Abstract
Bacteria of the genus Lactobacillus are microaerophilic or aerotolerant anaerobic Gram-positive non-spore-forming rods. They are considered essential members of the human gut microbiome; however, recent studies have revealed that these microorganisms are less predominant in the gut microbiome than initially thought. Lactobacillus spp. is mainly known for its use as a probiotic in foods and supplements to prevent and treat specific issues such as infectious diseases, irritable bowel syndrome, and diabetes mellitus. However, Lactobacillus spp. may occasionally cause infections such as bacteremia or infective endocarditis (IE). The present study aimed to review all cases of IE by Lactobacillus spp. and describe the epidemiology, microbiology, clinical characteristics, treatment, and outcomes of this infection by collecting relevant data from studies existing in Pubmed and Scopus until 28 September 2023. A total of 77 studies containing data for 82 patients were included. The median age was 56 years, and 69.6% were male. A prosthetic valve was present in 16% of patients, and 17.3% had previously been on probiotics. The aortic valve was the most commonly involved intracardiac site, followed by the mitral valve. Fever, embolic phenomena, sepsis, and heart failure were the most common clinical presentations. Aminoglycosides and penicillin were the most commonly used antimicrobials for definitive treatment. Surgery was performed in 53.7% of patients. Overall mortality was 17.1%. IE in prosthetic valves and presentation with shock were independently associated with overall mortality.
1. Introduction
Infective Endocarditis (IE) is an infection that involves the endocardium or prosthetic material in the heart, like prosthetic heart valves or cardiac implantable electronic devices (CIED, such as defibrillators and pacemakers). IE carries significant morbidity and mortality [1,2]. It is classically caused by aerobic Gram-positive cocci, such as streptococci, staphylococci, and enterococci, that may add up to 75% of the implicated microorganisms in patients with IE [3,4]. However, IE may also occur in the context of anaerobic bacteria, Gram-positive rods, or Gram-negative bacteria. The exact characteristics of IE by these pathogens have not been adequately described due to the rarity of the disease that they cause [5,6,7].
Bacteria of the genus Lactobacillus are microaerophilic or aerotolerant anaerobic Gram-positive non-spore-forming rods. They are considered essential members of the human gut microbiome, especially the oral cavity and ileum, and the female genital tract [8,9]; however, recent studies have revealed that these microorganisms are not as predominant in the gut microbiome as was initially thought [10]. Lactobacillus spp. is widely known for its use as a probiotic in foods and supplements to prevent and treat specific diseases [11,12]. These bacteria may also exert protective roles in the human microbiota, as shown in the case of the female genital tract, where women with a decrease in or absence of Lactobacillus spp. are more prone to developing bacterial vaginosis [13]. For example, there is ongoing research regarding these microorganisms’ benefits in infectious diseases, irritable bowel syndrome, inflammatory bowel disease, rheumatoid arthritis, type one diabetes, multiple sclerosis, obesity, type two diabetes, cancer, and cognitive development and behavior [10,11,12]. However, Lactobacillus spp. may occasionally cause infections, more commonly in patients with specific risk factors, such as diabetes, structural heart disease, cancer, total parenteral nutrition, chronic kidney disease, organ transplantation, and current antimicrobial use [14,15,16,17,18]. These infections include bacteremia, septic shock, urinary tract, central nervous system, prosthetic joint, and intra-abdominal infections as well as pneumonia, lung abscess, and IE, among others [19]. IE caused by Lactobacillus spp. is a rare infection with most evidence deriving from case reports and literature reviews [20].
The present study aimed to review all cases of IE by Lactobacillus spp. and describe the epidemiology, clinical characteristics, microbiology, treatment, and outcomes of this infection.
2. Methods
This narrative review is an effort to extract and collect existing data on IE by Lactobacillus species in humans. The primary aim of the present study was to provide information on the mortality of these infections. Secondary outcomes were to record data on (a) epidemiology, (b) the exact site of infection, (c) the patients’ clinical characteristics, (d) antimicrobial susceptibility, and (e) their treatment. For this review, PubMed/Medline and Scopus databases were searched for eligible articles reporting “Lactobacillus AND endocarditis” until 28 September 2023. This narrative review included original reports on infections, such as case reports and case series that provided information on epidemiology, microbiology, treatment, and outcomes of IE by Lactobacillus species in humans. Only articles in the English language were included. Reviews, systematic reviews, retrospective studies, and letters to the editor were excluded. Articles with no access to original data and studies referring to animal reports had to be excluded. Moreover, studies with insufficient data and articles without information on patients’ mortality and epidemiology were also excluded. The remaining articles’ references were also searched to assess potential studies.
Three investigators (I.G., A.G. and A.Z.) extracted information from all the eligible studies for this narrative review using a pre-defined template. The extracted data included study type, year of publication, and country; patient demographic data (age and gender); patients’ relevant medical history (previous cardiac surgery or cardiac valve replacement, time after cardiac valve replacement); infection data and microbiology (infection site, data regarding microorganism identification, presence of complications, presence of embolic phenomena); antimicrobial treatment administered (as definitive treatment); whether surgery was performed, and outcomes (i.e., cure or death). Data on microbiology and the association of mortality with index infection were reported by the original studies’ authors. Overall mortality was noted during admission and the months following patient discharge, as reported by the original studies’ authors. Information about patients’ previous history, oral hygiene, use of antimicrobials, and probiotics was collected as provided by the studies’ authors. If no such information was provided, the particular characteristic was considered as not being present in the patient’s history. Diagnosis of IE was confirmed by the investigators based on information provided by the authors and the modified Dukes’ criteria if the diagnosis was at least possible (at least one major and one minor criterion or at least three minor criteria) or if pathological data established a diagnosis of IE [21].
Data are presented as numbers (%) for categorical variables and mean (standard deviation) or median (interquartile range, IQR) for continuous variables. Categorical data were analyzed with Fisher’s exact test. Continuous variables were compared using the Mann–Whitney U-test for non-normally distributed variables or the t-test for normally distributed variables. All tests were two-tailed, and a p-value equal to or lower than 0.05 was considered significant. A univariate linear regression analysis was conducted to identify factors associated with all-cause mortality of patients with IE. More specifically, a univariate logistic regression was performed to identify any association between gender, age, presence of prosthetic cardiac valve, of poor teeth and oral hygiene or recent dental work, of previous episode of IE, rheumatic heart disease history, presence of IE at the aortic, mitral, pulmonary, tricuspid valve, presence of IE at multiple valves, presentation with fever, sepsis, embolic phenomena, development of heart failure, treatment with particular antimicrobials and performed surgical management with all-cause mortality. All statistics were calculated with GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA). A multivariate logistic regression analysis evaluated the effect of factors previously identified in the univariate analysis model associated with all-cause mortality with a p < 0.05. Multivariate analysis was performed using the SPSS version 23.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Included Studies’ Characteristics
A total of 485 articles from PubMed and Scopus were screened. Finally, 77 met the present study’s inclusion criteria [20,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. The 77 studies included in the current narrative review involved 82 patients in total. Among these studies, 37 were conducted in North and South America, 32 in Europe, 6 in Asia, 1 in Africa, and 1 in Oceania. There were 70 case reports and seven case series. Figure 1 shows the geographical distribution of Lactobacillus species worldwide and Figure 2 shows the flow diagram of study inclusion.
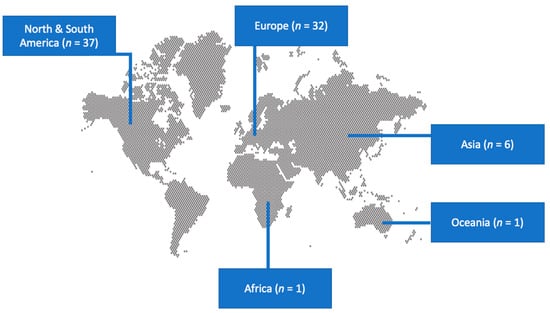
Figure 1.
Geographical distribution of studies on infective endocarditis by Lactobacillus species worldwide.
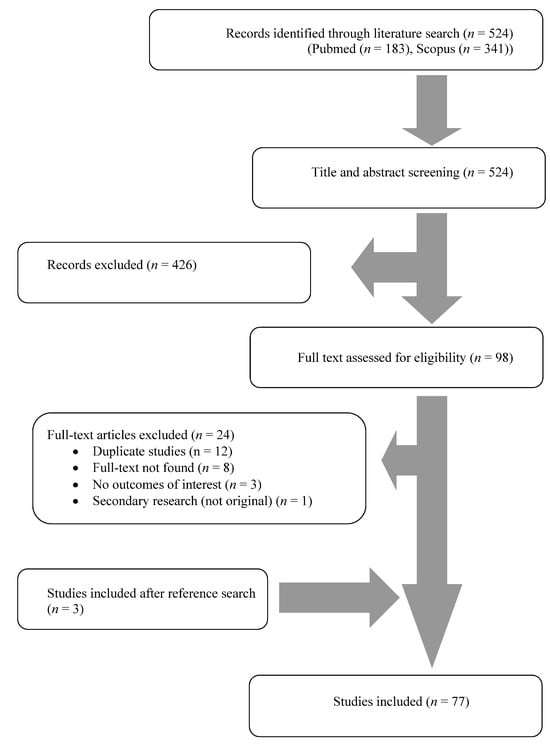
Figure 2.
Flow diagram of study inclusion.
3.2. Epidemiology of IE by Lactobacillus Species
The age of patients with IE by Lactobacillus species ranged from 2 to 85 years; the median age was 56 years, and 69.6% (55 out of 79 patients with available data) were male. Regarding predisposing factors, 41.5% (34/82) had poor teeth hygiene or recent dental work, 19% (15/79) had received antimicrobials within the last three months, 17.3% (14/81) had received probiotics during the previous three months, 16% (13/81) had a prosthetic cardiac valve, 9.8% (8/82) had a previous episode of IE, 8.5% (7/82) had history of congenital heart disease, 7.3% (6/82) had history of immunosuppression, 6.1% (5/82) had history of intravenous drug use (IVDU). Table 1 shows the patients’ characteristics.

Table 1.
Characteristics of patients with infective endocarditis by Lactobacillus species in total and regarding patients’ outcomes.
3.3. Microbiology and Diagnosis of IE by Lactobacillus Species
IE by Lactobacillus species was polymicrobial in 6.1% (five patients), with blood cultures being also positive for Streptococcus spp. in 2.4% (two), fungi in 2.4% (two), and Prevotella spp. in 1.2% (one). The isolated species from the 82 patients with IE were L. rhamnosus in 23.2% (19 patients), L. casei in 13.4% (11), L. jensenii in 13.4% (11), L. acidophilus in 13.4% (11), L. plantarum in 9.8% (8), L. paracasei in 8.5% (7), L. zeae in 2.4% (2), L. salicinius in 1.2% (1), L. garvieae in 1.2% (1), L. curvatus in 1.2% (1), L. salivarius in 1.2% (1). The species were not identified in 18.8% (12 patients). Antimicrobial resistance to penicillin was 8.5% (4 out of 47 patients with available data), and to aminoglycosides was 15.2% (5). Antimicrobial resistance to vancomycin was 86.2% (25/29).
The diagnosis was facilitated by transthoracic echocardiography in 45.3% (34/75), transesophageal echocardiography in 38.7% (34/75), autopsy in 6.3% (5/80), valve culture in 18.8% (15/80), and by foreign body culture in 6.3% (2/80) of patients. Blood cultures were positive in 97.6% (80/82).
3.4. Clinical Characteristics of IE by Lactobacillus Species
The infection affected the aortic valve in 50.7% (39 out of 77 patients with available data), the mitral in 48.1% (37/77), the tricuspid in 2.6% (2/77), the pulmonary valve in 2.6% (2/77), and a cardiac implanted electronic device in 1.2% (1/81). Multiple valves were infected in 5.2% (4/77).
The most common clinical symptoms included fever in 65% (52/80), sepsis in 27.8% (22/79), embolic phenomena in 34.6% (28/81), heart failure in 23.8% (19/80), and shock in 6.3% (5/79) of patients. Table 2 shows the clinical characteristics of patients with IE as well as the definitive treatment administered.

Table 2.
Clinical presentation and definitive treatment of patients with infective endocarditis by Lactobacillus species in total and regarding patients’ outcomes.
3.5. Treatment and Outcomes of IE by Lactobacillus Species
Definitive treatment of patients is summarized in Table 2 and detailed in Table S1. The median treatment among survivors was six weeks (interquartile range: 6–7 weeks). The most commonly used antimicrobials (as definitive treatment) were aminoglycosides in 67.1% (53 out of 79 patients with available data), penicillin in 59.5% (47), aminopenicillin in 39.2% (31), vancomycin in 24.1% (19), and cephalosporins in 20.3% (16) of cases. Surgical management in combination with antimicrobial treatment was performed in 53.7% (44/82) of cases. Overall mortality was 17.1% (14/82), and mortality directly attributed to the IE episode was 12.2% (10). Overall mortality occurred in a median of 12 days (interquartile range 1–46.5 days) after admission to the hospital. As shown in Figure S1, there was a trend for a relative reduction in mortality of patients with IE by Lactobacillus spp. as decades went by, with an overall mortality of 33.3% in the decade of 1970–1979 and an overall mortality of 11.8% in the current decade (2020 and on).
3.6. Statistical Analysis of IE by Lactobacillus Species
Table 1 and Table 2 compare patients with IE by Lactobacillus species who survived with those who died. Patients who died were more likely to have a prosthetic heart valve and were also more likely to develop shock in a statistically significant way.
Among the different parameters tested in the univariate regression analysis, IE in a prosthetic valve, polymicrobial IE, development of shock, as well as heart failure were positively associated with overall mortality. A multivariate logistic regression model identified IE in a prosthetic valve and the development of shock to be independently associated with overall mortality. Table 3 shows the results of the regression analysis.

Table 3.
Regression analysis of overall mortality in patients with infective endocarditis by Lactobacillus species.
4. Discussion
The present study described the characteristics of patients who developed IE by Lactobacillus species. The intracardiac site most commonly involved was the aortic valve, followed by the mitral valve. The most common clinical presentation included fever, embolic phenomena, sepsis, and the development of heart failure. Aminoglycosides and penicillin were the most commonly used antimicrobials for definitive treatment. Overall mortality was 17.1%.
The median age of patients diagnosed with IE by Lactobacillus spp. in the present study was 56 years, which is lower than the age in other cohorts of patients with IE, where the mean age is about 70 years [3,4,98]. A male predominance was noted, as is also the case in IE by other microorganisms [3,98]. A prosthetic valve was present in 16% of patients with IE by Lactobacillus spp., a rate that is comparable to that of other studies of IE, which at times may be as high as 50% [3,4,98]. A previous episode of IE was noted in 9.8% of patients in the present study, and the rate of patients with a history of rheumatic fever was 6.1%. Both these rates are similar to those of other studies of patients with IE [4,98]. Intravenous drug use was noted in 6.1% of the present patients, which is comparable to that in other studies of IE, where that rate is between 4% and 9.2% [3,4,98]. Congenital heart disease was noted in 8.5% in the present study, being comparable to the corresponding rate in another study of IE in the general population [4]. Notably, 19% of patients with IE by Lactobacillus spp. had a previous exposure to antimicrobials, which is consistent with the literature that shows that previous exposure to antimicrobials is a risk factor for the development of infection by Lactobacillus spp. [10]. Moreover, 17.3% of the present patients had a previous exposure to Lactobacillus spp. in the form of probiotics. Interestingly, the increase in probiotic and dairy consumption and the numerous cases of Lactobacillus spp. infections have led to questions regarding probiotics’ safety. The possible relationship between these agents and infection development remains a challenge that has to be proven, since Lactobacillus spp. constitutes part of the normal human flora [76]. Indeed, there is ongoing research on the safety of probiotics since, in some cases, mainly in patients with underlying conditions, infections due to probiotics may occur [19,99].
The most commonly infected intracardiac sites were the aortic in 50.7% and the mitral valves in 48.1%, which is similar to other studies of IE, where the aortic valve was the most commonly infected valve, followed by the mitral valve [3,98]. In line with the current literature, L. casei and L. rhamnosus were the most frequently identified pathogens. Regarding clinical presentation, the most common symptom was fever, occurring in 65% of patients, sepsis was evident only in 27.8%, while 6.3% developed shock. In other studies describing patients with IE, fever was present in 84% of patients [4], and shock was diagnosed in 9% [3]. However, fever is absent in almost 40% of Lactobacillus IE cases. Heart failure was diagnosed in 23.8% of the present patients, a rate lower than the corresponding rate in other studies evaluating patients with IE, where rates were within the range of 33% to 52% [3,98]. Embolic phenomena in IE by Lactobacillus spp. were diagnosed in 34.6% of the present patients, a rate similar to that of other studies with IE in the general population, where it ranged from 15% to 45% [3,4].
Lactobacillus species detection by conventional diagnostic methods remains challenging; early clinical suspicion is crucial for an efficient diagnostic approach. Laboratory findings are non-specific and can include thrombocytopenia, monoclonal gammopathy, or a positive rheumatoid factor [93]. In the majority of cases, the diagnostic algorithm Lactobacillus IE begins with standard procedures, including blood cultures, transthoracic echocardiogram (TTE), transesophageal echocardiogram (TEE), and application of the Duke’s criteria [91]. TEE constitutes a valuable tool for prompt imaging of valvular regurgitation or valve vegetations, although active infection versus healing vegetations is not easily differentiated. However, in some cases, TEE might not demonstrate findings of endocarditis and, as a result, should be combined with microbiological studies [82]. Valve culture, valves’ PCR, or valve histology also aid in the diagnosis. However, Lactobacillus spp. detection in most laboratories is demanding given the problematic culture growth of the pathogen; only 30–50% of the isolates can be identified by conventional methods [69]. Thus, advanced molecular techniques such as 16S rRNA combined with MALDI- TOF MS are required for accurate identification [71,92].
Regarding treating IE by Lactobacillus spp., there are no established susceptibility breakpoints; recommendations for antimicrobial administration rely on case series in the current literature. Antimicrobial therapy administered after susceptibility testing significantly decreases mortality rates [90]. Synergistic intravenous therapy with aminoglycosides and penicillin was the most commonly suggested. This is reasonable, considering that resistance to these two antimicrobials was 15.2% and 8.5%, respectively. Aminoglycosides have been used in the treatment of IE in many pathogens, classically in the context of combination therapy, mostly by Gram-positive pathogens, as well as in the case of Gram-negative pathogens [100,101,102,103]. However, there is currently a trend to reduce aminoglycoside use, since it is associated with high morbidity due to kidney injury, while its benefit in mortality is questionable [104,105,106,107,108]. Notably, in the present review, the statistical analysis among patients who survived and those who died did not reveal any statistically significant differences in terms of antimicrobial treatment in general, and for the use of aminoglycosides in the regimen in particular. Furthermore, the univariate linear regression analysis also did not find any association between aminoglycoside use and overall mortality. Thus, it would be tempting to state that aminoglycosides are not necessary in the treatment of this disease. However, only a randomized controlled trial could determine whether aminoglycosides provide any benefit in the treatment of IE by Lactobacillus spp. Moreover, this review includes a small number of patients; thus, it does not have adequate power to draw such solid conclusions.
Interestingly, resistance to vancomycin was 86.2%, which is in line with the literature suggesting that treatment of Lactobacillus spp. with vancomycin is considered generally ineffective, with most strains of Lactobacillus being inherently resistant to this drug [109]. Moreover, cases of resistance to tetracyclines, ciprofloxacin, or carbapenems have also been reported [78]. A possible mechanism of resistance to these agents is lactic acid production, leading to lower pH levels and decreased effectiveness of antimicrobials [71]. Duration of therapy should be per standard guidelines for IE treatment. In the present review, the median intravenous treatment duration was six weeks [78]. In some cases, surgical intervention, such as valve replacement or abscess drainage, should accompany antimicrobial therapy [90]. The presence of heart failure, large mobile vegetations, abscesses, or multi-drug resistant organisms constitutes strong indications for surgical intervention [93].
In the present review, overall mortality was 17.1%, while in 12.2% of all patients, death was directly attributed to the episode of IE. Overall mortality was comparable to the rates noted in other studies of IE, where it was within the range of 11–40% [3,4,98]. Mortality rates are generally attributed to inadequate treatment, polymicrobial infections, and the bacterial pathogenic potential [84]. Importantly, statistical analysis of the cases in the present study identified IE in a prosthetic valve and presentation with shock to be independently associated with overall mortality. Interestingly, a reduction in overall mortality was noted in the studies published more recently. In general, the management of IE has changed over the years, with surgery being more commonly indicated and performed in cases where conservative treatment is inadequate [110]. However, mortality remains high, at 15–30%, which could be attributed to changes in the epidemiology and the microbiology of the disease [110,111,112]. The relative reduction in the overall mortality in patients with IE by Lactobacillus spp. may be linked to a similar epidemiology of the disease over the years, as well as medical improvements in the diagnosis and the medical and surgical management of the disease [113,114].
This study has some limitations. First, it mainly consists of case reports; thus, the evidence may be low, since case reports and case series contain sufficient data the credibility of which mainly depends on the accurate record keeping of each institution. For example, the information on the past medical history oral hygiene of patients, the previous use of probiotics, and the previous use of antibiotics relied on a report by each study’s investigator. Inadequate referral could be associated with underreporting of the particular characteristic, leading to bias in the present study. In addition, the heterogeneity among institutions regarding surgical approaches and record-keeping affects information about outcomes and time-to-event analysis. However, given the rarity of this infection, an effort to conduct a prospective or retrospective study evaluating this condition could hardly enroll an adequate number of patients, even if it included cases over many years and from many centers. Finally, this is a narrative, not a systematic review.
5. Conclusions
To conclude, this narrative review describes the epidemiology, microbiology, clinical characteristics, treatment, and outcomes of IE by Lactobacillus spp. Penicillin and aminoglycosides were the most commonly used antimicrobials for definitive treatment since antimicrobial resistance was low to these antimicrobials. Prosthetic valve IE and presentation with shock were independently associated with mortality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13010053/s1, Table S1: Characteristics of the included studies. Figure S1: Overall mortality of patients with infective endocarditis by Lactobacillus species over time.
Author Contributions
Conceptualization, P.I.; methodology, P.I., A.Z., I.G., A.G. and S.B.; software, P.I.; validation, P.I. and S.B.; formal analysis, P.I., A.Z., A.G., I.G. and S.B.; investigation A.Z., I.G. and A.G.; data curation, P.I.; writing—original draft preparation, P.I.; writing—review and editing, S.B., A.Z., I.G., A.G. and G.S.; visualization, P.I. and S.B.; supervision, G.S.; project administration, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Gaca, J.G.; Chu, V.H. Management Considerations in Infective Endocarditis: A Review. JAMA 2018, 320, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Cresti, A.; Chiavarelli, M.; Scalese, M.; Nencioni, C.; Valentini, S.; Guerrini, F.; D’Aiello, I.; Picchi, A.; De Sensi, F.; Habib, G. Epidemiological and Mortality Trends in Infective Endocarditis, a 17-Year Population-Based Prospective Study. Cardiovasc. Diagn. Ther. 2017, 7, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.E.; Samonis, G.; Andrianaki, A.M.; Christofaki, M.; Dimopoulou, D.; Papadakis, J.; Gikas, A.; Kofteridis, D.P. Epidemiology, Microbiological and Clinical Features, Treatment, and Outcomes of Infective Endocarditis in Crete, Greece. Infect. Chemother. 2018, 50, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Endocarditis Due to Anaerobic Bacteria. Cardiology 2002, 98, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Infective Endocarditis Caused by Anaerobic Bacteria. Arch. Cardiovasc. Dis. 2008, 101, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Muñoz, P.; Burillo, A. Gram-Negative Endocarditis: Disease Presentation, Diagnosis and Treatment. Curr. Opin. Infect. Dis. 2021, 34, 672–680. [Google Scholar] [CrossRef]
- Vásquez, A.; Jakobsson, T.; Ahrné, S.; Forsum, U.; Molin, G. Vaginal Lactobacillus Flora of Healthy Swedish Women. J. Clin. Microbiol. 2002, 40, 2746–2749. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut Microbiome and Human Health: Exploring How the Probiotic Genus Lactobacillus Modulate Immune Responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in Health and Disease, a Driver or Just along for the Ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ziogou, A.; Ziogos, E.; Giannakodimos, I.; Giannakodimos, A.; Sifakis, S.; Ioannou, P.; Tsiodras, S. Bacterial Vaginosis and Post-Operative Pelvic Infections. Healthcare 2023, 11, 1218. [Google Scholar] [CrossRef]
- Husni, R.N.; Gordon, S.M.; Washington, J.A.; Longworth, D.L. Lactobacillus Bacteremia and Endocarditis: Review of 45 Cases. Clin. Infect. Dis. 1997, 25, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus Bacteremia, Clinical Significance, and Patient Outcome, with Special Focus on Probiotic L. Rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Z’Graggen, W.J.; Fankhauser, H.; Lammer, F.; Bregenzer, T.; Conen, D. Pancreatic Necrosis Infection Due to Lactobacillus paracasei in an Immunocompetent Patient. Pancreatology 2005, 5, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.P.; Lee, T.A.; Bolanos, J.T.; Danziger, L.H. Pathogenic Relevance of Lactobacillus: A Retrospective Review of over 200 Cases. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 31–40. [Google Scholar] [CrossRef]
- Rossi, F.; Amadoro, C.; Colavita, G. Members of the Lactobacillus Genus Complex (LGC) as Opportunistic Pathogens: A Review. Microorganisms 2019, 7, 126. [Google Scholar] [CrossRef]
- Rossi, F.; Amadoro, C.; Gasperi, M.; Colavita, G. Lactobacilli Infection Case Reports in the Last Three Years and Safety Implications. Nutrients 2022, 14, 1178. [Google Scholar] [CrossRef]
- Grazioli-Gauthier, L.; Rigamonti, E.; Leo, L.A.; Martinetti Lucchini, G.; Lo Priore, E.; Bernasconi, E. Lactobacillus jensenii Mitral Valve Endocarditis: Case Report, Literature Review and New Perspectives. IDCases 2022, 27, e01401. [Google Scholar] [CrossRef]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, 77, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J. Endocarditis Caused by Lactobacillus plantarum. Ann. Intern. Med. 1973, 78, 33. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, M.J. Lactobacillus casei Endocarditis. Ann. Intern. Med. 1975, 82, 539. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, S.; Min, K.W. Leukocytoclastic Angiitis in Subacute Bacterial Endocarditis. Arch. Dermatol. 1977, 113, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Chow, A.W.; Betts, D.; Guze, L.B. Lactobacillemia—Report of Nine Cases. Am. J. Med. 1978, 64, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Jawetz, H.I. Resection for Carcinoma of the Lung with Metastases. Chest 1980, 77, 713. [Google Scholar] [CrossRef] [PubMed]
- Shinar, E.; Leitersdorf, E.; Yevin, R. Lactobacillus plantarum Endocarditis. Klin. Wochenschr. 1984, 62, 1173–1174. [Google Scholar] [CrossRef]
- Sussman, J.I.; Baron, E.J.; Goldberg, S.M.; Kaplan, M.H.; Pizzarello, R.A. Clinical Manifestations and Therapy of Lactobacillus Endocarditis: Report of a Case and Review of the Literature. Clin. Infect. Dis. 1986, 8, 771–776. [Google Scholar] [CrossRef]
- Davies, A.J.; James, P.A.; Hawkey, P.M. Lactobacillus Endocarditis. J. Infect. 1986, 12, 169–174. [Google Scholar] [CrossRef]
- Fisher, T.R.; Carlisi, J.R.; Sautter, R.L. Lactobacillus Endocarditis. Clin. Microbiol. Newsl. 1988, 10, 150–151. [Google Scholar] [CrossRef]
- Struve, J.; Weiland, O.; Nord, C.E. Lactobacillus plantarum Endocarditis in a Patient with Benign Monoclonal Gammopathy. J. Infect. 1988, 17, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Naudé, W.D.; Swanepoel, A.; Böhmer, R.H.; Bolding, E. Endocarditis Caused by Lactobacillus casei Subspecies Rhamnosus. A Case Report. S. Afr. Med. J. 1988, 73, 612–614. [Google Scholar] [PubMed]
- Thangkhiew, I.; Gunstone, R.F. Association of Lactobacillus plantarum with Endocarditis. J. Infect. 1988, 16, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Stulz, P.; Zimmerli, W.; Mihatsch, J.; Grädel, E. Recurrent Infective Endocarditis in Idiopathic Hypertrophic Subaortic Stenosis. Thorac. Cardiovasc Surg. 1989, 37, 99–102. [Google Scholar] [CrossRef]
- Atkins, M.C.; Nicolson, L.; Harrison, G.A.J.; Paull, A.; Malnick, H.; Morrison, D. Lactobacillus jensenii Prosthetic Valve Endocarditis. J. Infect. 1990, 21, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Lim, H.S.; Lee, S.Y.; Cho, S.Y. Lactobacillus casei Subspecies Casei Endocarditis: A Case Report. Yonsei Med. J. 1991, 32, 69. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.K.; Daly, J.S.; Dodge, R.A. Two Cases of Endocarditis Due to Lactobacillus Species: Antimicrobial Susceptibility, Review, and Discussion of Therapy. Clin. Infect. Dis. 1992, 15, 250–255. [Google Scholar] [CrossRef]
- Sloss, J.M.; Cumberland, N.S. Deep Seated Infection Due to Lactobacillus caseii. J. R. Army Med. Corps 1993, 139, 25–26. [Google Scholar] [CrossRef]
- Olearchyk, A.S. Lactobacillus Endocarditis Necessitating Combined Mitral and Aortic Valve Replacement—A Case Report. Vasc. Surg. 1993, 27, 219–225. [Google Scholar] [CrossRef]
- Puleo, J.A.; Shammas, N.W.; Kelly, P.; Allen, M. Lactobacillus Isolated Pulmonic Valve Endocarditis with Ventricular Septal Defect Detected by Transesophageal Echocardiography. Am. Heart J. 1994, 128, 1248–1250. [Google Scholar] [CrossRef]
- Bessis, D.; Le Quellec, A.; Sotto, A.; Perez, C.; Ciurana, A.-J. Lactobacillus acidophilus Endocarditis After an Appendectomy. Clin. Infect. Dis. 1995, 20, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.W.; Paull, S.N. Effect of Biofilm Culture on Antibiotic Susceptibility of Lactobacilli Causing Endocarditis. J. Infect. 1995, 31, 80–81. [Google Scholar] [CrossRef]
- Amrikachi, M.; Lopez, L.; Cutler, D.; Boor, P.J. Lactobacillus Endocarditis: A Chronic Active Infection. Cardiovasc. Pathol. 1997, 6, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Vaghjimal, A.; Lutwick, L.I.; Chapnick, E.K. Endocarditis Caused by Lactobacillus. Postgrad. Med. J. 1997, 73, 61–62. [Google Scholar] [CrossRef]
- Mitchell, A.R.J.; Hayek, L.J. Lactobacillus Endocarditis. J. Infect. 1999, 38, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.D.; Taylor, M.B.; Kibbler, C.C.; Hamilton-Miller, J.M.T. Lactobacillus Endocarditis Caused by a Probiotic Organism. Clin. Microbiol. Infect. 1999, 5, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Avlami, A.; Kordossis, T.; Vrizidis, N.; Sipsas, N.V. Lactobacillus rhamnosus Endocarditis Complicating Colonoscopy. J. Infect. 2001, 42, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Presterl, E.; Kneifel, W.; Mayer, H.K.; Zehetgruber, M.; Makristathis, A.; Graninger, W. Endocarditis by Lactobacillus rhamnosus Due to Yogurt Ingestion? Scand. J. Infect. Dis. 2001, 33, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Schoevaerdts, D. Endocarditis in Older People. Age Ageing 2002, 31, 219–220. [Google Scholar] [CrossRef][Green Version]
- Wallet, F.; Dessein, R.; Armand, S.; Courcol, R.J. Molecular Diagnosis of Endocarditis Due to Lactobacillus casei Subsp. Rhamnosus. Clin. Infect. Dis. 2002, 35, e117–e119. [Google Scholar] [CrossRef]
- Beldner, S.; Bajwa, A.; Kaplan, B.; Rosen, S.; Steinberg, B.; Cacciabaudo, J. Septic Coronary Embolism. J. Interv. Cardiol. 2002, 15, 301–304. [Google Scholar] [CrossRef]
- Soleman, N.; Laferl, H.; Kneifel, W.; Tucek, G.; Budschedl, E.; Weber, H.; Pichler, H.; Mayer, H.K. How Safe Is Safe?—A Case of Lactobacillus paracasei ssp. Paracasei Endocarditis and Discussion of the Safety of Lactic Acid Bacteria. Scand. J. Infect. Dis. 2003, 35, 759–762. [Google Scholar] [CrossRef]
- Ze-Ze, L. Case of Aortic Endocarditis Caused by Lactobacillus casei. J. Med. Microbiol. 2004, 53, 451–453. [Google Scholar] [CrossRef]
- Khan, F.; Khakoo, R.; Failinger, C. Managing Embolic Myocardial Infarction in Infective Endocarditis: Current Options. J. Infect. 2005, 51, e101–e105. [Google Scholar] [CrossRef] [PubMed]
- Makaryus, A.N.; Yang, R.; Hahn, R.T.; Kort, S. A Rare Case of Lactobacillus acidophilus Presenting as Mitral Valve Bacterial Endocarditis. Echocardiography 2005, 22, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Salvana, E.M.T.; Frank, M. Lactobacillus Endocarditis: Case Report and Review of Cases Reported since 1992. J. Infect. 2006, 53, e5–e10. [Google Scholar] [CrossRef]
- See, J.R.H.; Czachor, J.S.; Brown, G.R. Lactobacillus Endocarditis: Case Report and Literature Review. Infect. Dis. Clin. Pract. 2006, 14, 134–136. [Google Scholar] [CrossRef]
- Khasnis, A.; Chick, D.; Havlichek, D. Mycotic Aneurysm of the Tibioperoneal Trunk as a Complication of Aortic Valve Endocarditis Due to Lactobacillus casei Infection: Case Report and Review of Literature. Infect. Dis. Clin. Pract. 2006, 14, 185–187. [Google Scholar] [CrossRef]
- Yagi, S.; Akaike, M.; Fujimura, M.; Ise, T.; Yoshida, S.; Sumitomo, Y.; Ikeda, Y.; Iwase, T.; Aihara, K.; Azuma, H.; et al. Infective Endocarditis Caused by Lactobacillus. Intern. Med. 2008, 47, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Wolz, M.; Schaefer, J. “Swiss Cheese-like” Brain Due to Lactobacillus rhamnosus. Neurology 2008, 70, 979. [Google Scholar] [CrossRef] [PubMed]
- Fradiani, P.A.; Petrucca, A.; Ascenzioni, F.; Di Nucci, G.; Teggi, A.; Bilancini, S.; Cipriani, P. Endocarditis Caused by Lactobacillus jensenii in an Immunocompetent Patient. J. Med. Microbiol. 2010, 59, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Gopalakrishna, K.V.; Maroo, P.; Iqbal, M.N. Lactobacillus-Cause of Death. Infect. Dis. Clin. Pract. 2010, 18, 219–220. [Google Scholar] [CrossRef]
- Nishijima, T.; Teruya, K.; Yanase, M.; Tamori, Y.; Mezaki, K.; Oka, S. Infectious Endocarditis Caused by Lactobacillus acidophilus in a Patient with Mistreated Dental Caries. Intern. Med. 2012, 51, 1619–1621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suárez-García, I.; Sánchez-García, A.; Soler, L.; Malmierca, E.; Gómez-Cerezo, J. Lactobacillus jensenii Bacteremia and Endocarditis after Dilatation and Curettage: Case Report and Literature Review. Infection 2012, 40, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.K.; Paez, A.P. Aortic Valve Endocarditis Due to Lactobacillus casei Complicated by Stroke and Vertebral Osteomyelitis: A Case Report. Infect. Dis. Clin. Pract. 2013, 21, 66–68. [Google Scholar] [CrossRef]
- Franko, B.; Vaillant, M.; Recule, C.; Vautrin, E.; Brion, J.-P.; Pavese, P. Lactobacillus paracasei Endocarditis in a Consumer of Probiotics. Médecine Et Mal. Infect. 2013, 43, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Botros, M.; Mukundan, D. Lactobacillus Endocarditis with Prosthetic Material: A Case Report on Non-Surgical Management with Corresponding Literature Review. Infect. Dis. Rep. 2014, 6, 5497. [Google Scholar] [CrossRef]
- Marciniak, A.; Karapanagiotidis, G.T.; Sarsam, M.; Sharma, R. Postpartum Lactobacillus jensenii Endocarditis in Patient with Bicuspid Aortic Valve. J. Thorac. Cardiovasc. Surg. 2014, 148, e219–e221. [Google Scholar] [CrossRef][Green Version]
- Patnaik, S.; Davila, C.D.; Chennupati, A.; Rubin, A. Endocarditis of the Native Aortic Valve Caused by Lactobacillus jensenii. Case Rep. 2015, 2015, bcr2014206288. [Google Scholar] [CrossRef]
- Gupta, A.; Harris, S.; Naina, H. Femoral Pseudoaneurysm as a Complication of Infective Endocarditis. J. Gen. Intern. Med. 2016, 31, 447–448. [Google Scholar] [CrossRef][Green Version]
- Felekos, I.; Lazaros, G.; Tsiriga, A.; Pirounaki, M.; Stavropoulos, G.; Paraskevas, J.; Toutouza, M.; Tousoulis, D. Lactobacillus rhamnosus Endocarditis: An Unusual Culprit in a Patient with Barlow’s Disease. Hell. J. Cardiol. 2016, 57, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Funabashi, N.; Takaoka, H.; Kohno, H.; Kishimoto, T.; Nakatani, Y.; Matsumiya, G.; Kobayashi, Y. Lactobacillus paracasei Endocarditis in a Consumer of Probiotics with Advanced and Severe Bicuspid Aortic Valve Stenosis Complicated with Diffuse Left Ventricular Mid-Layer Fibrosis. Int. J. Cardiol. 2016, 224, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, C.O.; Loranger, A.M.; Bharatkumar, A.G.; Almassi, G.H. Bacterial Endocarditis Caused by Lactobacillus acidophilus Leading to Rupture of Sinus of Valsalva Aneurysm. Tex. Heart Inst. J. 2016, 43, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Passera, M.; Pellicioli, I.; Corbellini, S.; Corno, M.; Vailati, F.; Bonanomi, E.; Farina, C. Lactobacillus casei Subsp. Rhamnosus Septicaemia in Three Patients of the Paediatric Intensive Care Unit. J. Hosp. Infect. 2016, 94, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Stroupe, C.; Pendley, J.; Isang, E.; Helms, B. Persistent Bacteremia Secondary to Delayed Identification of Lactobacillus in the Setting of Mitral Valve Endocarditis. IDCases 2017, 10, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Aaron, J.G.; Sobieszczyk, M.E.; Weiner, S.D.; Whittier, S.; Lowy, F.D. Lactobacillus rhamnosus Endocarditis After Upper Endoscopy. Open Forum Infect. Dis. 2017, 4, ofx085. [Google Scholar] [CrossRef] [PubMed]
- Groga-Bada, P.; Mueller, I.I.; Foschi, F.; Gawaz, M.; Eick, C. Mitral Valve Endocarditis Due to Lactobacillus. Case Rep. Med. 2018, 2018, 8613948. [Google Scholar] [CrossRef]
- Naqvi, S.S.B.; Nagendra, V.; Hofmeyr, A. Probiotic Related Lactobacillus rhamnosus Endocarditis in a Patient with Liver Cirrhosis. IDCases 2018, 13, e00439. [Google Scholar] [CrossRef]
- Lim, S.M.; Wong, B.; Cross, G.B.; Merchant, R. Lactobacillus Garvieae Endocarditis Presenting with Leg Cramps. IDCases 2018, 13, e00427. [Google Scholar] [CrossRef]
- Boumis, E.; Capone, A.; Galati, V.; Venditti, C.; Petrosillo, N. Probiotics and Infective Endocarditis in Patients with Hereditary Hemorrhagic Telangiectasia: A Clinical Case and a Review of the Literature. BMC Infect. Dis. 2018, 18, 65. [Google Scholar] [CrossRef]
- Ajam, M.; Adam, O.; Yeddi, A.; Kahlid, M.; Shokr, M.; Afonso, L. Prosthetic Aortic Valve Endocarditis in a Patient With Birt-Hogg-Dube Syndrome Due to Lactobacillus paracasei. Cardiol. Res. 2019, 10, 245–248. [Google Scholar] [CrossRef]
- Osman, A.; Taipale, M.; Najjar, M.; Osman, B. Lactobacillus paracasei Endocarditis of Bioprosthetic Aortic Valve Presenting with Recurrent Embolic Strokes. Access Microbiol. 2019, 1, e000038. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Tuchman, E.S.; Bruce, M.J.; Theodorou, M.E. Fatal Lactobacillus Endocarditis in a Patient with Transcatheter Aortic Valve Replacement. BMJ Case Rep. 2020, 13, e236835. [Google Scholar] [CrossRef] [PubMed]
- Antoun, M.; Hattab, Y.; Akhrass, F.-A.; Hamilton, L.D. Uncommon Pathogen, Lactobacillus, Causing Infective Endocarditis: Case Report and Review. Case Rep. Infect. Dis. 2020, 2020, 8833948. [Google Scholar] [CrossRef] [PubMed]
- Pasala, S.; Singer, L.; Arshad, T.; Roach, K. Lactobacillus Endocarditis in a Healthy Patient with Probiotic Use. IDCases 2020, 22, e00915. [Google Scholar] [CrossRef] [PubMed]
- Chukwurah, V.O.; Takang, C.; Uche, C.; Thomas, D.B.; El Masry, W.; Toka, H.R. Lactobacillus acidophilus Endocarditis Complicated by Pauci-Immune Necrotizing Glomerulonephritis. Case Rep. Med. 2020, 2020, 1607141. [Google Scholar] [CrossRef] [PubMed]
- Campagne, J.; Guichard, J.F.; Moulhade, M.C.; Kawski, H.; Maurier, F. Lactobacillus Endocarditis: A Case Report in France and Literature Review. IDCases 2020, 21, e00811. [Google Scholar] [CrossRef] [PubMed]
- Tavernese, A.; Stelitano, M.; Mauceri, A.; Mollace, R.; Uccello, G.; Romeo, F.; Cammalleri, V. Progression of Lactobacillus plantarum Prosthetic Valve Endocarditis Followed by Transesophageal Echocardiogram. Int. J. Infect. Dis. 2020, 97, 160–161. [Google Scholar] [CrossRef]
- Minto, T.; Bullock, N.; Deglurkar, I.; Hughes, O. Asymptomatic Bilateral Obstructing Ureteric Calculi Resulting in Lactobacillaemia and Endocarditis Requiring Emergency Aortic Valve Replacement. Urol. Case Rep. 2020, 32, 101218. [Google Scholar] [CrossRef]
- Ozer, M.; Goksu, S.Y.; Shahverdiani, A.; Mustafa, M. Lactobacillus acidophilus -Induced Endocarditis and Associated Splenic Abscess. Case Rep. Infect. Dis. 2020, 2020, 1382709. [Google Scholar] [CrossRef]
- Campbell, R.E.; Miller, A.; Afroze, A. Native Valve Endocarditis Secondary to Lactobacillus paracasei Bacteremia. Consultant 2020, 60, 27–28. [Google Scholar] [CrossRef]
- Bergas, A.; Rivera, S.; Torrecillas, M.; Cuervo, G. Native and Prosthetic Transcatheter Aortic Valve Infective Endocarditis Due to Lactobacillus rhamnosus. Enfermedades Infecc. Y Microbiol. Clínica 2022, 40, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Pischel, L.; Geirsson, A.; Magaldi, J.; Martinello, R.A.; Lee, A.I. A Brewing Back Pain. N. Engl. J. Med. 2021, 385, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Udongwo, N.; Fareen, N.; Abe, T.; Odak, M.; Saleh, S.; Zamel, L. Iatrogenic Infective Endocarditis with Septic Emboli: An Unusual Complication of Intracardiac Manipulation. J. Med. Cases 2022, 13, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bapna, M.; Maurer, J.; Ruddy, S.; Karnik, K.; Turett, G.; Urban, C.; Yoon, J.; Prasad, N.; Yung, L.; Lang, S.; et al. A Case of Lactobacillus jensenii Associated Native Valve Endocarditis. IDCases 2023, 32, e01806. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Alqaisi, S.; Nath, J. A Case of Lactobacillus casei Endocarditis Associated With Probiotic Intake in an Immunocompromised Patient. Cureus 2023, 15, e38049. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, E.; DePetrillo, J.; Qadeer, F. Meropenem Resistant Lactobacillus Endocarditis in an Immunocompetent Patient. SAGE Open Med. Case Rep. 2023, 11, 2050313X2311527. [Google Scholar] [CrossRef]
- Giannitsioti, E.; Skiadas, I.; Antoniadou, A.; Tsiodras, S.; Kanavos, K.; Triantafyllidi, H.; Giamarellou, H.; Hellenic Endocarditis Study Group. Nosocomial vs. Community-Acquired Infective Endocarditis in Greece: Changing Epidemiological Profile and Mortality Risk. Clin. Microbiol. Infect. 2007, 13, 763–769. [Google Scholar] [CrossRef]
- Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.; Vaara, M.; Valtonen, V. Safety of Probiotics That Contain Lactobacilli or Bifidobacteria. Clin. Infect. Dis. 2003, 36, 775–780. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Ioannou, P.; Vamvoukaki, R.; Kofteridis, D.P. Infective Endocarditis by Enterobacter Cloacae: A Systematic Review and Meta-Analysis. J. Chemother. 2021, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Miliara, E.; Baliou, S.; Kofteridis, D.P. Infective Endocarditis by Klebsiella Species: A Systematic Review. J. Chemother. 2021, 33, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Vougiouklakis, G. Infective Endocarditis by Proteus Species: A Systematic Review. Germs 2020, 10, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Fernández-Hidalgo, N.; Pilmis, B.; Tattevin, P.; Mainardi, J.-L. Aminoglycosides for Infective Endocarditis: Time to Say Goodbye? Clin. Microbiol. Infect. 2020, 26, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Pirracchio, R.; Rosa, A.; Petersen, M.L.; Van der Laan, M.; Fabiani, J.-N.; Fernandez-gerlinger, M.; Podglajen, I.; Safran, D.; Cholley, B.; et al. Incidence, Risk Factors and Prediction of Post-Operative Acute Kidney Injury Following Cardiac Surgery for Active Infective Endocarditis: An Observational Study. Crit. Care 2013, 17, R220. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hidalgo, N.; Almirante, B.; Gavaldà, J.; Gurgui, M.; Peña, C.; de Alarcón, A.; Ruiz, J.; Vilacosta, I.; Montejo, M.; Vallejo, N.; et al. Ampicillin plus Ceftriaxone Is as Effective as Ampicillin plus Gentamicin for Treating Enterococcus Faecalis Infective Endocarditis. Clin. Infect. Dis. 2013, 56, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.; Rasmussen, R.V.; Bundgaard, H.; Hassager, C.; Bruun, L.E.; Lauridsen, T.K.; Moser, C.; Sogaard, P.; Arpi, M.; Bruun, N.E. Enterococcus Faecalis Infective Endocarditis: A Pilot Study of the Relationship between Duration of Gentamicin Treatment and Outcome. Circulation 2013, 127, 1810–1817. [Google Scholar] [CrossRef]
- Buchholtz, K.; Larsen, C.T.; Hassager, C.; Bruun, N.E. In Infectious Endocarditis Patients Mortality Is Highly Related to Kidney Function at Time of Diagnosis: A Prospective Observational Cohort Study of 231 Cases. Eur. J. Intern. Med. 2009, 20, 407–410. [Google Scholar] [CrossRef]
- Campedelli, I.; Mathur, H.; Salvetti, E.; Clarke, S.; Rea, M.C.; Torriani, S.; Ross, R.P.; Hill, C.; O’Toole, P.W. Genus-Wide Assessment of Antibiotic Resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2019, 85, e01738-18. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Guimarães-Júnior, M.H.; Murta Pinto, P.H.O.; Coelho, R.M.P.; Souza Barros, T.L.; de Faleiro Maia, N.P.A.; Madureira, D.A.; Padilha Reis, R.C.; Costa, P.H.N.; Bráulio, R.; et al. Outcomes of Infective Endocarditis in the Current Era: Early Predictors of a Poor Prognosis. Int. J. Infect. Dis. 2018, 68, 102–107. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective Endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Wallace, S.M.; Walton, B.I.; Kharbanda, R.K.; Hardy, R.; Wilson, A.P.; Swanton, R.H. Mortality from Infective Endocarditis: Clinical Predictors of Outcome. Heart 2002, 88, 53–60. [Google Scholar] [CrossRef]
- Cimmino, G.; Bottino, R.; Formisano, T.; Orlandi, M.; Molinari, D.; Sperlongano, S.; Castaldo, P.; D’Elia, S.; Carbone, A.; Palladino, A.; et al. Current Views on Infective Endocarditis: Changing Epidemiology, Improving Diagnostic Tools and Centering the Patient for Up-to-Date Management. Life 2023, 13, 377. [Google Scholar] [CrossRef]
- Mills, M.T.; Al-Mohammad, A.; Warriner, D.R. Changes and Advances in the Field of Infective Endocarditis. Br. J. Hosp. Med. 2022, 83, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).