Multidrug-Resistant Sepsis: A Critical Healthcare Challenge
Abstract
1. Background
2. Epidemiology and Burden of MDR Sepsis
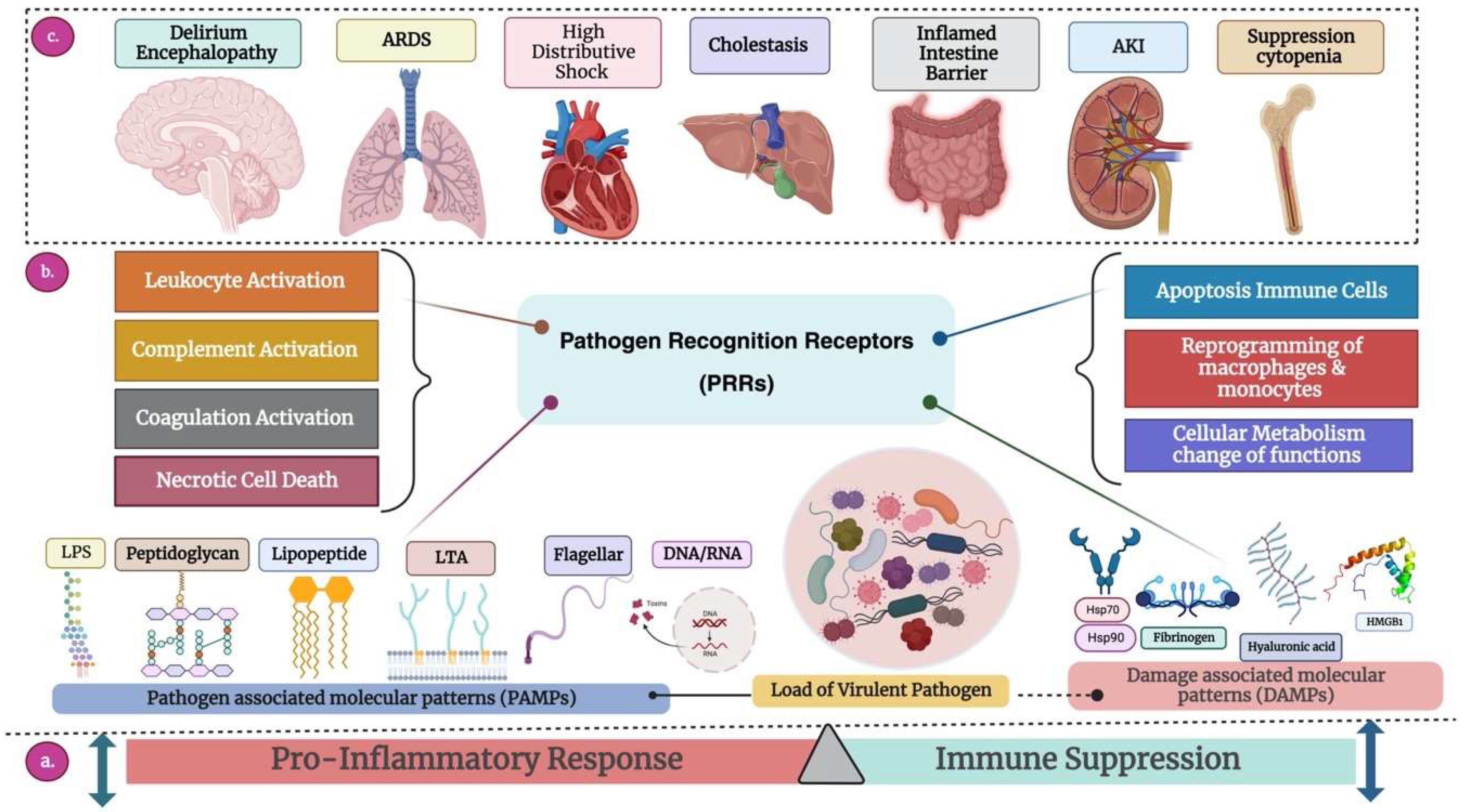
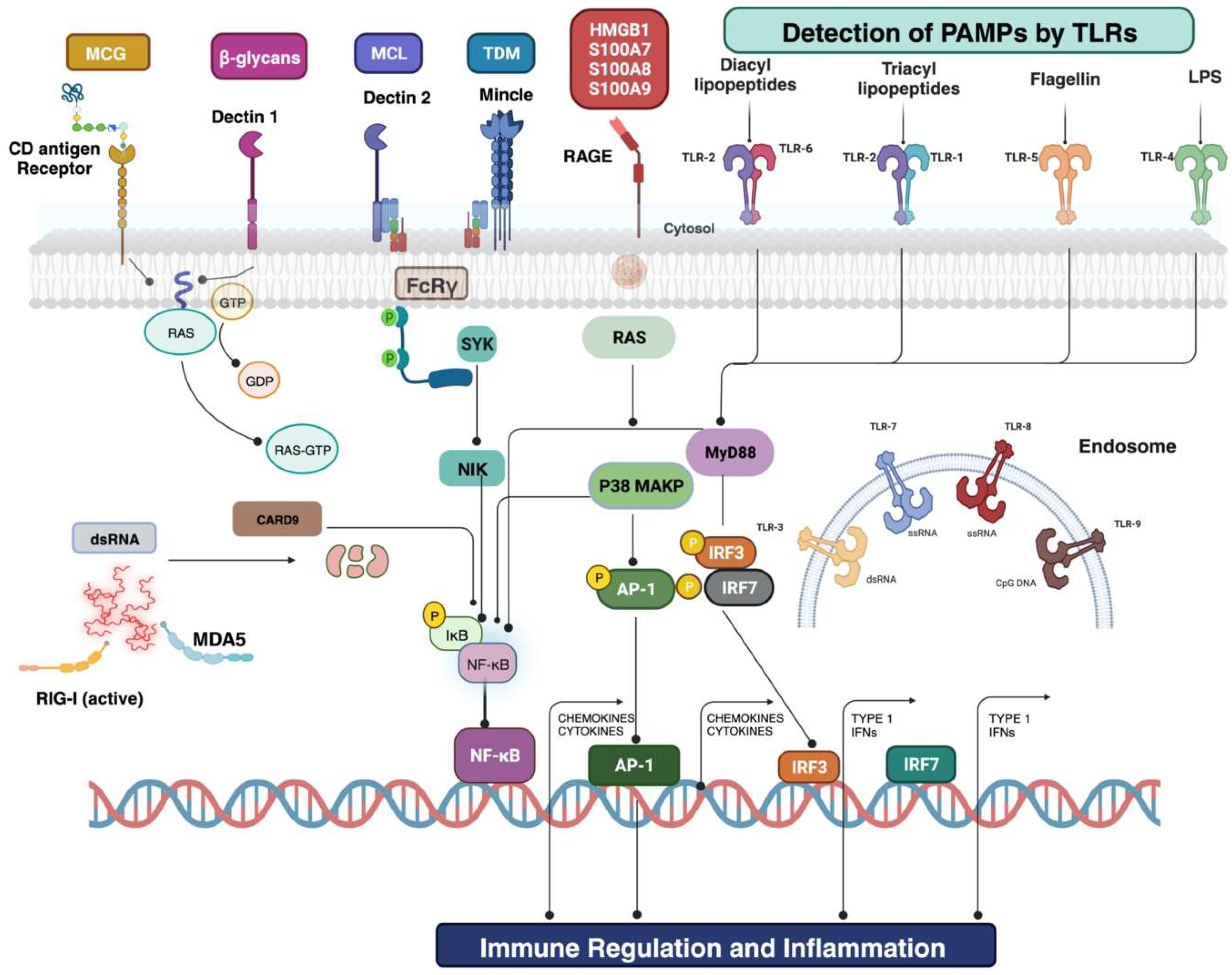
3. Pathogenesis and Mechanisms of Drug Resistance
4. Common Pathogens Involved in MDR Sepsis
5. Diagnostic Challenges and Innovations
6. Clinical Management of MDR Sepsis
7. Impact of MDR Sepsis on Critical Care
8. Frequency and Causes of Readmission in Sepsis Patients
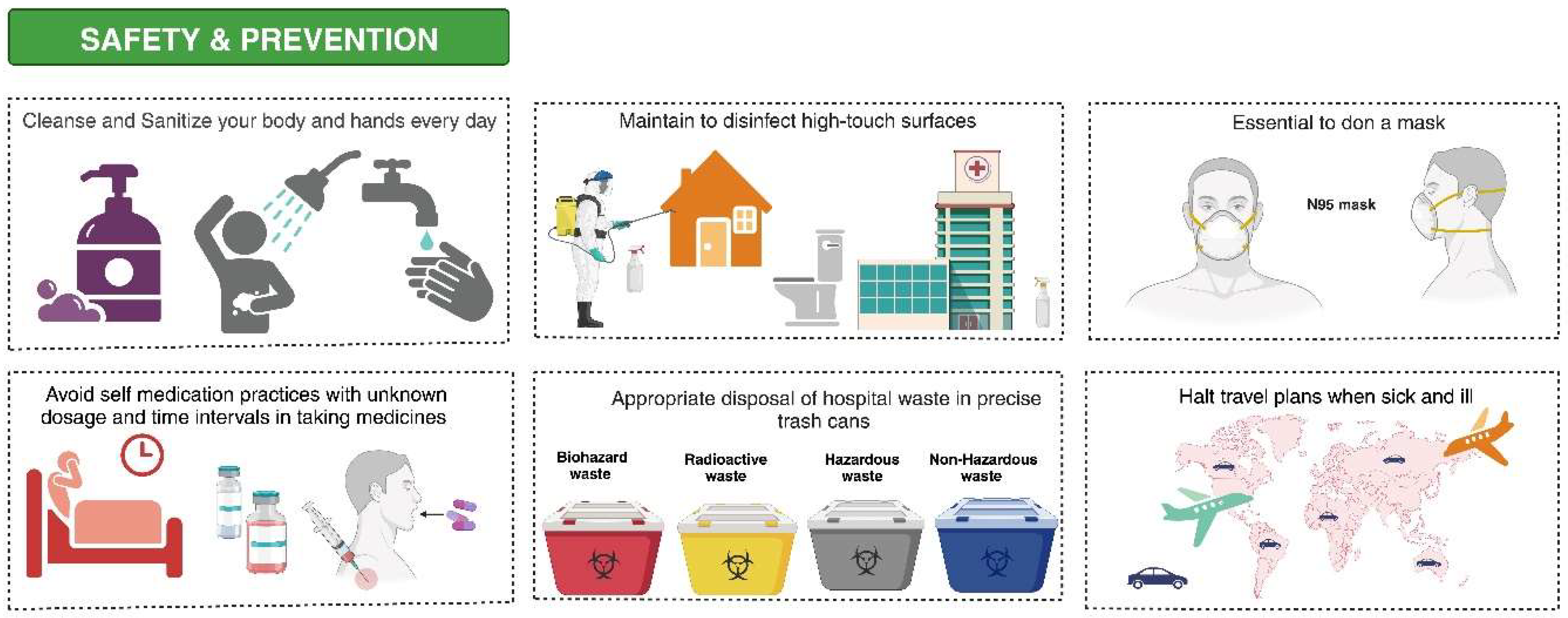
9. Preventive Measures and Infection Control
10. Global Efforts and Collaborations
11. Discussion
12. Strengths and Limitations
13. Future Directions in Research and Therapeutics
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| MDROs | Multidrug resistance organisms |
| MDR | Multidrug-resistant |
| POCT | Point-of-care testing |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| CRE | Carbapenem-resistant Enterobacteriaceae |
| ICU | Intensive care unit |
| WHO | World Health Organization |
| NMSS | National Mortality Surveillance System |
| ESBLs | Extended spectrum-beta-lactamase |
| COVID-19 | Coronavirus disease 2019 |
| AMR | Antimicrobial resistance |
| ESKAPE | Enterococcus faecium, S. aureus, Acinetobacter baumannii, Klebsiella pneumonia, Enterobacter species, and Pseudomonas aeruginosa |
| AMEs | Aminoglycoside modifying enzymes |
| PBPs | penicillin-binding proteins |
| ARDS | Acute respiratory distress syndrome |
| AKI | Acute kidney injury |
| DAMPs | Danger-associated molecular patterns |
| DNA | Deoxyribonucleic |
| RNA | Ribonucleic acid |
| HMGB1 | High-mobility group box-1 protein |
| HSPs | Heat shock proteins |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| PAMPs | Pathogen-associated molecular patterns |
| APCs | Antigen-presenting cells |
| PPR | Pattern recognition receptors |
| TLRs | Toll-like receptors |
| IFNs | Interferons |
| NF-κΒ | Nuclear factor-κΒ |
| IRF | Interferon regulatory factor |
| TNF | Tumor necrosis factor |
| IL | Interleukins |
| BSI | Bloodstream infection |
| MERS | Middle East respiratory syndrome-related |
| SARS | Severe acute respiratory syndrome |
| PCT | Procalcitonin |
| CRP | C-reactive protein |
| WBC | White blood cells |
| SIRS | Systemic Inflammatory Response Syndrome |
| AUROC | Area under the receiver operating characteristic |
| SERS | Surface-enhanced Raman spectroscopy |
| MALDI-TOF MS | Matrix-assisted laser desorption ionization time-of-flight mass spectrometry |
| PCR | Polymerase chain reaction |
| MS | Mass spectrometry |
| SCC | Surviving Sepsis Campaign |
| AS | Antimicrobial stewardship |
| IDSA | Infectious Diseases Society of America |
| ESICM | European Society of Intensive Care Medicine |
| VAP | Ventilator-associated pneumonia |
| HAP | hospital-acquired pneumonia |
| ASP | Antimicrobial Stewardship Program |
| IPC | Infection prevention and control |
| CDC | Centers for Disease Control and Prevention |
| AwaRe | Access, Watch, and Reserve |
| HCPs | Healthcare providers |
| VRE | Vancomycin-resistant Enterobacterales |
| IFNγ | interferon-gamma |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| mHLA-DR | monocyte human leukocyte antigen-DR |
| CARB | Combating Antibiotic-Resistant Bacteria |
| GSA | Global Sepsis Alliance |
| WHA | World Health Assembly |
| WSD | World Sepsis Day |
| SSCM | Society of Critical Care Medicine |
| LMIC | Low and middle-income countries |
References
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine Storm and Sepsis Disease Pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.; Osuchowski, M.F.; Shankar-Hari, M.; Skirecki, T.; Winkler, M.S.; Lachmann, G.; La Rosée, P.; Monneret, G.; Venet, F.; Bauer, M.; et al. Current Gaps in Sepsis Immunology: New Opportunities for Translational Research. Lancet. Infect. Dis. 2019, 19, e422–e436. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and Septic Shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Fernández, A.; Almansa, R.; Carrasco, E.; Heredia, M.; Lajo, C.; Goncalves, L.; Gómez-Herreras, J.I.; de Lejarazu, R.O.; Bermejo-Martin, J.F. Pro- and Anti-Inflammatory Responses Are Regulated Simultaneously from the First Moments of Septic Shock. Eur. Cytokine Netw. 2011, 22, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Andaluz-Ojeda, D.; Bobillo, F.; Iglesias, V.; Almansa, R.; Rico, L.; Gandía, F.; Resino, S.; Tamayo, E.; de Lejarazu, R.O.; Bermejo-Martin, J.F. A Combined Score of Pro- and Anti-Inflammatory Interleukins Improves Mortality Prediction in Severe Sepsis. Cytokine 2012, 57, 332–336. [Google Scholar] [CrossRef]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role Of Cytokines As A Double-Edged Sword In Sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Tang, B.M.; Huang, S.J.; McLean, A.S. Genome-Wide Transcription Profiling of Human Sepsis: A Systematic Review. Crit. Care 2010, 14, R237. [Google Scholar] [CrossRef]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. J. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Torio, C.M.; Moore, B.J. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2016.
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-Term Cognitive Impairment and Functional Disability among Survivors of Severe Sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Juneja, D.; Singh, O.; Dang, R.; Arora, V. Severe Sepsis and Its Impact on Outcome in Elderly and Very Elderly Patients Admitted in Intensive Care Unit. J. Intensive Care Med. 2012, 27, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Wan Muhd Shukeri, W.F.; Mat Nor, M.B.; Md Ralib, A. Sepsis and Its Impact on Outcomes in Elderly Patients Admitted to a Malaysian Intensive Care Unit. Malays. J. Med. Sci. MJMS 2022, 29, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Plata-Menchaca, E.P.; Ferrer, R.; Ruiz Rodríguez, J.C.; Morais, R.; Póvoa, P. Antibiotic Treatment in Patients with Sepsis: A Narrative Review. Hosp. Pract. 2022, 50, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, W.E.; Yang, Q. Clinical Perspective of Antimicrobial Resistance in Bacteria. Infect. Drug Resist. 2022, 15, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, M.C.; Shariff, M.; Nnajide, C.M.; Beri, K.; Okezie, U.M.; Iroha, I.R.; Esimone, C.O. Phenotypic and Molecular Characterization of β-Lactamases among Enterobacterial Uropathogens in Southeastern Nigeria. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 5843904. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Dutescu, I.A.; Hillier, S.A. Encouraging the Development of New Antibiotics: Are Financial Incentives the Right Way Forward? A Systematic Review and Case Study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef]
- Saleem, N. Antibiotics Modulate Variable Immunological Responses in Sepsis—A Narrative Review. Preprints 2022, 2022100218. [Google Scholar]
- Efunshile, A.M.; Ezeanosike, O.; Nwangwu, C.C.; König, B.; Jokelainen, P.; Robertson, L.J. Apparent Overuse of Antibiotics in the Management of Watery Diarrhoea in Children in Abakaliki, Nigeria. BMC Infect. Dis. 2019, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Belachew, S.A.; Hall, L.; Selvey, L.A. Non-Prescription Dispensing of Antibiotic Agents among Community Drug Retail Outlets in Sub-Saharan African Countries: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Suy, S.; Rego, S.; Bory, S.; Chhorn, S.; Phou, S.; Prien, C.; Heng, S.; Wu, S.; Legido-Quigley, H.; Hanefeld, J.; et al. Invisible Medicine Sellers and Their Use of Antibiotics: A Qualitative Study in Cambodia. BMJ Glob. Health 2019, 4, e001787. [Google Scholar] [CrossRef] [PubMed]
- Tangcharoensathien, V.; Chanvatik, S.; Sommanustweechai, A. Complex Determinants of Inappropriate Use of Antibiotics. Bull. World Health Organ. 2018, 96, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Garau, J. Impact of Antibiotic Restrictions: The Ethical Perspective. Clin. Microbiol. Infect. 2006, 12, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Legrand, M. Epidemiology of Sepsis and Septic Shock. Curr. Opin. Anaesthesiol. 2021, 34, 71–76. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2015, 193, 259–272. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.-N.; Vincent, J.-L.; et al. Sepsis in Intensive Care Unit Patients: Worldwide Data from the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Todi, S.; Chatterjee, S.; Bhattacharyya, M. Epidemiology of Severe Sepsis in India. Crit. Care 2007, 11, P65. [Google Scholar] [CrossRef]
- Anand, A.; Kumar, N.; Gambhir, I. Clinicomicrobiological Profile of the Indian Elderly with Sepsis. Ann. Trop. Med. Public Health 2016, 9, 316–320. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, M.; Todi, S.K. Epidemiology of Adult-Population Sepsis in India: A Single Center 5 Year Experience. Indian J. Crit. Care Med. 2017, 21, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Prabhudev, P.; Ramamoorthi, K.; Acharya, R.V. A Clinical and Demographic Profile of Elderly (>65 Years) in the Medical Intensive Care Units of a Tertiary Care Center. Indian J. Crit. Care Med. 2023, 27, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Zeng, X.Y.; Yin, P.; Wang, L.J.; Wang, C.Y.; Jiang, W.; Zhou, M.G.; Du, B. Sepsis-Related Mortality in China: A Descriptive Analysis. Intensive Care Med. 2018, 44, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Xu, Y.; Yin, P.; Wang, Y.; Chen, Y.; Liu, W.; Li, S.; Peng, J.-M.; Dong, R.; Hu, X.-Y.; et al. National Incidence and Mortality of Hospitalized Sepsis in China. Crit. Care 2023, 27, 84. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and Mortality of Hospital- and ICU-Treated Sepsis: Results from an Updated and Expanded Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Marchaim, D.; Gottesman, T.; Schwartz, O.; Korem, M.; Maor, Y.; Rahav, G.; Karplus, R.; Lazarovitch, T.; Braun, E.; Sprecher, H.; et al. National Multicenter Study of Predictors and Outcomes of Bacteremia upon Hospital Admission Caused by Enterobacteriaceae Producing Extended-Spectrum Beta-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 5099–5104. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Cauda, R.; Tumbarello, M. Detecting Risk and Predicting Patient Mortality in Patients with Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae Bloodstream Infections. Future Microbiol. 2012, 7, 1173–1189. [Google Scholar] [CrossRef]
- Sibila, O.; Rodrigo-Troyano, A.; Shindo, Y.; Aliberti, S.; Restrepo, M.I. Multidrug-Resistant Pathogens in Patients with Pneumonia Coming from the Community. Curr. Opin. Pulm. Med. 2016, 22, 219–226. [Google Scholar] [CrossRef]
- Paul, M.; Shani, V.; Muchtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic Review and Meta-Analysis of the Efficacy of Appropriate Empiric Antibiotic Therapy for Sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Zilberberg, M.D.; Shorr, A.F.; Vo, L.; Schein, J.; Micek, S.T.; Kim, M. Epidemiology, Microbiology and Outcomes of Healthcare-Associated and Community-Acquired Bacteremia: A Multicenter Cohort Study. J. Infect. 2011, 62, 130–135. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control; World Health Organization Regional Office for Europe. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Capsoni, N.; Bellone, P.; Aliberti, S.; Sotgiu, G.; Pavanello, D.; Visintin, B.; Callisto, E.; Saderi, L.; Soldini, D.; Lardera, L.; et al. Prevalence, Risk Factors and Outcomes of Patients Coming from the Community with Sepsis Due to Multidrug Resistant Bacteria. Multidiscip. Respir. Med. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital Deaths in Patients with Sepsis from 2 Independent Cohorts. JAMA 2014, 312, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Rubens, M.; Saxena, A.; Ramamoorthy, V.; Das, S.; Khera, R.; Hong, J.; Armaignac, D.; Veledar, E.; Nasir, K.; Gidel, L. Increasing Sepsis Rates in the United States: Results From National Inpatient Sample, 2005 to 2014. J. Intensive Care Med. 2020, 35, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Krishak, R.; Shukla, D.K. NICU in a Community Level Hospital. Indian J. Pediatr. 2005, 72, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, R.; Ramakrishnan, N. Cost of Intensive Care in India. Indian J. Crit. Care Med. 2008, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Farrah, K.; McIntyre, L.; Doig, C.J.; Talarico, R.; Taljaard, M.; Krahn, M.; Fergusson, D.; Forster, A.J.; Coyle, D.; Thavorn, K. Sepsis-associated Mortality, Resource Use, and Healthcare Costs: A Propensity-Matched Cohort Study. J. Crit. Care Med. 2021, 49, 215–227. [Google Scholar] [CrossRef]
- Oami, T.; Imaeda, T.; Nakada, T.a.; Abe, T.; Takahashi, N.; Yamao, Y.; Nakagawa, S.; Ogura, H.; Shime, N.; Umemura, Y.; et al. Temporal Trends of Medical Cost and Cost-Effectiveness in Sepsis Patients: A Japanese Nationwide Medical Claims Database. J. Intensive Care 2022, 10, 33. [Google Scholar] [CrossRef]
- Lewis, K. The science of antibiotic discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef]
- Lebreton, F.; Manson, A.L.; Saavedra, J.T.; Straub, T.J.; Earl, A.M.; Gilmore, M.S. Tracing the Enterococci from Paleozoic Origins to the Hospital. Cell 2017, 169, 849–861.e813. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; Van Veen, H.W.; Pos, K.M.; Piddock, L.J.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. J Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.W. Control of Hospital Acquired Infections and Antimicrobial Resistance in Europe: The Way to Go. Wien. Med. Wochenschr. 2019, 169, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mishra, S.K.; Shrestha, A. Characterisation of ESKAPE Pathogens with Special Reference to Multidrug Resistance and Biofilm Production in a Nepalese Hospital. Infect. Drug Resist. 2021, 14, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Recio, R.; Mancheño, M.; Viedma, E.; Villa, J.; Orellana, M.; Lora-Tamayo, J.; Chaves, F. Predictors of Mortality in Bloodstream Infections Caused by Pseudomonas aeruginosa and Impact of Antimicrobial Resistance and Bacterial Virulence. Antimicrob. Agents Chemother. 2020, 64, e01759-19. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Timsina, R.; Shrestha, U.; Singh, A.; Timalsina, B. Inducible Clindamycin Resistance and Erm Genes in Staphylococcus aureus in School Children in Kathmandu, Nepal. Future Sci. OA 2020, 7, Fso361. [Google Scholar] [CrossRef]
- Boomer, J.S.; Green, J.M.; Hotchkiss, R.S. The Changing Immune System in Sepsis: Is Individualized Immuno-Modulatory Therapy the Answer? Virulence 2014, 5, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Monneret, G. Advances in the Understanding and Treatment of Sepsis-Induced Immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Girardis, M.; Cossarizza, A. Early Alterations of B Cells in Patients with Septic Shock: Another Piece in the Complex Puzzle of the Immune Response in Sepsis. Crit. Care 2013, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Vakkalanka, J.P.; Harland, K.K.; Swanson, M.B.; Mohr, N.M. Clinical and Epidemiological Variability in Severe Sepsis: An Ecological Study. J. Epidemiol. Community Health 2018, 72, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Varghese, D.; Ishida, C.; Haseer Koya, H. Polypharmacy; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Macias, J.; Kahly, O.; Edward, R.; Khan, S.; Qureshi, A.; Shaik, A.; Shala, A.; Shah, D. Sepsis: A Systematic Review of Antibiotic Resistance and Antimicrobial Therapies. J. Mod. Res. Inflamm. 2022, 11, 9–23. [Google Scholar] [CrossRef]
- Reinhart, K. How Antimicrobial Resistance Amplifies the Burden of Sepsis. Available online: https://www.news-medical.net/health/How-Antimicrobial-Resistance-Amplifies-the-Burden-of-Sepsis.aspx (accessed on 15 August 2023).
- Hurst, J. Invasive Fungal Infections Can Lead to Sepsis—And Have a High Mortality Rate. Available online: https://www.biomerieuxconnection.com/2019/09/24/invasive-fungal-infections-can-lead-to-sepsis-and-have-a-high-mortality-rate/ (accessed on 15 August 2023).
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and Clinical Management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Lewis, C.A.; Nisbet, R.; Mannino, D.M.; Martin, G.S. The Role of Infection and Comorbidity: Factors that Influence Disparities in Sepsis. Crit. Care Med. 2006, 34, 2576–2582. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Wilson, J.W.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Incidence of Pseudomonas aeruginosa bacteremia: A Population-Based Study. Am. J. Med. 2008, 121, 702–708. [Google Scholar] [CrossRef]
- Laupland, K.B.; Gregson, D.B.; Church, D.L.; Ross, T.; Pitout, J.D. Incidence, Risk Factors and Outcomes of Escherichia coli Bloodstream Infections in a Large Canadian Region. Clin. Microbiol. Infect. 2008, 14, 1041–1047. [Google Scholar] [CrossRef]
- Ulu Kilic, A.; Alp, E.; Cevahir, F.; Ture, Z.; Yozgat, N. Epidemiology and Cost Implications of Candidemia, a 6-Year Analysis from a Developing Country. Mycoses 2017, 60, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.H.; Webster, K.M. Epidemiology and Outcomes of Candidemia in 2019 Patients: Data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Degoricija, V.; Sharma, M.; Legac, A.; Gradiser, M.; Sefer, S.; Vucicević, Z. Survival Analysis of 314 Episodes of Sepsis in Medical Intensive Care Unit in University Hospital: Impact of Intensive Care Unit Performance and Antimicrobial Therapy. Croat. Med. J. 2006, 47, 385–397. [Google Scholar] [PubMed]
- Garnacho-Montero, J.; Ortiz-Leyba, C.; Herrera-Melero, I.; Aldabó-Pallás, T.; Cayuela-Dominguez, A.; Marquez-Vacaro, J.A.; Carbajal-Guerrero, J.; Garcia-Garmendia, J.L. Mortality and Morbidity Attributable to Inadequate Empirical Antimicrobial Therapy in Patients Admitted to the ICU with Sepsis: A Matched Cohort Study. J. Antimicrob. Chemother. 2008, 61, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Pradipta, I.S.; Sodik, D.C.; Lestari, K.; Parwati, I.; Halimah, E.; Diantini, A.; Abdulah, R. Antibiotic Resistance in Sepsis Patients: Evaluation and Recommendation of Antibiotic Use. North Am. J. Med. Sci. 2013, 5, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Guerrero, J.; Cayuela-Domínguez, A.; Fernández-García, E.; Aldabó-Pallás, T.; Márquez-Vácaro, J.A.; Ortiz-Leyba, C.; Garnacho-Montero, J. Epidemiology and Long-Term Outcome of Sepsis in Elderly Patients. Med. Intensiv. 2014, 38, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, M.W.; Inthorn, D.; Andress, H.J.; Schildberg, F.W. Incidence and Mortality of Severe Sepsis in Surgical Intensive Care Patients: The Influence of Patient Gender on Disease Process and Outcome. Intensive Care Med. 2000, 26, 167–172. [Google Scholar] [CrossRef]
- Adrie, C.; Azoulay, E.; Francais, A.; Clec’h, C.; Darques, L.; Schwebel, C.; Nakache, D.; Jamali, S.; Goldgran-Toledano, D.; Garrouste-Orgeas, M.; et al. Influence of Gender on the Outcome of Severe Sepsis: A Reappraisal. Chest 2007, 132, 1786–1793. [Google Scholar] [CrossRef]
- Berkowitz, D.M.; Martin, G.S. Sepsis and Sex: Can We Look beyond Our Hormones? Chest 2007, 132, 1725–1727. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Yang, H.; Li, H.; Shen, Y.; Zhang, D. Comparison of Culture-Negative and Culture-Positive Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. Crit. Care 2021, 25, 167. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.M.; Muller-Pebody, B.; Planche, T.; Johnson, A.; Hopkins, S.; Sharland, M.; Kennea, N.; Heath, P.T. Neonatal Sepsis--Many Blood Samples, Few Positive Cultures: Implications for Improving Antibiotic Prescribing. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F487–F488. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Brun-Buisson, C.; Torres, A.; Jorgensen, J. Diagnosis of Infection in Sepsis: An Evidence-Based Review. Crit. Care Med. 2004, 32, S466–S494. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Stenstrom, R.; Paquette, K.; Stabler, S.N.; Akhter, M.; Davidson, A.C.; Gavric, M.; Lawandi, A.; Jinah, R.; Saeed, Z.; et al. Blood Culture Results Before and After Antimicrobial Administration in Patients With Severe Manifestations of Sepsis: A Diagnostic Study. Ann. Intern. Med. 2019, 171, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Scheer, C.S.; Fuchs, C.; Gründling, M.; Vollmer, M.; Bast, J.; Bohnert, J.A.; Zimmermann, K.; Hahnenkamp, K.; Rehberg, S.; Kuhn, S.O. Impact of Antibiotic Administration on Blood Culture Positivity at the Beginning of Sepsis: A Prospective Clinical Cohort Study. Clin. Microbiol. Infect. 2019, 25, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Marik, P.E.; Stephenson, E. The Ability of Procalcitonin, Lactate, White Blood Cell Count and Neutrophil-Lymphocyte Count Ratio to Predict Blood Stream Infection. Analysis of a Large Database. J. Crit. Care 2020, 60, 135–139. [Google Scholar] [CrossRef]
- Seigel, T.A.; Cocchi, M.N.; Salciccioli, J.; Shapiro, N.I.; Howell, M.; Tang, A.; Donnino, M.W. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J. Emerg. Med. 2012, 42, 254–259. [Google Scholar] [CrossRef]
- Van den Bruel, A.; Thompson, M.J.; Haj-Hassan, T.; Stevens, R.; Moll, H.; Lakhanpaul, M.; Mant, D. Diagnostic value of laboratory tests in identifying serious infections in febrile children: Systematic review. BMJ 2011, 342, d3082. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulou, I.G.; Parashar, D.; Lin, R.; Antonowicz, S.; Wells, A.D.; Bajwa, F.M.; Krijgsman, B. The diagnostic value of white cell count, C-reactive protein and bilirubin in acute appendicitis and its complications. Ann. R. Coll. Surg. Engl. 2013, 95, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Camacho-Rivera, M.; Taioli, E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS ONE 2014, 9, e112361. [Google Scholar] [CrossRef] [PubMed]
- Ljungström, L.; Pernestig, A.K.; Jacobsson, G.; Andersson, R.; Usener, B.; Tilevik, D. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS ONE 2017, 12, e0181704. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Westerdijk, K.; Simons, K.S.; Zegers, M.; Wever, P.C.; Pickkers, P.; de Jager, C.P.C. The value of the neutrophil-lymphocyte count ratio in the diagnosis of sepsis in patients admitted to the Intensive Care Unit: A retrospective cohort study. PLoS ONE 2019, 14, e0212861. [Google Scholar] [CrossRef]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell. Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef]
- Stirling, A.D.; Moran, N.R.; Kelly, M.E.; Ridgway, P.F.; Conlon, K.C. The predictive value of C-reactive protein (CRP) in acute pancreatitis—Is interval change in CRP an additional indicator of severity? HPB 2017, 19, 874–880. [Google Scholar] [CrossRef]
- Khanna, A.K.; Meher, S.; Prakash, S.; Tiwary, S.K.; Singh, U.; Srivastava, A.; Dixit, V.K. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013, 2013, 367581. [Google Scholar] [CrossRef]
- Póvoa, P.; Coelho, L.; Almeida, E.; Fernandes, A.; Mealha, R.; Moreira, P.; Sabino, H. Early identification of intensive care unit-acquired infections with daily monitoring of C-reactive protein: A prospective observational study. Crit. Care 2006, 10, R63. [Google Scholar] [CrossRef] [PubMed]
- Assicot, M.; Gendrel, D.; Carsin, H.; Raymond, J.; Guilbaud, J.; Bohuon, C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kamat, I.S.; Ramachandran, V.; Eswaran, H.; Guffey, D.; Musher, D.M. Procalcitonin to Distinguish Viral From Bacterial Pneumonia: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2020, 70, 538–542. [Google Scholar] [CrossRef]
- Joo, K.; Park, W.; Lim, M.J.; Kwon, S.R.; Yoon, J. Serum procalcitonin for differentiating bacterial infection from disease flares in patients with autoimmune diseases. J. Korean Med. Sci. 2011, 26, 1147–1151. [Google Scholar] [CrossRef]
- El-Sayed, D.; Grotts, J.; Golgert, W.A.; Sugar, A.M. Sensitivity and specificity of procalcitonin in predicting bacterial infections in patients with renal impairment. Open Forum Infect. Dis. 2014, 1, ofu068. [Google Scholar] [CrossRef]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.B. The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef]
- Jeong, K.; Stanwix, P.L.; May, E.F.; Aman, Z.M. Surface-Enhanced Raman Scattering Imaging of Cetylpyridinium Chloride Adsorption to a Solid Surface. J Anal. Chem. 2022, 94, 14169–14176. [Google Scholar] [CrossRef]
- Delport, J.A.; Strikwerda, A.; Armstrong, A.; Schaus, D.; John, M. MALDI-ToF short incubation identification from blood cultures is associated with reduced length of hospitalization and a decrease in bacteremia associated mortality. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1181–1186. [Google Scholar] [CrossRef]
- Beganovic, M.; Costello, M.; Wieczorkiewicz, S.M. Effect of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) Alone versus MALDI-TOF MS Combined with Real-Time Antimicrobial Stewardship Interventions on Time to Optimal Antimicrobial Therapy in Patients with Positive Blood Cultures. J. Clin. Microbiol. 2017, 55, 1437–1445. [Google Scholar] [CrossRef]
- Patel, T.S.; Kaakeh, R.; Nagel, J.L.; Newton, D.W.; Stevenson, J.G. Cost Analysis of Implementing Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Plus Real-Time Antimicrobial Stewardship Intervention for Bloodstream Infections. J. Clin. Microbiol. 2017, 55, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Luethy, P.M.; Johnson, J.K. The Use of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) for the Identification of Pathogens Causing Sepsis. J. Appl. Lab. Med. 2019, 3, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Westh, H.; Lisby, G.; Breysse, F.; Böddinghaus, B.; Chomarat, M.; Gant, V.; Goglio, A.; Raglio, A.; Schuster, H.; Stuber, F.; et al. Multiplex real-time PCR and blood culture for identification of bloodstream pathogens in patients with suspected sepsis. Clin. Microbiol. Infect. 2009, 15, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.I.; Al-Marzooq, F.; How, S.H.; Kuan, Y.C.; Ng, T.H. The use of multiplex real-time PCR improves the detection of the bacterial etiology of community acquired pneumonia. Trop. Biomed. 2011, 28, 531–544. [Google Scholar] [PubMed]
- van de Groep, K.; Bos, M.P.; Varkila, M.R.J.; Savelkoul, P.H.M.; Ong, D.S.Y.; Derde, L.P.G.; Juffermans, N.P.; van der Poll, T.; Bonten, M.J.M.; Cremer, O.L. Moderate positive predictive value of a multiplex real-time PCR on whole blood for pathogen detection in critically ill patients with sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Jupe, J.; Mack, H.; Coleman, T.P.; Lawrence, S.M.; Fraley, S.I. Emerging Technologies for Molecular Diagnosis of Sepsis. Clin. Microbiol. Rev. 2018, 31, e00089-17. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Gesten, F.C.; Phillips, G.S.; Terry, K.M.; Seymour, C.W.; Prescott, H.C.; Friedrich, M.; Iwashyna, T.J.; Osborn, T.; Lemeshow, S. Mortality Changes Associated with Mandated Public Reporting for Sepsis. The Results of the New York State Initiative. Am. J. Respir. Crit. Care Med. 2018, 198, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int. Immunopharmacol. 2018, 58, 173–185. [Google Scholar] [CrossRef]
- Rello, J.; van Engelen, T.S.R.; Alp, E.; Calandra, T.; Cattoir, V.; Kern, W.V.; Netea, M.G.; Nseir, S.; Opal, S.M.; van de Veerdonk, F.L.; et al. Towards precision medicine in sepsis: A position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2018, 24, 1264–1272. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Fornai, M.; Pacher, P.; Lee, H.T.; Haskó, G. P2X4 receptors, immunity, and sepsis. Curr. Opin. Pharmacol. 2019, 47, 65–74. [Google Scholar] [CrossRef]
- Mirasoli, M.; Bonvicini, F.; Lovecchio, N.; Petrucci, G.; Zangheri, M.; Calabria, D.; Costantini, F.; Roda, A.; Gallinella, G.; Caputo, D.; et al. On-chip LAMP-BART reaction for viral DNA real-time bioluminescence detection. Sens. Actuators B Chem. 2018, 262, 1024–1033. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.F.; Douglas, I.S.; Finfer, S.; Gårdlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Plata-Menchaca, E.P.; Ferrer, R. Life-support tools for improving performance of the Surviving Sepsis Campaign Hour-1 bundle. Med. Intensiv. 2018, 42, 547–550. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Kumar, A.; Ellis, P.; Arabi, Y.; Roberts, D.; Light, B.; Parrillo, J.E.; Dodek, P.; Wood, G.; Kumar, A.; Simon, D.; et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009, 136, 1237–1248. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef]
- Singer, M. Antibiotics for Sepsis: Does Each Hour Really Count, or Is It Incestuous Amplification? Am. J. Respir. Crit. Care Med. 2017, 196, 800–802. [Google Scholar] [CrossRef]
- Mi, M.Y.; Klompas, M.; Evans, L. Early Administration of Antibiotics for Suspected Sepsis. N. Engl. J. Med. 2019, 380, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Nuvials, X.; Palomar, M.; Masclans, J.R.; Rello, J. Appropriateness is critical. Crit. Care Clin. 2011, 27, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Rhodes, A.; Phillips, G.S.; Townsend, S.R.; Schorr, C.A.; Beale, R.; Osborn, T.; Lemeshow, S.; Chiche, J.D.; Artigas, A.; et al. Surviving Sepsis Campaign: Association between performance metrics and outcomes in a 7.5-year study. Crit. Care Med. 2015, 43, 3–12. [Google Scholar] [CrossRef]
- Coz Yataco, A.; Jaehne, A.K.; Rivers, E.P. Protocolized Early Sepsis Care Is Not Only Helpful for Patients: It Prevents Medical Errors. Crit. Care Med. 2017, 45, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Gutiérrez-Pizarraya, A.; Escoresca-Ortega, A.; Fernández-Delgado, E.; López-Sánchez, J.M. Adequate antibiotic therapy prior to ICU admission in patients with severe sepsis and septic shock reduces hospital mortality. Crit. Care 2015, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, R.; Winters, M.E.; Vilke, G.M.; Wardi, G. Does Early and Appropriate Antibiotic Administration Improve Mortality in Emergency Department Patients with Severe Sepsis or Septic Shock? J. Emerg. Med. 2017, 53, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Suberviola Cañas, B.; Jáuregui, R.; Ballesteros, M.; Leizaola, O.; González-Castro, A.; Castellanos-Ortega, Á. Effects of antibiotic administration delay and inadequacy upon the survival of septic shock patients. Med. Intensiv. 2015, 39, 459–466. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Vazquez-Guillamet, C.; Kollef, M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596. [Google Scholar] [CrossRef]
- Ferrer, R.; Martínez, M.L.; Gomà, G.; Suárez, D.; Álvarez-Rocha, L.; de la Torre, M.V.; González, G.; Zaragoza, R.; Borges, M.; Blanco, J.; et al. Improved empirical antibiotic treatment of sepsis after an educational intervention: The ABISS-Edusepsis study. Crit. Care 2018, 22, 167. [Google Scholar] [CrossRef]
- Green, R.S.; Gorman, S.K. Emergency department antimicrobial considerations in severe sepsis. Emerg. Med. Clin. N. Am. 2014, 32, 835–849. [Google Scholar] [CrossRef]
- Hamandi, B.; Holbrook, A.M.; Humar, A.; Brunton, J.; Papadimitropoulos, E.A.; Wong, G.G.; Thabane, L. Delay of adequate empiric antibiotic therapy is associated with increased mortality among solid-organ transplant patients. Am. J. Transplant. 2009, 9, 1657–1665. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; García-Cabrera, E.; Diaz-Martín, A.; Lepe-Jiménez, J.A.; Iraurgi-Arcarazo, P.; Jiménez-Alvarez, R.; Revuelto-Rey, J.; Aznar-Martín, J. Determinants of outcome in patients with bacteraemic pneumococcal pneumonia: Importance of early adequate treatment. Scand. J. Infect. Dis. 2010, 42, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Harmankaya, M.; Oreskov, J.O.; Burcharth, J.; Gögenur, I. The impact of timing of antibiotics on in-hospital outcomes after major emergency abdominal surgery. Eur. J. Trauma Emerg. Surg. 2020, 46, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Puskarich, M.A.; Trzeciak, S.; Shapiro, N.I.; Arnold, R.C.; Horton, J.M.; Studnek, J.R.; Kline, J.A.; Jones, A.E. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit. Care Med. 2011, 39, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Micek, S.T.; Kollef, M.H. Time to Appropriate Antibiotic Therapy Is an Independent Determinant of Postinfection ICU and Hospital Lengths of Stay in Patients With Sepsis. Crit. Care Med. 2015, 43, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Lapinsky, S.; Dial, S.; Arabi, Y.; Dodek, P.; Wood, G.; Ellis, P.; Guzman, J.; Marshall, J.; Parrillo, J.E.; et al. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009, 35, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Iscimen, R.; Cartin-Ceba, R.; Yilmaz, M.; Khan, H.; Hubmayr, R.D.; Afessa, B.; Gajic, O. Risk factors for the development of acute lung injury in patients with septic shock: An observational cohort study. Crit. Care Med. 2008, 36, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Shin, J.; Jo, I.J.; Park, J.E.; Yoon, H.; Cha, W.C.; Sim, M.S.; Shin, T.G. Delayed Antibiotic Therapy and Organ Dysfunction in Critically Ill Septic Patients in the Emergency Department. J. Clin. Med. 2019, 8, 222. [Google Scholar] [CrossRef]
- Labelle, A.; Juang, P.; Reichley, R.; Micek, S.; Hoffmann, J.; Hoban, A.; Hampton, N.; Kollef, M. The determinants of hospital mortality among patients with septic shock receiving appropriate initial antibiotic treatment. Crit. Care Med. 2012, 40, 2016–2021. [Google Scholar] [CrossRef]
- van Paridon, B.M.; Sheppard, C.; Joffe, A.R.; Alberta Sepsis Network. Timing of antibiotics, volume, and vasoactive infusions in children with sepsis admitted to intensive care. Crit. Care 2015, 19, 293. [Google Scholar] [CrossRef]
- Alam, N.; Oskam, E.; Stassen, P.M.; Exter, P.V.; van de Ven, P.M.; Haak, H.R.; Holleman, F.; Zanten, A.V.; Leeuwen-Nguyen, H.V.; Bon, V.; et al. Prehospital antibiotics in the ambulance for sepsis: A multicentre, open label, randomised trial. Lancet Respir. Med. 2018, 6, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sterling, S.A.; Miller, W.R.; Pryor, J.; Puskarich, M.A.; Jones, A.E. The Impact of Timing of Antibiotics on Outcomes in Severe Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. Crit. Care Med. 2015, 43, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.N.B.; Park, J.; Doi, S.A.; Sharman, V.; Clark, J.; Robinson, J.; Crilly, J. Effect of Immediate Administration of Antibiotics in Patients With Sepsis in Tertiary Care: A Systematic Review and Meta-analysis. Clin. Ther. 2017, 39, 190–202.e196. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Bassetti, M.; François, B.; Karam, G.; Chastre, J.; Torres, A.; Roberts, J.A.; Taccone, F.S.; Rello, J.; Calandra, T.; et al. Advances in antibiotic therapy in the critically ill. Crit. Care 2016, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Force, I.S.T. Infectious Diseases Society of America (IDSA) POSITION STATEMENT: Why IDSA Did Not Endorse the Surviving Sepsis Campaign Guidelines. Clin. Infect. Dis. 2017, 66, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J.; Coopersmith, C.M.; Whittle, J.; Hale, C.P.; Fishman, N.O.; Kim, T.J. Sepsis National Hospital Inpatient Quality Measure (SEP-1): Multistakeholder Work Group Recommendations for Appropriate Antibiotics for the Treatment of Sepsis. Clin. Infect. Dis. 2017, 65, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, P.Y.; Boussekey, N.; Devos, P.; Alfandari, S.; Turbelin, C.; Chiche, A.; Meybeck, A.; Georges, H.; Leroy, O. Impact of combination therapy with aminoglycosides on the outcome of ICU-acquired bacteraemias. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2293–2299. [Google Scholar] [CrossRef]
- Micek, S.T.; Welch, E.C.; Khan, J.; Pervez, M.; Doherty, J.A.; Reichley, R.M.; Kollef, M.H. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: A retrospective analysis. Antimicrob. Agents Chemother. 2010, 54, 1742–1748. [Google Scholar] [CrossRef]
- Kumar, A.; Safdar, N.; Kethireddy, S.; Chateau, D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: A meta-analytic/meta-regression study. Crit. Care Med. 2010, 38, 1651–1664. [Google Scholar] [CrossRef]
- Ong, D.S.Y.; Frencken, J.F.; Klein Klouwenberg, P.M.C.; Juffermans, N.; van der Poll, T.; Bonten, M.J.M.; Cremer, O.L. Short-Course Adjunctive Gentamicin as Empirical Therapy in Patients With Severe Sepsis and Septic Shock: A Prospective Observational Cohort Study. Clin. Infect. Dis. 2017, 64, 1731–1736. [Google Scholar] [CrossRef]
- Klompas, M. Monotherapy Is Adequate for Septic Shock Due to Gram-Negative Organisms. Crit. Care Med. 2017, 45, 1930–1932. [Google Scholar] [CrossRef]
- Coopersmith, C.M.; De Backer, D.; Deutschman, C.S.; Ferrer, R.; Lat, I.; Machado, F.R.; Martin, G.S.; Martin-Loeches, I.; Nunnally, M.E.; Antonelli, M.; et al. Surviving Sepsis Campaign: Research Priorities for Sepsis and Septic Shock. Crit. Care Med. 2018, 46, 1334–1356. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.K.; McGuire, W.C.; Malhotra, A. Prone Positioning in Acute Respiratory Distress Syndrome. NEJM Evid. 2022, 1, EVIDra2100046. [Google Scholar] [CrossRef]
- Spadaro, S. Multidrug Resistance in Critically Ill Patients: An Unresolved Issue. Microorganisms 2023, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Dünser, M.W.; Festic, E.; Dondorp, A.; Kissoon, N.; Ganbat, T.; Kwizera, A.; Haniffa, R.; Baker, T.; Schultz, M.J. Recommendations for sepsis management in resource-limited settings. Intensive Care Med. 2012, 38, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Branson, R.; Eichenwald, E.C.; Greene, L.R.; Howell, M.D.; Lee, G.; Magill, S.S.; Maragakis, L.L.; Priebe, G.P.; Speck, K.; et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014, 35, 915–936. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Berselli, A.; Fogagnolo, A.; Capuzzo, M.; Ragazzi, R.; Marangoni, E.; Bertacchini, S.; Volta, C.A. Evaluation of a protocol for vancomycin administration in critically patients with and without kidney dysfunction. BMC Anesthesiol. 2015, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Schinas, G.; Polyzou, E.; Spernovasilis, N.; Gogos, C.; Dimopoulos, G.; Akinosoglou, K. Preventing Multidrug-Resistant Bacterial Transmission in the Intensive Care Unit with a Comprehensive Approach: A Policymaking Manual. Antibiotics 2023, 12, 1255. [Google Scholar] [CrossRef]
- Norman, B.C.; Cooke, C.R.; Ely, E.W.; Graves, J.A. Sepsis-Associated 30-Day Risk-Standardized Readmissions: Analysis of a Nationwide Medicare Sample. Crit. Care Med. 2017, 45, 1130–1137. [Google Scholar] [CrossRef]
- Liu, V.; Lei, X.; Prescott, H.C.; Kipnis, P.; Iwashyna, T.J.; Escobar, G.J. Hospital readmission and healthcare utilization following sepsis in community settings. J. Hosp. Med. 2014, 9, 502–507. [Google Scholar] [CrossRef]
- DeMerle, K.M.; Royer, S.C.; Mikkelsen, M.E.; Prescott, H.C. Readmissions for Recurrent Sepsis: New or Relapsed Infection? Crit. Care Med. 2017, 45, 1702–1708. [Google Scholar] [CrossRef]
- Goodwin, A.J.; Rice, D.A.; Simpson, K.N.; Ford, D.W. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit. Care Med. 2015, 43, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C. Variation in Postsepsis Readmission Patterns: A Cohort Study of Veterans Affairs Beneficiaries. Ann. Am. Thorac. Soc. 2017, 14, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ortego, A.; Gaieski, D.F.; Fuchs, B.D.; Jones, T.; Halpern, S.D.; Small, D.S.; Sante, S.C.; Drumheller, B.; Christie, J.D.; Mikkelsen, M.E. Hospital-based acute care use in survivors of septic shock. Crit. Care Med. 2015, 43, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Netzer, G.; Small, D.S.; Hanish, A.; Fuchs, B.D.; Gaieski, D.F.; Mikkelsen, M.E. Association Between Index Hospitalization and Hospital Readmission in Sepsis Survivors. Crit. Care Med. 2016, 44, 478–487. [Google Scholar] [CrossRef]
- Arshad, A.; Ayaz, A.; Haroon, M.A.; Jamil, B.; Hussain, E. Frequency and Cause of Readmissions in Sepsis Patients Presenting to a Tertiary Care Hospital in a Low Middle Income Country. Crit. Care Explor. 2020, 2, e0080. [Google Scholar] [CrossRef] [PubMed]
- Liaskou, M.; Duggan, C.; Joynes, R.; Rosado, H. Pharmacy’s role in antimicrobial resistance and stewardship. Pharm. J. 2018, 10. [Google Scholar] [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef]
- Bondarenka, C.M.; Bosso, J.A. Successful Implementation of an Antimicrobial Stewardship Program at an Academic Medical Center. Hosp. Pharm. 2017, 52, 508–513. [Google Scholar] [CrossRef]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M.; et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull. World Health Organ. 2023, 101, 290–296. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef] [PubMed]
- Ostrowsky, B.; Banerjee, R.; Bonomo, R.A.; Cosgrove, S.E.; Davidson, L.; Doron, S.; Gilbert, D.N.; Jezek, A.; Lynch, J.B., 3rd; Septimus, E.J.; et al. Infectious Diseases Physicians: Leading the Way in Antimicrobial Stewardship. Clin. Infect. Dis. 2018, 66, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.K.; Pollack, L.A. The role of public health in antimicrobial stewardship in healthcare. Clin. Infect. Dis. 2014, 59 (Suppl. S3), S101–S103. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H.; Page, S.W. Antimicrobial Stewardship in Veterinary Medicine. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Davidson, L.E. Antimicrobial stewardship. Mayo Clin. Proc. 2011, 86, 1113–1123. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Bertollo, L.G.; Lutkemeyer, D.S.; Levin, A.S. Are antimicrobial stewardship programs effective strategies for preventing antibiotic resistance? A systematic review. Am. J. Infect. Control 2018, 46, 824–836. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, J.F.; Bardán-García, B.; Peña-Rodríguez, M.F.; Álvarez-Díaz, H.; Mariño-Callejo, A. Meropenem antimicrobial stewardship program: Clinical, economic, and antibiotic resistance impact. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zequinao, T.; Gasparetto, J.; Oliveira, D.D.S.; Silva, G.T.; Telles, J.P.; Tuon, F.F. A broad-spectrum beta-lactam-sparing stewardship program in a middle-income country public hospital: Antibiotic use and expenditure outcomes and antimicrobial susceptibility profiles. Braz. J. Infect. Dis. 2020, 24, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.; Htoutou Sedlakova, M.; Urbanek, K.; Mlynarcik, P.; Roderova, M.; Hricova, K.; Mezerova, K.; Kucova, P.; Zapletalova, J.; Fiserova, K.; et al. Implementation of Antibiotic Stewardship in a University Hospital Setting. Antibiotics 2021, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Strich, J.R.; Palmore, T.N. Preventing Transmission of Multidrug-Resistant Pathogens in the Intensive Care Unit. Infect. Dis. Clin. N. Am. 2017, 31, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.R.; Callery, S.; Vearncombe, M.; Simor, A.E. The role of colonization pressure in nosocomial transmission of methicillin-resistant Staphylococcus aureus. Am. J. Infect. Control 2009, 37, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Masse, J.; Elkalioubie, A.; Blazejewski, C.; Ledoux, G.; Wallet, F.; Poissy, J.; Preau, S.; Nseir, S. Colonization pressure as a risk factor of ICU-acquired multidrug resistant bacteria: A prospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 797–805. [Google Scholar] [CrossRef]
- Shams, A.M.; Rose, L.J.; Edwards, J.R.; Cali, S.; Harris, A.D.; Jacob, J.T.; LaFae, A.; Pineles, L.L.; Thom, K.A.; McDonald, L.C.; et al. Assessment of the Overall and Multidrug-Resistant Organism Bioburden on Environmental Surfaces in Healthcare Facilities. Infect. Control Hosp. Epidemiol. 2016, 37, 1426–1432. [Google Scholar] [CrossRef]
- Lyons, A.; Rose, L.; Noble-Wang, J.A. Survival of Healthcare Pathogens on Hospital Surfaces; SHEA: St. Louis, MO, USA, 2017. [Google Scholar]
- Ohl, M.; Schweizer, M.; Graham, M.; Heilmann, K.; Boyken, L.; Diekema, D. Hospital privacy curtains are frequently and rapidly contaminated with potentially pathogenic bacteria. Am. J. Infect. Control 2012, 40, 904–906. [Google Scholar] [CrossRef]
- Snitkin, E.S.; Zelazny, A.M.; Thomas, P.J.; Stock, F.; Henderson, D.K.; Palmore, T.N.; Segre, J.A. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 2012, 4, 148ra116. [Google Scholar] [CrossRef]
- Otter, J.A.; Mepham, S.; Athan, B.; Mack, D.; Smith, R.; Jacobs, M.; Hopkins, S. Terminal decontamination of the Royal Free London’s high-level isolation unit after a case of Ebola virus disease using hydrogen peroxide vapor. Am. J. Infect. Control 2016, 44, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Kotay, S.; Chai, W.; Guilford, W.; Barry, K.; Mathers, A.J. Spread from the Sink to the Patient: In Situ Study Using Green Fluorescent Protein (GFP)-Expressing Escherichia coli To Model Bacterial Dispersion from Hand-Washing Sink-Trap Reservoirs. Appl. Environ. Microbiol. 2017, 83, e03327-16. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.M.; Armbruster, C.R.; Arduino, M.J. Plumbing of hospital premises is a reservoir for opportunistically pathogenic microorganisms: A review. Biofouling 2013, 29, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, F.; Payne, D.J. Reaction: Alternative Modalities to Address Antibiotic-Resistant Pathogens. Chem 2017, 3, 369–372. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2019; Control and Prevention Centers for Disease and Division of Healthcare Quality Promotion; Antibiotic Resistance and Coordination Strategy Unit National Center for Emerging Zoonotic and Infectious Diseases: Atlanta, GA, USA, 2019.
- The White House. National Action Plan for Combating Antibiotic-Resistant Bacteria; The White House: Washington, DC, USA, 2015.
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Bosserman, R.E.; Kwon, J.H. Know your Microbe Foes: The Role of Surveillance in Combatting Antimicrobial Resistance. Yale J. Biol. Med. 2022, 95, 517–523. [Google Scholar] [PubMed]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs. US Department of Health and Human Services, CDC. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 14 August 2023).
- Turcotte, J.; Sanford, Z.; Broda, A.; Patton, C. Centers for Medicare & Medicaid Services Hierarchical Condition Category score as a predictor of readmission and reoperation following elective inpatient spine surgery. J. Neurosurg. Spine 2019, 31, 600–606. [Google Scholar] [CrossRef]
- Pulcini, C. Antibiotic stewardship: A European perspective. FEMS Microbiol. Lett. 2017, 364, fnx230. [Google Scholar] [CrossRef]
- Carter, R.R.; Sun, J.; Jump, R.L. A Survey and Analysis of the American Public’s Perceptions and Knowledge About Antibiotic Resistance. Open Forum Infect. Dis. 2016, 3, ofw112. [Google Scholar] [CrossRef]
- Hayes, J.F. Fighting Back against Antimicrobial Resistance with Comprehensive Policy and Education: A Narrative Review. Antibiotics 2022, 11, 644. [Google Scholar] [CrossRef]
- WHO. Sepsis, Infection Prevention and Control. Available online: https://www.who.int/teams/integrated-health-services/infection-prevention-control/sepsis (accessed on 14 August 2023).
- Stehr, S.N.; Reinhart, K. Sepsis as a Global Health Problem—Why We Need a Global Sepsis Alliance. Shock 2013, 39, 3–4. [Google Scholar] [CrossRef]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care 2018, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Kissoon, N. Challenges and Solutions in translating sepsis guidelines into practice in resource-limited settings. Transl. Pediatr. 2021, 10, 2646–2665. [Google Scholar] [CrossRef]
- The Lancet Respiratory, M. The future of critical care: Lessons from the COVID-19 crisis. Lancet Respir. Med. 2020, 8, 527. [Google Scholar] [CrossRef]
- Tiskumara, R.; Fakharee, S.H.; Liu, C.Q.; Nuntnarumit, P.; Lui, K.M.; Hammoud, M.; Lee, J.K.; Chow, C.B.; Shenoi, A.; Halliday, R.; et al. Neonatal infections in Asia. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F144–F148. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.H.; Kim, S.D.; Kim, H.S.; Kim, E.S.; Lee, H.J.; Lee, J.A.; Choi, C.W.; Kim, E.K.; Choi, E.H.; Kim, B.I.; et al. Trends in epidemiology of neonatal sepsis in a tertiary center in Korea: A 26-year longitudinal analysis, 1980–2005. J. Korean Med. Sci. 2011, 26, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Mintz, A.; Mor, M.; Klinger, G.; Scheuerman, O.; Pirogovsky, A.; Sokolover, N.; Bromiker, R. Changing epidemiology and resistance patterns of pathogens causing neonatal bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Investigators of the Delhi Neonatal Infection Study (DeNIS) Collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: A cohort study. Lancet Glob. Health 2016, 4, e752–e760. [Google Scholar] [CrossRef] [PubMed]
- Kalın, G.; Alp, E.; Chouaikhi, A.; Roger, C. Antimicrobial Multidrug Resistance: Clinical Implications for Infection Management in Critically Ill Patients. Microorganisms 2023, 11, 2575. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Burnham, J.P.; Lane, M.A.; Kollef, M.H. Impact of Sepsis Classification and Multidrug-Resistance Status on Outcome Among Patients Treated With Appropriate Therapy. Crit. Care Med. 2015, 43, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.P.; Chen, Y.C.; Chen, S.Y.; Chen, S.Y.; Chang, S.C. Risk for subsequent infection and mortality after hospitalization among patients with multidrug-resistant gram-negative bacteria colonization or infection. Antimicrob. Resist. Infect. Control 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-G. Whole-genome sequencing in the prediction of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2016, 14, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Forging a Link Between Biofilms and Disease. Science 1999, 283, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Gaind, R.; Chellani, H.; Agarwal, P. Extended-spectrum beta lactamase-producing gram-negative bacteria: Clinical profile and outcome in a neonatal intensive care unit. Ann. Trop. Paediatr. 2007, 27, 45–54. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Xue, M.; Yang, C.; Huang, Y.; Chihade, D.B.; Liu, L.; Yang, Y.; Qiu, H. Early- and Late-Onset Bloodstream Infections in the Intensive Care Unit: A Retrospective 5-Year Study of Patients at a University Hospital in China. J. Infect. Dis. 2020, 221, S184–S192. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: Oxford, UK, 2023. [Google Scholar]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Cohen, J. Non-antibiotic strategies for sepsis. Clin. Microbiol. Infect. 2009, 15, 302–307. [Google Scholar] [CrossRef]
- Polat, G.; Ugan, R.A.; Cadirci, E.; Halici, Z. Sepsis and Septic Shock: Current Treatment Strategies and New Approaches. Eurasian J. Med. 2017, 49, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.; Guido, B.; Carlotta, R.; Fiorenza, F.; Michele, G.; Marco, P.; Giuseppe, R.; GiVi, T.I.G.I.p.l.V.d.I.i.T.I.i.a.i.c. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: A multicenter randomised controlled clinical trial. BMJ Open 2014, 4, e003536. [Google Scholar] [CrossRef]
- Górski, A.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Borysowski, J. The Potential of Phage Therapy in Sepsis. Front. Immunol. 2017, 8, 1783. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; O’Dea, K.; Gordon, A. Immune therapy in sepsis: Are we ready to try again? J. Intensive Care Soc. 2018, 19, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Delsing, C.E.; Gresnigt, M.S.; Leentjens, J.; Preijers, F.; Frager, F.A.; Kox, M.; Monneret, G.; Venet, F.; Bleeker-Rovers, C.P.; van de Veerdonk, F.L.; et al. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: A case series. BMC Infect. Dis. 2014, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Flohé, S.; Lendemans, S.; Selbach, C.; Waydhas, C.; Ackermann, M.; Schade, F.U.; Kreuzfelder, E. Effect of granulocyte-macrophage colony-stimulating factor on the immune response of circulating monocytes after severe trauma. Crit. Care Med. 2003, 31, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Meisel, C.; Schefold, J.C.; Pschowski, R.; Baumann, T.; Hetzger, K.; Gregor, J.; Weber-Carstens, S.; Hasper, D.; Keh, D.; Zuckermann, H.; et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: A double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 2009, 180, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef]
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S. Therapeutic antibodies for infectious diseases. Bull. World Health Organ. 2017, 95, 235–237. [Google Scholar] [CrossRef]
- Peters van Ton, A.M.; Kox, M.; Abdo, W.F.; Pickkers, P. Precision Immunotherapy for Sepsis. Front. Immunol. 2018, 9, 1926. [Google Scholar] [CrossRef]
| Gram-Positive Bacteria | ||
|---|---|---|
| Bacterial Species | Mechanisms of Multidrug Resistance | Association with Sepsis |
| Staphylococcus aureus (including MRSA) | Altered penicillin-binding proteins (PBP2a) | Increased severity of infections, including skin and soft tissue infections, pneumonia, and bloodstream infections. |
| Efflux pumps | MRSA is commonly associated with healthcare-associated infections. | |
| Biofilm formation | Virulence factors contribute to pathogenicity. | |
| Enterococcus faecium/faecalis (including VRE) | Altered target site (D-Ala-D-Ala to D-Ala-D-Lac) | Frequent in healthcare-associated infections, especially in immunocompromised patients. |
| Biofilm formation | High resistance to vancomycin, a crucial antibiotic. | |
| Gram-Negative Bacteria | ||
| Escherichia coli (Including ESBL-producing) | Production of extended-spectrum beta-lactamases | High resistance to beta-lactam antibiotics, leading to challenging treatment |
| Plasmid-mediated resistance | Common in urinary tract, respiratory, and bloodstream infections. | |
| Porin mutations | Associated with nosocomial infections, which can progress to sepsis. | |
| Klebsiella pneumoniae (Including CRE strains) | Production of carbapenemases | Limited treatment options due to resistance to broad-spectrum antibiotics. |
| Plasmid-mediated resistance | High mortality rates associated with bloodstream infections. | |
| Reduced permeability of the outer membrane | Commonly found in healthcare settings. | |
| Acinetobacter baumannii | Efflux pumps | Common cause of healthcare-associated infections, especially in ICUs. |
| Biofilm formation | Associated with high mortality rates in bloodstream infections | |
| Intrinsic resistance mechanisms | Often involved in ventilator-associated pneumonia and septicemia. | |
| Pseudomonas aeruginosa | Efflux pumps | Commonly implicated in hospital-acquired infections, including sepsis. |
| Biofilm formation | Infections associated with a higher risk of treatment failure. | |
| Sl No | Aspect | Conventional Methods | Molecular Methods |
|---|---|---|---|
| 1 | Sample Type | Limited range of sample types | More adaptable to various sample types |
| 2 | Identification Speed | Relatively slow, it may take days to provide results. | Rapid results, often within hours. |
| 3 | Sensitivity and Specificity | It may have lower sensitivity and specificity. | Generally, higher sensitivity and specificity |
| 4 | Range of Pathogens Detected | Limited to certain pathogens (Genera of the Pathogen) | Broad range, capable of detecting various pathogens (exact Species of the Pathogen) |
| 5 | Type of Information | Phenotypic information (e.g., growth inhibition). | Genotypic information (specific genes or mutations). |
| 6 | Multiplexing Capability | Limited ability to test for multiple resistance genes | High multiplexing capability, detecting multiple targets in a single test |
| 7 | Equipment Required | Often requires specialized equipment and expertise | Requires specific equipment but can be more accessible |
| 8 | Ease of Use | It may require trained personnel and specialized equipment. | User-friendly protocols, less technical expertise needed |
| 9 | Accuracy | Subject to human handling error | Less prone to human error, higher accuracy |
| 10 | Resistance Detection Method | Culture-based methods, susceptibility testing | DNA sequencing, PCR, genotypic assays |
| 11 | Cost | Lower initial cost in some cases | Higher initial cost, but potentially cost-effective over time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.R.; Balraj, T.A.; Kempegowda, S.N.; Prashant, A. Multidrug-Resistant Sepsis: A Critical Healthcare Challenge. Antibiotics 2024, 13, 46. https://doi.org/10.3390/antibiotics13010046
Kumar NR, Balraj TA, Kempegowda SN, Prashant A. Multidrug-Resistant Sepsis: A Critical Healthcare Challenge. Antibiotics. 2024; 13(1):46. https://doi.org/10.3390/antibiotics13010046
Chicago/Turabian StyleKumar, Nishitha R., Tejashree A. Balraj, Swetha N. Kempegowda, and Akila Prashant. 2024. "Multidrug-Resistant Sepsis: A Critical Healthcare Challenge" Antibiotics 13, no. 1: 46. https://doi.org/10.3390/antibiotics13010046
APA StyleKumar, N. R., Balraj, T. A., Kempegowda, S. N., & Prashant, A. (2024). Multidrug-Resistant Sepsis: A Critical Healthcare Challenge. Antibiotics, 13(1), 46. https://doi.org/10.3390/antibiotics13010046





