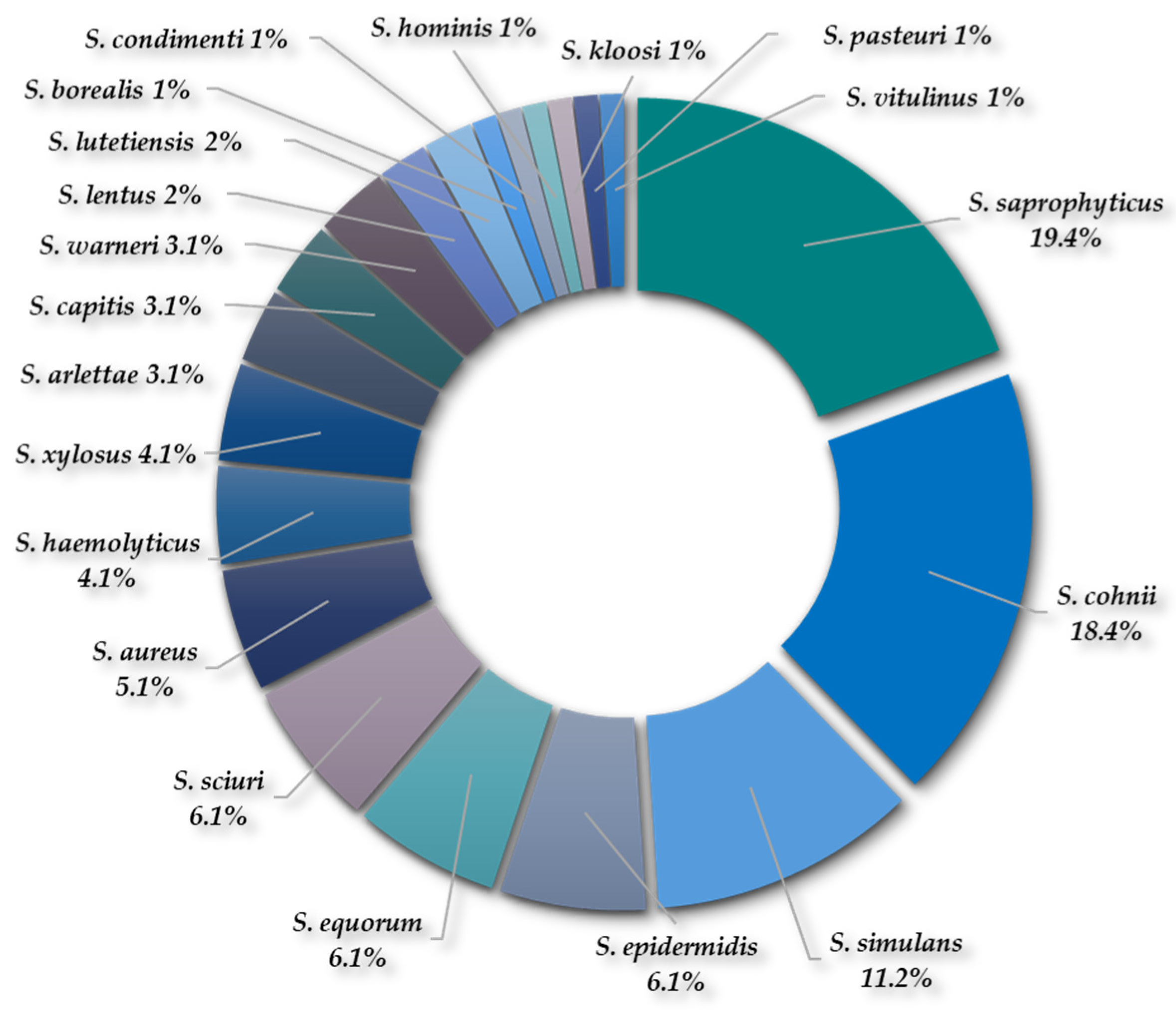
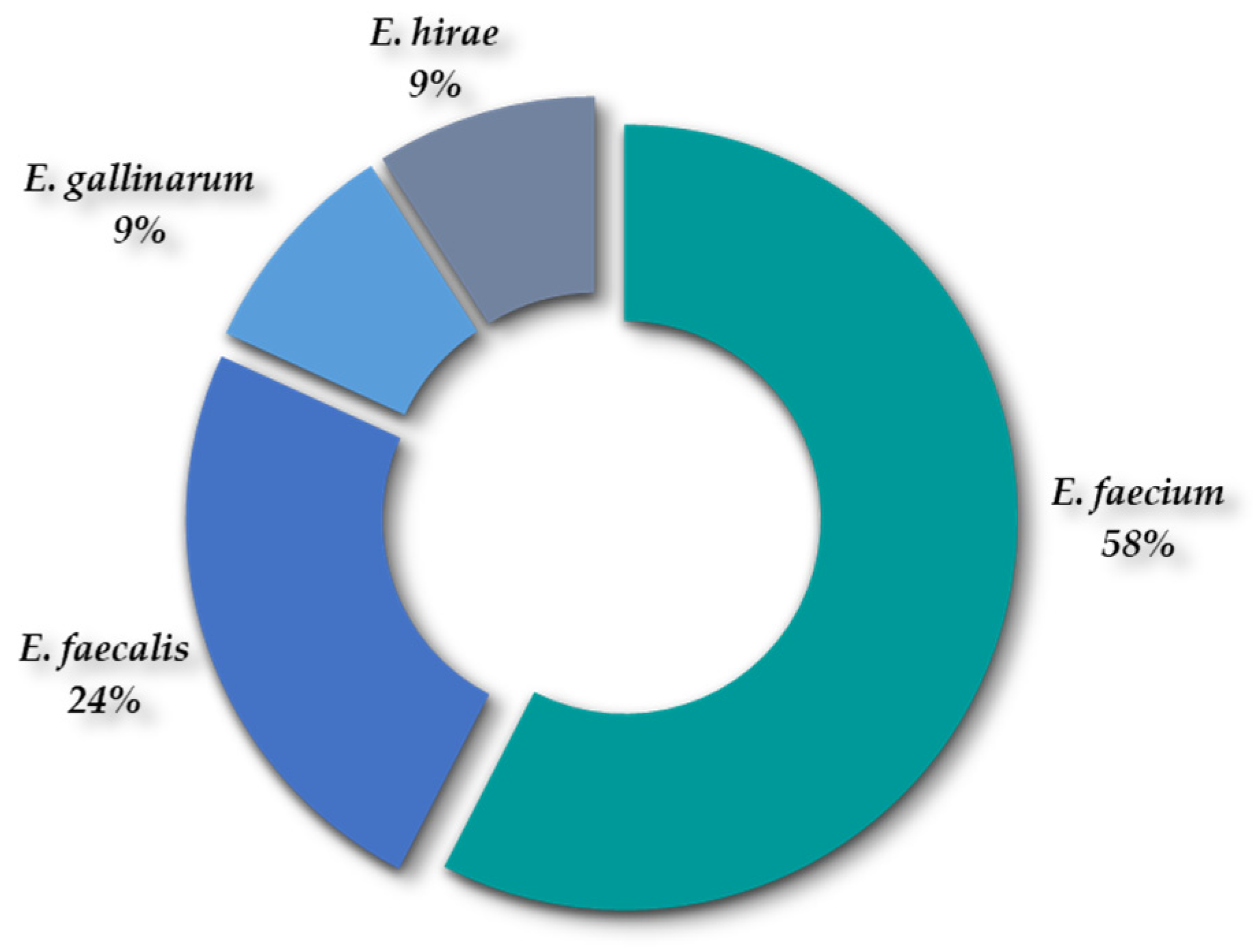
2.1.3. Diversity of Species of Staphylococcus spp. and Enterococcus spp.
2.2. Detection of Clinically Relevant Antimicrobial-Resistant Bacteria
2.2.1. Cefotaxime-Resistant Enterobacteriaceae (CTXR-E)
2.2.2. ESBL-Producing E. coli/K. pneumoniae (ESBL-Ec/Kp) Isolates
2.2.3. Carbapenem-Resistant Enterobacteriaceae (CR-E)
2.2.4. Enterobacteriaceae Isolates Recovered from COLR Agar Plates
2.2.5. Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus faecium/faecalis
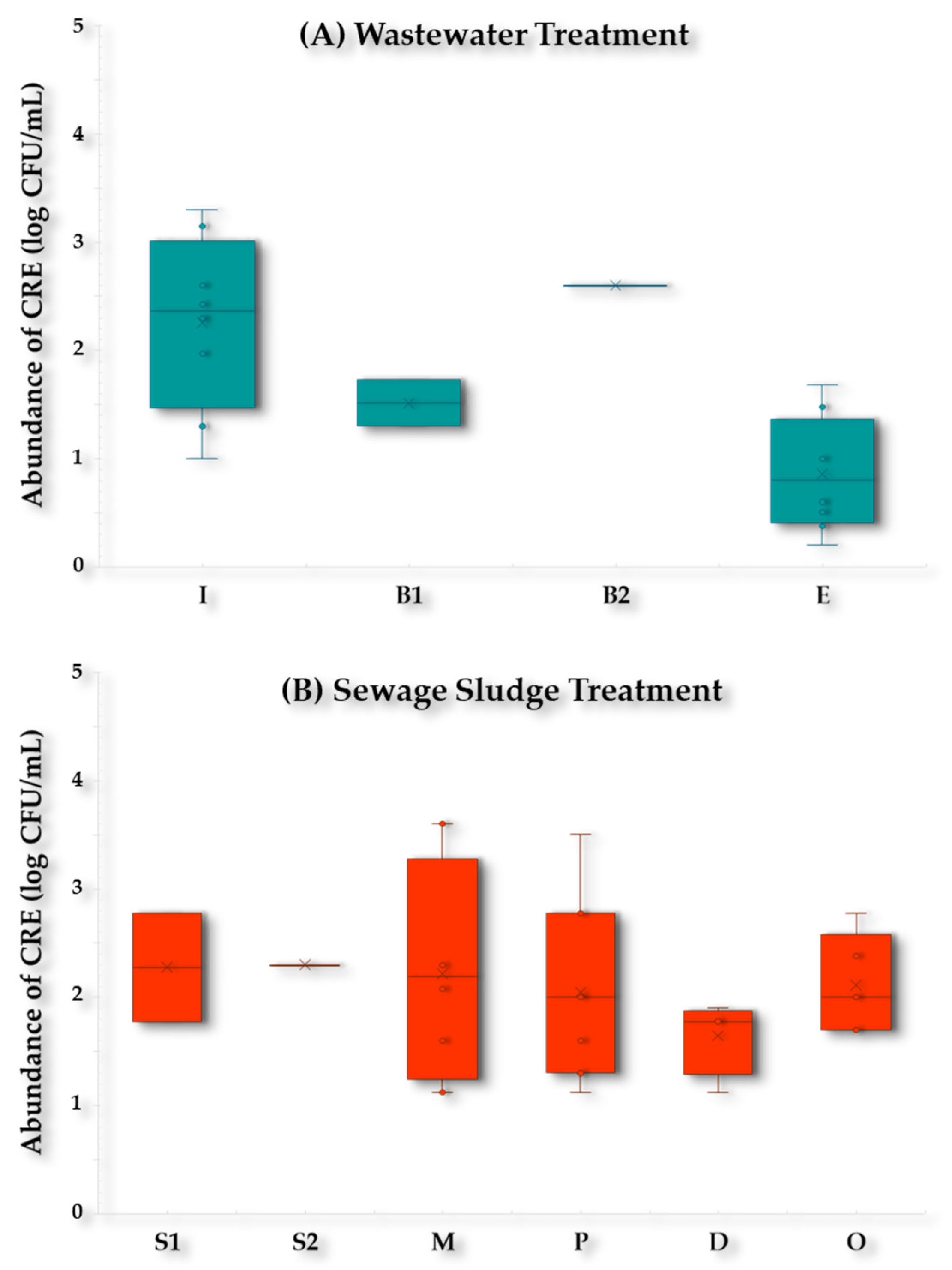
2.3. Microbiological Impact of the WWTP-2 Collectors
3. Materials and Methods
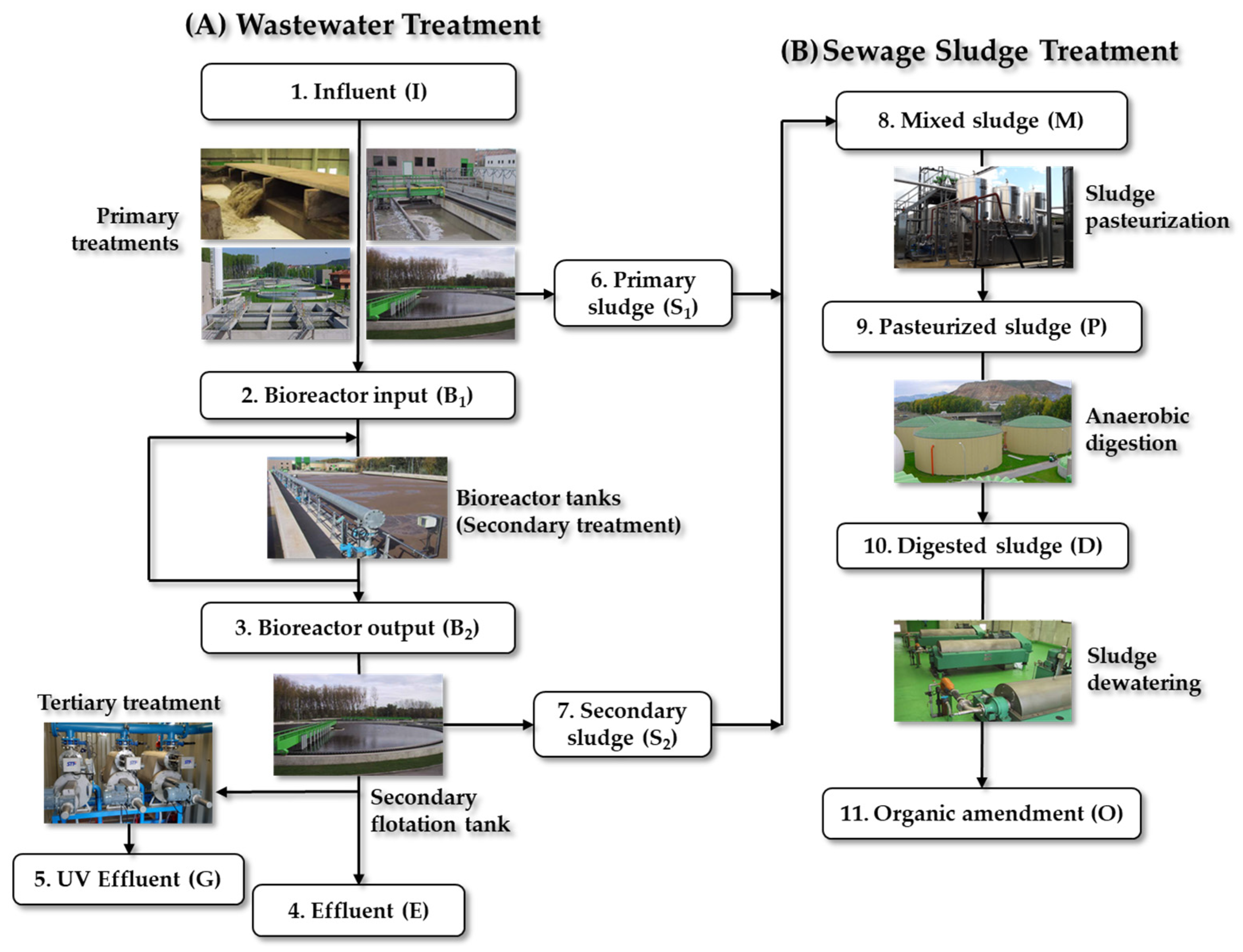
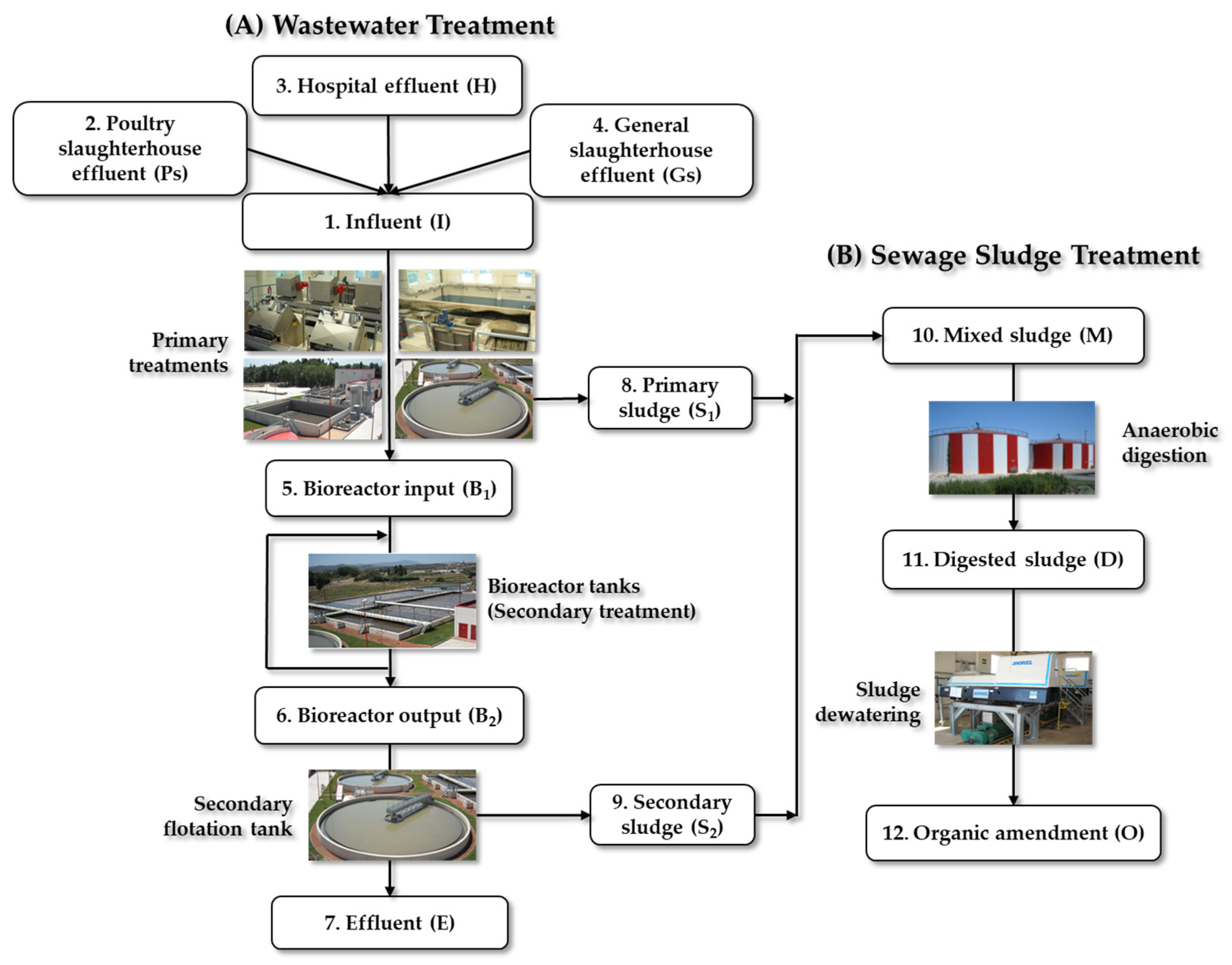
3.1. Criteria for the Selection of WWTP Sampling
3.1.1. Characteristics of WWTP-1
3.1.2. Characteristics of WWTP-2
3.2. Samples Collection
3.3. Samples Processing
3.4. Culture Media and Bacterial Isolation
3.5. Identification and Preservation of Bacterial Isolates
3.6. Confirmation of Relevant Resistance Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| ARB | Antimicrobial-resistant bacteria |
| ARGs | Antimicrobial resistance genes |
| COLR-E | Colistin-resistant Enterobacteriaceae |
| CR-E | Carbapenem-resistant Enterobacteriaceae |
| ESBL-Ec/Kp | Extended-Spectrum β-Lactamase-producing E. coli/K. pneumoniae |
| IPMR | Imipenem resistant |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| VRE | Vancomycin-resistant Enterococcus spp. |
| VR-E. faecium/faecalis | Vancomycin-resistant E. faecium/E. faecalis |
| WWTPs | Wastewater treatment plants |
References
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microb. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H. Strategic approach for combating antimicrobial resistance (AMR). Glob. Health Med. 2019, 1, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Medic. 2023, 20, e1004264. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.; Lozano, C.; Benito, D.; Estepa, V.; Tenorio, C.; Zarazaga, M.; Torres, C. Characterization of staphylococci in urban wastewater treatment plants in Spain, with detection of methicillin resistant Staphylococcus aureus ST398. Environ. Pollut. 2016, 212, 71–76. [Google Scholar] [CrossRef]
- Manaia, C.M.; Rocha, J.; Scaccia, N.; Marano, R.; Radu, E.; Biancullo, F.; Cerqueira, F.; Fortunato, G.; Iakovides, I.C.; Zammit, I.; et al. Antibiotic resistance in wastewater treatment plants: Tackling the black box. Environ. Internat. 2018, 115, 312–324. [Google Scholar] [CrossRef]
- Harnisz, M.; Kiedrzyńska, E.; Kiedrzyński, M.; Korzeniewska, E.; Czatzkowska, M.; Koniuszewska, I.; Jóźwik, A.; Szklarek, S.; Niestępski, S.; Zalewski, M. The impact of WWTP size and sampling season on the prevalence of antibiotic resistance genes in wastewater and the river system. Sci. Total Environ. 2020, 741, 140466. [Google Scholar] [CrossRef]
- Manaia, C.M. Framework for establishing regulatory guidelines to control antibiotic resistance in treated effluents. Crit. Rev. Environ. Sci. Tech. 2023, 53, 754–779. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Zhao, K.; Song, G.; Zhao, S.; Liu, R. Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic resistant bacteria (ARB) during sewage sludge treatment and disposal: A review. Sci. Total Environ. 2023, 877, 162772. [Google Scholar] [CrossRef]
- Rorat, A.; Courtois, P.; Vandenbulcke, F.; Lemiere, S. Sanitary and environmental aspects of sewage sludge management. In Industrial and Municipal Sludge; Butterworth-Heinemann: Oxford, UK, 2019; pp. 155–180. [Google Scholar]
- Styszko, K.; Durak, J.; Kończak, B.; Głodniok, M.; Borgulat, A. The impact of sewage sludge processing on the safety of its use. Scient. Rep. 2022, 12, 12227. [Google Scholar] [CrossRef]
- Börjesson, S.; Matussek, A.; Melin, S.; Löfgren, S.; Lindgren, P.E. Methicillin-resistant Staphylococcus aureus (MRSA) in municipal wastewater: An uncharted threat? J. Appl. Microbiol. 2010, 108, 1244–1251. [Google Scholar] [CrossRef]
- Biavasco, F.; Foglia, G.; Paoletti, C.; Zandri, G.; Magi, G.; Guaglianone, E.; Sundsfjord, A.; Pruzzo, C.; Donelli, G.; Facinelli, B. VanA-Type Enterococci from Humans, Animals, and Food: Species Distribution, Population Structure, Tn 1546 Typing and Location, and Virulence Determinants. Appl. Environ. Microbiol. 2007, 73, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Devane, M.L.; Moriarty, E.; Weaver, L.; Cookson, A.; Gilpin, B. Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res. 2020, 185, 116204. [Google Scholar] [CrossRef] [PubMed]
- Makowska, N.; Bresa, K.; Koczura, R.; Philips, A.; Nowis, K.; Mokracka, J. Urban wastewater as a conduit for pathogenic Gram-positive bacteria and genes encoding resistance to β-lactams and glycopeptides. Sci. Total Environ. 2021, 765, 144176. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Samarghandi, M.R.; Shokoohi, R.; Godini, K.; Arabestani, M.R. Prevalence and Removal Efficiency of Enterococcal Species and Vancomycin-resistant Enterococci of a Hospital Wastewater Treatment Plant. Avic. J. Environ. Health Eng. 2016, 3. [Google Scholar]
- Silva, V.; Ribeiro, J.; Rocha, J.; Manaia, C.M.; Silva, A.; Pereira, J.E.; Maltez, L.; Capelo, J.L.; Igrejas, G.; Poeta, P. High Frequency of the EMRSA-15 Clone (ST22-MRSA-IV) in Hospital Wastewater. Microorganisms 2022, 10, 147. [Google Scholar] [CrossRef]
- Ben Said, M.; Abbassi, M.S.; Gómez, P.; Ruiz-Ripa, L.; Sghaier, S.; Ibrahim, C.; Torres, C.; Hassen, A. Staphylococcus aureus isolated from wastewater treatment plants in Tunisia: Occurrence of human and animal associated lineages. J. Water Health. 2017, 15, 638–643. [Google Scholar] [CrossRef]
- Schmiege, D.; Zacharias, N.; Sib, E.; Falkenberg, T.; Moebus, S.; Evers, M.; Kistemann, T. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase-producing Escherichia coli in urban community wastewater. Sci. Total Environ. 2021, 785, 147269. [Google Scholar] [CrossRef]
- Savin, M.; Bierbaum, G.; Schmithausen, R.M.; Heinemann, C.; Kreyenschmidt, J.; Schmoger, S.; Akbaba, I.; Käsbohrer, A.; Hammerl, J.A. Slaughterhouse wastewater as a reservoir for extended-spectrum β-lactamase (ESBL)-producing, and colistin-resistant Klebsiella spp. and their impact in a “One Health” perspective. Sci. Total Environ. 2022, 804, 150000. [Google Scholar] [CrossRef]
- Hassen, B.; Abbassi, M.S.; Ruiz-Ripa, L.; Mama, O.; Ibrahim, C.; Benlabidi, S.; Hassen, A.; Torres, C.; Hammami, S. Genetic characterization of extended-spectrum β-lactamase-producing Enterobacteriaceae from a biological industrial wastewater treatment plant in Tunisia with detection of the colistin-resistance mcr-1 gene. FEMS Microb. Ecol. 2021, 97, fiaa231. [Google Scholar] [CrossRef]
- Alouache, S.; Estepa, V.; Messai, Y.; Ruiz, E.; Torres, C.; Bakour, R. Characterization of ESBLs and Associated Quinolone Resistance in Escherichia coli and Klebsiella pneumoniae Isolates from an Urban Wastewater Treatment Plant in Algeria. Microb. Drug Resist. 2014, 20, 30–38. [Google Scholar] [CrossRef]
- Rocha, J.; Ferreira, C.; Mil-Homens, D.; Busquets, A.; Fialho, A.M.; Henriques, I.; Gomila, M.; Manaia, C.M. Third generation cephalosporin-resistant Klebsiella pneumoniae thriving in patients and in wastewater: What do they have in common? BMC Genom. 2022, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Galler, H.; Feierl, G.; Petternel, C.; Reinthaler, F.F.; Haas, D.; Grisold, A.J.; Luxner, J.; Zarfel, G. KPC-2 and OXA-48 carbapenemase-harbouring Enterobacteriaceae detected in an Austrian wastewater treatment plant. Clinic. Microb. Infect. 2014, 20, O132–O134. [Google Scholar] [CrossRef] [PubMed]
- Hoelle, J.; Johnson, J.; Johnston, B.; Kinkle, B.; Boczek, L.; Ryu, H.; Hayes, S. Survey of US wastewater for carbapenem-resistant Enterobacteriaceae. J. Water Health 2019, 17, 219–226. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef]
- Picão, R.C.; Cardoso, J.P.; Campana, E.H.; Nicoletti, A.G.; Petrolini, F.V.; Assis, D.M.; Juliano, L.; Gales, A.C. The route of antimicrobial resistance from the hospital effluent to the environment: Focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn. Microb. Infect. Dis. 2013, 76, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Bondarczuk, K.; Piotrowska-Seget, Z. Microbial diversity and antibiotic resistance in a final effluent-receiving lake. Sci. Total Environ. 2019, 650, 2951–2961. [Google Scholar] [CrossRef]
- Cahill, N.; O’Connor, L.; Mahon, B.; Varley, Á.; McGrath, E.; Ryan, P.; Cormican, M.; Brehony, C.; Jolley, K.A.; Maiden, M.C.; et al. Hospital effluent: A reservoir for carbapenemase-producing Enterobacterales? Sci. Total Environ. 2019, 672, 618–624. [Google Scholar] [CrossRef]
- Onalenna, O.; Rahube, T.O. Assessing bacterial diversity and antibiotic resistance dynamics in wastewater effluent-irrigated soil and vegetables in a microcosm setting. Heliyon 2022, 8, e09089. [Google Scholar] [CrossRef]
- Azuma, T.; Uchiyama, T.; Zhang, D.; Usui, M.; Hayashi, T. Distribution and characteristics of carbapenem-resistant and extended-spectrum β-lactamase (ESBL) producing Escherichia coli in hospital effluents, sewage treatment plants, and river water in an urban area of Japan. Sci. Total Environ. 2022, 839, 156232. [Google Scholar] [CrossRef]
- Chen, K.; Liang, J.; Wang, Y.; Tao, Y.; Lu, Y.; Wang, A. A global perspective on microbial risk factors in effluents of wastewater treatment plants. J. Environ. Sci. 2024, 138, 227–235. [Google Scholar] [CrossRef]
- Araújo, S.; Sousa, M.; Tacão, M.; Baraúna, R.A.; Silva, A.; Ramos, R.; Alves, A.; Manaia, C.M.; Henriques, I. Carbapenem-resistant bacteria over a wastewater treatment process: Carbapenem-resistant Enterobacteriaceae in untreated wastewater and intrinsically-resistant bacteria in final effluent. Sci. Total Environ. 2021, 782, 146892. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Harnisz, M. Culture-Dependent and Culture-Independent Methods in Evaluation of Emission of Enterobacteriaceae from Sewage to the Air and Surface Water. Water Air Soil Pollut. 2012, 223, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, M.; Nucleo, E.; Spalla, M.; Zara, F.; Novazzi, F.; Marchetti, V.M.; Piazza, A.; Bitar, I.; De Cicco, M.; Paolucci, S.; et al. Occurrence of Extended Spectrum β-Lactamases, KPC-Type, and MCR-1.2-Producing Enterobacteriaceae from Wells, River Water, and Wastewater Treatment Plants in Oltrepò Pavese Area, Northern Italy. Front. Microb. 2017, 8, 2232. [Google Scholar] [CrossRef]
- Hocquet, D.; Muller, A.; Bertrand, X. What happens in hospitals does not stay in hospitals: Antibiotic-resistant bacteria in hospital wastewater systems. J. Hosp. Infect. 2016, 93, 395–402. [Google Scholar] [CrossRef]
- Marutescu, G.; Popa, M.; Gheorghe-Barbu, I.; Barbu, I.C.; Rodríguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.-F.; Kemper, A.; Spießberger, B.; et al. Wastewater treatment plants, an “escape gate” for ESCAPE pathogens. Front. Microb. 2023, 14, 1193907. [Google Scholar] [CrossRef]
- Hölzel, C.; Schwaiger, K.; Harms, K.; Küchenhoff, H.; Kunz, A.; Meyer, K.; Müller, C.; Bauer, J. Sewage sludge and liquid pig manure as possible sources of antibiotic resistant bacteria. Environ. Res. 2010, 110, 318–326. [Google Scholar] [CrossRef]
- Zurfluh, K.; Bagutti, C.; Brodmann, P.; Alt, M.; Schulze, J.; Fanning, S.; Stephan, R.; Nüesch-Inderbinen, M. Wastewater is a reservoir for clinically relevant carbapenemase- and 16s rRNA methylase-producing Enterobacteriaceae. Int. J. Antimicrob. Agent. 2017, 50, 436–440. [Google Scholar] [CrossRef]
- Rolbiecki, D.; Harnisz, M.; Korzeniewska, E.; Buta, M.; Hubeny, J.; Zieliński, W. Detection of carbapenemase-producing, hypervirulent Klebsiella spp. in wastewater and their potential transmission to river water and WWTP employees. Intern. J. Hyg. Environ. Health. 2021, 237, 113831. [Google Scholar] [CrossRef]
- Hrenovic, J.; Ivankovic, T.; Ivekovic, D.; Repec, S.; Stipanicev, D.; Ganjto, M. The fate of carbapenem-resistant bacteria in a wastewater treatment plant. Water Res. 2017, 126, 232–239. [Google Scholar] [CrossRef]
- Blaak, H.; Kemper, M.A.; Man, H.; Van Leuken, J.P.G.; Schijven, J.F.; Van Passel, M.W.J.; Schmitt, H.; de Roda Husman, A.M. Nationwide surveillance reveals frequent detection of carbapenemase-producing Enterobacterales in Dutch municipal wastewater. Sci. Total Environ. 2021, 776, 145925. [Google Scholar] [CrossRef]
- Yao, Y.; Lazaro-Perona, F.; Falgenhauer, L.; Valverde, A.; Imirzalioglu, C.; Dominguez, L.; Cantón, R.; Mingorance, J.; Chakraborty, T. Insights into a Novel blaKPC-2-Encoding IncP-6 Plasmid Reveal Carbapenem-Resistance Circulation in Several Enterobacteriaceae Species from Wastewater and a Hospital Source in Spain. Front Microb. 2017, 8, 1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, L.; Zhu, L.; Lu, G.; Guo, H.; Guan, J.; Jing, J.; Sun, S.; Wang, Y.; Wang, Z.; et al. Characteristics of Carbapenem-resistant Klebsiella pneumoniae in sewage from a tertiary hospital in Jilin Province, China. PLoS ONE 2023, 18, e0285730. [Google Scholar] [CrossRef] [PubMed]
- Ekwanzala, M.D.; Dewar, J.B.; Kamika, I.; Momba, M.N. Genome sequence of carbapenem-resistant Citrobacter koseri carrying blaOXA-181 isolated from sewage sludge. J. Glob. Antim. Resist. 2020, 20, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, W.A. A spotlight on Raoultella ornithinolytica: A newly emerging life-threatening zoonotic pathogen. Int. J. One Health 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Chi, X.; Li, Y.; Kou, Z.; Hou, P.; Xie, H.; Bi, Z.; Zheng, B. Emergence of NDM-1- and CTX-M-3-Producing Raoultella ornithinolytica in Human Gut Microbiota. Front. Microb. 2019, 10, 2678. [Google Scholar] [CrossRef]
- Iovleva, A.; Mettus, R.T.; McElheny, C.L.; Griffith, M.P.; Mustapha, M.M.; Pasculle, A.W.; Shields, R.K.; Cooper, V.S.; Doi, Y. High-Level High-Level Carbapenem Resistance in OXA-232-Producing Raoultella ornithinolytica Triggered by Ertapenem Therapy. Antim. Agents Chemother. 2019, 64, 10–1128. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, J.; Xu, H.; Yu, X.; Shen, P.; Ji, J.; Ying, C.; Zheng, B.; Xiao, Y. Emergence of KPC-2-Producing Raoultella ornithinolytica Isolated from a Hospital Wastewater Treatment Plant. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Castanheira, M.; Deshpande, L.; DiPersio, J.; Kang, J.; Weinstein, M.; Jones, R. First Descriptions of blaKPC in Raoultella spp. (R. planticola and R. ornithinolytica): Report from the SENTRY Antimicrobial Surveillance Program. J. Clin. Microb. 2009, 47, 4129–4130. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, J.; Ji, J.; Fang, Y.; Shen, P.; Ying, C.; Lv, J.; Xiao, Y.; Li, L. Emergence of Raoultella ornithinolytica Coproducing IMP-4 and KPC-2 Carbapenemases in China. Antim. Agents Chemother. 2015, 59, 7086–7089. [Google Scholar] [CrossRef][Green Version]
- Zou, H.; Berglund, B.; Wang, S.; Zhou, Z.; Gu, C.; Zhao, L.; Meng, C.; Li, X. Emergence of blaNDM-1, blaNDM-5, blaKPC-2 and blaIMP-4 carrying plasmids in Raoultella spp. in the environment. Environ. Pollut. 2022, 306, 119437. [Google Scholar] [CrossRef]
- Boopathy, R. Presence of Methicillin Resistant Staphylococcus aureus (MRSA) in sewage treatment plant. Bioresource Tech. 2017, 240, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Gündoğdu, A.; Stratton, H.; Katouli, M. Antibiotic resistant Staphylococcus aureus in hospital wastewaters and sewage treatment plants with special reference to methicillin-resistant Staphylococcus aureus (MRSA). J. Appl. Microb. 2013, 114, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Chou, C.C. Spreading of β-lactam resistance gene (mecA) and methicillin-resistant Staphylococcus aureus through municipal and swine slaughterhouse wastewaters. Water Res. 2014, 64, 288–295. [Google Scholar] [CrossRef]
- Börjesson, S.; Melin, S.; Matussek, A.; Lindgren, P. A seasonal study of the mecA gene and Staphylococcus aureus including methicillin-resistant S. aureus in a municipal wastewater treatment plant. Water Res. 2009, 43, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.E.; Micallef, S.A.; Gibbs, S.G.; Davis, J.A.; He, X.; George, A.; Kleinfelter, L.M.; Schreiber, N.A.; Mukherjee, S.; Sapkota, A.; et al. Methicillin-Resistant Staphylococcus aureus (MRSA) Detected at Four, U.S. Wastewater Treatment Plants. Environ. Health Perspect. 2012, 120, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.; Tiago, I.; Verissimo, A.; Boaventura, R.; Nunes, O.; Manaia, C. Antibiotic resistance of enterococci and related bacteria in an urban wastewater treatment plant. FEMS Microb. Ecol. 2006, 55, 322–329. [Google Scholar] [CrossRef]
- Torres, C.; Reguera, J.A.; Sanmartin, M.J.; Pérez-Díaz, M.C.; Baquero, F. vanA-Mediated vancomycin-resistant Enterococcus spp. in sewage. J. Antim. Chemoth. 1994, 33, 553–561. [Google Scholar] [CrossRef]
- Sahlström, L.; Rehbinder, V.; Albihn, A.; Aspan, A.; Bengtsson, B. Vancomycin resistant enterococci (VRE) in Swedish sewage sludge. Acta Vet Scand. 2009, 51, 24. [Google Scholar] [CrossRef]
- Varela, A.R.; Ferro, G.; Vredenburg, J.; Yanık, M.; Vieira, L.; Rizzo, L.; Lameiras, C.; Manaia, C.M. Vancomycin resistant enterococci: From the hospital effluent to the urban wastewater treatment plant. Scienc Total Environ. 2013, 450–451, 155–161. [Google Scholar] [CrossRef]
- Pérez-Etayo, L.; González, D.; Leiva, J.; Vitas, A.I. Multidrug-Resistant Bacteria Isolated from Different Aquatic Environments in the North of Spain and South of France. Microorganisms 2020, 8, 1425. [Google Scholar] [CrossRef]
- Sib, E.; Lenz-Plet, F.; Barabasch, V.; Klanke, U.; Savin, M.; Hembach, N.; Schallenberg, A.; Kehl, K.; Albert, C.; Gajdiss, M.; et al. Bacteria isolated from hospital, municipal and slaughterhouse wastewaters show characteristic, different resistance profiles. Sci. Total Environ. 2020, 746, 140894. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.; Bierbaum, G.; Hammerl, J.A.; Heinemann, C.; Parcina, M.; Sib, E.; Voigt, A.; Kreyenschmid, J. ESKAPE Bacteria and Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolated from Wastewater and Process Water from German Poultry Slaughterhouses. Appl. Environ. Microb. 2020, 86, e02748-19. [Google Scholar] [CrossRef]
- Ferreira, C.; Otani, S.; Aarestrup, F.M.; Manaia, C.M. Quantitative PCR versus metagenomics for monitoring antibiotic resistance genes: Balancing high sensitivity and broad coverage. FEMS Microbes 2023, 4, xtad008. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino-Hurtado, M.S.; Fernández-Fernández, R.; Campaña-Burguet, A.; González-Azcona, C.; Lozano, C.; Zarazaga, M.; Torres, C. A Surveillance Study of Culturable and Antimicrobial-Resistant Bacteria in Two Urban WWTPs in Northern Spain. Antibiotics 2024, 13, 955. https://doi.org/10.3390/antibiotics13100955
Pino-Hurtado MS, Fernández-Fernández R, Campaña-Burguet A, González-Azcona C, Lozano C, Zarazaga M, Torres C. A Surveillance Study of Culturable and Antimicrobial-Resistant Bacteria in Two Urban WWTPs in Northern Spain. Antibiotics. 2024; 13(10):955. https://doi.org/10.3390/antibiotics13100955
Chicago/Turabian StylePino-Hurtado, Mario Sergio, Rosa Fernández-Fernández, Allelen Campaña-Burguet, Carmen González-Azcona, Carmen Lozano, Myriam Zarazaga, and Carmen Torres. 2024. "A Surveillance Study of Culturable and Antimicrobial-Resistant Bacteria in Two Urban WWTPs in Northern Spain" Antibiotics 13, no. 10: 955. https://doi.org/10.3390/antibiotics13100955
APA StylePino-Hurtado, M. S., Fernández-Fernández, R., Campaña-Burguet, A., González-Azcona, C., Lozano, C., Zarazaga, M., & Torres, C. (2024). A Surveillance Study of Culturable and Antimicrobial-Resistant Bacteria in Two Urban WWTPs in Northern Spain. Antibiotics, 13(10), 955. https://doi.org/10.3390/antibiotics13100955








