Structure–Activity Relationships of Cationic Lipidoids against Escherichia coli
Abstract
1. Introduction
2. Results
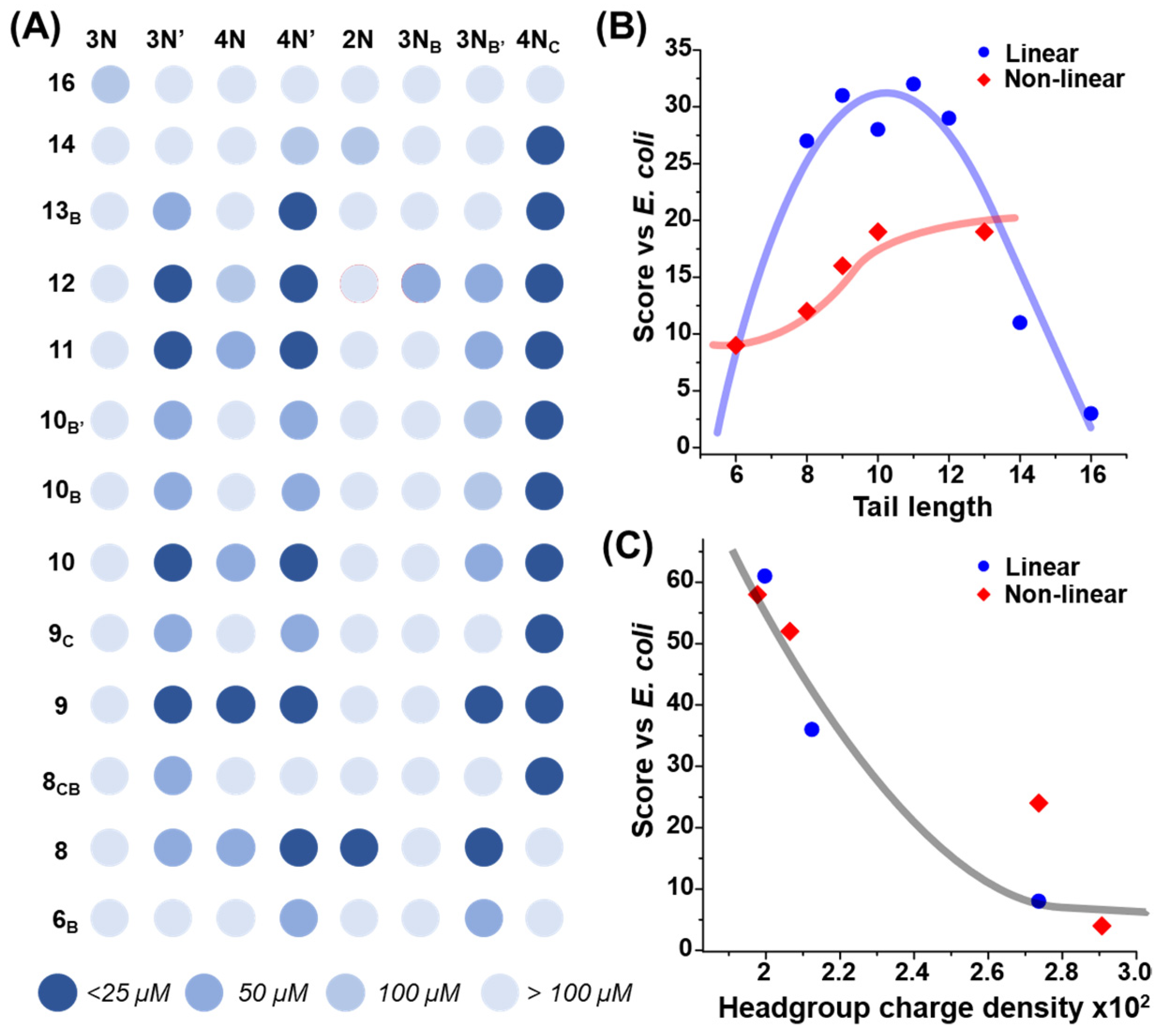
2.1. Combinatorial Antimicrobial Activity Screening
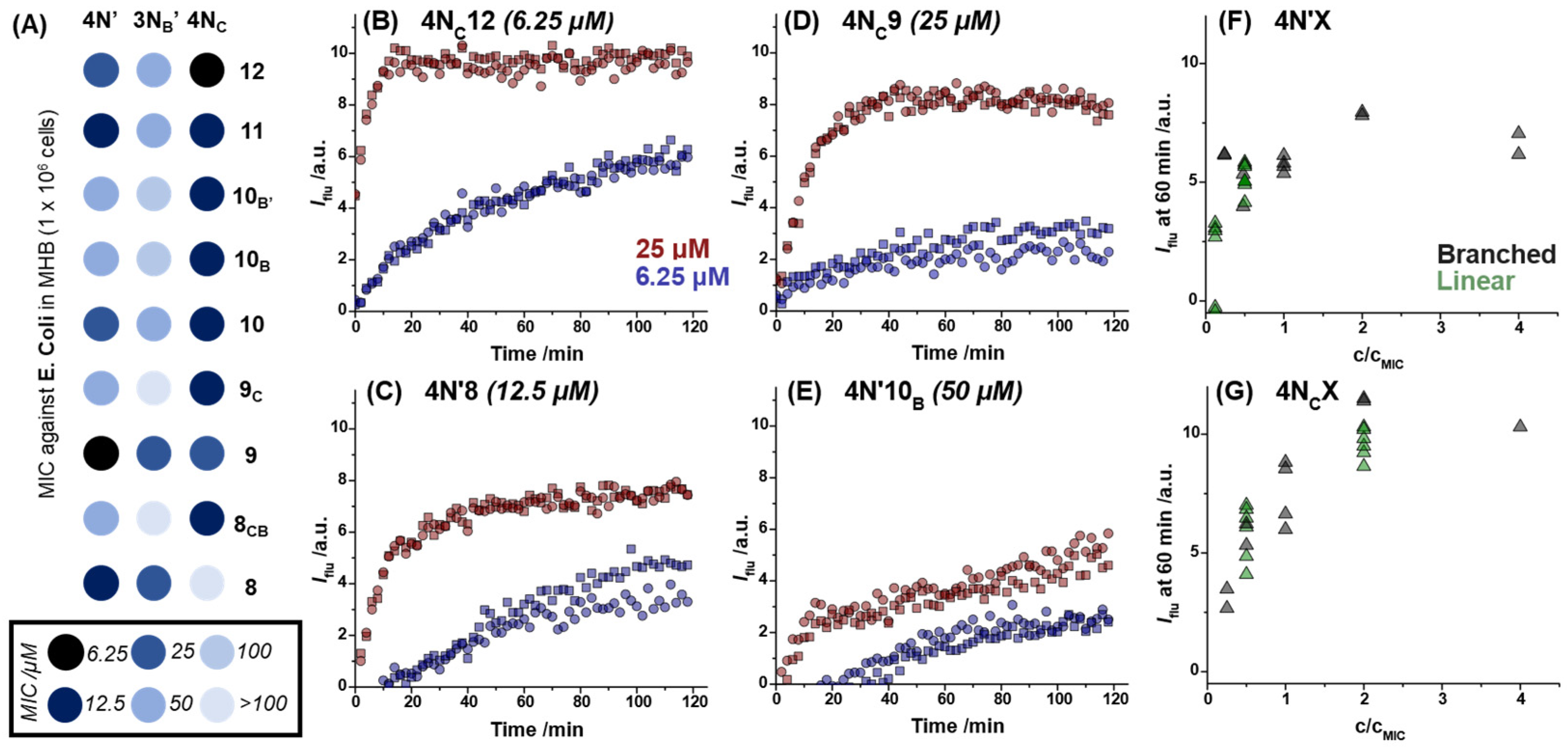
2.2. Dye Permeabilization Assay
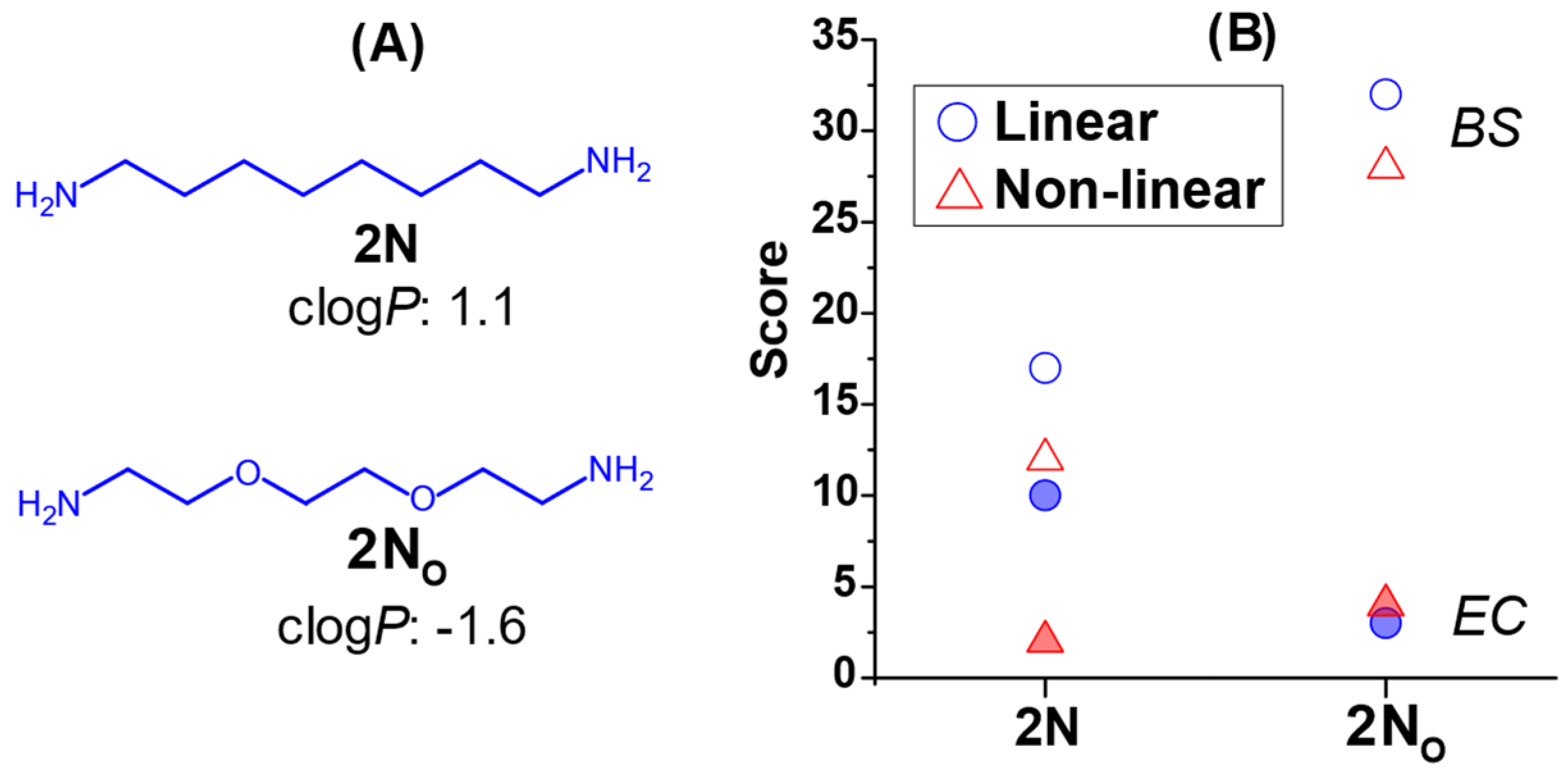
2.3. The Effect of Headgroup Hydrophilicity on Antimicrobial Activity
3. Discussion
4. Materials and Methods
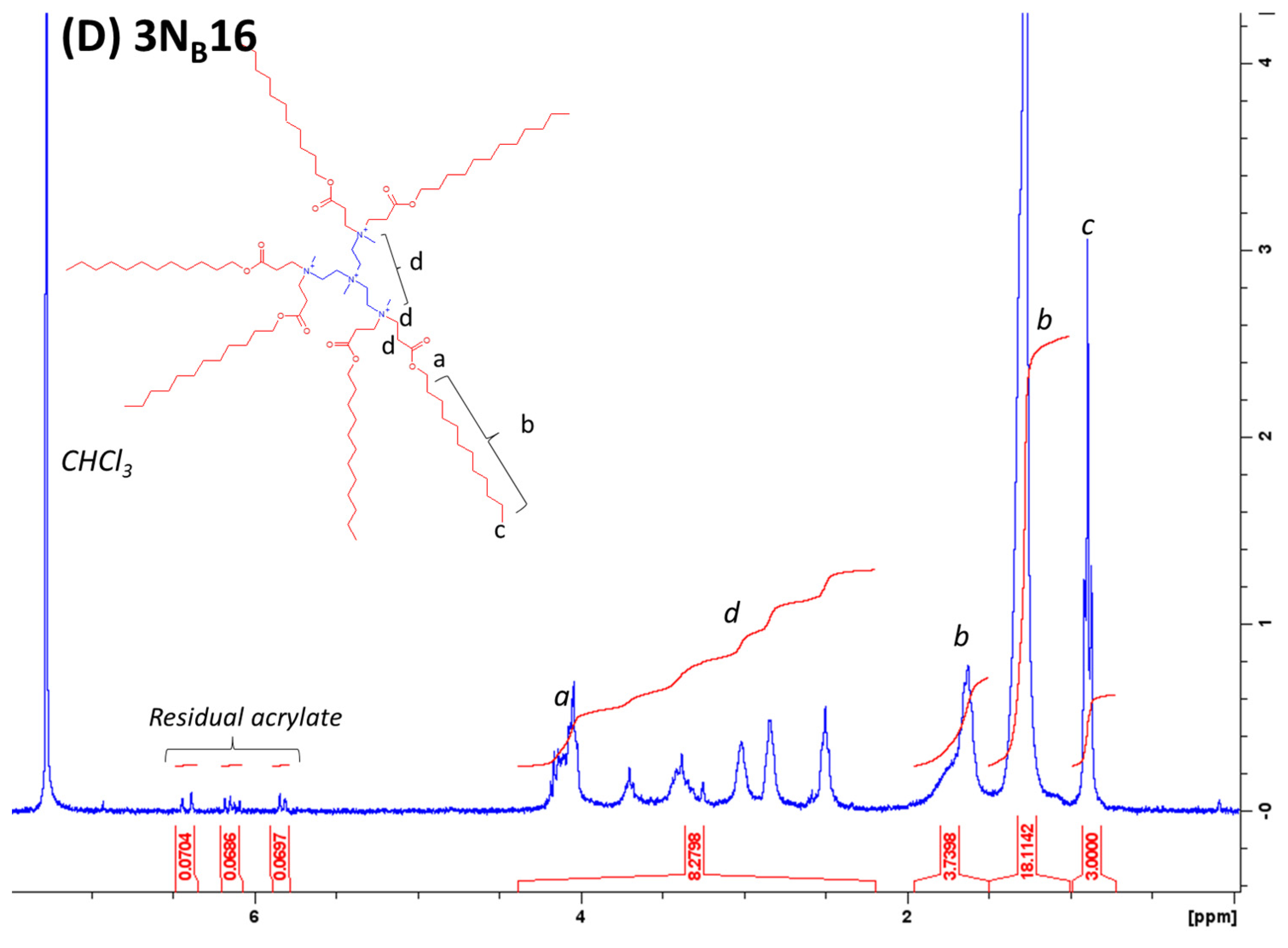
4.1. Lipidoid Synthesis
4.2. Analysis of Purity
4.3. Bacterial Culture Preparation
4.4. Lipidoid Preparation for MIC Assays
4.5. Bacterial Growth Calibration Curve
4.6. Propidium Iodide Permeation Assay
4.7. Antimicrobial Performance Scoring Criteria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The Multifaceted Nature of Antimicrobial Peptides: Current Synthetic Chemistry Approaches and Future Directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Rathinakumar, R.; Wimley, W.C. Biomolecular Engineering by Combinatorial Design and High-Throughput Screening: Small, Soluble Peptides That Permeabilize Membranes. J. Am. Chem. Soc. 2008, 130, 9849–9858. [Google Scholar] [CrossRef] [PubMed]
- Rathinakumar, R.; Walkenhorst, W.F.; Wimley, W.C. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009, 131, 7609–7617. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.D.F.; Hancock, R.E.W. Alternative Mechanisms of Action of Cationic Antimicrobial Peptides on Bacteria. Expert Rev. Anti. Infect. Ther. 2007, 5, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Lohner, K. Membrane-Active Antimicrobial Peptides as Template Structures for Novel Antibiotic Agents. Curr. Top. Med. Chem. 2017, 17, 508–519. [Google Scholar] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Kang, X.; Dong, F.; Shi, C.; Liu, S.; Sun, J.; Chen, J.; Li, H.; Xu, H.; Lao, X.; Zheng, H. DRAMP 2.0, an Updated Data Repository of Antimicrobial Peptides. Sci. Data 2019, 6, 148. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable Cationic Antibacterial Amphiphiles: Synthesis, Mechanism of Action, and Cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, D.; Cao, M.; Chen, Y.; Liu, Z.; Wu, C.; Xu, H.; Wang, S.; Wang, Y. Self-Aggregation, Antibacterial Activity, and Mildness of Cyclodextrin/Cationic Trimeric Surfactant Complexes. ACS Appl. Mater. Interfaces 2016, 8, 30811–30823. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Vijayasarathy, P.; Sin, A.; Nam, H.; Khan, S.; Parambath, J.B.M.; Mohamed, A.A.; Han, C. Antimicrobial Activity of Quaternary Ammonium Salts: Structure-Activity Relationship. Med. Chem. Res. 2022, 31, 1663–1678. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, F.; Chen, H.; Li, M.; Qiao, F.; Liu, Z.; Hou, Y.; Wu, C.; Fan, Y.; Liu, L.; et al. Selective Antimicrobial Activities and Action Mechanism of Micelles Self-Assembled by Cationic Oligomeric Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P.C. Building a Better Quaternary Ammonium Compound (QAC): Branched Tetracationic Antiseptic Amphiphiles. ChemMedChem 2016, 11, 1401–1405. [Google Scholar] [CrossRef]
- Jennings, J.; Dunja, A.; Semeraro, E.; Lohner, K.; Malanovic, N. Combinatorial Screening of Cationic Lipidoids Reveals How Molecular Conformation Affects Membrane-Targeting Antimicrobial Activity. ACS Appl. Mat. Interf. 2023; just accepted. [Google Scholar] [CrossRef]
- Jennings, J.; Carter, M.C.D.; Son, C.Y.; Cui, Q.; Lynn, D.M.; Mahanthappa, M.K. Protonation-Driven Aqueous Lyotropic Self-Assembly of Synthetic Six-Tail Lipidoids. Langmuir 2020, 36, 8240–8252. [Google Scholar] [CrossRef]
- Jennings, J.; Pabst, G. Multiple Routes to Bicontinuous Cubic Liquid Crystal Phases Discovered by High-Throughput Self-Assembly Screening of Multi-Tail Lipidoids. Small 2023, 19, 2206747. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Boyce, J.M. Quaternary Ammonium Disinfectants and Antiseptics: Tolerance, Resistance and Potential Impact on Antibiotic Resistance. Antimicrob. Resist. Infect. Control 2023, 12, 32. [Google Scholar] [CrossRef]
- Nell, M.J.; Tjabringa, G.S.; Wafelman, A.R.; Verrijk, R.; Hiemstra, P.S.; Drijfhout, J.W.; Grote, J.J. Development of Novel LL-37 Derived Antimicrobial Peptides with LPS and LTA Neutralizing and Antimicrobial Activities for Therapeutic Application. Peptides 2006, 27, 649–660. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-Positive Bacterial Cell Envelopes: The Impact on the Activity of Antimicrobial Peptides. Biochim. Biophys. Acta Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-like Materials for Delivery of RNAi Therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, M.; Knupp, S.A.; Soto-Feliciano, Y.; Hu, X.; Kaplan, D.L.; Langer, R.; Anderson, D.G.; Xu, Q. Combinatorial Library of Lipidoids for in Vitro Dna Delivery. Bioconjug. Chem. 2012, 23, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (Qacs) and Ionic Liquids (Ils) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Jones, G.L. From Petri Dish to Patient: Bioavailability Estimation and Mechanism of Action for Antimicrobial and Immunomodulatory Natural Products. Front. Microbiol. 2019, 10, 2470. [Google Scholar] [CrossRef]
- Wu, E.L.; Fleming, P.J.; Yeom, M.S.; Widmalm, G.; Klauda, J.B.; Fleming, K.G.; Im, W.E. Coli Outer Membrane and Interactions with OmpLA. Biophys. J. 2014, 106, 2493–2502. [Google Scholar] [CrossRef]
- Sangster, J.M. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; John Wiley & Sons: Chichester, UK, 1997; Volume 2, ISBN 0471973971. [Google Scholar]
- Bishop, D.G.; Rutberg, L.; Samuelsson, B. The Chemical Composition of the Cytoplasmic Membrane of Bacillus Subtilis. Eur. J. Biochem. 1967, 2, 448–453. [Google Scholar] [CrossRef]
- Leitgeb, B.; Szekeres, A.; Manczinger, L.; Vágvölgyi, C.; Kredics, L. The History of Alamethicin: A Review of the Most Extensively Studied Peptaibol. Chem. Biodivers. 2007, 4, 1027–1051. [Google Scholar] [CrossRef]
- Chang, T.-W.; Lin, Y.-M.; Wang, C.-F.; Liao, Y.-D. Outer Membrane Lipoprotein Lpp Is Gram-Negative Bacterial Cell Surface Receptor for Cationic Antimicrobial Peptides*. J. Biol. Chem. 2012, 287, 418–428. [Google Scholar] [CrossRef]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U.; et al. Small Cationic Antimicrobial Peptides Delocalize Peripheral Membrane Proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–E1418. [Google Scholar] [CrossRef]
- Scheinpflug, K.; Wenzel, M.; Krylova, O.; Bandow, J.E.; Dathe, M.; Strahl, H. Antimicrobial Peptide CWFW Kills by Combining Lipid Phase Separation with Autolysis. Sci. Rep. 2017, 7, 44332. [Google Scholar] [CrossRef]
- Malanovic, N.; Buttress, J.A.; Vejzovic, D.; Ön, A.; Piller, P.; Kolb, D.; Lohner, K.; Strahl, H. Disruption of the Cytoplasmic Membrane Structure and Barrier Function Underlies the Potent Antiseptic Activity of Octenidine in Gram-Positive Bacteria. Appl. Environ. Microbiol. 2022, 88, e0018022. [Google Scholar] [CrossRef] [PubMed]
- Marx, L.; Semeraro, E.F.; Mandl, J.; Kremser, J.; Frewein, M.P.; Malanovic, N.; Lohner, K.; Pabst, G. Bridging the Antimicrobial Activity of Two Lactoferricin Derivatives in E. coli and Lipid-Only Membranes. Front. Med. Technol. 2021, 3, 625975. [Google Scholar] [CrossRef] [PubMed]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]


| Score | Condition |
|---|---|
| 0 | No inhibition at any studied concentration |
| 1 | Delayed growth at 100 μM only |
| 2 | Delayed growth > 200 min at 100 μM only |
| 3 | No growth at 100 μM only |
| 4 | Delayed growth > 200 min at 50 μM |
| 5 | No growth at 50 μM |
| 6 | Delayed > 200 min or no growth at 25 μM |
| 2NO | 2N | 3N | 3N′ | 4N | 4N′ | 3NB | 3NB′ | 4NC | Tail Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 6B | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 4 | 0 | 9 |
| 8 | 0 | 6 | 0 | 4 | 4 | 6 | 0 | 6 | 1 | 27 |
| 8CB | 2 | 1 | 0 | 5 | 0 | 0 | 0 | 0 | 6 | 12 |
| 9 | 0 | 1 | 0 | 6 | 6 | 6 | 0 | 6 | 6 | 31 |
| 9C | 0 | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 6 | 16 |
| 10 | 1 | 0 | 0 | 6 | 5 | 6 | 0 | 5 | 6 | 28 |
| 10B | 1 | 0 | 0 | 5 | 0 | 5 | 0 | 3 | 6 | 19 |
| 10B′ | 0 | 0 | 0 | 5 | 1 | 5 | 0 | 2 | 6 | 19 |
| 11 | 1 | 0 | 1 | 6 | 5 | 6 | 3 | 5 | 6 | 32 |
| 12 | 1 | 1 | 0 | 6 | 2 | 6 | 4 | 4 | 6 | 29 |
| 13B | 1 | 1 | 0 | 4 | 1 | 6 | 0 | 1 | 6 | 19 |
| 14 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 6 | 11 |
| 16 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Head total | 7 | 12 | 4 | 52 | 24 | 58 | 8 | 36 | 61 |
| Tail | Score |
|---|---|
| 6B | 0 |
| 8 | 0 |
| 8CB | 6 |
| 9 | 5 |
| 9C | 5 |
| 10 | 6 |
| 10B | 5 |
| 10B′ | 6 |
| 11 | 6 |
| 12 | 6 |
| 13B | 6 |
| 14 | 3 |
| 16 | 6 |
| Head total | 60 |
| MW | clogP | |
|---|---|---|
| 6B | 128.2 | 2.02 |
| 8 | 128.2 | 2.39 |
| 8CB | 208.3 | 4.22 |
| 9 | 142.2 | 2.92 |
| 9C | 206.3 | 3.69 |
| 10 | 156.2 | 3.45 |
| 10B | 198.3 | 4.49 |
| 10B′ | 184.3 | 4.33 |
| 11 | 170.3 | 3.98 |
| 12 | 184.3 | 4.5 |
| 13B | 212.3 | 5.39 |
| 14 | 212.3 | 5.57 |
| 16 | 240.4 | 6.64 |
| N/MW # (Charge Density) | clogP * | |
|---|---|---|
| 2N | 0.0139 | 1.1 |
| 2NO | 0.0135 | −1.59 |
| 3N | 0.0291 | −1.87 |
| 3N′ | 0.0206 | −0.84 |
| 4N | 0.0274 | −2.18 |
| 4N′ | 0.0198 | −0.96 |
| 3NB | 0.0274 | −2.68 |
| 3NB′ | 0.0212 | −1.01 |
| 4NC | 0.0200 | −0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jennings, J.; Ašćerić, D.; Malanovic, N.; Pabst, G. Structure–Activity Relationships of Cationic Lipidoids against Escherichia coli. Antibiotics 2023, 12, 1300. https://doi.org/10.3390/antibiotics12081300
Jennings J, Ašćerić D, Malanovic N, Pabst G. Structure–Activity Relationships of Cationic Lipidoids against Escherichia coli. Antibiotics. 2023; 12(8):1300. https://doi.org/10.3390/antibiotics12081300
Chicago/Turabian StyleJennings, James, Dunja Ašćerić, Nermina Malanovic, and Georg Pabst. 2023. "Structure–Activity Relationships of Cationic Lipidoids against Escherichia coli" Antibiotics 12, no. 8: 1300. https://doi.org/10.3390/antibiotics12081300
APA StyleJennings, J., Ašćerić, D., Malanovic, N., & Pabst, G. (2023). Structure–Activity Relationships of Cationic Lipidoids against Escherichia coli. Antibiotics, 12(8), 1300. https://doi.org/10.3390/antibiotics12081300






