2.1. Extraction Yield
The extraction yield of essential oil (TaEO), crude methanolic (TaME) and hydro-methanolic (TaHME) extracts of
T. annuum aerial parts are presented in
Table 1. The obtained TaEO has a very dark blue color with a fruity and herbaceous aroma. Therein, the extraction yield of TaEO was on average 0.7% (wt/wt) of dry material, which was higher than the ones found previously with a percentage ranging between 0.23% and 0.5% [
6,
10,
11], while our yield was noted to be lower than the studies by Greche et al. [
12] and Zaim et al. [
13] with the values of 0.8% and 1.2%, respectively.
Regarding the crude extracts, six extracts were prepared using three parts, including the stems, leaves and flowers, and two solvents, which were methanol (100%) and hydro-methanol (80:20,
v/
v). Generally, the results showed that hydro-methanol was the better solvent to obtain a high extraction yield in the three parts; in addition, the extraction yields of TaME and TaHME were closely similar to each other in the cases of flowers (26.66% and 28.90%) and leaves (20.07% and 21.89%), while in case of stems, the extraction yield of TaME was significantly lower than TaHME with values of 8.87% and 16.89%, respectively. These results demonstrated that extraction yields were affected by the plant part and solvent polarity used (
Table 1). The polarity of solvents plays an important role in increasing extraction yield; whereas, the solvent with a higher polarity, such as water, has the ability of solubility and absorbance of bioactive compounds more than solvents with less polarity such as methanol, ethanol, etc. [
14,
15,
16]. These confirmed the present results that showed a higher extraction yield using a combination of methanol with water than with methanol alone.
2.2. Bioactive Compounds Contents
The present work was the first study to estimate the bioactive compounds including total phenolic content (TPC), total flavonoid content (TFC) and carotenoid contents (lycopene (TLC) and β-carotene (Tβ-CC)) of the medicinal plant
T. annuum. As presented in
Table 2, the three parts of
T. annuum contained important bioactive compounds, in particular, TPC and TFC in both crude extracts TaME and TaHME with values varying between 51.32 and 116.32 mg/g of dry crude extract (DCE). Similar to the extraction yield, the hydro-methanolic extracted higher bioactive compounds than the absolute methanol, with a significant difference between the values of the two solvent extracts in each part.
The TPC of
T. annuum was observed to be significantly higher in the leaves than in the flowers and stems. The highest amount was found in the leaves TaHME and the lowest was in stems TaME with values of 116.32 and 51.32 mg of gallic acid equivalents (GAEs)/g of DCE, respectively. The TPC value in leaves TaME in the present work was higher than the one in
Tanacetum erzincanense (64.4 mg GAEs/g DCE) previously reported by Yapıcı et al. [
17]; in addition, the TPCs of the present study were higher than several
Tanacetum species methanolic extracts, with their values varying from 32.15 to 47.11 mg GAEs/mg DCE [
18]. However, Devrnja et al. [
19] reported that methanolic extracts of
Tanacetum vulgare from Serbia contained higher TPC than our finding in the three parts stems, leaves and flowers with the values of 83.60, 112.60 and 96.20 mg GAEs/g of DCE, respectively.
Concerning TFC, the highest content was observed in the leaves TaHME with the value of 91.54 mg of catechin equivalents (CEs)/g of DCE, and the lowest content was in the leaves and stems TaME with the amounts 58.81 and 58.63 mg/g of DCE, respectively. Flowers contained significantly higher TFC value in the case of TaME (64.04 mg CEs/g DCE) and the leaves in the case of TaHME (91.54 mg CEs/g DCE). In addition, there was no significant difference in the comparison between stems and leaves in the case of TaME and between stems and flowers in the case of TaHME. In comparison with the previous studies, our TFC values in
T. annuum were observed to be higher than several other
Tanacetum species that were reported to contain TFC values ranging from 18.54 to 55.40 mg/g of DCE, using different parts of the plants [
18,
20]. Yapıcı et al. reported that the TFC value of the methanolic extract of
T. erzincanense was 62.20 mg of quercetin equivalents per g of DCE, which was higher than TaME and lower than TaHME of the leaves TFC of the current study [
17].
Regarding the carotenoids (
Table 2), lycopene was determined in the three parts of
T. annuum with significantly different amounts from each other; whereas, stems contained the highest TLC, followed by leaves and flowers with the contents of 0.74, 0.69 and 0.19 µg/g of dry weight (DW), respectively. On the contrary, β-carotene was determined only in flowers with the value of 0.61 µg/g of DW.
Overall, the obtained results demonstrated that all parts of
T. annuum contain an important amount of bioactive compounds, particularly total phenolic and total flavonoid, which possess a strong antioxidant activity and are responsible for many other biological activities. Moreover, the results demonstrated that bioactive compound contents were affected by solvent polarity and varied from one part to another. This verified what is described in the literature, the solvent with higher polarity extracted more bioactive compounds that are responsible for several bioactivities [
14,
15,
16].
2.3. Chemical Composition of Essential Oil by GC-MS
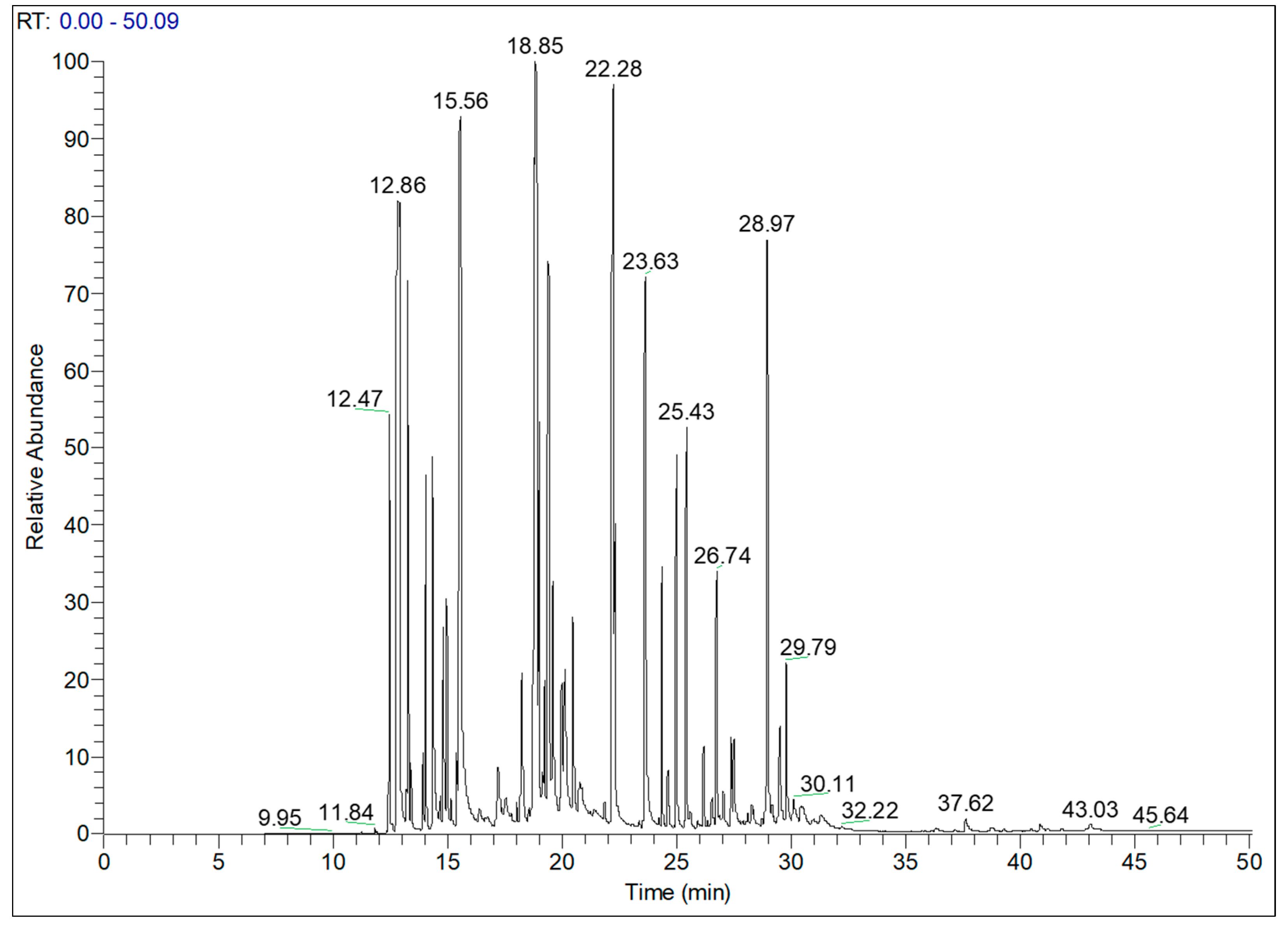
The chemical composition of TaEO of mixed aerial parts was analysed by GC-MS, and the results are illustrated in the chromatogram in
Figure 1 containing 39 peaks which correspond to 39 identified compounds, representing 99.92% of total volatile essential oil compounds in
T. annuum. These 39 biologically active molecules are divided into three classes of terpenoids: namely, monoterpenoids (85.22%), sesquiterpenoids (14.21%) and diterpenoids (0.49%). The main compounds identified were camphor (16.69%), α-pinene (12.37%), bornyl acetate (11.97%), limonene (11.10%), borneol (6.33%), α-terpinyl acetate (4.62%) and chamazulene (3.49%). The GC-MS results of TaEO are listed in
Table 3.
The main compounds identified in
T. annuum in the present study are already known for several biological activities. The predominant compound camphor, which possesses anti-inflammatory and antimicrobial activities is widely used in the cosmetic and pharmaceutical industries [
6]; in addition, this compound showed strong activity against several phytopathogens of
Fusarium species [
21]. The second main substance, α-pinene, was noted to have antioxidant [
22], anticancer, antimicrobial (including against
F. oxysporum [
23]) and anti-inflammatory activities [
24]. Bornyl acetate has also been recorded to have antioxidant, antimicrobial, anti-inflammatory, anti-tumour and insecticidal properties [
25]. Furthermore, the compound limonene is widely used in cosmetics, medicine and agriculture due to its activities such as antioxidant, anti-inflammatory, anticancer, insecticidal, antibacterial and herbicidal activity, and it is registered as a pesticide and a fungicide in China [
26]. Moreover, borneol, α-terpinyl acetate and chamazulene were reported to possess antioxidant, anti-inflammatory and antifungal properties [
6,
24,
25,
27]. Additionally, in essential oils, even minor compounds can exert biological effects due to synergistic effects between chemical classes [
25].
Several research works have investigated the chemical compositions of
T. annuum essential oils using GC-MS analysis, and the majority of these works were from the northern region of Morocco, such as a study conducted by Greche et al., which indicated that sabinene (22.3%), camphor (13.2%) and β-pinene (10.1%) were the major components obtained from
T. annuum aerial parts essential oil [
9]. Zaim et al. reported that TaEO of aerial parts (leaves and flowers) collected from the same area of the study by Greche et al. [
9] (Larache, northern Morocco) was dominated by the substances myrcene (13.67%), camphor (12.67%), sabinene (9.49%) and β-pinene (7.70%) [
13]. In addition, chamazulene (17.74%), sabinene (14.39%) and camphor (14.21%) were the main compounds identified in TaEO by Belcadi et al. [
6]. Moreover, a study using industrial TaEO from Germany found that the essential oil was rich in sabinene (14%), camphor (13.6%), myrcene (8%), β-pinene (7.7%), chamazulene (6.9%), α-phellandrene (6.5%) and
p-cymene (5%) [
5]. Similar to the GC-MS results of this study, all of the previous studies reported that camphor was one of the main compounds identified in TaEO. Overall, many factors can typically affect the quantity and chemical composition of the essential oil, including site and period of collection, stage of growth, and methods and conditions of drying, storage and extraction [
28].
2.4. Characterization of Phenolic Compounds by HPLC-MS
Phenolic compounds are natural bioactive compounds extracted mainly from plants that have demonstrated interesting biological activities, including antioxidant, anti-inflammatory, antimicrobial, and antiproliferative activities, among others, which has led to great interest in their use by numerous industries [
29]. Therefore, the current study investigated, for the first time, the phenolic composition of the
T. annuum plant using HPLC-MS. The individual phenolic compounds in the samples were identified and quantified based on their UV-Vis spectra, mass spectra, retention times and their areas by commercial standards [
30].
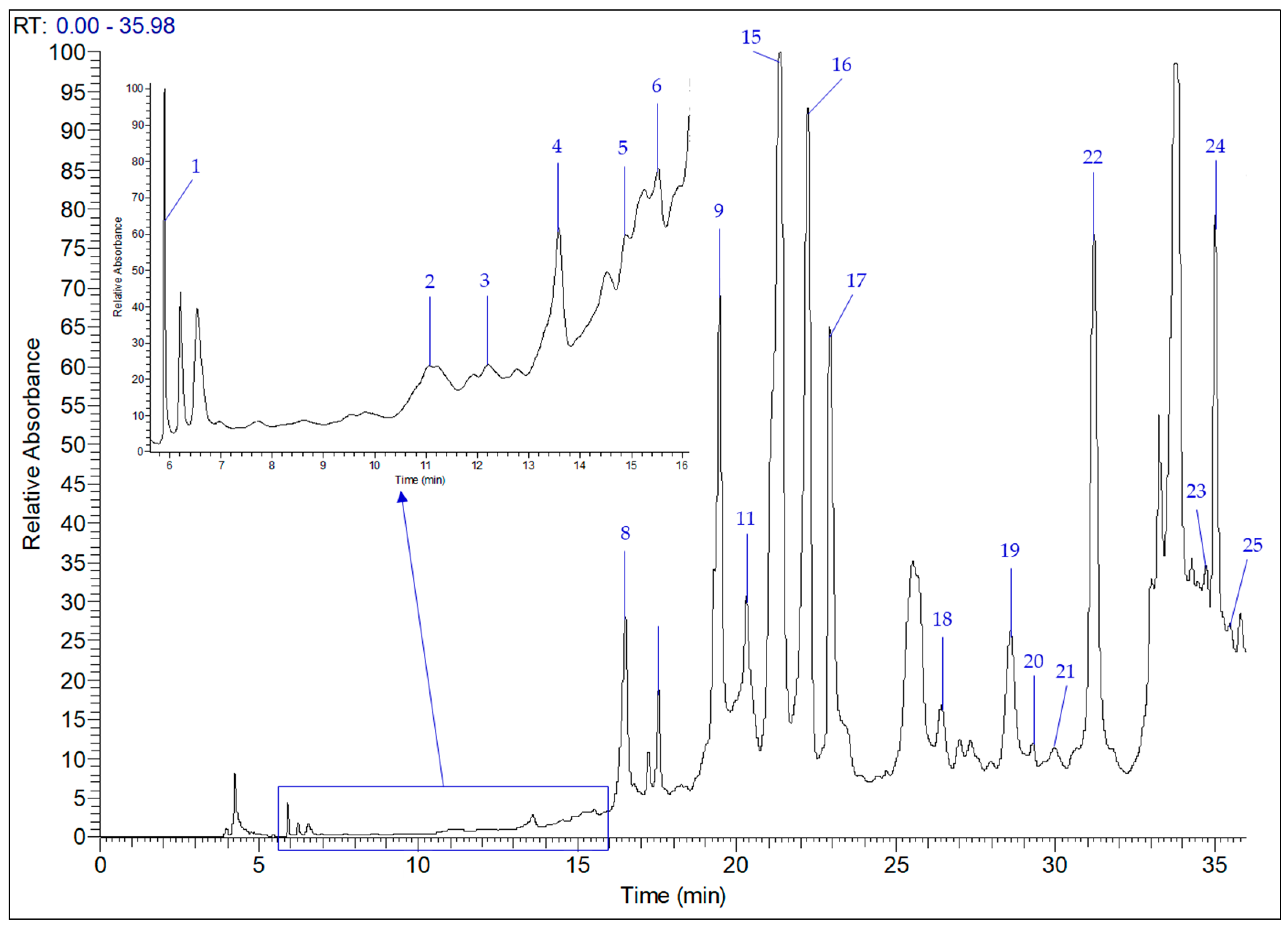
Figure 2 demonstrates the HPLC-MS chromatogram illustrating the peaks of phenolic compounds present in the
T. annuum aerial part, which were identified, quantified and presented in
Table 4. From 19 identified compounds, 10 were phenolic acids (gallic, protocatechuic, chlorogenic,
p-hydroxybenzoic, caffeic, syringic, ferulic acid, methylparaben, rosmarinic and salicylic acids) and 9 were flavonoids (catechin, rutin, luteolin 7-glucoside, apigenin 7-glucoside, luteolin, quercetin, apigenin and kaempferol).
T. annum extract showed great content of individual polyphenols, which were mainly predominated by apigenin 7-glucoside (4540 µg/g DW), luteolin 7-glucoside (2804 µg/g), salicylic acid (1878 µg/g), rutin (786.40 µg/g), kaempferol (592.30 µg/g) and rosmarinic acid (579.9 µg/g), while gallic and caffeic acids were detected in the lowest amounts in the plant extract with the values of 11.29 and 7.46 µg/g, respectively. However, vanillic acid, ellagic acid,
p-coumaric acid, vanillin, naringin and cinnamic acid were not detected in
T. annuum hydro-methanolic extract.
The main compounds detected in the Blue Tansy (Moroccan Blue Chamomile) are well-known for several biological effects; for instance, the major flavonoid identified, apigenin 7-glucoside, a widely distributed food flavone in the genus of
Tanacetum, has shown numerous biological properties, including antioxidants, anti-inflammatory, antiviral, antifungal and hepatoprotective [
31,
32]. The following flavonoid luteolin 7-glucoside, a primary active component of luteolin, has demonstrated multiple biological activities such as antimicrobial, antioxidant, anti-inflammatory and antitumor [
33,
34]. In addition, the third main identified compound salicylic acid is a phenolic acid well-known for its medicinal impact on a variety of skin disorders and contributes to skin exfoliation and removal of dead cells [
35,
36].
As there is no literature on phenolic compounds of
T. annuum, the HPLC-MS results of this work will be compared with
T. vulgare, which is a species closer to ours, and also compared with other
Tanacetum such as
T. balsamita and
T. parthenium. Herein, the studied plant was more rich and diversified in polyphenols than the
Tanacetum species cited above [
20,
34,
37]. Bączek et al. found that
T. vulgare and
T. balsamita contained a higher concentration of phenolic acids than flavonoids which is contrary to our findings; however, apigenin-7-O-glucoside and luteolin 7-O-glucoside was observed as the main flavonoids in
T. balsamita, which agrees with this study [
37]. In the study by Babich et al., chlorogenic acid, ferulic acid and rosmarinic acid were the main phenolic compounds identified in
T. vulgare from Russia [
34]. The leaves of
Tanacetum species
T. vulgare,
T. macrophyllum, and
T. corymbosum as well as the aerial parts of
T. parthenium have recently been found to have high levels of chlorogenic acid [
20,
38]. Generally, the diversity and richness of phenolic compounds differ from one plant to another. Overall, the aerial part of the investigated plant
T. annuum can be considered a great natural source of polyphenols, which are important for the development of several agricultural and pharmaceutical products.
2.5. Antifungal Activity and Synergistic Effect
The antifungal activity of
T. annuum crude extracts and essential oil was carried out against the fungal pathogen
Fusarium oxysporum f. sp.
albedinis (
Foa) that causes Bayoud disease, vascular wilt in date palm, which is considered a major constraint that impedes the development of the date palm sector in North Africa and Sahara, and more specifically in Morocco [
39]. This research revealed that all
T. annuum extracts tested effectively inhibited the growth of
Foa, indicating the presence of strong antifungal compounds. The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values of different tested samples are listed in
Table 5; whereas the concentrations required to cause growth inhibition of
Foa ranged from 3.33 to 9.17 mg/mL by crude extracts, and from 0.5 to 4 mg/mL by commercial fungicides and its combination with essential oil. Moreover, the best growth inhibition result was obtained by essential oil alone with MIC and MFC values of 3.33 and 4.58 µL/mL, respectively. Concerning the crude extracts, the leaves showed the highest anti-
Foa property with an MIC of 3.33 mg/mL for both solvents and an MFC of 7.50 and 8.33 mg/mL for TaHME and TaME, respectively. The anti-
Foa activity of extracts from the stems and flowers was slightly similar. In addition, generally speaking, there were no significant differences between TaME and TaHME of each part, these could be due to their similarity and no higher difference in the amounts of bioactive compounds such as total phenolic and total flavonoids or individual polyphenols, which are already known for their antimicrobial activity [
29]. Therefore, as there was a highly similar amount of total phenolic and total flavonoids between the three parts and by using the two solvents, the major individual phenolic compounds (namely, apigenin 7-glucoside, luteolin 7-glucoside, salicylic acid, rutin, kaempferol, rosmarinic acid, ferulic acid, quercetin, syringic acid and catechin) found in the mixed aerial parts of
T. annuum could be the main phytochemical contributors for the growth inhibition of the harmful pathogen
Foa by acting alone or in synergy. In terms of mechanisms of action, phenolic acids and flavonoids can exhibit antifungal activity by disrupting cell division, hyphal formation and/or triggering severe oxidative stress leading to cell death. This may be attributed to the direct impact of active phytochemical ingredients on fungal cells or due to the induction of defense mechanisms in the host plant [
40,
41].
Regarding commercial fungicides which are used against Bayoud diseases, the results demonstrated that SAAF 75% inhibited the growth of
Foa better than carbendazim 50% with a half dose. As presented in
Table 6, the results obtained on the synergistic effects of TaEO in combination with the two used synthetic fungicides showed a synergistic interaction in the association with carbendazim 50% with an FICI value of 0.4, while showing an additive interaction in the case of the combination between TaEO and SAAF 75% with an FICI value of 0.65. The combination of TaEO (3 µL/mL) with the used fungicides (1 mg/mL) reduced the dose for inhibition of the pathogen in half for SAAF 75% and by two thirds for carbendazim 50% (
Table 5). The combination of individual compounds, essential oil and other antifungal agents possess particular benefits for antimicrobial activity, and their mechanism of action has multiple targets on microorganisms such as cell wall destruction, increasing permeability, damaging of the cytoplasmic membrane, membrane protein damage, and coagulation of cytoplasm resulting in metabolic damage and cell death [
42]. Generally, this combination could be an interesting alternative to combat Bayoud diseases.
The strong antifungal activity of
T. annuum essential oil against
Foa could be attributed to its major compounds such as camphor which possessed several biological activities [
6], in particular, a strong activity against several phytopathogens of the
Fusarium species [
21] and the major substance α-pinene noted to have activity against
F. oxysporum [
23]. Additionally, bornyl acetate and limonene have been recorded as having antimicrobial and insecticidal properties, and limonene is used as a fungicide [
25,
26]. In addition to that, the other main compounds, borneol, α-terpinyl acetate and chamazulene, have been reported to have the ability of growth inhibition of fungal pathogens [
6,
24,
25,
27], and not forgetting that in essential oil, even the minor compounds can exert biological effects due to synergistic effects between chemical classes [
25]. Concerning the mechanism of action of essential oil, the antifungal activity of TaEO might be caused by the properties of terpenes/terpenoids that—due to their highly lipophilic nature and low molecular weight—are capable of disrupting the cell membrane, causing cell death or inhibiting the sporulation and germination of food spoilage fungi [
43,
44], which also can be caused due to synergistic effects between chemical classes [
25]. Furthermore, several studies on antifungal activity reported that
T. annuum essential oil has been able to inhibit the growth of numerous fungal species at different concentrations, including
Alternaria solani,
Botrytis cinerea,
Helminthosporium oryzae,
Pyricularia oryzae,
Verticillium dahliae [
9],
Penicillium expansum [
6],
Aspergillus niger,
Candida albicans and
Cryptococcus neoformans [
45].
Several research works investigated the antifungal activity of numerous plants’ crude extracts and essential oils against
F. oxysporum f. sp.
albedinis [
1,
2,
3,
46,
47]. The study by Rahmouni et al. investigated the antifungal activity of essential oils of five medicinal and aromatic plants collected from northern Morocco against the same pathogen isolate
F. oxysporum f. sp.
Albedinis, and the results demonstrated that
Origanum compactum (MIC = 2.5 and MFC = 5 µL/mL) showed better activity than the four plants
Myrtus communis,
Thymus satureioides,
Lavandula dentata and
Rosmarinus officinalis with MIC and MFC ranges between 10 and 40 µL/mL; in addition, these last four plants were also less active against
Foa than the present essential oil [
3]. On the other hand, Rahmouni et al. also evaluated the activity of the major components of its plant which included three molecules present in the current analysed essential oil as major compounds, namely, α-pinene, borneol and with a small percentage of α-terpineol, and these compounds possessed an anti-
Fusarium effect [
3]. The previous studies by Chibane et al. [
47] and Chibane et al. [
1] cited, respectively, that the essential oil of
Cladanthus eriolepis and
Asteriscus graveolens showed growth inhibition values of 86.20% and 100% against
Foa at the concentration of 4 µL/mL, which is slightly higher than our concentration. Concerning the crude extracts, Bouhlali et al., previously determined the antifungal activity of five plant extracts, namely,
Acacia cyanophylla,
Cupressus atlantica,
Eucalyptus torquata,
Nerium oleander and
Schinus molle, against
Foa, and results showed that all extracts possessed antifungal activity, where the extracts of
E. torquata and
C. atlantica showed the strongest antifungal effect resulting in the inhibition of mycelial growth, sporulation and spore germination in a dose-dependent manner [
2].
Overall, the crude extracts and essential oil of T. annuum showed a strong antifungal activity due to the ability of their major compounds to inhibit the growth of the pathogen. These compounds will be our future work to separately evaluate their anti-Fusarium activity and to understand the mechanisms and their connection with their antifungal action.
2.6. Antioxidant Activity
The antioxidant activity of the crude extracts TaME and TaHME of the
T. annuum parts (stems, leaves and flowers) was evaluated using the DPPH (2,2-Diphenyl-1-picrylhydrazyl) assay, which is valid, accurate, quick, easy, economic and one of the most widely used methods for assessing the antioxidant properties of the extracts or purified compounds [
48].
Figure 3 illustrates the increase of DPPH radical activities of the six extracts with the increase of the tested samples’ concentration (ranging from 0.25 to 4 mg/mL). Based on radical-scavenging activity inhibition (%), IC
50 values of the samples were graphically determined by their concentration providing 50% inhibition values (
Figure 3 and
Table 7). Each high IC
50 value indicates a lower antiradical potential [
49].
As shown in
Table 7, all the crude extracts exhibited a great antioxidant capacity as expected, considering the high amounts of total phenolic and total flavonoid contents in each part extract. The IC
50 values ranged between 0.22 and 0.65 mg/mL. The highest antioxidant effect was shown by TaHME (stems) with a value of 0.22 mg/mL, and the lowest one was observed in TaME (leaves) with a concentration of 0.65 mg/mL; in addition, there was no significant difference between the leaves’ and flowers’ antioxidant capacity in the two extract cases, TaME and TaHME. However, the antioxidant activity of the TaHME extracts was slightly better than TaME in the three parts (
Table 7). Ascorbic acid was used as a positive control and showed stronger antioxidant properties with the IC
50 value equal to 0.036 mg/mL. The phenolic compounds including phenolic acids and flavonoids are considered primary antioxidants, which have the ability to neutralize the free radicals or inhibit the production of free radicals from hydroperoxides by donating hydrogen atoms to lipid radicals [
50]. The mechanism of antioxidant actions involved either hydrogen atom transfer, transfer of a single electron, sequential proton loss electron transfer, or chelation of transition metals [
51].
To our knowledge, the antioxidant potential of different parts of
T. annuum has not been evaluated by any previous studies. For this reason, comparisons will be made with other
Tanacetum species. Herin, several studies investigated the antiradical activity of
Tanacetum species using aerial parts mixed or separated and different solvents; therefore, their results varied from one sample to another. Baranauskienė et al. performed the antioxidant activity of
T. vulgare, which gave the best result using water extract with EC
50 = 1.33 mg/mL (EC = effect concentration); this finding was lower than the one obtained in the current study [
52].
T. vulgare was also the objective of a study by Devrnja et al., investigating the antioxidant activity of methanolic extracts of stems, leaves and flowers, with obtained IC
50 values of 0.080, 0.077 and 0.058 mg/mL, respectively [
19]. Furthermore, Esmaeili et al. [
18] evaluated the antioxidant properties of the ethanolic extracts of the aerial part of six
Tanacetum species (
T. budjnurdense,
T. hololeucum,
T. chiliophyllum,
T. sonboli,
T. tabrisianum and
T. kotschyi), and the results showed IC
50 values ranged between 0.060 and 0.157 mg/mL, which were better than our findings. Overall, the Moroccan
T. annuum could be considered a good source of antioxidant compounds.