Metronidazole Potentiation by Panax Ginseng and Symphytum officinale: A New Strategy for P. gingivalis Infection Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultures
2.2. Plant Extracts and Antibiotic Working Concentrations
2.3. Biofilm Inhibition Assay
2.4. Detection of Acylated Homoserine Lactones (AHLs)
2.5. Statistical Analysis
3. Results
3.1. Antibacterial Activity of Plant Extracts and Metronidazole
3.2. Biofilm Inhibition Rate of Bacterial Standard Strain and Isolates
3.3. Acylated Homoserine Lactones (AHLs) Production
3.4. Dose-Dependent Effects of G, S, and F
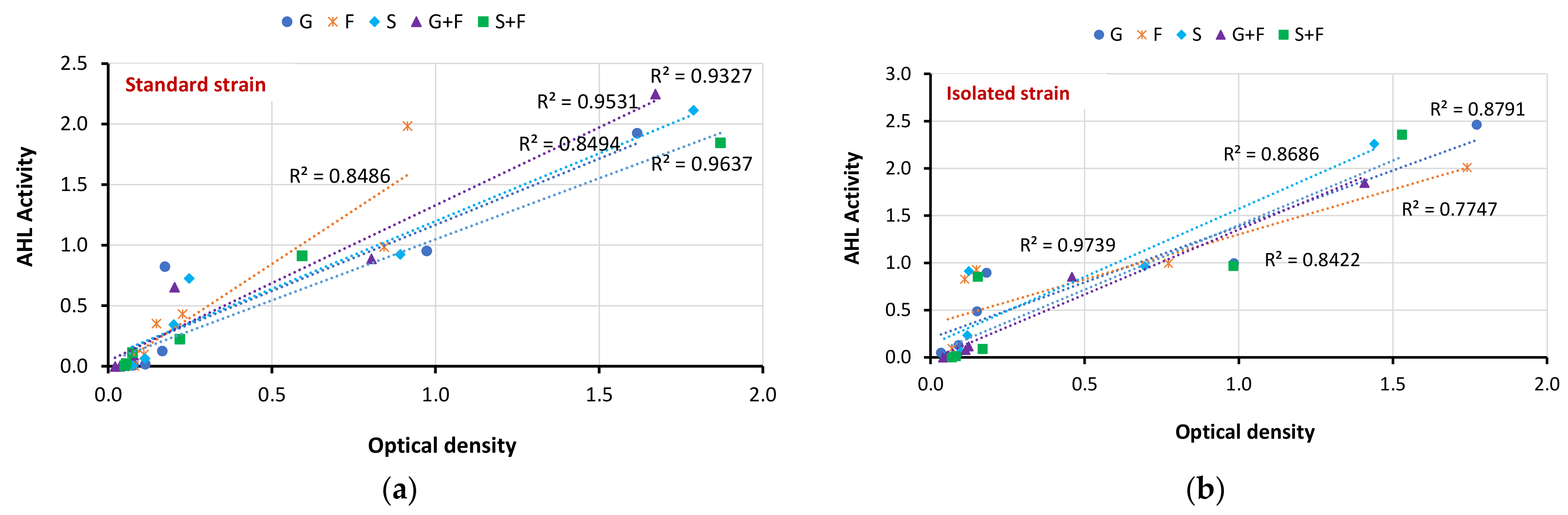
3.5. Correlation between Biofilm Inhibition and AHL Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, J.; Jentsch, H.; Stingu, C.S.; Sack, U. General immune status and oral microbiology in patients with different forms of periodontitis and healthy control subjects. PLoS ONE 2014, 9, e109187. [Google Scholar] [CrossRef]
- Ray, R.R.; Pattnaik, S. Contribution of phytoextracts in challenging the biofilms of pathogenic bacteria. Biocatal. Agric. Biotechnol. 2023, 48, 102642. [Google Scholar] [CrossRef]
- Alwan, A.M.; Rokaya, D.; Kathayat, G.; Afshari, J.T. Onco-immunity and therapeutic application of amygdalin: A review. J. Oral Biol. Craniofacial Res. 2022, 13, 155–163. [Google Scholar] [CrossRef]
- Ali, A.; Saliem, S.; Abdulkareem, A.; Radhi, H.; Gul, S. Evaluation of the efficacy of lycopene gel compared with minocycline hydrochloride microspheres as an adjunct to nonsurgical periodontal treatment: A randomised clinical trial. J. Dent. Sci. 2021, 16, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Narayanasamy, M.; Feussner, K.-D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Fitzsimonds, Z.R.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontol. 2000 2022, 89, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Kadir, M.Q.; AL-Ani, N.E.; Alwan, S.M. Synthesis of New Cyclic Amines-Linked Metronidazole Derivatives as Possible Prodrugs. Iraqi J. Pharm. Sci. 2017, 18, 1–7. [Google Scholar] [CrossRef]
- Kareem, H.H.; Al-Ghurabi, B.H.; Albadri, C. Molecular Detection of Porphyromonas gingivalis in COVID-19 Patients. J. Baghdad Coll. Dent. 2022, 34, 52–61. [Google Scholar] [CrossRef]
- Alwan, A.M.; Afshari, J.T. In Vivo Growth Inhibition of Human Caucasian Prostate Adenocarcinoma in Nude Mice Induced by Amygdalin with Metabolic Enzyme Combinations. BioMed Res. Int. 2022, 2022, 4767621. [Google Scholar] [CrossRef]
- Di Salle, A.; Spagnuolo, G.; Conte, R.; Procino, A.; Peluso, G.; Rengo, C. Effects of various prophylactic procedures on titanium surfaces and biofilm formation. J. Periodontal Implant Sci. 2018, 48, 373–382. [Google Scholar] [CrossRef]
- Khorshid, M. Point of Care testing: The future of periodontal dis-ease diagnosis and monitoring. J. Baghdad Coll. Dent. 2022, 34, 44–50. [Google Scholar] [CrossRef]
- Khalel, A.M.; Fadhil, E. Histological and Immunohistochemical Study of Osteocalcin to Evaluate The Effect of Local Application of Symphytum officinale Oil on Bone Healing on Rat. Diyala J. Med. 2020, 18, 71–78. [Google Scholar] [CrossRef]
- Serbanescu, M.A.; Oveisi, M.; Sun, C.; Fine, N.; Bosy, A.; Glogauer, M. Metronidazole enhances killing of Porphyromonas gingivalis by human PMNs. Front. Oral Health 2022, 3, 933997. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Dell’agli, M.; Badea, M.; Dima, L.; Colombo, E.; Sangiovanni, E.; Restani, P.; Bosisio, E. Plant food supplements with anti-inflammatory properties: A systematic review (II). Crit. Rev. Food Sci. Nutr. 2013, 53, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Egra, S.; Kuspradini, H.; Kusuma, I.W.; Batubara, I.; Yamauchi, K.; Mitsunaga, T. Garcidepsidone B from Garcinia parvifolia: Antimicrobial activities of the medicinal plants from East and North Kalimantan against dental caries and periodontal disease pathogen. Med. Chem. Res. 2023, 1–8. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Aabed, K.; Benabdelkamel, H.; Shami, A.; Alotaibi, M.O.; Alanazi, M.; Alfadda, A.A.; Rahman, I. Proteomic Profiling Reveals Cytotoxic Mechanisms of Action and Adaptive Mechanisms of Resistance in Porphyromonas gingivalis: Treatment with Juglans regia and Melaleuca alternifolia. ACS Omega 2023, 8, 12980–12991. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Minno, A.D.; Filippis, A.D.; Sommella, E.; Buccato, D.G.; Lellis, L.F.D.; El-Seedi, H.R.; Khalifa, S.A.; Piccinocchi, R.; Galdiero, M.; et al. In Vitro Antimicrobial and Antibiofilm Properties and Bioaccessibility after Oral Digestion of Chemically Characterized Extracts Obtained from Cistus × incanus L., Scutellaria lateriflora L., and Their Combination. Foods 2023, 12, 1826. [Google Scholar] [CrossRef]
- Mizutani, K.; Buranasin, P.; Mikami, R.; Takeda, K.; Kido, D.; Watanabe, K.; Takemura, S.; Nakagawa, K.; Kominato, H.; Saito, N.; et al. Effects of antioxidant in adjunct with periodontal therapy in patients with type 2 diabetes: A systematic review and meta-analysis. Antioxidants 2021, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Shanan, Z.J.; Hadi, S.M.; Shanshool, S.K. Structural analysis of chemical and green synthesis of cuo nanoparticles and their effect on biofilm formation. Baghdad Sci. J. 2018, 15, 211–216. [Google Scholar] [CrossRef]
- Berghänel, A.; Heistermann, M.; Schülke, O.; Ostner, J. Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc. Natl. Acad. Sci. USA 2017, 114, E10658–E10666. [Google Scholar] [CrossRef]
- Ibraheem, D.R.; Hussein, N.N.; Sulaiman, G.M. Antibacterial Activity of Silver nanoparticles against Pathogenic Bacterial Isolates from Diabetic Foot Patients. Iraqi J. Sci. 2023, 64, 2223–2239. [Google Scholar] [CrossRef]
- Nakayama, M.; Inoue, T.; Naito, M.; Nakayama, K.; Ohara, N. Attenuation of the phosphatidylinositol 3-kinase/Akt signaling pathway by Porphyromonas gingivalis gingipains RgpA, RgpB, and Kgp. J. Biol. Chem. 2015, 290, 5190–5202. [Google Scholar] [CrossRef] [PubMed]
- Fredua-Agyeman, M.; Gaisford, S. Real time microcalorimetric profiling of prebiotic inulin metabolism. Food Hydrocoll. Health 2023, 4, 100141. [Google Scholar] [CrossRef]
- Bakir, S.H.; Ali, F.A. Comparison of different methods for detection of biofilm production in multi-drug resistance bacteria causing pharyngotonsillitis. Int. J. Res. H Pharm. Biosci. 2016, 3, 13–22. [Google Scholar]
- Wei, E.S.; Kavitha, R.; Sa’ad, M.A.; Lalitha, P.; Fuloria, N.K.; Ravichandran, M.; Fuloria, S. Development of a Simple Protocol for Zymogram-Based Isolation and Characterization of Gingipains from Porphyromonas gingivalis: The Causative Agent of Periodontitis. Appl. Sci. 2023, 13, 4314. [Google Scholar] [CrossRef]
- Baldiris, R.; TeherÃ, V.; Vivas-Reyes, R.; Montes, A.; Arzuza, O. Anti-biofilm activity of ibuprofen and diclofenac against some biofilm producing Escherichia coli and Klebsiella pneumoniae uropathogens. Afr. J. Microbiol. Res. 2016, 10, 1675–1684. [Google Scholar]
- De Boer, C.G.; Hughes, T.R. YeTFaSCo: A database of evaluated yeast transcription factor sequence specificities. Nucleic Acids Res. 2012, 40, D169–D179. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Kannappan, A.; Thiyagarajan, S.; Srinivasan, R.; Jeyapragash, D.; Paul, J.B.J.; Velmurugan, P.; Ravi, A.V. AHL-Lactonase producing Psychrobacter sp. from Palk Bay sediment mitigates quorum sensing-mediated virulence production in Gram negative bacterial pathogens. Front. Microbiol. 2021, 12, 634593. [Google Scholar] [CrossRef]
- García-Castillo, M.; Morosini, M.-I.; Gálvez, M.; Baquero, F.; del Campo, R.; Meseguer, M.-A. Differences in biofilm development and antibiotic susceptibility among clinical Ureaplasma urealyticum and Ureaplasma parvum isolates. J. Antimicrob. Chemother. 2008, 62, 1027–1030. [Google Scholar] [CrossRef]
- Pellegrini, M.C.; Ponce, A.G. Beet (Beta vulgaris) and Leek (Allium porrum) leaves as a source of bioactive compounds with anti-quorum sensing and anti-biofilm activity. Waste Biomass Valorization 2020, 11, 4305–4313. [Google Scholar] [CrossRef]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]



| Dilution | Total Concentrations (mg/mL) | ||||
|---|---|---|---|---|---|
| G | S | F | G+F | S+F | |
| C: Control | 1000 | 330 | 500 | 300 | 166 |
| D1: first dilution | 500 | 165 | 250 | 150 | 83 |
| D2: second dilution | 250 | 82.5 | 125 | 75 | 41.5 |
| D3: third dilution | 125 | 41.25 | 62.5 | 37.5 | 20.75 |
| D4: fourth dilution | 62.5 | 20.625 | 31.25 | 18.75 | 10.375 |
| D5: fifth dilution | 31.25 | 10.312 | 15.625 | 9.375 | 5.187 |
| D6: sixth dilution | 15.625 | 5.156 | 7.812 | 4.687 | 2.593 |
| Microorganisms | Concentration | Mean Inhibition-Zone Diameter, ± SD (mm) | F-Test | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| G | F | S | G+F | S+F | ||||
| Strain (ATCC 33277) | C | 20.5120 ± 0.014 | 20.4119 ± 0.114 | 18.6111 ± 0.147 | 24.0125 ± 0.321 | 22.0367 ± 0.014 | 132.25 | 0.000 |
| D1 | 20.2476 ± 0.214 | 18.2778 ± 0.0251 | 18.2110 ± 0.202 | 22.5019 ± 0.052 | 20.8970 ± 0.023 | 129.63 | 0.005 | |
| D2 | 18.3017 ± 0.105 | 18.2100 ± 0.221 | 17.1556 ± 0.054 | 21.000 ± 0.014 | 18.0000 ± 0.514 | 141.11 | 0.003 | |
| D3 | 16.1506 ± 0.221 | 13.6321 ± 0.074 | 15.0556 ± 0.015 | 17.8642 ± 0.0162 | 15.6410 ± 0.0363 | 128.63 | 0.001 | |
| D4 | 12.000 ± 0.0241 | Zero | Zero | 12.2590 ± 0.0669 | 12.3780 ± 0.0554 | 99.52 | 0.142 | |
| D5 | Zero | Zero | Zero | Zero | Zero | - | - | |
| Isolate | C | 18.3889 ± 0.041 | 20.1359 ± 0.014 | 18.5124 ± 0.025 | 22.8690 ± 0.0021 | 20.1056 ± 0.0145 | 138.28 | 0.0025 |
| D1 | 18.2778 ± 0.25 | 18.0189 ± 0.0036 | 16.2531 ± 0.021 | 21.9810 ± 0.084 | 20.0245 ± 0.0126 | 129.69 | 0.0015 | |
| D2 | 16.5000 ± 0.019 | 14.5890 ± 0.033 | 14.4376 ± 0.033 | 18.4587 ± 0.0415 | 19.0849 ± 0.0235 | 137.18 | 0.0032 | |
| D3 | 13.6111 ± 0.014 | 12.3801 ± 0.74 | 12.1400 ± 0.039 | 15.1398 ± 0.0963 | 16.8941 ± 0.0229 | 98.69 | 0.0015 | |
| D4 | Zero | Zero | Zero | 13.6782 ± 0.254 | 14.7901 ± 0.0325 | 91.57 | 0.521 | |
| D5 | Zero | Zero | Zero | Zero | Zero | - | - | |
| Microorganism | Concentration | Mean Optical Density (OD) of Biofilm ±SD | F-Test | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| G | F | S | G+F | S+F | ||||
| Standard strain (ATCC 33277) | C | 2.846 | 2.846 | 2.846 | 2.846 | 2.846 | 124.69 | 0.0041 |
| D1 | 1.616 ± 0.071 | 0.914 ± 0.14 | 1.788 ± 0.002 | 1.672 ± 0.302 | 1.870 ± 0.078 | 125.23 | 0.0062 | |
| D2 | 0.973 ± 0.062 | 0.843 ± 0.092 | 0.892 ± 0.056 | 0.804 ± 0.041 | 0.592 ± 0.041 | 133.36 | 0.0024 | |
| D3 | 0.173 ± 0.035 | 0.227 ± 0.047 | 0.247 ± 0.105 | 0.203 ± 0.004 | 0.219 ± 0.009 | 122.56 | 0.0093 | |
| D4 | 0.165 ± 0.030 | 0.147 ± 0.081 | 0.200 ± 0.010 | 0.077 ± 0.092 | 0.074 ± 0.106 | 136.63 | 0.004 | |
| D5 | 0.113 ± 0.025 | 0.109 ± 0.037 | 0.112 ± 0.053 | 0.035 ± 0.105 | 0.055 ± 0.003 | 115.96 | 0.0082 | |
| D6 | 0.077 ± 0.008 | 0.079 ± 0.069 | 0.073 ± 0.006 | 0.021 ± 0.038 | 0.050 ± 0.014 | 121.52 | 0.0093 | |
| Clinical isolate | C | 3.186 | 3.186 | 3.186 | 3.186 | 3.186 | 119.52 | 0.004 |
| D1 | 1.772 ± 0.080 | 1.530 ± 0.079 | 1.440 ± 0.250 | 1.741 ± 0.002 | 1.408 ± 0.004 | 126.39 | 0.005 | |
| D2 | 0.985 ± 0.068 | 0.983 ± 0.130 | 0.695 ± 0.091 | 0.772 ± 0.031 | 0.459 ± 0.045 | 127.25 | 0.000 | |
| D3 | 0.182 ± 0.034 | 0.154 ± 0.056 | 0.125 ± 0.003 | 0.149 ± 0.003 | 0.124 ± 0.290 | 132.78 | 0.0063 | |
| D4 | 0.151 ± 0.045 | 0.169 ± 0.085 | 0.120 ± 0.018 | 0.111 ± 0.092 | 0.114 ± 0.007 | 133.03 | 0.0025 | |
| D5 | 0.091 ± 0.028 | 0.082 ± 0.101 | 0.098 ± 0.004 | 0.070 ± 0.013 | 0.052 ± 0.098 | 132.96 | 0.0014 | |
| D6 | 0.034 ± 0.013 | 0.069 ± 0.040 | 0.045 ± 0.013 | 0.056 ± 0.032 | 0.041 ± 0.002 | 128.55 | 0.0069 | |
| Microorganism | Concentration | AHL Activity ± SD | F-Test | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| G | F | S | G+F | S+F | ||||
| Standard strain (ATCC 33277) | C | 1.926 ± 0.379 | 1.984 ± 0.630 | 2.114 ± 0.049 | 2.248 ± 0.801 | 1.845 ± 0.778 | 88.95 | 0.0014 |
| D1 | 0.953 ± 0.078 | 0.987 ± 0.122 | 0.925 ± 0.500 | 0.887 ± 0.201 | 0.914 ± 0.058 | 41.24 | 0.0036 | |
| D2 | 0.824 ± 0.135 | 0.432 ± 0.004 | 0.725 ± 0.001 | 0.654 ± 0.015 | 0.226 ± 0.031 | 51.14 | 0.000 | |
| D3 | 0.127 ± 0.039 | 0.354 ± 0.062 | 0.344 ± 0.023 | 0.092 ± 0.002 | 0.114 ± 0.106 | 44.56 | 0.0025 | |
| D4 | 0.017 ± 0.025 | 0.098 ± 0.100 | 0.065 ± 0.013 | 0.001 ± 0.005 | 0.023 ± 0.010 | 39.23 | 0.0051 | |
| D5 | 0.009 ± 0.001 | 0.006 ± 0.004 | 0.006 ± 0.003 | 0.000 | 0.005 ± 0.001 | 33.54 | 0.0052 | |
| Clinical isolate | C | 2.462 ± 0.980 | 2.009 ± 0.001 | 2.259 ± 0.979 | 1.845 ± 0.002 | 2.357 ± 0.368 | 29.68 | 0.0041 |
| D1 | 0.997 ± 0.060 | 0.995 ± 0.029 | 0.965 ± 0.019 | 0.854 ± 0.031 | 0.967 ± 0.002 | 36.05 | 0.002 | |
| D2 | 0.895 ± 0.143 | 0.927 ± 0.101 | 0.912 ± 0.103 | 0.115 ± 0.003 | 0.854 ± 0.251 | 41.98 | 0.0042 | |
| D3 | 0.487 ± 0.009 | 0.827 ± 0.058 | 0.235 ± 0.072 | 0.078 ± 0.010 | 0.089 ± 0.050 | 42.56 | 0.003 | |
| D4 | 0.129 ± 0.020 | 0.094 ± 0.010 | 0.059 ± 0.002 | 0.012 ± 0.020 | 0.012 ± 0.021 | 40.25 | 0.000 | |
| D5 | 0.05 ± 0.003 | 0.009 ± 0.013 | 0.001 ± 0.010 | 0.000 | 0.002 ± 0.001 | 40.14 | 0.009 | |
| Microorganism | Concentration | Percentage Inhibition of Biofilm Formation | ||||
|---|---|---|---|---|---|---|
| G | F | S | G+F | S+F | ||
| Standard strain (ATCC 33277) | D1 | 98.1 | 97.8 | 98 | 98.2 | 98.7 |
| D2 | 96.1 | 97.4 | 96.9 | 97.8 | 98.3 | |
| D3 | 95.2 | 94.6 | 95.2 | 96.5 | 96.4 | |
| D4 | 94.2 | 95.1 | 94 | 95.3 | 96.1 | |
| D5 | 69 | 69.1 | 78.1 | 80.7 | 85.5 | |
| D6 | 44.3 | 51.9 | 54.8 | 45.3 | 55.8 | |
| Clinical isolate | D1 | 97.2 | 97.2 | 97.4 | 99.2 | 98.2 |
| D2 | 96 | 96.1 | 96.1 | 98.7 | 98 | |
| D3 | 94.2 | 94.8 | 92.9 | 97.2 | 97.3 | |
| D4 | 93.9 | 92 | 91.3 | 92.8 | 92.1 | |
| D5 | 69.9 | 70.3 | 68.6 | 71.7 | 79.1 | |
| D6 | 43.2 | 67.8 | 37.1 | 41.2 | 34.2 | |
| X2 | 3.254 | |||||
| p-value | 0.00014 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.M.; Al-Mizraqchi, A.S.; Haider, J. Metronidazole Potentiation by Panax Ginseng and Symphytum officinale: A New Strategy for P. gingivalis Infection Control. Antibiotics 2023, 12, 1288. https://doi.org/10.3390/antibiotics12081288
Ibrahim SM, Al-Mizraqchi AS, Haider J. Metronidazole Potentiation by Panax Ginseng and Symphytum officinale: A New Strategy for P. gingivalis Infection Control. Antibiotics. 2023; 12(8):1288. https://doi.org/10.3390/antibiotics12081288
Chicago/Turabian StyleIbrahim, Salah M., Abbas S. Al-Mizraqchi, and Julfikar Haider. 2023. "Metronidazole Potentiation by Panax Ginseng and Symphytum officinale: A New Strategy for P. gingivalis Infection Control" Antibiotics 12, no. 8: 1288. https://doi.org/10.3390/antibiotics12081288
APA StyleIbrahim, S. M., Al-Mizraqchi, A. S., & Haider, J. (2023). Metronidazole Potentiation by Panax Ginseng and Symphytum officinale: A New Strategy for P. gingivalis Infection Control. Antibiotics, 12(8), 1288. https://doi.org/10.3390/antibiotics12081288









