A Multifaceted Intervention and Its Effects on Antibiotic Usage in Norwegian Nursing Homes
Abstract
1. Introduction
2. Results
2.1. Measures and Implementation of Intervention Material
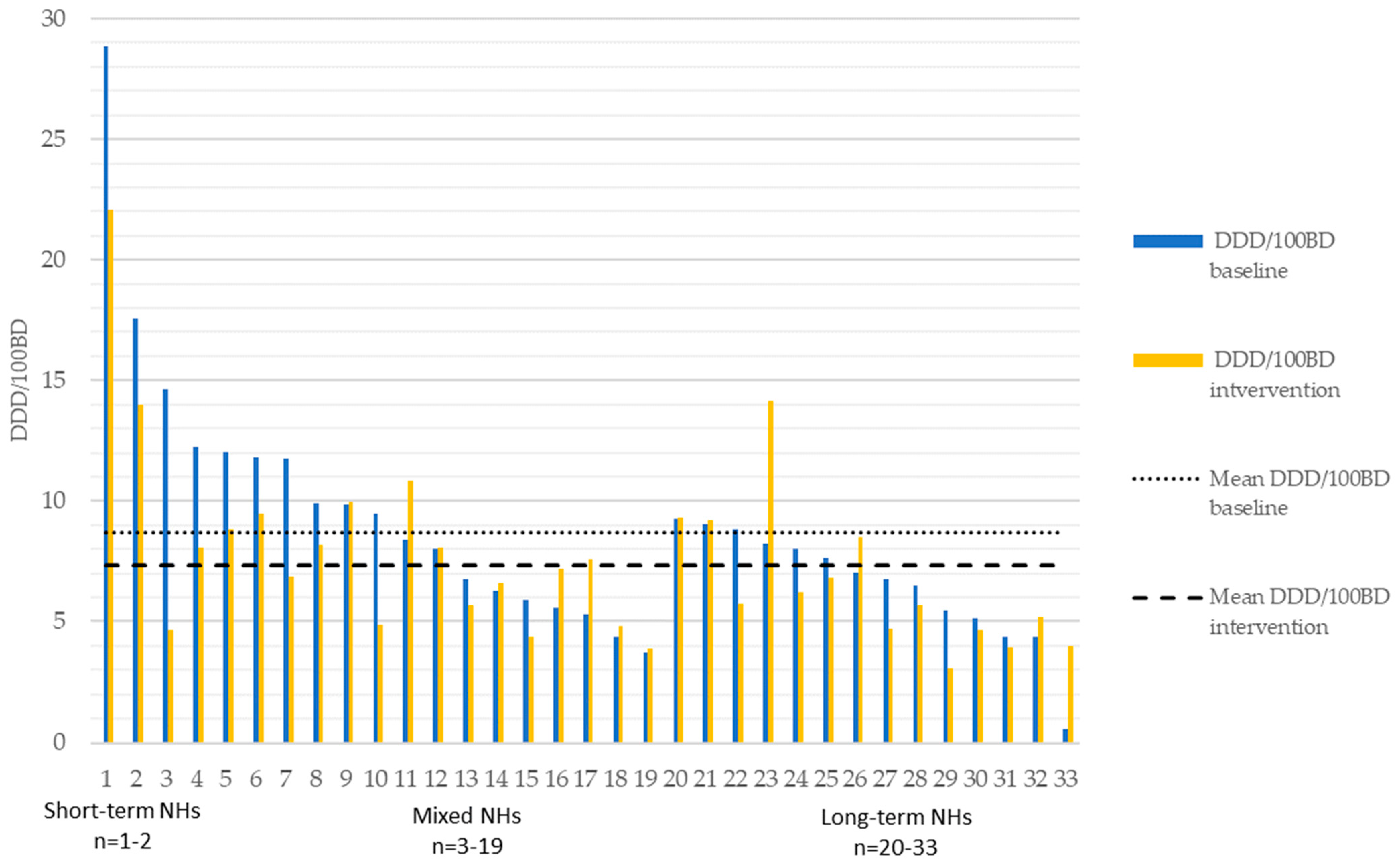
2.2. Antibiotic Use (Excluding Methenamine)
2.3. Methenamine Use
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
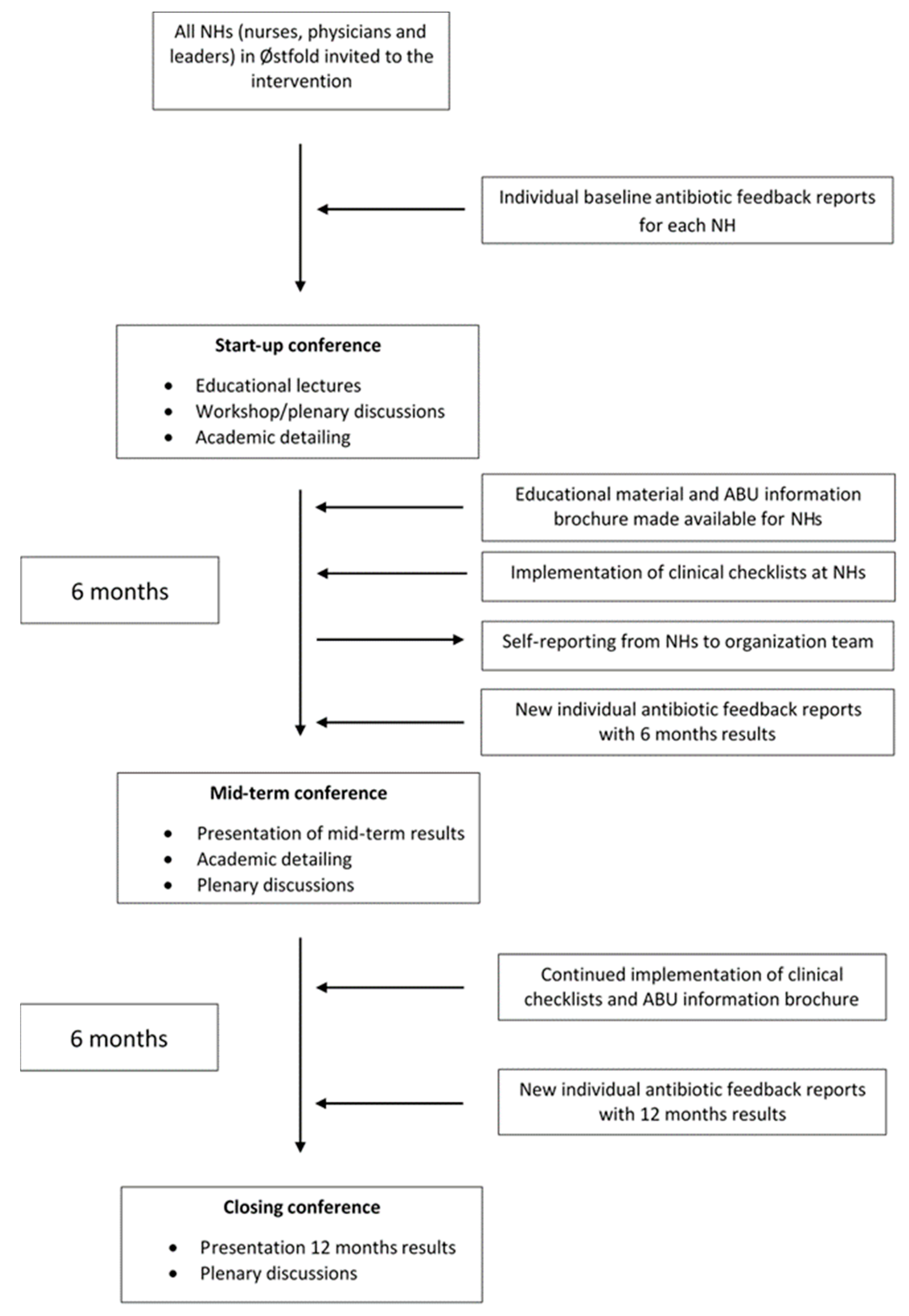
4.2. Intervention
4.3. Conferences and Antibiotic Feedback Reports
4.4. Training, Clinical Decision Support Tools and Academic Detailing
4.5. Resource Persons
4.6. Train-the-Trainer Model, Educational Material and Written Resources
4.7. Data Collection, Antibiotic Classification, and Nursing Home Characteristics
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NORM/NORM-VET 2021. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Available online: https://unn.no/Documents/Kompetansetjenester,%20-sentre%20og%20fagr%C3%A5d/NORM%20-%20Norsk%20overv%C3%A5kingssystem%20for%20antibiotikaresistens%20hos%20mikrober/Rapporter/NORM%20NORM-VET%202021.pdf (accessed on 6 July 2023).
- Blix, H.S.; Roed, J.; Sti, M.O. Large variation in antibacterial use among Norwegian nursing homes. Scand. J. Infect. Dis. 2007, 39, 536–541. [Google Scholar] [CrossRef]
- Eriksen, H.M.; Saether, A.R.; Viktil, K.K.; Andberg, L.; Munkerud, M.W.; Willoch, K.; Blix, H.S. Use of antibiotics in nursing homes--surveillance with different methods. Tidsskr. Nor. Laegeforen. 2013, 133, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Harbin, N.J.; Haug, J.B.; Romøren, M.; Lindbæk, M. Oral and parenteral antibiotic use in Norwegian nursing homes: Are primary care institutions becoming our new local hospitals? JAC-Antimicrob. Resist. 2020, 2, dlaa093. [Google Scholar] [CrossRef]
- Hepper, H.J.; Sieber, C.; Walger, P.; Bahrmann, P.; Singler, K. Infections in the elderly. Crit. Care. Clin. 2013, 29, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Harbin, N.J.; Lindbæk, M.; Romøren, M. Barriers and facilitators of appropriate antibiotic use in primary care institutions after an antibiotic quality improvement program—A nested qualitative study. BMC Geriatr. 2022, 22, 458. [Google Scholar] [CrossRef]
- Røen, I.; Selbæk, G.; Kirkevold, Ø.; Engedal, K.; Testad, I.; Bergh, S. Resourse Use and Disease Couse in dementia—Nursing Home (REDIC-NH), a longitudinal cohort study; design and patient characteristics at admission to Norwegian nursing homes. BMC Health. Serv. Res. 2017, 17, 365. [Google Scholar] [CrossRef]
- Fog, A.F.; Mdala, I.; Engedal, K.; Straand, J. Variation between nursing homes in drug use and in drug-related problems. BMC Geriatr. 2020, 20, 336. [Google Scholar] [CrossRef]
- Van Buul, L.W.; van der Steen, J.T.; Doncker, S.M.; Achterberg, W.P.; Schellevis, F.G.; Veenhuizen, R.B.; Hertogh, C.M. Factors influencing antibiotic prescribing in long-term care facilities: A qualitative in-depth study. BMC Geriatr. 2014, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Blix, H.S.; Bergman, J.; Schjott, J. How are antibacterials used in nursing homes? Results from a point-prevalence prescription study in 44 Norwegian nursing homes. Pharmacoepidemiol. Drug. Saf. 2010, 19, 1025–1030. [Google Scholar] [CrossRef]
- Alberg, T.; Holen, O.; Blix, H.S.; Lindbaek, M.; Bentele, H.; Eriksen, H.M. Antibiotic use and infections in nursing homes. Tidsskr. Nor. Laegeforen. 2017, 137, 357–361. [Google Scholar] [CrossRef]
- Kabbani, S.; Wang, S.W.; Ditz, L.L.; Gouin, K.A.; Palms, D.; Rowe, T.A.; Hyun, D.Y.; Chi, N.W.; Stone, N.D.; Hicks, L.A. Description of antibiotic use variability among US nursing homes using electronic health record data. Antimicrob. Steward. Healthc. Epidemiol. 2021, 1, e58. [Google Scholar] [CrossRef]
- Latour, K.; Catry, B.; Broex, E.; Vankerckhoven, V.; Muller, A.; Stroobants, R.; Goossens, H.; Jans, B. Indications for antimicrobial prescribing in European nursing homes: Results from a point prevalence survey. Pharmacoepidemiol. Drug. Saf. 2012, 21, 937–944. [Google Scholar] [CrossRef]
- Ricchizzi, E.; Latour, K.; Karki, T.; Buttazzi, R.; Jans, B.; Moro, M.L.; Nakitanda, O.A.; Plachouras, D.; Monnet, D.L.; Suetens, C.; et al. Antimicrobial use in European long-term care facilities: Results from the third point prevalence survey of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro. Surveill. 2018, 23, 1800394. [Google Scholar] [CrossRef]
- Rowe, T.A.; Juthani-Mehta, M. Urinary tract infection in older adults. Aging. Health. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Leihof, R.F.; Nielsen, K.L.; Frimodt-Møller, N. Asymptomatic Bacteriuria (ABU) in Elderly: Prevalence, Virulence, Phylogeny, Antibiotic Resistance and Complement C3 in Urine. Microorganisms 2021, 9, 390. [Google Scholar] [CrossRef]
- Van Buul, L.W.; Veenhuizen, R.B.; Achterberg, W.P.; Schellevis, F.G.; Essink, R.T.; de Greeff, S.C.; Natsch, S.; van der Steen, J.T.; Hertogh, C.M. Antibiotic prescribing in Dutch nursing homes: How appropriate is it? J. Am. Med. Dir. Assoc. 2015, 16, 229–237. [Google Scholar] [CrossRef]
- D’Agata, E.; Loeb, M.B.; Mitchell, S.L. Challenges in assessing nursing home residents with advanced dementia for suspected urinary tract infections. J. Am. Geriatr Soc. 2013, 61, 62–66. [Google Scholar] [CrossRef]
- Rotjanapan, P.; Dosa, D.; Thomas, K.S. Potentially inappropriate treatment of urinary tract infections in two Rhode Island nursing homes. Arch. Intern. Med. 2011, 171, 438–443. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on antimicrobial Resistance. Available online: http://www.scielo.org.za/scielo.php?pid=S0256-95742015000500001&script=sci_arttext&tlng=es (accessed on 6 July 2023).
- European Comission, Public Health. EU One Health Action Plan against AMR. Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf (accessed on 6 July 2023).
- World Health Organization. Library of AMR National Action Plans. Available online: https://www.who.int/teams/surveillance-prevention-control-AMR/national-action-plan-monitoring-evaluation/library-of-national-action-plans (accessed on 6 July 2023).
- Norwegian Ministry of Health and Care Services. Action Plan to Reduce Antibiotics Resistance in the Health Services. Available online: https://www.regjeringen.no/contentassets/915655269bc04a47928fce917e4b25f5/handlingsplan-antibiotikaresistens.pdf (accessed on 6 July 2023).
- Van Buul, L.W.; Monnier, A.A.; Sundvall, P.D.; Ulleryd, P.; Godycki-Cwirko, M.; Kowalczyk, A.; Lindbaek, M.; Hertogh, C. Antibiotic Stewardship in European Nursing Homes: Experiences From the Netherlands, Norway, Poland, and Sweden. J. Am. Med. Dir. Assoc. 2020, 21, 34–40.e31. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Ministry of Health and Care Services. The Coordination Reform. Proper treatment—At the Right Place and Right Time. Available online: https://www.regjeringen.no/contentassets/d4f0e16ad32e4bbd8d8ab5c21445a5dc/en-gb/pdfs/stm200820090047000en_pdfs.pdf (accessed on 6 July 2023).
- Crespo-Rivas, J.C.; Guisado-Gil, A.B.; Peñalva, G.; Rodríguez-Villodres, Á.; Martín-Gandul, C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Are antimicrobial stewardship interventions effective and safe in long-term care facilities? A systematic review and meta-analysis. Clin. Microbiol Infect. 2021, 27, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Tandan, M.; Thapa, P.; Maharjan, P.; Bhandari, B. Impact of antimicrobial stewardship program on antimicrobial-resistance and prescribing in nursing homes: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2022, 29, 74–87. [Google Scholar] [CrossRef]
- Pasay, D.K.; Guirguis, M.S.; Shkrobot, R.C.; Slobodan, J.P.; Wagg, A.S.; Sadowski, C.A.; Conly, J.M.; Saxinger, L.M.; Bresee, L.C. Antimicrobial stewardship in rural nursing homes: Impact of interprofessional education and clinical decision tool implementation on urinary tract infection treatment in a cluster randomized trial. Infect. Control Hosp. Epidemiol. 2019, 40, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Gjelstad, S.; Høye, S.; Straand, J.; Brekke, M.; Dalen, I.; Lindbæk, M. Improving antibiotic prescribing in acute respiratory tract infections: Cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ 2013, 347, f4403. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Langford, B.J.; Daneman, N.; Friedrich, J.O.; Garber, G. Antimicrobial Stewardship Programs in Long-Term Care Settings: A Meta-Analysis and Systematic Review. J. Am. Geriatr Soc. 2019, 67, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Ipe, D.S.; Sundac, L.; Benjamin, W.H., Jr.; Moore, K.H.; Ulett, G.C. Asymptomatic bacteriuria: Prevalence rates of causal microorganisms, etiology of infection in different patient populations, and recent advances in molecular detection. FEMS Microbiol. Lett. 2013, 346, 1–10. [Google Scholar] [CrossRef]
- Eure, T.; LaPlace, L.L.; Melchreit, R.; Maloney, M.; Lynfield, R.; Whitten, T.; Warnke, L.; Dumyati, G.; Quinlan, G.; Concannon, C.; et al. Measuring Antibiotic Appropriateness for Urinary Tract Infections in Nursing Home Residents. Infect. Control Hosp. Epidemiol. 2017, 38, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- McArthur, C.; Bai, Y.; Hewston, P.; Giangregorio, L.; Straus, S.; Papaioannou, A. Barriers and facilitators to implementing evidence-based guidelines in long-term care: A qualitative evidence synthesis. Implement. Sci. 2021, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Mills, W.L.; Pimentel, C.B.; Snow, A.L.; Allen, R.S.; Wewiorski, N.J.; Palmer, J.A.; Clark, V.; Roland, T.M.; McDannold, S.E.; Hartmann, C.W. Nursing Home Staff Perceptions of Barriers and Facilitators to Implementing a Quality Improvement Intervention. J. Am. Med. Dir. Assoc. 2019, 20, 810–815. [Google Scholar] [CrossRef]
- Westbye, S.F.; Rostoft, S.; Romøren, M.; Thoresen, L.; Wahl, A.K.; Pedersen, R. Barriers and facilitators to implementing advance care planning in naïve contexts—Where to look when plowing new terrain? BMC Geriatr. 2023, 23, 387. [Google Scholar] [CrossRef]
- Bergs, J.; Lambrechts, F.; Simons, P.; Vlayen, A.; Marneffe, W.; Hellings, J.; Cleemput, I.; Vandijck, D. Barriers and facilitators related to the implementation of surgical safety checklists: A systematic review of the qualitative evidence. BMJ Qual. Saf. 2015, 24, 776–786. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P t 2015, 40, 277–283. [Google Scholar]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Romøren, M.; Pedersen, R.; Førde, R. One patient, two worlds—Coordination between nursing home and hospital doctors. Tidsskr. Nor. Laegeforen. 2017, 137, 193–197. [Google Scholar] [CrossRef][Green Version]
- Harding, C.; Chadwick, T.; Homer, T.; Lecouturier, J.; Mossop, H.; Carnell, S.; King, W.; Abouhajar, A.; Vale, L.; Watson, G.; et al. Methenamine hippurate compared with antibiotic prophylaxis to prevent recurrent urinary tract infections in women: The Altar non-inferiority RCT. Health Technol. Assess. 2022, 26, 1–172. [Google Scholar] [CrossRef]
- Rui, L.; Lindbaek, M.; Gjelstad, S. Preventive effect of methenamine in women with recurrent urinary tract infections—A case-control study. Scand. J. Prim. Health Care 2022, 40, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Bakhit, M.; Krzyzaniak, N.; Hilder, J.; Clark, J.; Scott, A.M.; Mar, C.D. Use of methenamine hippurate to prevent urinary tract infections in community adult women: A systematic review and meta-analysis. Br. J. Gen. Pract. 2021, 71, e528–e537. [Google Scholar] [CrossRef] [PubMed]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs.Atlanta, GA: US Department of Health and Human Services, CDC. 2019. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 8 August 2023).
- Kirkevold, M. Teaching nursing homes: The Norwegian experience 20 years on. J. Res. Nurs. 2018, 23, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Yu, H.; Grabowski, D.C. High Nursing Staff Turnover In Nursing Homes Offers Important Quality Information. Health Aff. 2021, 40, 384–391. [Google Scholar] [CrossRef]
- Eldridge, S.; Kerry, S.; Torgerson, D.J. Bias in identifying and recruiting participants in cluster randomised trials: What can be done? BMJ 2009, 339, b4006. [Google Scholar] [CrossRef]
- Brierley, G.; Brabyn, S.; Torgerson, D.; Watson, J. Bias in recruitment to cluster randomized trials: A review of recent publications. J. Eval. Clin. Pract. 2012, 18, 878–886. [Google Scholar] [CrossRef]
- Nace, D.A.; Hanlon, J.T.; Crnich, C.J.; Drinka, P.J.; Schweon, S.J.; Anderson, G.; Perera, S. A Multifaceted Antimicrobial Stewardship Program for the Treatment of Uncomplicated Cystitis in Nursing Home Residents. JAMA Intern. Med. 2020, 180, 944–951. [Google Scholar] [CrossRef]
- Pettersson, E.; Vernby, A.; Mölstad, S.; Lundborg, C.S. Can a multifaceted educational intervention targeting both nurses and physicians change the prescribing of antibiotics to nursing home residents? A cluster randomized controlled trial. J. Antimicrob. Chemother. 2011, 66, 2659–2666. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.A.R.; van de Pol, A.C.; Heltveit-Olsen, S.R.; Lindbæk, M.; Høye, S.; Lithén, S.S.; Sundvall, P.D.; Sundvall, S.; Arnljots, E.S.; Gunnarsson, R.; et al. Effect of a multifaceted antibiotic stewardship intervention to improve antibiotic prescribing for suspected urinary tract infections in frail older adults (ImpresU): Pragmatic cluster randomised controlled trial in four European countries. BMJ 2023, 380, e072319. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 6 July 2023).
- StataCorp. Stata Statistical Software: Release 17; StataCorp LP: College Station, TX, USA, 2022. [Google Scholar]
| Intervention | Control 1 | Control 2 | Control 3 | Control 4 | Controls (Total) | |
|---|---|---|---|---|---|---|
| County population 2016 | 289,267 | 277,684 | 298,487 | 172,494 | 244,967 | 993,632 |
| Number of NHs | 33 | 35 | 46 | 25 | 30 | 136 |
| Number of beds | 1807 | 1477 | 1845 | 1090 | 1561 | 5973 |
| Controls (mean) | ||||||
| Doctor hours/bed/week | 0.46 | 0.54 | 0.43 | 0.6 | 0.45 | 0.49 |
| Beds/NH | 55 (r: 13–120) | 42 (r: 13–120) | 40 (r: 8–91) | 44 (r: 16–100) | 52 (r: 13–126) | 44 (r: 8–126) |
| Bed days/NH/year | 19,840 | 14,802 | 14,177 | 14,967 | 18,695 | 15,480 |
| Size of NHs | ||||||
| 0–40 beds | 9 | 19 | 27 | 13 | 11 | 17.5 |
| 41–69 beds | 15 | 14 | 12 | 9 | 11 | 10.75 |
| 70+ beds | 9 | 2 | 7 | 3 | 8 | 5 |
| NH category | ||||||
| Short-term NHs | 2 | 3 | 1 | 2 | 3 | 2.75 |
| Long term NHs | 14 | 8 | 17 | 6 | 12 | 10.75 |
| Mixed NHs | 17 | 24 | 28 | 17 | 15 | 21 |
| Estimated Difference β | 95% CI | p | ||
|---|---|---|---|---|
| Change in total antibiotic use in control counties combined | ref. | |||
| Comparison of change in total antibiotic use between intervention and controls combined | −0.75 | −1.91–0.41 | 0.207 | |
| Nursing home category: | ||||
| Mixed Long-term Short-term | ref. −1.56 12.45 | −2.51–−0.62 6.86–17.96 | 0.001 <0.001 | |
| Size of nursing home: | ||||
| Small | ref. | |||
| Medium | −1.83 | −3.22–−0.43 | 0.01 | |
| Large | −2.33 | −4.15–−0.52 | 0.012 | |
| Doctor hours/bed/week | 0.03 | −0.01–0.07 | 0.112 |
| Antibiotic Indication Group | County | DDD/100 BD Baseline | DDD/100 BD Intervention | Estimated Change β | 95% CI | p |
|---|---|---|---|---|---|---|
| Oral UTI | Intervention | 4.08 | 2.74 | −1.34 | −1.85–−0.84 | <0.001 |
| Control | 3.69 | 3.25 | −0.45 | −0.74–−0.15 | 0.003 | |
| Oral RTI | Intervention | 2.29 | 2.49 | 0.2 | −0.17–0.56 | 0.29 |
| Control | 3.15 | 3.17 | 0.01 | −0.3–0.31 | 0.97 | |
| Oral SSTI | Intervention | 0.70 | 0.63 | −0.07 | −0.33–0.2 | 0.62 |
| Control | 0.61 | 0.51 | −0.1 | −0.2–0.01 | 0.08 | |
| Other oral antibiotics | Intervention | 0.36 | 0.38 | 0.03 | −0.11–0.18 | 0.65 |
| Control | 0.32 | 0.27 | −0.05 | −0.15–0.05 | 0.34 | |
| Intravenous | Intervention | 1.20 | 1.03 | −0.16 | −0.37–0.05 | 0.15 |
| Control | 0.76 | 0.72 | −0.04 | −0.2–0.11 | 0.59 | |
| Total | Intervention | 8.68 | 7.31 | −1.38 | −2.35–−0.41 | 0.005 |
| Control | 8.53 | 7.93 | −0.63 | −1.27–0.01 | 0.054 |
| Estimated Difference β | 95% CI | p | |
|---|---|---|---|
| Change in UTI-AB use in combined control counties | ref. | ||
| Comparison between changes in UTI-AB use within intervention and combined controls | −0.9 | −1.28–−0.31 | 0.003 |
| Nursing home category: | |||
| Mixed | ref. | ||
| Long-term | −0.83 | −1.3–−0.36 | 0.001 |
| Short-term | 5.43 | 2.8–8.06 | <0.001 |
| Size of nursing home: | |||
| Small | ref. | ||
| Medium | −0.61 | −1.27–0.06 | 0.073 |
| Large | −0.75 | −1.58–0.09 | 0.081 |
| Doctor hours/bed/week | 0.005 | −0.01–0.02 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harbin, N.J.; Haug, J.B.; Lindbæk, M.; Akselsen, P.E.; Romøren, M. A Multifaceted Intervention and Its Effects on Antibiotic Usage in Norwegian Nursing Homes. Antibiotics 2023, 12, 1372. https://doi.org/10.3390/antibiotics12091372
Harbin NJ, Haug JB, Lindbæk M, Akselsen PE, Romøren M. A Multifaceted Intervention and Its Effects on Antibiotic Usage in Norwegian Nursing Homes. Antibiotics. 2023; 12(9):1372. https://doi.org/10.3390/antibiotics12091372
Chicago/Turabian StyleHarbin, Nicolay Jonassen, Jon Birger Haug, Morten Lindbæk, Per Espen Akselsen, and Maria Romøren. 2023. "A Multifaceted Intervention and Its Effects on Antibiotic Usage in Norwegian Nursing Homes" Antibiotics 12, no. 9: 1372. https://doi.org/10.3390/antibiotics12091372
APA StyleHarbin, N. J., Haug, J. B., Lindbæk, M., Akselsen, P. E., & Romøren, M. (2023). A Multifaceted Intervention and Its Effects on Antibiotic Usage in Norwegian Nursing Homes. Antibiotics, 12(9), 1372. https://doi.org/10.3390/antibiotics12091372







