Anti-Salmonella Activity of a Novel Peptide, KGGDLGLFEPTL, Derived from Egg Yolk Hydrolysate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antimicrobial Activity
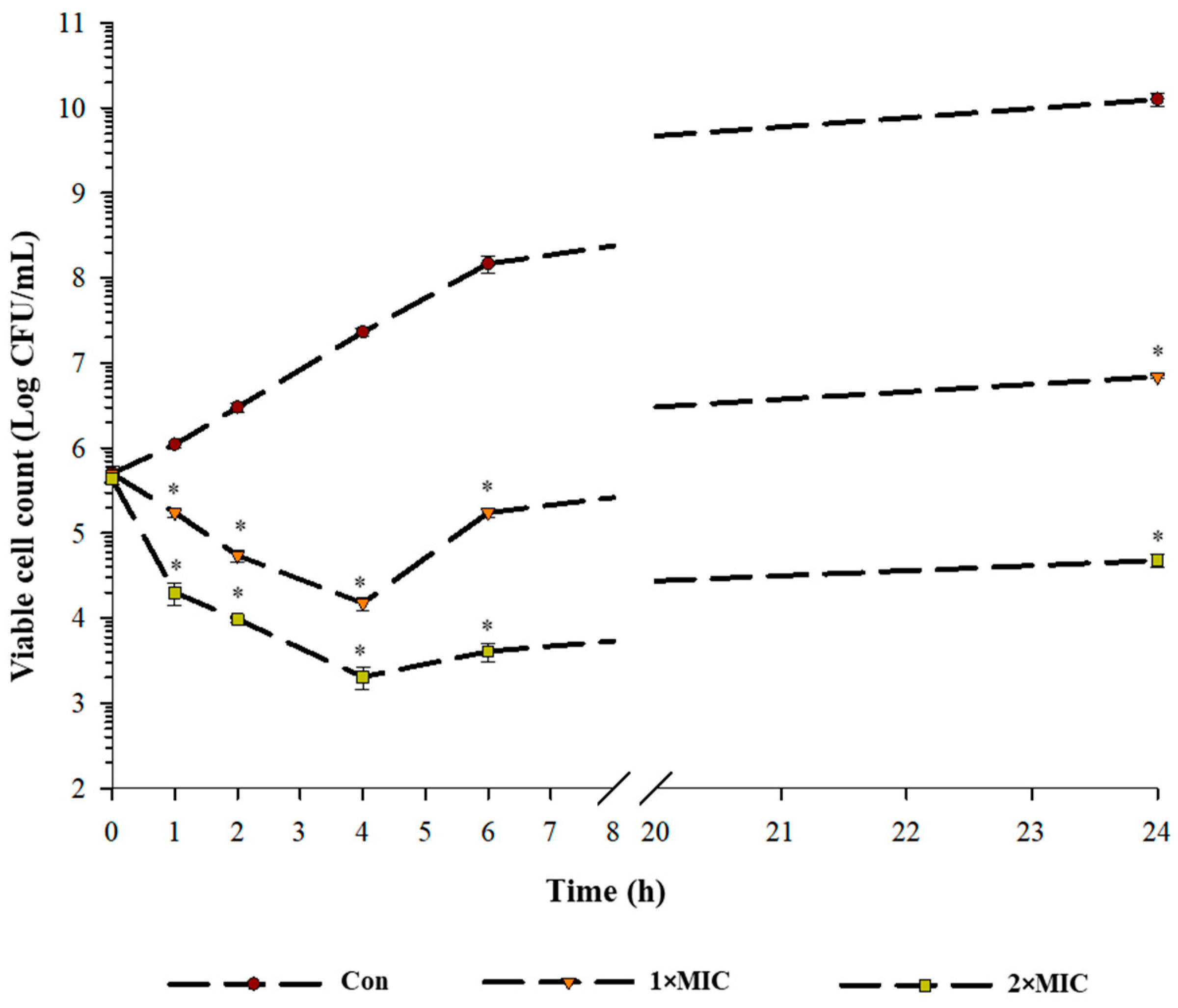
2.2. Time Kill Kinetics
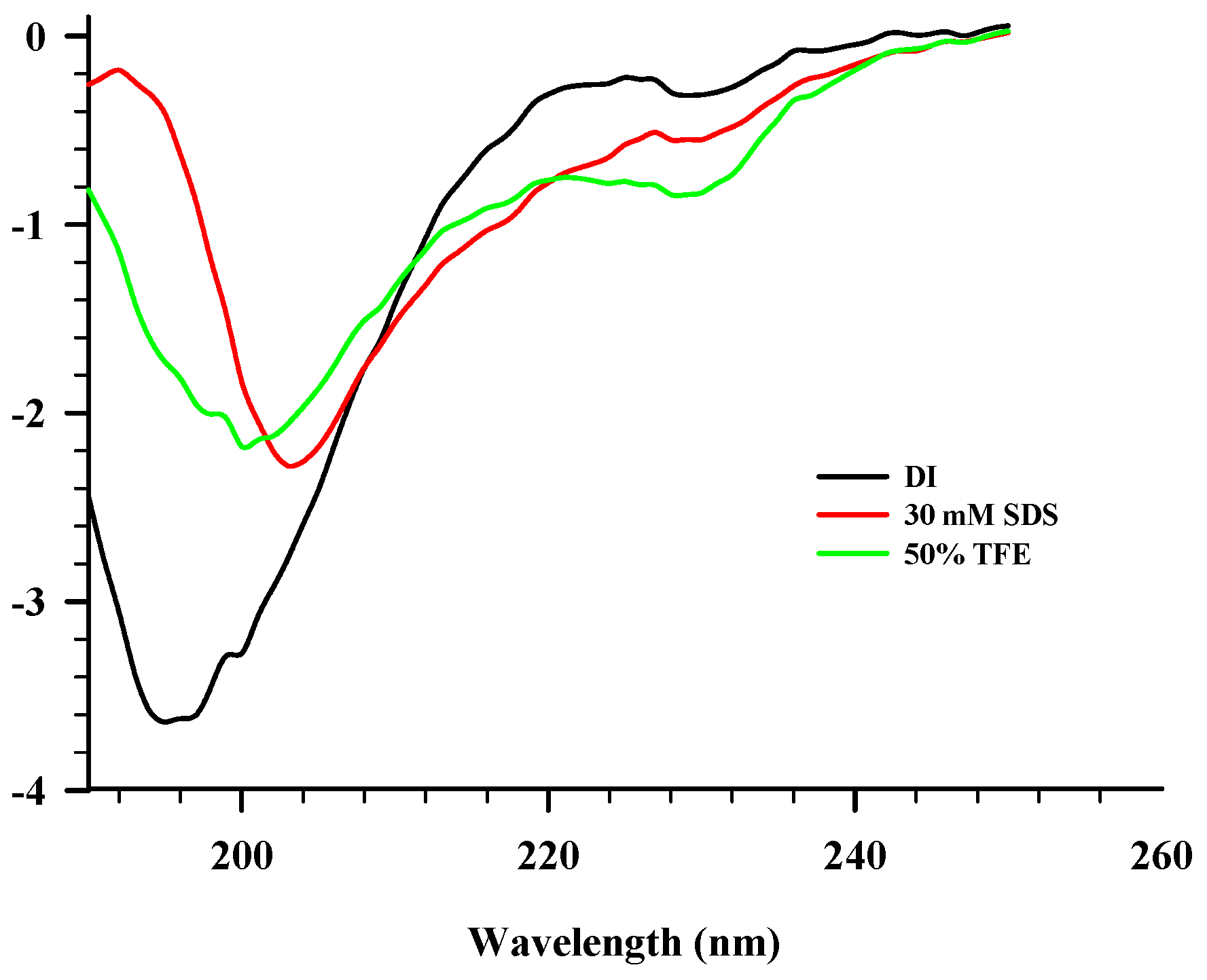
2.3. CD Spectroscopy
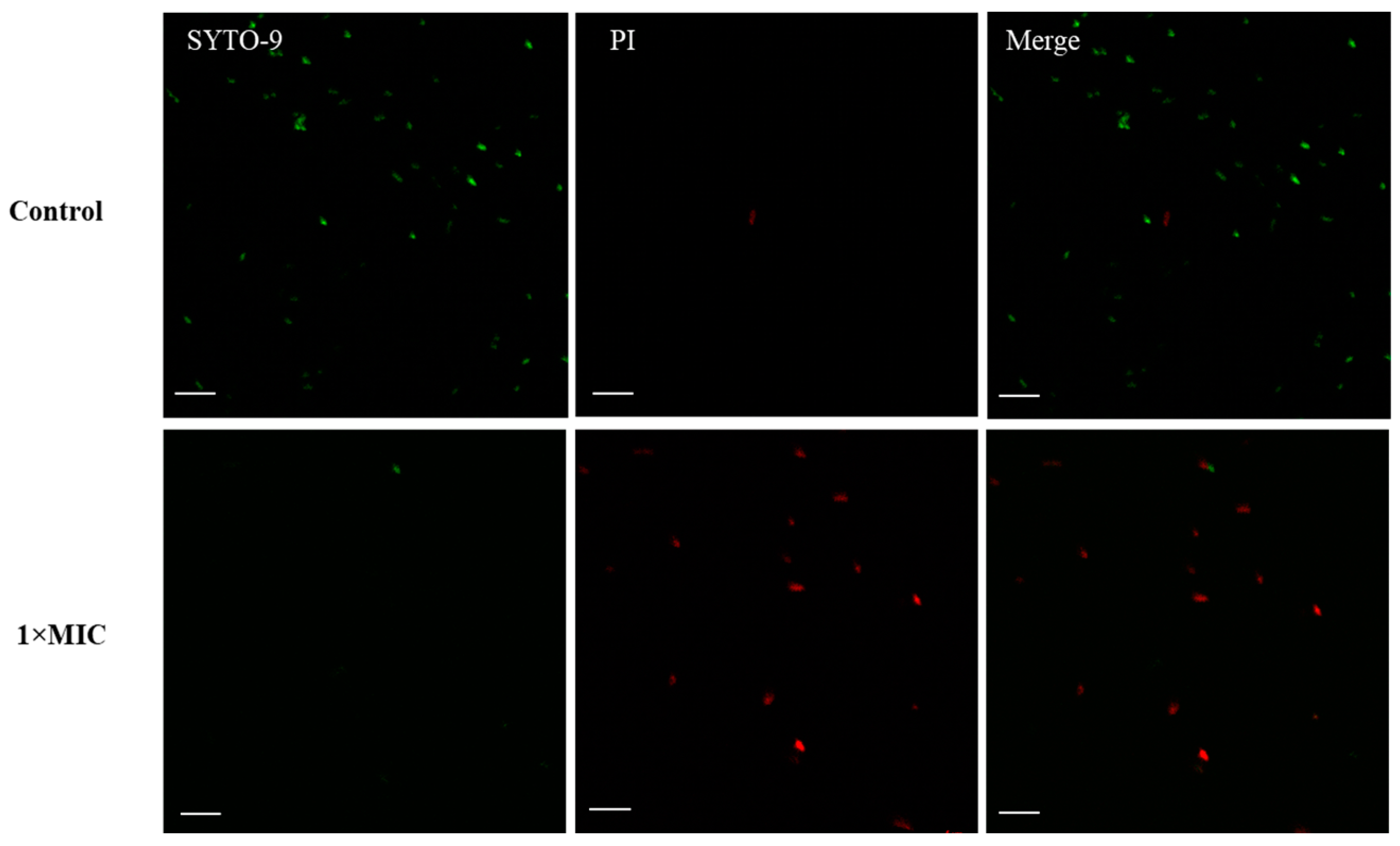
2.4. Membrane Disruption
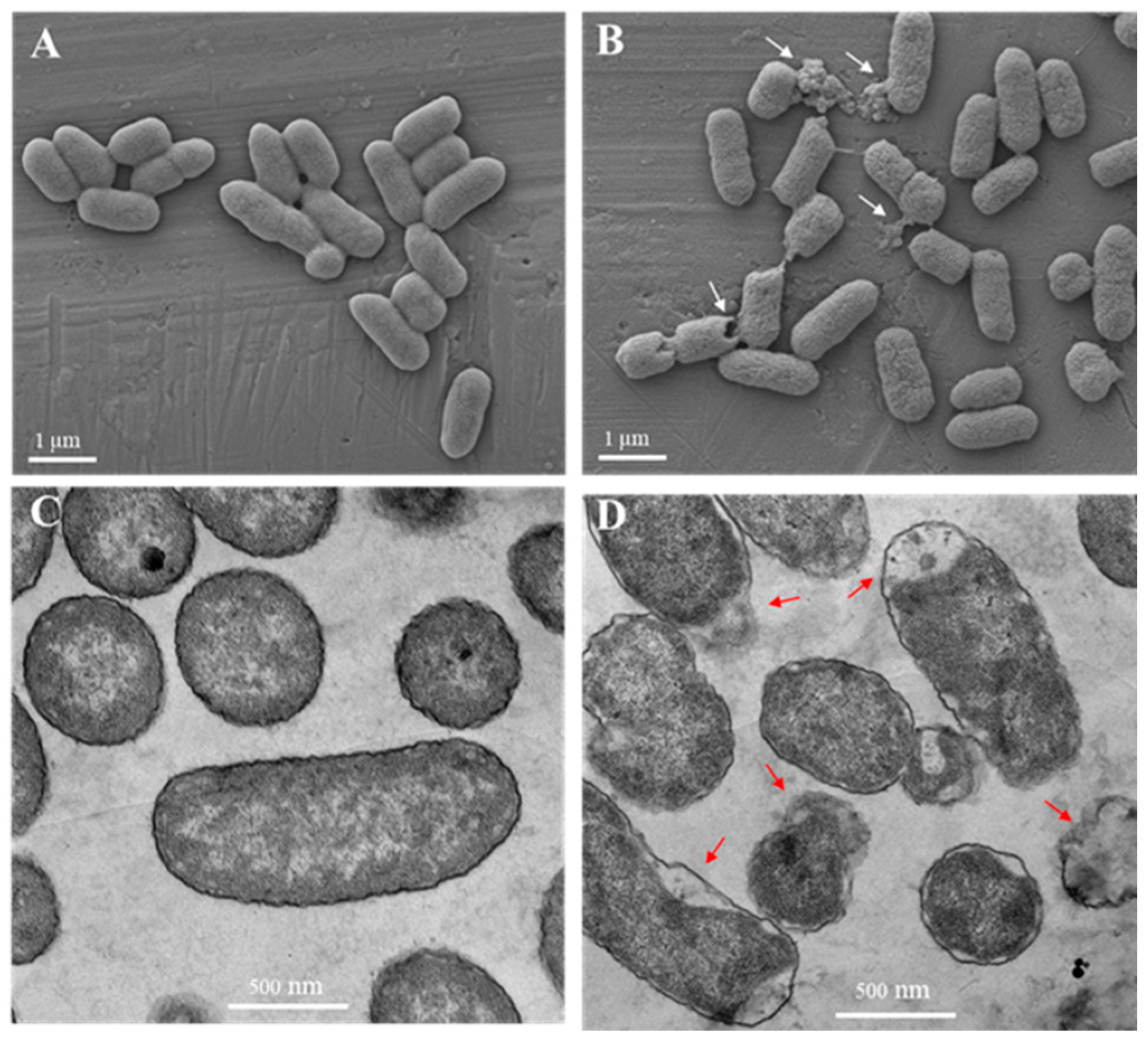
2.5. Electron Microscopy
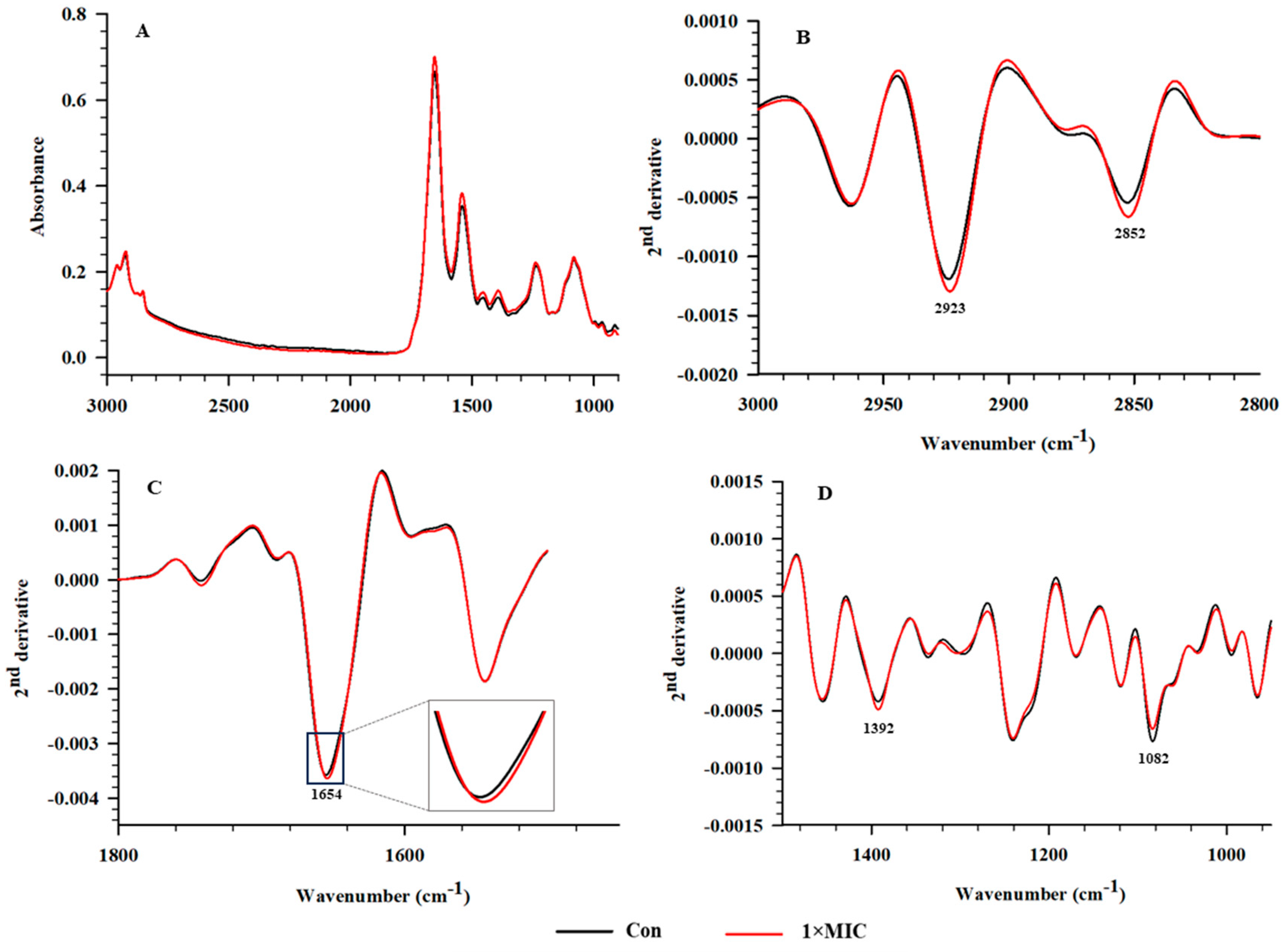
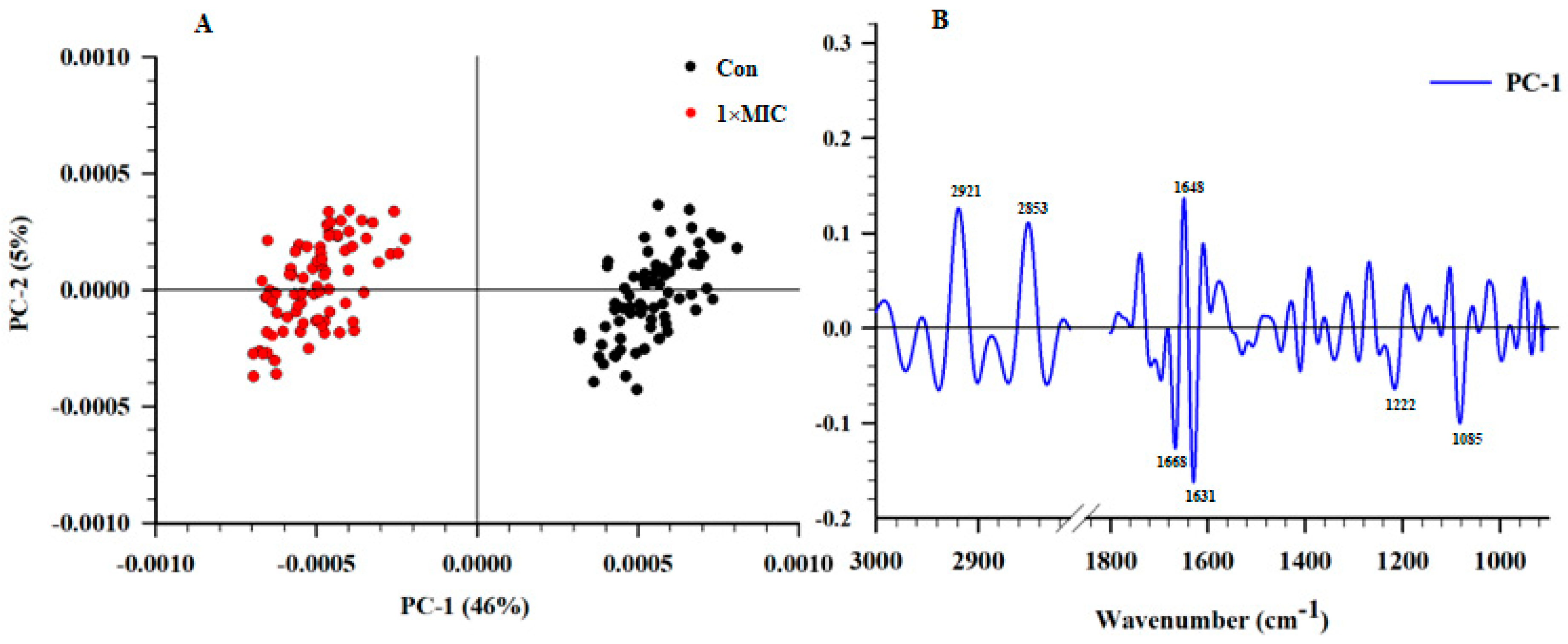
2.6. SR–FTIR Microspectroscopy
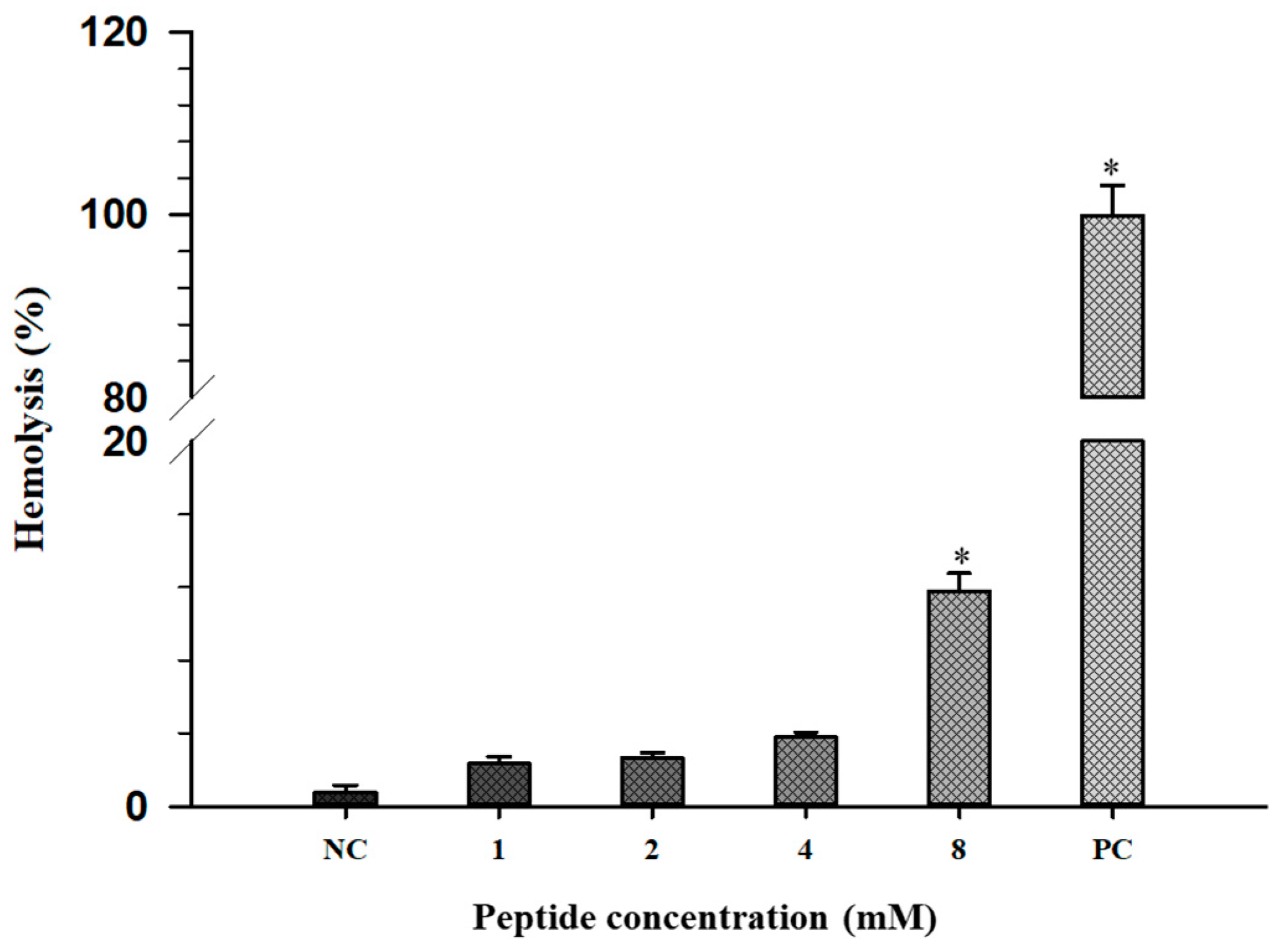
2.7. Hemolytic Activity
3. Materials and Methods
3.1. Peptide Synthesis
3.2. Antimicrobial Activity Assay
3.3. Time Killing Assay
3.4. Circular Dichroism (CD) Analysis
3.5. Leakage of Nucleic Acids
3.6. Electron Microscopy
3.7. Confocal Laser Scanning Microscopy (CLSM)
3.8. Synchrotron–Fourier–Transform Infrared Spectroscopy (SR–FTIR)
3.9. Hemolysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Food Safety. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 14 July 2023).
- Todd, E. Foodborne diseases: Overview of biological hazards and foodborne diseases. Encycl. Food Saf. 2014, 1, 221–242. [Google Scholar]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.; Conte-Junior, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Wessels, K.; Rip, D.; Gouws, P. Salmonella in chicken meat: Consumption, outbreaks, characteristics, current control methods and the potential of bacteriophage use. Foods 2021, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. Antimicrobial peptides stage a comeback: Better understanding of the mechanisms of action, modification and synthesis of antimicrobial peptides is reigniting commercial development. Nat. Biotechnol. 2013, 31, 379–383. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bam, M.; Luat, E.; Jui, M.S.; Ganewatta, M.S.; Shokfai, T.; Nagarkatti, M.; Decho, A.W.; Tang, C. Macromolecular-clustered facial amphiphilic antimicrobials. Nat. Commun. 2018, 9, 5231. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Clinical application of AMPs. Adv. Exp. Med. Biol. 2019, 1117, 281–298. [Google Scholar]
- Luo, X.; Song, Y.; Cao, Z.; Qin, Z.; Dessie, W.; He, N.; Wang, Z.; Tan, Y. Evaluation of the antimicrobial activities and mechanisms of synthetic antimicrobial peptide against food-borne pathogens. Food Biosci. 2022, 49, 101903. [Google Scholar] [CrossRef]
- Ning, Y.; Han, P.; Ma, J.; Liu, Y.; Fu, Y.; Wang, Z.; Jia, Y. Characterization of brevilaterins, multiple antimicrobial peptides simultaneously produced by Brevibacillus laterosporus S62-9, and their application in real food system. Food Biosci. 2021, 42, 101091. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.K.; Baranwal, D.; Patel, A.R.; Shah, N.; Utama, G.L.; Niamah, A.K. Bacteriocins as antimicrobial and preservative agents in food: Biosynthesis, separation and application. Food Biosci. 2022, 46, 101594. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Acharya, A.; Hegde, V.; Prakash, B. Rational design of hyperstable antibacterial peptides for food preservation. NPJ Sci. Food 2021, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, J.; Aweya, J.J.; Yuan, Z.; Weng, W.; Zhang, Y.; Liu, G.-M. Antimicrobial mechanism of Larimichthys crocea whey acidic protein-derived peptide (LCWAP) against Staphylococcus aureus and its application in milk. Int. J. Food Microbiol. 2020, 335, 108891. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Reig, M.; Toldrá, F. Bioactive peptides generated from meat industry by-products. Food Res. Int. 2014, 65, 344–349. [Google Scholar] [CrossRef]
- Bersi, G.; Barberis, S.E.; Origone, A.L.; Adaro, M.O. Bioactive peptides as functional food ingredients. In Role of Materials Science in Food Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–186. [Google Scholar]
- Rivero-Pino, F.; Leon, M.J.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Antimicrobial plant-derived peptides obtained by enzymatic hydrolysis and fermentation as components to improve current food systems. Trends Food Sci. Technol. 2023, 135, 32–42. [Google Scholar] [CrossRef]
- Arulrajah, B.; Muhialdin, B.J.; Zarei, M.; Hasan, H.; Saari, N. Lacto-fermented Kenaf (Hibiscus cannabinus L.) seed protein as a source of bioactive peptides and their applications as natural preservatives. Food Control 2020, 110, 106969. [Google Scholar] [CrossRef]
- Bi, J.; Tian, C.; Jiang, J.; Zhang, G.-L.; Hao, H.; Hou, H.-M. Antibacterial activity and potential application in food packaging of peptides derived from turbot viscera hydrolysate. J. Agric. Food Chem. 2020, 68, 9968–9977. [Google Scholar] [CrossRef] [PubMed]
- Moscoso-Mujica, G.; Zavaleta, A.I.; Mujica, A.; Arnao, I.; Moscoso-Neira, C.; Santos, M.; Sánchez, J. Antimicrobial peptides purified from hydrolysates of kanihua (Chenopodium pallidicaule Aellen) seed protein fractions. Food Chem. 2021, 360, 129951. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Zambrowicz, A.; Pokora, M.; Setner, B.; Dąbrowska, A.; Szołtysik, M.; Szewczuk, Z.; Polanowski, A.; Trziszka, T.; Chrzanowska, J. Egg-yolk protein by-product as a source of ACE-inhibitory peptides obtained with using unconventional proteinase from Asian pumpkin (Cucurbita ficifolia). J. Proteom. 2014, 110, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Pimchan, T.; Tian, F.; Thumanu, K.; Rodtong, S.; Yongsawatdigul, J. Isolation, identification, and mode of action of antibacterial peptides derived from egg yolk hydrolysate. Poult. Sci. 2023, 102, 102695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qaiyumi, S.; Friedman, S.; Singh, R.; Foley, S.; White, D.; McDermott, P.; Donkar, T.; Bolin, C.; Munro, S. Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J. Clin. Microbiol. 2003, 41, 5366–5371. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Howe, J.; Morton, L.H.; Brandenburg, K.; Harris, F.; Phoenix, D.A. Interactions of an anionic antimicrobial peptide with Staphylococcus aureus membranes. Biochem. Biophys. Res. Commun. 2006, 347, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, L.; Huang, Z.; Zhao, Q.; Fan, J.; Tian, Y.; Huang, A. Isolation, identification and characterization of a novel antimicrobial peptide from Moringa oleifera seeds based on affinity adsorption. Food Chem. 2023, 398, 133923. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Wang, X.; Huang, A. Characterization of a novel antimicrobial peptide from buffalo casein hydrolysate based on live bacteria adsorption. J. Dairy Sci. 2020, 103, 11116–11128. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-L.; Shi, Y.-H.; Zhao, W.; Hao, G.; Le, G.-W. Insertion mode of a novel anionic antimicrobial peptide MDpep5 (Val-Glu-Ser-Trp-Val) from Chinese traditional edible larvae of housefly and its effect on surface potential of bacterial membrane. J. Pharm. Biomed. Anal. 2008, 48, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Almarwani, B.; Phambu, N.; Hamada, Y.Z.; Sunda-Meya, A. Interactions of an anionic antimicrobial peptide with Zinc (II): Application to bacterial mimetic membranes. Langmuir 2020, 36, 14554–14562. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; He, L.; Wang, X.; Ding, X.; Li, L.; Tian, Y.; Huang, A. Characterization of a novel antimicrobial peptide isolated from Moringa oleifera seed protein hydrolysates and its membrane damaging effects on Staphylococcus aureus. J. Agric. Food Chem. 2022, 70, 6123–6133. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Shi, Q.; Yang, P.; Wei, R. Identification of antibacterial peptides from Maillard reaction products of half-fin anchovy hydrolysates/glucose via LC-ESI-QTOF-MS analysis. J. Funct. Foods. 2017, 36, 387–395. [Google Scholar] [CrossRef]
- Torres, M.D.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Souza, P.F.; Marques, L.S.; Oliveira, J.T.; Lima, P.G.; Dias, L.P.; Neto, N.A.; Lopes, F.E.; Sousa, J.S.; Silva, A.F.; Caneiro, R.F. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 2020, 175, 132–145. [Google Scholar] [CrossRef]
- Baltutis, V.; O’Leary, P.D.; Martin, L.L. Self-assembly of linear, natural antimicrobial peptides: An evolutionary perspective. ChemPlusChem 2022, 87, e202200240. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Bjørnestad, V.A.; Lund, R. Resolving the structural interactions between antimicrobial peptides and lipid membranes using small-angle scattering methods: The case of indolicidin. Soft Matter 2018, 14, 8750–8763. [Google Scholar] [CrossRef]
- Zhou, L.; Lian, K.; Wang, M.; Jing, X.; Zhang, Y.; Cao, J. The antimicrobial effect of a novel peptide LL-1 on Escherichia coli by increasing membrane permeability. BMC Microbiol. 2022, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kong, X.; Hua, Y.; Chen, Y.; Zhang, C.; Chen, Y. Identification of antibacterial peptides generated from enzymatic hydrolysis of cottonseed proteins. LWT 2020, 125, 109199. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Z.; Cao, S.; Wang, H.; Jiang, C.; Hussain, M.A.; Pang, S. Broad-Spectrum antimicrobial activity and low cytotoxicity against human cells of a peptide derived from bovine αS1-casein. Molecules 2018, 23, 1220. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Rodtong, S.; Thumanu, K.; Hua, Y.; Roytrakul, S.; Yongsawatdigul, J. Molecular insights into the mode of action of antibacterial peptides derived from chicken plasma hydrolysates. Foods 2022, 11, 3564. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Anionic antimicrobial peptides from eukaryotic organisms. Curr. Protein Pept. Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Naumann, D. Infrared spectroscopy in microbiology. In Encyclopedia of Analytical Chemistry; Meyers, R., Ed.; Wiley: Chichester, UK, 2000; pp. 102–131. [Google Scholar]
- Xiao, J.; Zhang, H.; Niu, L.; Wang, X. Efficient screening of a novel antimicrobial peptide from Jatropha curcas by cell membrane affinity chromatography. J. Agric. Food Chem. 2011, 59, 1145–1151. [Google Scholar] [CrossRef]
- Che, Q.; Zhou, Y.; Yang, H.; Li, J.; Xu, X.; Lai, R. A novel antimicrobial peptide from amphibian skin secretions of Odorrana grahami. Peptides 2008, 29, 529–535. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, R.; Li, Q.; Li, B. Isolation and characterization of an antibacterial peptide from protein hydrolysates of Spirulina platensis. Eur. Food Res. Technol. 2016, 242, 685–692. [Google Scholar] [CrossRef]
- Nakajima, Y.; Ishibashi, J.; Yukuhiro, F.; Asaoka, A.; Taylor, D.; Yamakawa, M. Antibacterial activity and mechanism of action of tick defensin against Gram-positive bacteria. Biochim. Biophys. Acta Gen. Subj. 2003, 1624, 125–130. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, X.; Xu, L.; Wang, Y. Design of hybrid β-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 2013, 34, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhang, F.; Liao, X.; Hu, X.; Chen, Y.; Deng, L. Analysis of Escherichia coli cell damage induced by HPCD using microscopies and fluorescent staining. Int. J. Food Microbiol. 2010, 144, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.; Liu, L.-P.; Deber, C.M. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob. Agents Chemother. 2002, 46, 3585–3590. [Google Scholar] [CrossRef] [PubMed]

| Serotype | MIC (mM) |
|---|---|
| S. Typhimurium TISTR 292 | 2 |
| S. Typhimurium ATCC 14028 | 4 |
| S. Enteritidis DMST 15679 | 2 |
| S. Enteritidis ATCC 13076 | 4 |
| S. Newport ATCC 6962 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimchan, T.; Tian, F.; Thumanu, K.; Rodtong, S.; Yongsawatdigul, J. Anti-Salmonella Activity of a Novel Peptide, KGGDLGLFEPTL, Derived from Egg Yolk Hydrolysate. Antibiotics 2024, 13, 19. https://doi.org/10.3390/antibiotics13010019
Pimchan T, Tian F, Thumanu K, Rodtong S, Yongsawatdigul J. Anti-Salmonella Activity of a Novel Peptide, KGGDLGLFEPTL, Derived from Egg Yolk Hydrolysate. Antibiotics. 2024; 13(1):19. https://doi.org/10.3390/antibiotics13010019
Chicago/Turabian StylePimchan, Thippawan, Fu Tian, Kanjana Thumanu, Sureelak Rodtong, and Jirawat Yongsawatdigul. 2024. "Anti-Salmonella Activity of a Novel Peptide, KGGDLGLFEPTL, Derived from Egg Yolk Hydrolysate" Antibiotics 13, no. 1: 19. https://doi.org/10.3390/antibiotics13010019
APA StylePimchan, T., Tian, F., Thumanu, K., Rodtong, S., & Yongsawatdigul, J. (2024). Anti-Salmonella Activity of a Novel Peptide, KGGDLGLFEPTL, Derived from Egg Yolk Hydrolysate. Antibiotics, 13(1), 19. https://doi.org/10.3390/antibiotics13010019








