Abstract
Antimicrobial resistance (AMR) has emerged as an urgent global public health issue that requires immediate attention. Methicillin-resistant staphylococci (MRS) is a major problem, as it may cause serious human and animal infections, eventually resulting in death. This study determined the proportional distribution, genetic characteristics, and antimicrobial susceptibility of mecA- or mecC-carrying staphylococci isolated from food chain products. A total of 230 samples were taken from meat, food, fermented food, and food containers. Overall, 13.9% (32/230) of the samples were identified to have Staphylococcus aureus isolates; of those, 3.9% (9/230) were MRS, with eight mecA-positive and one mecC-positive samples, and 1.3% (3/230) methicillin-resistant Staphylococcus aureus (MRSA). MRSA strains belonging to three sequence types (ST9, ST22, and a newly identified ST), three different spa types (T005, t526, and a newly identified type), and three different SCCmec types (IV, V, and an unidentified SCCmec) were detected. Additionally, eight mecA-positive staphylococcal isolates were identified as S. haemolyticus, S. sciuri, S. simulans, and S. warneri, while the mecC-harboring isolate was S. xylosus. The enterotoxin gene, SEm, was detected at 1.56% in S. aureus, whereas SEq was detected at 0.31%, and SEi was also found in MRSA. Our study emphasizes the importance of enhanced hygiene standards in reducing the risk of occupational and foodborne MRSA infections associated with the handling or consumption of meat, food, and preserved food products.
1. Introduction
Staphylococci are natural inhabitants of the skin and mucous membranes in both humans and various animals. They are typically classified into two groups based on their ability to produce coagulase: coagulase-positive (CoPS) and coagulase-negative staphylococci (CoNS) [1]. Staphylococcus aureus (S. aureus), a CoPS member, is widely recognized as a major causative agent of food poisoning and infections in both clinical and community settings [2,3,4,5]. The production of coagulase by S. aureus promotes blood clotting, and the resulting fibrin coat on the bacterial surface may facilitate the evasion of the immune system. CoNS consist of numerous species, including opportunistic pathogens such as S. epidermidis, S. capitis, S. hominis, S. haemolyticus, S. saccharolyticus, S. warneri, S. lugdunensis, S. saprophyticus, and S. cohnii. Although CoNS lack the ability to produce coagulase, they possess species and strain-specific virulence factors that contribute to their role as notorious opportunistic pathogens. One significant pathogenicity mechanism employed by CoNS is their ability to form biofilms, allowing them to colonize both abiotic surfaces of medical devices and biotic surfaces such as host tissues coated with host factors [6].
This opportunistic pathogen is capable of infecting both humans and other mammals, resulting in a broad spectrum of diseases. These include food poisoning, which manifests as abdominal pains, diarrhea, nausea, and vomiting, as well as more serious conditions such as endocarditis, pneumonia, osteomyelitis, toxic shock syndrome, septicemia, and soft tissue and skin infections [7,8]. In addition, S. aureus is frequently found in animal-derived foods such as undercooked meat and dairy products [9,10,11]. Its ability to survive in a variety of environments and to cause such a wide range of diseases highlights the need for effective prevention and control measures concerning public health.
The pathogenicity of S. aureus is attributed to a combination of factors that contribute to its invasive nature, the production of extracellular factors, and its antibiotic resistance. In order to enhance the process of pathogenesis and facilitate udder infection, S. aureus has developed a range of virulence factors. These factors include various extracellular enzymes such as lipases, proteases, amylases, hyaluronidase, DNases, coagulase, lactamase, hemolysins, and capsules [12]. Additionally, S. aureus produces enterotoxins (SEs: SEA to SEE) and non-classical SE-like toxins (SEl: SEG to SEU) that are associated with food poisoning. Notably, these toxins are resistant to heat, proteolytic enzymes, and low pH conditions. Furthermore, S. aureus is known to produce toxic shock syndrome toxin 1 (TSST-1), a potent superantigenic toxin. The presence of TSST-1 can lead to severe symptoms such as high fever, rash, shock syndrome, hypotension, and the inflammation of the blood system, as well as to the Panton–Valentine leucocidin (PVL), which causes leukocytosis along with necrosis on the skin or mucosa surface, and TSST-1 is capable of inducing lysis of human neutrophils and enhances the adherence of S. aureus to the extracellular matrix [12,13,14,15].
The emergence of methicillin-resistant staphylococci (MRS) presents a significant and concerning threat, as these strains exhibit resistance to all beta-lactam antibiotics, thereby compromising treatment options and increasing the risk of life-threatening infections. It is important to recognize that other coagulase-positive (S. aureus, S. schleiferi, S. delphini, S. intermedius, S pseudintermedius, and S. lutrae) and coagulase-negative MRS species (S. cohnii, S. epidermidis, S. haemolyticus, S. hominis, S. lentus, S. lugdunensis, S. sciuri, and S. xylosus) have gained significance in recent years. These species have been implicated in a variety of opportunistic infections, particularly among immunocompromised patients [16]. The development of methicillin resistance is primarily attributed to the presence of the mecA gene, a pivotal genetic element located on the mobile genetic element known as the staphylococcal cassette chromosome mec (SCCmec). This genetic element encodes an altered penicillin-binding protein (PBP2a), which imparts resistance to methicillin and other beta-lactam antibiotics. In addition to mecA, the presence of other mec genes, including mecB and mecC, has also been recognized as being associated with beta-lactam resistance [1]. This gene is widespread in S. aureus and coagulase-negative staphylococci (CoNS) from both human and animal origin [17,18]. The widespread consumption of antibiotics in the livestock sector has led to their persistent release into the environment and increased antibiotic-resistant bacteria. Numerous studies have demonstrated the presence of mecA-positive methicillin-resistant Staphylococcus aureus (MRSA) in various food sources, such as retail meat, fish, poultry, pork, beef, ready-to-eat foods, and even vegetables [11,19,20,21]. Additionally, CoNS carrying mecA, which are known for their increasing rates of methicillin resistance, have been detected in milk at a rate of 0.6% in Brazil and 6.7% in Tunisia [22,23], in ready-to-eat foods at a rate of 16.4% in Poland [24], and in meat at a rate 2.3–8% in Egypt [25,26], which raises additional concerns about the spread of resistance. In Thailand, the prevalence of MRA was found to be 20.5% in the university environment and 52.3% in the hospital environment [27]. The prevalence of MRSA in meat has been reported as 44.8–50% [28,29]. However, a lower prevalence of MRSA (3.8%) in non-human isolates was reported [30].
The presence of staphylococci in meat is oftentimes because personnel participating in the production process engage in unhygienic behaviors during the processing, shipping, slicing, storage, and point-of-sale stages throughout the production process. By evaluating these factors, valuable insights into the potential transmission of antibiotic resistance and virulence factors through the food chain can be obtained. In light of these findings, the purpose of the present study was to investigate the distribution of methicillin-resistant staphylococci in various food types, with a concentration on characterizing their SCCmec types, spa types, and the presence of enterotoxin genes.
2. Results
2.1. Distribution of Methicillin-Resistant Staphylococci
From the 230 samples, 666 staphylococcal isolates were identified comprising 3 MRSA isolates from 3 samples (1 pork and 2 beef; 3/230, 1.30%); 8 MRS carrying mecA from 8 samples (8/230, 3.47%) consisting of pork (n = 4), beef (n = 2), and chicken (n = 2); 1 MRS harboring mecC from pork (1/230, 0.43%); and 70 S. aureus (methicillin-susceptible) isolates from 32 samples (32/230, 13.91%) consisting of pork (n = 6), beef (n = 5), chicken (n = 19), and fermented food (n = 2). (Table 1)

Table 1.
The number of mecA- and mecC-positive strains, S. aureus and MRSA in different types in this study.
The mecA-harboring MRS (n = 8) were identified as three S. haemolyticus strains, two S. sciuri strains, two S. warneri strains, and one S. stimulans strain. We identified one MRS harboring mecC as S. xylosus (n = 1). These are summarized in Table 2. The proportion of methicillin-resistant staphylococci (MRSA and MRS) present in foods was 12/320, 5.21% in the current study.

Table 2.
Genetic characteristics and resistance profiles of MRSA and mecA- and mecC-positive isolates.
As shown in Table 2, MRSA strain no. B(3)1.2 belonged to SCCmec type IV, ST22, spa t005, and carried the pvl gene. MRSA strain no. P(2)12.4 showed unidentified SCCmec types, ST9, and spa t526, whereas MRSA strain no. B22.5 was SCCmec type V, a new spa type and a new ST; however, it is closely related to ST1455, which is isolated from the lung aspirate of a Chinese patient, as shown in Figure 1. Among the eight mecA-harboring MRS isolates, the most common SCCmec type was an unidentified SCCmec type (5/8, 62.5%), followed by SCCmec types V (2/8, 25%) and III (1/8, 12.5%), as shown in Table 2. Finally, the mecC-harboring S. xylosus carried an unidentified SCCmec type (Table 2).
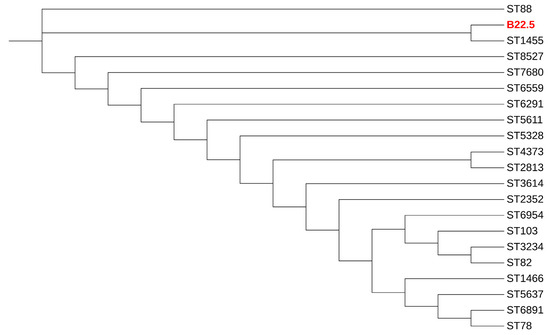
Figure 1.
Dendrogram of concatenated sequences of seven MLST loci of MRSA strain B22.5 (red color), as a new ST isolate, and its related STs.
2.2. Antimicrobial Resistance
All three of the MRSA isolates were multidrug-resistant (MDR), with resistance to erythromycin, oxacillin, cefoxitin, gentamycin, azithromycin, and ciprofloxacin, while two isolates (strain no. P(2)12.4 and B(3)1.2) could induce clindamycin resistance. The mecA- and mecC-carrying staphylococci were classified as MDR in seven strains except for S. haemolyticus strain no.C49.2 and S. xylosus strain no P20.3, as shown in Table 2. However, all isolates were susceptible to vancomycin, linezolid, and rifampin.
2.3. Detection of Foodborne Staphylococcus Aureus Enterotoxin Genes
In total, 73 S. aureus isolates, including 3 MRSA, were detected with five enterotoxin genes (SEj, SEl, Seq, Sem, and SEr). The SEm gene was found in S. aureus (5/73, 6.85%), and the SEq and SEj genes were found in MRSA strain no B22.5 (1/73, 1.37%).
3. Discussion
Antibiotic resistance bacteria are a major global health problem, emerging in a variety of environmental samples. To better comprehend the dissemination of methicillin-resistant staphylococci in food product chains in northeast Thailand, we characterized staphylococcal isolates from foods, food containers, and meat. Our study showed that the proportion of S. aureus (10%) was lower than in other studies in Thailand, for example, 83% (87/105) of S. aureus present in ready-to-eat food samples in Songkhla Province [31] and 60% (36/60) in fermented pork sausage in Amnatcharoen Province [32]. In other countries, S. aureus has been found in retail raw meat samples: 21.23% (96/452) in Tukey [33], 21.81% (89/408) in India [34], 16.9–35% in China [35,36,37], 33.9% (165/487) in Chile [38], and 13.8% (22/160) in Greece [39]. In contrast, in the current study, the proportion of MRSA in food samples was also low (0.94%; 3/320), which was less than for the proportions reported in other regions of Thailand, such as 20% (2/10) [28] and 44.8% (55/116) [29], both in retail pork samples. However, some studies showed a low prevalence of MRSA in non-human samples, for example, 2.2% from retail food and food handlers’ gloves, 1.7% in beef, 1.2–1.9% in pork, 0.3% in chicken, 3.5% in turkey, 1.86% in secondary school environments, and 1.58% from environmental contamination in railway stations and coach stations [30]. Differences in the sampling period, sample size, sampling site, sampling techniques, isolation method, single enrichment step, the frequency of MRSA in different samples, or geographical locations could partially explain the variation in prevalence. However, these results highlight the necessity to mitigate the risk of S. aureus and MRSA transmission via meat products to humans in the food supply chain.
The current study revealed that SCCmec types IV and V were detected concordant with several studies in retail meat products worldwide [40,41,42,43,44]. One MRSA in the current study was ST9, which is predominant in most Asian countries, including Taiwan, Hong Kong, Malaysia, and Thailand [45,46,47,48,49]. The ST9 strains are generally MDR, with >80% resistance to erythromycin, ciprofloxacin, gentamicin, tetracycline, and clindamycin [50], which was similar to our strain in the current study. Our MRSA ST22 strain is the epidemic clone EMRSA-15, and it is a hospital-associated pathogen, typically resistant to ciprofloxacin and erythromycin [51,52,53]. However, some studies have reported MRSA ST22 isolated from animals [54,55]. It is interesting that a novel ST of MRSA was identified from beef samples in the current study. This ST was closely related to ST 1455, which was isolated from a human patient’s bronchoalveolar lavage [56]. Therefore, this novel ST should be subjected to monitoring and surveillance.
The mecA-carrying staphylococcal isolates other than MRSA in the current study belonged to five species of coagulase-negative staphylococci, namely S. haemolyticus (37.5%), S. sciuri (25%), S. warneri (25%), and S. simulans (12.5%), while the mecC-harboring isolate was S. xylosus. In Egypt, Osman et al. detected S. hyicus (30%), S. intermedius (15%), S. epidermidis (5%), S. hemilyticus (5%), S. hominis (5%), S. lugdumenis (15%), S. simulants (5%), and S. scuri (20%) in imported beef meat [25]. Boamah et al. identified S. gallinarum (32%); S. saprophyticus (20%); S. chromogens (20%); S. warneri (12%); S. hominis (8%); S. caprae and S. epidermidis (4%); S. sciuri (42.97%); S. lentus (35.94%); S. xylosus (4.30%); S. haemolyticus (3.91%); S. saprophyticus (1.95%); and S. cohnii (0.39%) in poultry in Ghana [57]. Pimenta et al. found S. gallinarum (35.2%); S. simulans (17%); S. sciuri (10.2%); S. lentus (4.5%); and S. cohnii and S. xylosus (2.2%) in broiler chicken products in Brazil [58]. Moreover, in Korea, S. agnetis (19.4%), S. saprophyticus (19%), S. chromogens (14.5%), S hyicus (12.9%), and S. sciuri (13.8%) were detected in retail chicken meat [1]. These findings suggest that the frequent occurrence of non-aureus staphylococci in meat may be a hazard associated with food and public health safety. Some of them can cause foodborne infections [59,60], contribute to antibiotic resistance transmission [61], and lead to zoonotic infections [62]. Therefore, close monitoring should be carefully considered.
Regarding the risk of foodborne intoxication, numerous surveys of staphylococcal enterotoxins (SEs) have been reported, which have identified five classical enterotoxin types, SEa to SEe [63], and many new types of SEs have been reported: SEg, SEh, SEi, SEk, SEl, SEm, SEn, SEo, SEp, SEq, SEr, and SEu [64]. One of the limitations of this study is that we did not detect the classical enterotoxin genes; therefore, these classical enterotoxin genes could not be ruled out in our S. aureus isolates. Hu et al. showed five new types of enterotoxin genes, namely SEj, SEl, SEq, SEm, and SEr. Of these, SEj and SEr were detected in 16.6% and 14.3%, respectively [65]. Additionally, SEi (97.2%) and SEm (86.1%) were frequently detected in retail foods in China [11]. In contrast, our study revealed SEm in S. aureus (6.1%, 5/82) and SEq and SEi in MRSA (1.2%, 1/82). There has been a rise in the number of foodborne staphylococcal isolates, especially MRSA, which is linked to novel enterotoxins; therefore, these data indicate that we should pay attention to both types of toxins. In addition, the five new enterotoxin genes were extensively present in proteins of animal origin compared with that from other origins. This is related to the animal characteristics and interaction with the living environment, operation environment for food processing, and storage environment for finished products [65].
4. Materials and Methods
4.1. Ethical Statement
Ethical review and approval were not required because this study did not involve human subjects.
4.2. Sample Collection, Isolation, and Presumptive Deification
From June to December 2019, a total of 230 samples were taken from various foods and storage containers located in rural northeastern Thailand. A variety of samples, including food containers, meat, pork, chicken, beef, and pickled food, were gathered. The samples included 80 food container samples, 30 food samples, 90 meat samples (30 pork, 30 chicken, and 30 beef), and 30 pickled food samples. The collection of samples was performed in sterile conditions. Storage containers were swabbed using sterile cotton that was immediately placed in 1 mL of Mannitol salt broth (MSB) (HiMedia Laboratories Pvt. Ltd.; Nashik, India). Food product samples were collected in accordance with Sorour et al. [26]. The samples were transferred to the laboratory in sterile plastic bags. A 10 g amount of each food sample was diluted with 90 mL of buffer peptone water (BPW) (HiMedia Laboratories Pvt. Ltd.; Nashik, India), incubated overnight at 37 °C under aerobic conditions, and then streaked on mannitol salt agar medium (MSA) (HiMedia Laboratories Pvt. Ltd.; Nashik, India) before incubating at 37 °C and examined after 24 h to 48 h. There was a presumption that the colonies on MSA, which were colored yellow and pink, were staphylococci. Following the preliminary fundamental phenotypic examination (which included a microscopic inspection, Gram staining, catalase production, and coagulase tube test utilizing rabbit plasma), these isolates were identified at the species level via either PCR or DNA sequencing, as will be detailed in subsequent sections.
4.3. Microbiology and Molecular Characterization of S. Aureus and MRSA
In accordance with the protocol provided by the manufacturer, total genomic DNA was extracted using a ZymoBIOMICsTM DNA Miniprep Kit (Zymo Research; Irvine, CA, USA). The quantity and purity of DNA were determined using a NanoDropTM 2000 Spectrometer (Thermo Fisher Scientific; Waltham, MA, USA), and the DNA sample was stored at −20 °C for further study.
Multiplex PCR was performed to detect femA genes specific for S. aureus species, and the mecA, mecC, and lukS genes following a previously established protocol [66,67]. The sequence primers are shown in Table 3. DNA amplification was carried out in 25 μL of a PCR mixture that contained 12.5 μL of 2x JumpStart™ REDTaq® ReadyMix™ Reaction Mix (SIGMA; Saint Louis, MO, USA), 0.4 μM of each primer, 100 ng of the DNA sample, and sterile deionized water. PCR was carried out using the following thermal cyclic conditions: initial denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, an extension at 72 °C for 30 s, a final extension of 72 °C for 5 min, and cooling to 4 °C.

Table 3.
Sequences primers of target genes in the current study.
4.4. Enterotoxin Genes and PVL Detection
S. aureus isolates were subjected to PCR for the identification of five enterotoxin genes, namely SEj, SEl, SEq, SEm, and SEr, as described elsewhere [65]. Briefly, the total reaction volume was 25 μL and included the following: 12.5 μL 2x MytaqTM HS Red Mix (Bioline Reagents Ltd.; London, UK), sterile deionized water, 1 μM of each primer, and 100 ng DNA template. The PCR conditions were as follows: pre-denaturation at 94 °C, 40 s; annealing at 52 °C, 40 s; and extension at 72 °C, 1 min for a total of 35 cycles; and final extension for at 72 °C, 10 min. This procedure was used for all genes except SEj, for which the annealing temperature was 55 °C.
4.5. Sequencing of mecA-or mecC-Harboring Staphylococci
Sequencing was carried out as described by Poyart et al. [68]. The DNA samples were amplified for the sodA gene with the primer sodA-F (5′ CCITAYICITAYGAYGCIYTIGARCC-3′) and sodA-R (5′-ARRTARTAIGCRT GYTCCCAIACRTC-3′). Briefly, 50 µL of the reaction mixture was used, which contained 25 µL of 2x MytaqTM HS Red Mix (Bioline Reagents Ltd.; London, UK), sterile deionized water, 0.75 µM of each primer, and 100 ng of bacterial DNA sample. Thermal cycling reaction conditions consisted of initial denaturing at 95 °C for 3 min and then being subjected to 35 cycles of amplification, denaturation at 95 °C for 30 s, annealing at 37 °C for 60 s, and elongation at 72 °C for 45 s. The PCR amplicons were purified using a GF-1 AmbiClean Kit (Vivantis Technologies Sdn Bhd; Kuala Lumpur, Malaysia) and then sequenced at 1st BASE products and services company, Malaysia. The Basic Local Alignment Search Tool (BLASTN) was used to identify species of staphylococci using a cut-off value of ≥97% [69].
4.6. Molecular Typing
To determine the Staphylococcal Chromosomal Cassette (SCCmec) type, a multiplex PCR (M-PCR) was performed according to the method described by Kondo et al. [68]. M-PCR 1, designed for the ccr type assignment, employed two primers for mecA detection and eight primers for the identification of five ccr genes. Within these eight primers, there were four primers that included a forward primer shared by ccrB1-3 and three reverse primers specific to ccrA1, ccrA2, and ccrA3. This allowed for the identification of ccr1-3 based on the differences in the ccrA genes. Additionally, two primers were utilized for identifying ccr4 and two for identifying ccr5. In M-PCR 2, which aimed to assign mec classes, four primers were employed to identify the gene lineages of mecA-mecI (class A mec), mecA-IS1272 (class B mec), and mecA-IS431 (class C mec). The PCR reaction mixture for both M-PCR 1 and M-PCR 2 consisted of 100 ng of DNA extract in a total volume of 25 µL. This mixture included 12.5 μlx of 2× JumpStart™ REDTaq® ReadyMix™ PCR Reaction Mix (SIGMA; Saint Louis, MO, USA) and a concentration of 0.2 µM for each primer. The thermal cycling conditions involved an initial denaturation step at 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 2 min, annealing at 57 °C for 1 min, and extension at 72 °C for 2 min. The amplification process concluded with a final extension step at 72 °C for 2 min.
Multilocus sequence typing (MLST) was performed following the protocol described elsewhere [70]. Seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) were amplified using PCR. The PCRs were carried out with 50 μL reaction volumes containing 12.5 μL 2x MytaqTM HS Red Mix (Bioline Reagents Ltd.; London, UK), 2.5 µM of each primer, 100 ng bacterial DNA sample, and sterile deionized water. PCR amplification was performed with thermal cycling reaction conditions consisting of initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min, and the cycle was completed with a single extension at 72 °C for 5 min. The PCR amplicons were purified using a GF-1 AmbiClean Kit (Vivantis Technologies Sdn Bhd; Kuala Lumpur, Malaysia) and then sequenced at 1st BASE products and services company, Malaysia. The alleles and sequence types (STs) were identified using the scheme published in multilocus sequence typing databases (https://pubmlst.org/organisms/staphylococcus-aureus, (accessed on 20 February 2023).
The spa typing was performed via the amplification of polymorphic X region of the S. aureus protein A gene (spa) using the standard primers spa-1095F (5′-AGACGATCCTTCGGTGAGC3′) and spa-1517R (5′-GCTTTTGCAATGTCATTTACTG3′) and a PCR program described elsewhere [71]. Briefly, 50 µL of the reaction mixture was used, which contained 25 μL 2X MytaqTM HS Red Mix (Bioline Reagents Ltd., London, UK), sterile deionized water, and 100 ng of the bacterial DNA sample. Thermal cycling reaction conditions consisted of initial denaturation at 80 °C, 5 min; 35 cycles of denaturation at 94 °C, 45 s; annealing at 60 °C, 45 s; and extension at 72 °C, 90 s; and finally, a single extension at 72 °C, 10 min. The PCR amplicons were purified using a GF-1 AmbiClean Kit (Vivantis Technologies Sdn Bhd; Kuala Lumpur, Malaysia) and then sequenced at 1st BASE products and services company, Malaysia. Spa types were determined with the Spa Typer website http://spatyper.fortinbras.us, (accessed on 20 February 2023).
4.7. Electrophoretic Analysis of PCR Products
After amplification, 5 μL of PCR product was subjected to analysis on 2% agarose gel (Bioline Reagents Ltd., London, UK) in 0.5X TBE buffer (Omega BioTek, Inc; Norcross, Georgia) to determine the molecular weight of the amplified DNA fragment. The 5 μL GeneRuler 100 bp Plus DNA ladder (Thermo Scientific; Vilnius, Lithuania) was loaded onto the same agarose gel as a molecular weight standard. Subsequently, the gel was stained with ethidium bromide (Wako, Wako Pure Chemical Industries, Ltd.; Tokyo, Japan) and destained by soaking it in water. Electrophoresis was performed on horizontal electrophoresis equipment (Mupid-Exu; Chuo-ku, Japan) for 30 min at a constant 100 Volts. Subsequently, the gel was visualized using a UV Transilluminator (SynGene; Cambridge, UK), enabling a comparison between the migration patterns of the DNA ladder bands and the PCR products.
4.8. Analysis of New STs
The construction of the phylogenetic tree for STs that are closely related to strain B22.5, a new ST, was performed in this study via Phylogeny.fr [72]. The phylogenetic tree was visualized using the Interactive Tree of Life (iTOL) (http://itol.embl.de, (accessed on 7 July 2023) [73].
4.9. Antimicrobial Susceptibility Testing
The antimicrobial susceptibility test was performed using disk diffusion in Mueller–Hinton agar (Merck; Darmstadt, Germany) according to the 2023 Clinical and Laboratory Standards Institute guidelines, using 13 antimicrobials of different classes including cefoxitin (FOX, 30 µg), oxacillin (OX, 1 µg), tetracycline (TE, 30 µg), erythromycin (E, 15 µg), azithromycin (AZM, 15 µg), chloramphenicol (C, 30 µg), ciprofloxacin (CIP, 5 µg), trimethoprim/sulfamethoxazole (SXT, 25 µg), gentamycin (CN, 10 µg), rifampin (RA, 5 µg), linezolid (LZD, 30 µg), and clindamycin (DA, 2 µg) sourced from OXOID Ltd. (Hampshire, UK), while vancomycin was determined with minimum inhibitory concentrations (MICs). S. aureus ATCC 25,923 was used as a quality control strain for antimicrobial susceptibility testing. The plates were incubated at 37 °C for 18–24 h. After overnight incubation, the zone of inhibition was measured and interpreted as susceptible, intermediate, and resistant based on the recommendation of CLSI (2023) [74]. All antimicrobial susceptibility tests were repeated three times. Multi-drug resistance patterns of the isolates were identified according to the guideline described by Magiorakos et al. [75].
The D test method was carried out in accordance with Chavez-Bueno S et al. [76] in order to determine whether or not inducible resistance to clindamycin develops. In Brief, the bacterial isolates were plated on a Mueller–Hinton agar plate at a MacFarland concentration of 0.5 to evenly cover the agar surface. Clindamycin and erythromycin disks, containing 2 μg and 15 μg of each antibiotic, were placed in the middle of the plate separated by a distance of 1.5 cm between the edges. Plates were incubated at 37 °C for 24 h. Inducible resistance to clindamycin was defined as the blunting of the clear circular area of no growth surrounding the clindamycin disk on the side adjacent to the erythromycin disk, and a positive D test result indicated that this type of resistance had been induced. It was determined the D test was negative since there was no evidence of a blunted zone of inhibition, which demonstrates that the strain is in fact susceptible to clindamycin.
5. Conclusions
Through the course of our research, we were able to present a comprehensive analysis that shed light on the proportional distribution of S. aureus, methicillin-resistant staphylococcus aureus (MRSA), and coagulase-negative staphylococci carrying mecA or mecC genes in various food categories such as meat, general food items, and pickled foods, as well as in food containers across the rural landscape of northeastern Thailand. These findings bring to light possible concerns with regard to public health, more notably those concerning the environmental contamination of staphylococci that are present within the food chain. It is essential to understand that the existence of these bacteria in meat and other food products may serve as a possible source of antimicrobial resistance and enterotoxin genes, leading to cross-contamination between the population and livestock. As a result, it is necessary to give careful consideration to the control of these bacteria and take appropriate preventative actions in order to limit the risks associated with their presence.
Author Contributions
Conceptualization, A.K. and P.C.; formal analysis, R.K.; funding acquisition, P.C.; investigation, R.K., P.B. and R.H.; methodology, R.K., W.K. and P.C.; resources, A.K.; supervision, A.K.; validation, W.K. and P.C.; writing—original draft preparation, R.K. and P.C.; writing—review and editing, A.K. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported in part by the Graduate Program Scholarship from The Graduate School, Kasetsart University, Bangkok, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand, provided English editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, S.I.; Kim, S.D.; Park, J.H.; Yang, S.J. Species Distribution, Antimicrobial Resistance, and Enterotoxigenicity of Non- aureus Staphylococci in Retail Chicken Meat. Antibiotics 2020, 9, 809. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Sudjarwo, S.A.; Effendi, M.H.; Ramandinianto, S.C.; Gelolodo, M.A.; Widodo, A.; Riwu, K.H.P.; Kurniawati, D.A. Pet animals as reservoirs for spreading methicillin-resistant Staphylococcus aureus to human health. J. Adv. Vet. Anim. Res. 2023, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Ren, Q.; Liao, G.; Wu, Z.; Lv, J.; Chen, W. Prevalence and characterization of Staphylococcus aureus isolates from subclinical bovine mastitis in southern Xinjiang, China. J. Dairy Sci. 2020, 103, 3368–3380. [Google Scholar] [CrossRef]
- Becker, K.; Both, A.; Weißelberg, S.; Heilmann, C.; Rohde, H. Emergence of coagulase-negative staphylococci. Expert Rev. Anti-Infect. Ther. 2020, 18, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.G.; Feil, E.J.; Lindsay, J.A.; Peacock, S.J.; Day, N.P.J.; Enright, M.C.; Foster, T.J.; Moore, C.E.; Hurst, L.; Atkin, R.; et al. Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 2004, 101, 9786–9791. [Google Scholar] [CrossRef] [PubMed]
- Urmi, M.R.; Ansari, W.K.; Islam, M.S.; Sobur, M.A.; Rahman, M.; Rahman, M.T. Antibiotic resistance patterns of Staphylococcus spp. isolated from fast foods sold in different restaurants of Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 2021, 8, 274–281. [Google Scholar] [CrossRef]
- Sadat, A.; Shata, R.R.; Farag, A.M.M.; Ramadan, H.; Alkhedaide, A.; Soliman, M.M.; Elbadawy, M.; Abugomaa, A.; Awad, A. Prevalence and Characterization of PVL-Positive Staphylococcus aureus Isolated from Raw Cow’s Milk. Toxins 2022, 14, 97. [Google Scholar] [CrossRef]
- Al-Ashmawy, M.A.; Sallam, K.I.; Abd-Elghany, S.M.; Elhadidy, M.; Tamura, T. Prevalence, Molecular Characterization, and Antimicrobial Susceptibility of Methicillin-Resistant Staphylococcus aureus Isolated from Milk and Dairy Products. Foodborne Pathog. Dis. 2016, 13, 156–162. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, F.; Wu, Q.; Zhang, J.; Pang, R.; Zeng, H.; Yang, X.; Chen, M.; Wang, J.; et al. Prevalence and Characterization of Food-Related Methicillin-Resistant Staphylococcus aureus (MRSA) in China. Front. Microbiol. 2019, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Ramos, C.; Monteiro, V.; Santos, J.; Fernandes, P. Virulence Potential and Antibiotic Susceptibility of S. aureus Strains Isolated from Food Handlers. Microorganisms 2022, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Papadopoulos, T.; Angelidis, A.S.; Kotzamanidis, C.; Zdragas, A.; Papa, A.; Filioussis, G.; Sergelidis, D. Prevalence, antimicrobial susceptibility and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus isolated from dairy industries in north-central and north-eastern Greece. Int. J. Food Microbiol. 2019, 291, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Zhang, Q.; Trongtorsak, A.; Pyakuryal, B.; Egoryan, G.; Sous, M.; Ahmed, R.; Trelles-Garcia, D.P.; Yanez-Bello, M.A.; Trelles-Garcia, V.P.; et al. An overlooked cause of septic shock: Staphylococcal Toxic Shock Syndrome secondary to an axillary abscess. IDCases 2020, 23, e01039. [Google Scholar] [CrossRef]
- Schaumburg, F.; Ngoa, U.A.; Kösters, K.; Köck, R.; Adegnika, A.A.; Kremsner, P.G.; Lell, B.; Peters, G.; Mellmann, A.; Becker, K. Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin. Microbiol. Infect. 2011, 17, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Buz On-Dur, L.; Capita, R.; Alonso-Calleja, C. Antibiotic susceptibility of methicillin-resistant staphylococci (MRS) of food origin: A comparison of agar disc diffusion method and a commercially available miniaturized test. Food Microbiol. 2017, 72, 220–224. [Google Scholar] [CrossRef]
- Huber, H.; Ziegler, D.; Pflüger, V.; Vogel, G.; Zweifel, C.; Stephan, R. Prevalence and characteristics of methicillin-resistant coagulase-negative staphylococci from livestock, chicken carcasses, bulk tank milk, minced meat, and contact persons. BMC Vet. Res. 2011, 7, 6. [Google Scholar] [CrossRef]
- Nemeghaire, S.; Vanderhaeghen, W.; Angeles Argudín, M.; Haesebrouck, F.; Butaye, P. Characterization of methicillin-resistant Staphylococcus sciuri isolates from industrially raised pigs, cattle and broiler chickens. J. Antimicrob. Chemother. 2014, 69, 2928–2934. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Beshiru, A.; Igbinosa, I.H.; Ogofure, A.G.; Ekundayo, T.C.; Okoh, A.I. Prevalence, multiple antibiotic resistance and virulence profile of methicillin-resistant Staphylococcus aureus (MRSA) in retail poultry meat from Edo, Nigeria. Front. Cell. Infect. Microbiol. 2023, 13, 183. [Google Scholar] [CrossRef]
- Bernier-Lachance, J.; Arsenault, J.; Usongo, V.; Parent, E.; Labrie, J.; Jacques, M.; Malouin, F.; Archambault, M. Prevalence and characteristics of Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada. PLoS ONE 2020, 15, e0227183. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Yu, S.; Wu, Q.; Guo, W.; Huang, J.; Cai, S. Prevalence of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus in Retail Ready-to-Eat Foods in China. Front. Microbiol. 2016, 7, 816. [Google Scholar] [CrossRef] [PubMed]
- Klibi, A.; Maaroufi, A.; Torres, C.; Jouini, A. Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia. Int. J. Antimicrob. Agents 2018, 52, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Fernandes dos Santos, F.; Mendonça, L.C.; Reis, D.R.d.L.; Guimarães, A.d.S.; Lange, C.C.; Ribeiro, J.B.; Machado, M.A.; Brito, M.A.V.P. Presence of mecA-positive multidrug-resistant Staphylococcus epidermidis in bovine milk samples in Brazil. J. Dairy Sci. 2016, 99, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Chajecka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Laniewska-Trokenheim, L. Coagulase-negative staphylococci (CoNS) isolated from ready-to-eat food of animal origin--phenotypic and genotypic antibiotic resistance. Food Microbiol. 2015, 46, 222–226. [Google Scholar] [CrossRef]
- Osman, K.; Alvarez-Ordóñez, A.; Ruiz, L.; Badr, J.; ElHofy, F.; Al-Maary, K.S.; Moussa, I.M.I.; Hessain, A.M.; Orabi, A.; Saad, A.; et al. Antimicrobial resistance and virulence characterization of Staphylococcus aureus and coagulase-negative staphylococci from imported beef meat. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 35. [Google Scholar] [CrossRef]
- Sorour, H.K.; Shalaby, A.G.; Abdelmagid, M.A.; Hosny, R.A. Characterization and pathogenicity of multidrug-resistant coagulase-negative Staphylococci isolates in chickens. Int. Microbiol. 2023, 1–12. [Google Scholar] [CrossRef]
- Seng, R.; Kitti, T.; Thummeepak, R.; Kongthai, P.; Leungtongkam, U.; Wannalerdsakun, S.; Sitthisak, S. Biofilm formation of methicillin-resistant coagulase negative staphylococci (MR-CoNS) isolated from community and hospital environments. PLoS ONE 2017, 12, e0184172. [Google Scholar] [CrossRef]
- Vestergaard, M.; Cavaco, L.M.; Sirichote, P.; Unahalekhaka, A.; Dangsakul, W.; Svendsen, C.A.; Aarestrup, F.M.; Hendriksen, R.S. SCCmec Type IX Element in Methicillin Resistant Staphylococcusaureusspa Type t337 (CC9) Isolated from Pigs and Pork in Thailand. Front. Microbiol. 2012, 3, 103. [Google Scholar] [CrossRef]
- Tanomsridachchai, W.; Changkaew, K.; Changkwanyeun, R.; Prapasawat, W.; Intarapuk, A.; Fukushima, Y.; Yamasamit, N.; Kapalamula, T.F.; Nakajima, C.; Suthienkul, O.; et al. Antimicrobial Resistance and Molecular Characterization of Methicillin-Resistant Staphylococcus aureus Isolated from Slaughtered Pigs and Pork in the Central Region of Thailand. Antibiotics 2021, 10, 206. [Google Scholar] [CrossRef]
- Saenhom, N.; Kansan, R.; Chopjitt, P.; Boueroy, P.; Hatrongjit, R.; Kerdsin, A. Evaluation of in-house cefoxitin screening broth to determine methicillin-resistant staphylococci. Heliyon 2022, 8, ee08950. [Google Scholar] [CrossRef]
- Sukhumungoon, P.; Bunnueang, N.; Kongpheng, S.; Singkhamanan, K.; Saengsuwan, P.; Rattanachuay, P.; Dangsriwan, S. Methicillin-Resistant Staphylococcus aureus from Ready-to-Eat Foods in a Hospital Canteen, Southern Thailand: Virulence Characterization And Genetic Relationship. Southeast Asian J. Trop. Med. Public Health 2015, 46, 86. [Google Scholar]
- Sankomkai, W.; Boonyanugomol, W.; Kraisriwattana, K.; Nutchanon, J.; Boonsam, K.; Kaewbutra, S.; Wongboot, W. Characterisation of Classical Enterotoxins, Virulence Activity, and Antibiotic Susceptibility of Staphylococcus aureus Isolated from Thai Fermented Pork Sausages, Clinical Samples, and Healthy Carriers in Northeastern Thailand. J. Vet. Res. 2020, 64, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Şanlıbaba, P. Prevalence, antibiotic resistance, and enterotoxin production of Staphylococcus aureus isolated from retail raw beef, sheep, and lamb meat in Turkey. Int. J. Food Microbiol. 2022, 361, 109461. [Google Scholar] [CrossRef]
- Zehra, A.; Gulzar, M.; Singh, R.; Kaur, S.; Gill, J.P.S. Prevalence, multidrug resistance and molecular typing of methicillin-resistant Staphylococcus aureus (MRSA) in retail meat from Punjab, India. J. Glob. Antimicrob. Resist. 2019, 16, 152–158. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Wu, Q.; Zhang, J.; Zhang, F.; Yang, X.; Wu, H.; Zeng, H.; Chen, M.; Ding, Y.; et al. Staphylococcus aureus Isolated From Retail Meat and Meat Products in China: Incidence, Antibiotic Resistance and Genetic Diversity. Front. Microbiol. 2018, 9, 2767. [Google Scholar] [CrossRef]
- Ou, C.; Shang, D.; Yang, J.; Chen, B.; Chang, J.; Jin, F.; Shi, C. Prevalence of multidrug-resistant Staphylococcus aureus isolates with strong biofilm formation ability among animal-based food in Shanghai. Food Control 2020, 112, 107106. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, X.; Chen, X.; Zou, G.; Huang, Q.; Meng, X.; Pei, X.; Chen, Z.; Zhou, R.; Hu, D.; et al. Prevalence and Virulence Determinants of Staphylococcus aureus in Wholesale and Retail Pork in Wuhan, Central China. Foods 2022, 11, 4114. [Google Scholar] [CrossRef]
- Velasco, V.; Vergara, J.L.; Bonilla, A.M.; Muñoz, J.; Mallea, A.; Vallejos, D.; Quezada-Aguiluz, M.; Campos, J.; Rojas-García, P. Prevalence and Characterization of Staphylococcus aureus Strains in the Pork Chain Supply in Chile. Foodborne Pathog. Dis. 2018, 15, 262–268. [Google Scholar] [CrossRef]
- Komodromos, D.; Kotzamanidis, C.; Giantzi, V.; Pappa, S.; Papa, A.; Zdragas, A.; Angelidis, A.; Sergelidis, D. Prevalence, Infectious Characteristics and Genetic Diversity of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus (MRSA) in Two Raw-Meat Processing Establishments in Northern Greece. Pathog. 2022, 11, 1370. [Google Scholar] [CrossRef]
- Feßler, A.T.; Kadlec, K.; Hassel, M.; Hauschild, T.; Eidam, C.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of methicillin-resistant Staphylococcus aureus isolates from food and food products of poultry origin in Germany. Appl. Environ. Microbiol. 2011, 77, 7151–7157. [Google Scholar] [CrossRef]
- Boost, M.V.; Wong, A.; Ho, J.; O’Donoghue, M. Isolation of methicillin-resistant Staphylococcus aureus (MRSA) from retail meats in Hong Kong. Foodborne Pathog. Dis. 2013, 10, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Wang, X.; Donabedian, S.; Zervos, M.; da Rocha, L.; Zhang, Y. Methicillin-resistant Staphylococcus aureus in retail meat, Detroit, Michigan, USA. Emerg. Infect. Dis. 2011, 17, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.M.; Dressler, A.E.; Harper, A.L.; Scheibel, R.P.; Wardyn, S.E.; Roberts, L.K.; Kroeger, J.S.; Smith, T.C. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) on retail meat in Iowa. J. Infect. Public Health 2011, 4, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Benito, D.; Gómez, P.; Lozano, C.; Estepa, V.; Gómez-Sanz, E.; Zarazaga, M.; Torres, C. Genetic lineages, antimicrobial resistance, and virulence in Staphylococcus aureus of meat samples in Spain: Analysis of immune evasion cluster (IEC) genes. Foodborne Pathog. Dis. 2014, 11, 354–356. [Google Scholar] [CrossRef]
- Lo, Y.P.; Wan, M.T.; Chen, M.M.; Su, H.Y.; Lauderdale, T.L.; Chou, C.C. Molecular characterization and clonal genetic diversity of methicillin-resistant Staphylococcus aureus of pig origin in Taiwan. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 513–521. [Google Scholar] [CrossRef]
- Guardabassi, L.; O’donoghue, M.; Moodley, A.; Ho, J.; Boost, M. Novel Lineage of Methicillin-Resistant Staphylococcus aureus, Hong Kong. Emerg. Infect. Dis. 2009, 15, 1998. [Google Scholar] [CrossRef]
- Neela, V.; Zafrul, A.M.; Mariana, N.S.; Van Belkum, A.; Liew, Y.K.; Rad, E.G. Prevalence of ST9 methicillin-resistant Staphylococcus aureus among pigs and pig handlers in Malaysia. J. Clin. Microbiol. 2009, 47, 4138–4140. [Google Scholar] [CrossRef]
- Anukool, U.; O’Neill, C.E.; Butr-Indr, B.; Hawkey, P.M.; Gaze, W.H.; Wellington, E.M.H. Meticillin-resistant Staphylococcus aureus in pigs from Thailand. Int. J. Antimicrob. Agents 2011, 38, 86–87. [Google Scholar] [CrossRef]
- Larsen, J.; Imanishi, M.; Hinjoy, S.; Tharavichitkul, P.; Duangsong, K.; Davis, M.F.; Nelson, K.E.; Larsen, A.R.; Skov, R.L. Methicillin-resistant Staphylococcus aureus ST9 in pigs in Thailand. PLoS ONE 2012, 7, e31245. [Google Scholar] [CrossRef]
- Chuang, Y.Y.; Huang, Y.C. Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: An emerging issue? Int. J. Antimicrob. Agents 2015, 45, 334–340. [Google Scholar] [CrossRef]
- Silva, V.; Almeida, F.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Tejedor-Junco, M.T.; Caniça, M.; Igrejas, G.; Poeta, P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bonura, C.; Plano, M.R.A.; Di Carlo, P.; Calà, C.; Cipolla, D.; Corsello, G.; Mammina, C. MRSA ST22-IVa (EMRSA-15 clone) in Palermo, Italy. J. Infect. Public Health 2010, 3, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Niek, W.K.; Teh, C.S.J.; Idris, N.; Thong, K.L.; Ponnampalavanar, S. Predominance of ST22-MRSA-IV Clone and Emergence of Clones for Methicillin-Resistant Staphylococcus aureus Clinical Isolates Collected from a Tertiary Teaching Hospital Over a Two-Year Period. Jpn. J. Infect. Dis. 2019, 72, 228–236. [Google Scholar] [CrossRef]
- Coelho, C.; Torres, C.; Radhouani, H.; Pinto, L.; Lozano, C.; Gómez-Sanz, E.; Zaragaza, M.; Igrejas, G.; Poeta, P. Molecular detection and characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolates from dogs in Portugal. Microb. Drug Resist. 2011, 17, 333–337. [Google Scholar] [CrossRef]
- Costa, S.S.; Ribeiro, R.; Serrano, M.; Oliveira, K.; Ferreira, C.; Leal, M.; Pomba, C.; Couto, I. Staphylococcus aureus Causing Skin and Soft Tissue Infections in Companion Animals: Antimicrobial Resistance Profiles and Clonal Lineages. Antibiotics 2022, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z.; Chen, Y.S.; Yang, J.P.; Zhang, W.; Hu, C.P.; Li, J.S.; Mu, L.; Hu, Y.H.; Geng, R.; Hu, K.; et al. Preliminary molecular epidemiology of the Staphylococcus aureus in lower respiratory tract infections: A multicenter study in China. Chin. Med. J. 2011, 124, 687–692. [Google Scholar] [CrossRef]
- Boamah, V.E.; Agyare, C.; Odoi, H.; Adu, F.; Gbedema, S.Y.; Dalsgaard, A. Prevalence and antibiotic resistance of coagulase-negative Staphylococci isolated from poultry farms in three regions of Ghana. Infect. Drug Resist. 2017, 10, 175–183. [Google Scholar] [CrossRef]
- Pimenta, R.L.; de Melo, D.A.; Bronzato, G.F.; de Salles Souza, V.R.; Holmström, T.C.N.; de Oliveira Coelho, S.D.M.; da Silva Coelho, I.; de Souza, M.M.S. Characterization of staphylococcus spp. isolates and β-lactam resistance in broiler chicken production. Rev. Bras. Med. Vet. 2021, 43, e00720. [Google Scholar] [CrossRef]
- Rall, V.L.M.; Sforcin, J.M.; De Deus, M.F.R.; De Sousa, D.C.; Camargo, C.H.; Godinho, N.C.; Galindo, L.A.; Soares, T.C.S.; Araújo, J.P. Polymerase Chain Reaction Detection of Enterotoxins Genes in Coagulase-Negative Staphylococci Isolated from Brazilian Minas Cheese. Foodborne Pathog. Dis. 2010, 7, 1121–1123. [Google Scholar] [CrossRef]
- Podkowik, M.; Park, J.Y.; Seo, K.S.; Bystroń, J.; Bania, J. Enterotoxigenic potential of coagulase-negative staphylococci. Int. J. Food Microbiol. 2013, 163, 34–40. [Google Scholar] [CrossRef]
- Silva, V.; Caniça, M.; Ferreira, E.; Vieira-Pinto, M.; Saraiva, C.; Pereira, J.E.; Capelo, J.L.; Igrejas, G.; Poeta, P. Multidrug-Resistant Methicillin-Resistant Coagulase-Negative Staphylococci in Healthy Poultry Slaughtered for Human Consumption. Antibiotics 2022, 11, 365. [Google Scholar] [CrossRef]
- El-Deeb, W.; Cave, R.; Fayez, M.; Alhumam, N.; Quadri, S.; Mkrtchyan, H.V. Methicillin Resistant Staphylococci Isolated from Goats and Their Farm Environments in Saudi Arabia Genotypically Linked to Known Human Clinical Isolates: A Pilot Study. Microbiol. Spectr. 2022, 10, e00387-22. [Google Scholar] [CrossRef]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Liao, W.W.; Fan, C.M.; Pai, W.Y.; Chiou, C.S.; Tsen, H.Y. PCR detection of Staphylococcal enterotoxins (SEs) N, O, P, Q, R, U, and survey of SE types in Staphylococcus aureus isolates from food-poisoning cases in Taiwan. Int. J. Food Microbiol. 2008, 121, 66–73. [Google Scholar] [CrossRef]
- Hu, W.D. Distribution of food-borne Staphylococcus aureus enterotoxin genes. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-Talib, H.; Yean, C.Y.; Al-Khateeb, A.; Hassan, H.; Singh, K.K.B.; Al-Jashamy, K.; Ravichandran, M. A pentaplex PCR assay for the rapid detection of methicillin-resistant Staphylococcus aureus and Panton-Valentine Leucocidin. BMC Microbiol. 2009, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Stegger, M.; Andersen, P.S.; Kearns, A.; Pichon, B.; Holmes, M.A.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA(LGA251). Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef]
- Petti, C.A.; Bosshard, P.P.; Brandt, M.E.; Clarridge, J.E.; Feldblyum, T.V.; Foxall, P.; Furtado, M.R.; Pace, N.; Procop, G. Interpretive Criteria for Identification of Bacteria and Fungi by DNA Target Sequencing; Approved Guideline. Clin. Lab. Stand. Inst. (CLSI) Doc. 2008, 28, 19087–19898. [Google Scholar]
- Enright, M.C.; Day, N.P.J.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef]
- Shopsin, B.; Gomez, M.; Waddington, M.; Riehman, M.; Kreiswirth, B.N. Use of coagulase gene (coa) repeat region nucleotide sequences for typing of methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 2000, 38, 3453–3456. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, 465–469. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; M100; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Chavez-Bueno, S.; Bozdogan, B.; Katz, K.; Bowlware, K.L.; Cushion, N.; Cavuoti, D.; Ahmad, N.; McCracken, G.H.; Appelbaum, P.C. Inducible clindamycin resistance and molecular epidemiologic trends of pediatric community-acquired methicillin-resistant Staphylococcus aureus in Dallas, Texas. Antimicrob. Agents Chemother. 2005, 49, 2283–2288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).