Adsorptive–Photocatalytic Performance for Antibiotic and Personal Care Product Using Cu0.5Mn0.5Fe2O4
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Cu0.5Mn0.5Fe2O4 Nanoparticles
2.3. Chemical and Material Analyses
2.4. Varying Dosage of H2O2 or Catalysts
2.5. OTC Adsorption Study
2.5.1. Adsorption Kinetics
2.5.2. Adsorption Isotherms
2.5.3. Isotherm Models
2.5.4. Model Evaluation
2.6. Evaluating Effects of Treated Water on Seed Germination and Root Anatomy
3. Results and Discussion
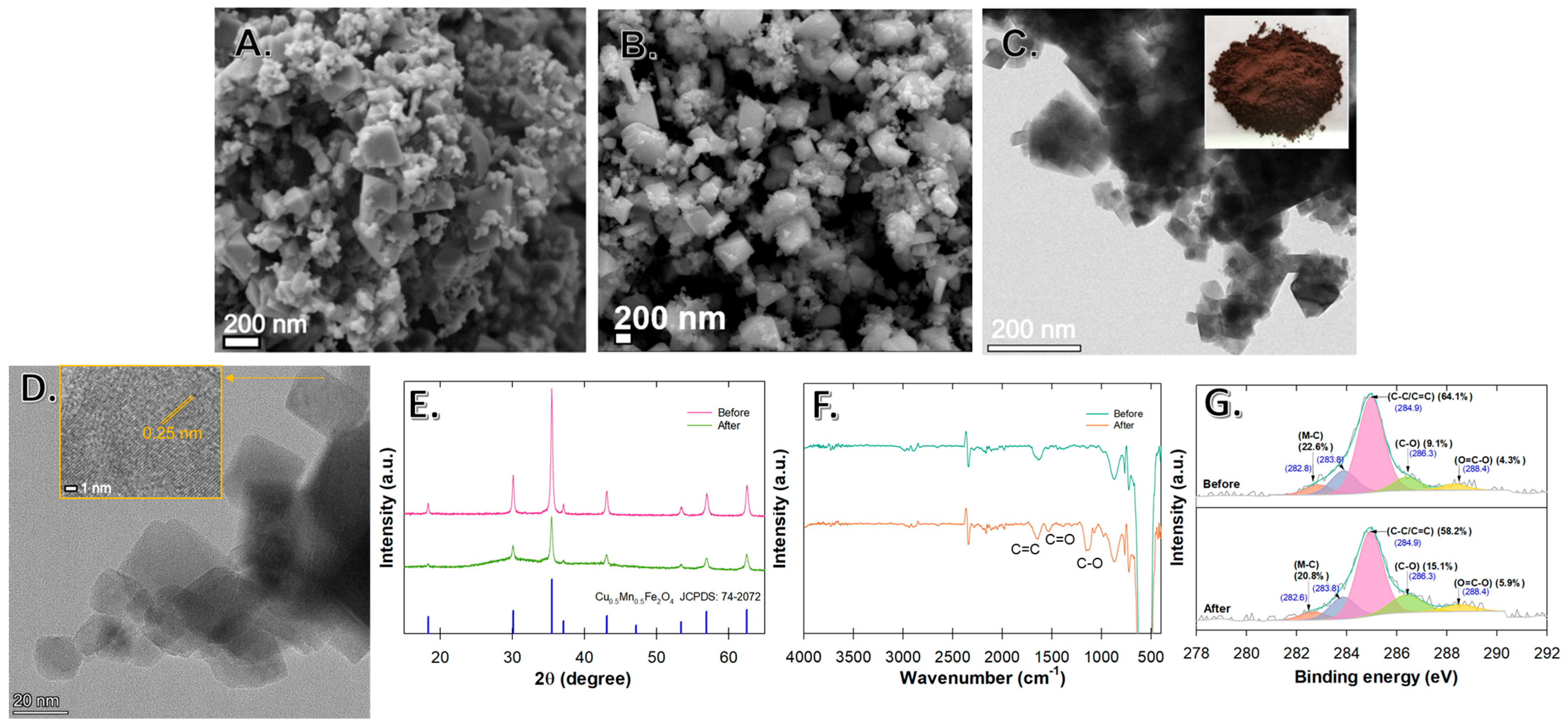
3.1. Cu0.5Mn0.5Fe2O4 Characteristics
3.2. Photocatalytic Performance
3.2.1. Paraben Degradation Efficiency
3.2.2. Oxytetracycline (OTC) Degradation Efficiency
3.3. Adsorptive Performance
3.4. OTC Degradation Products
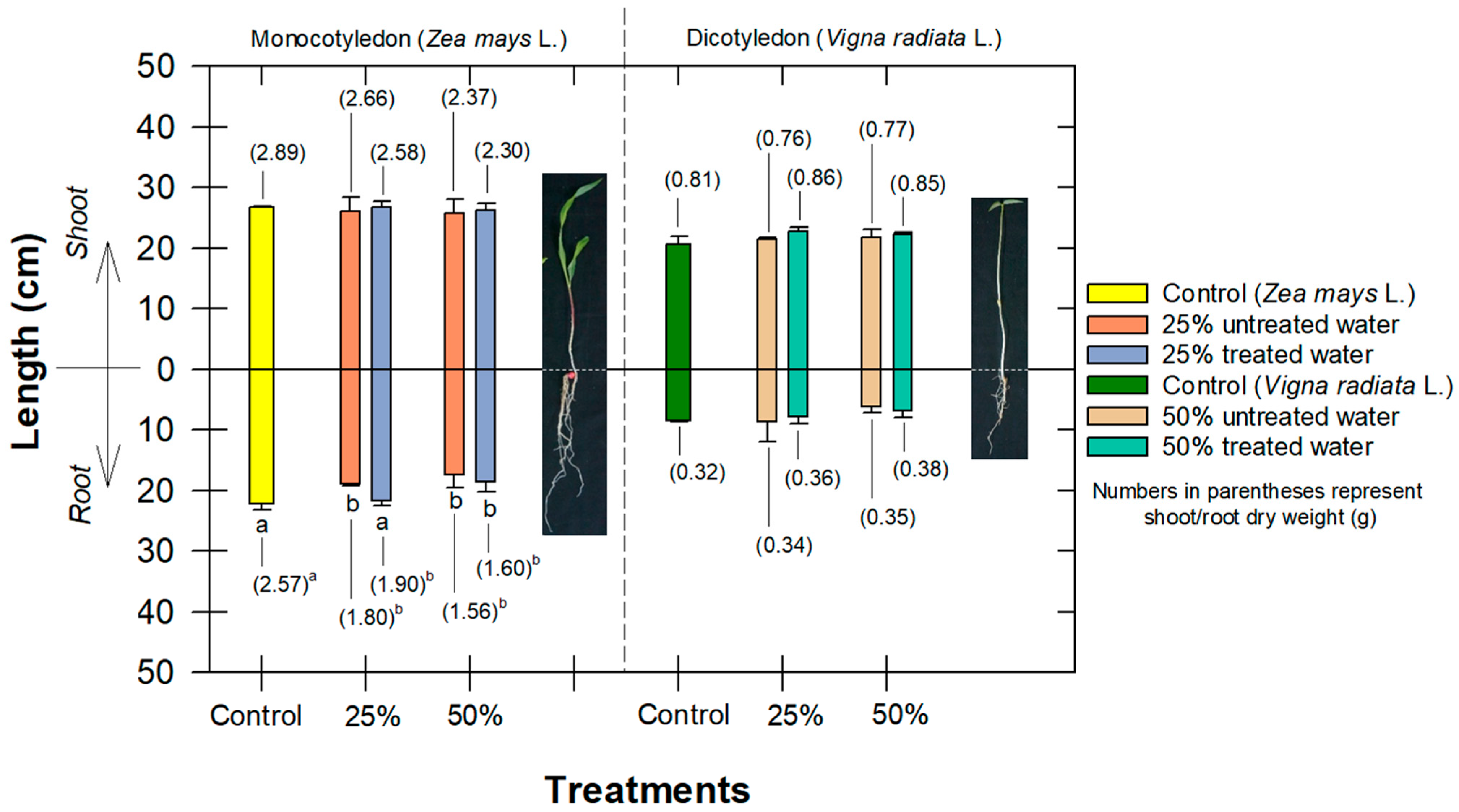
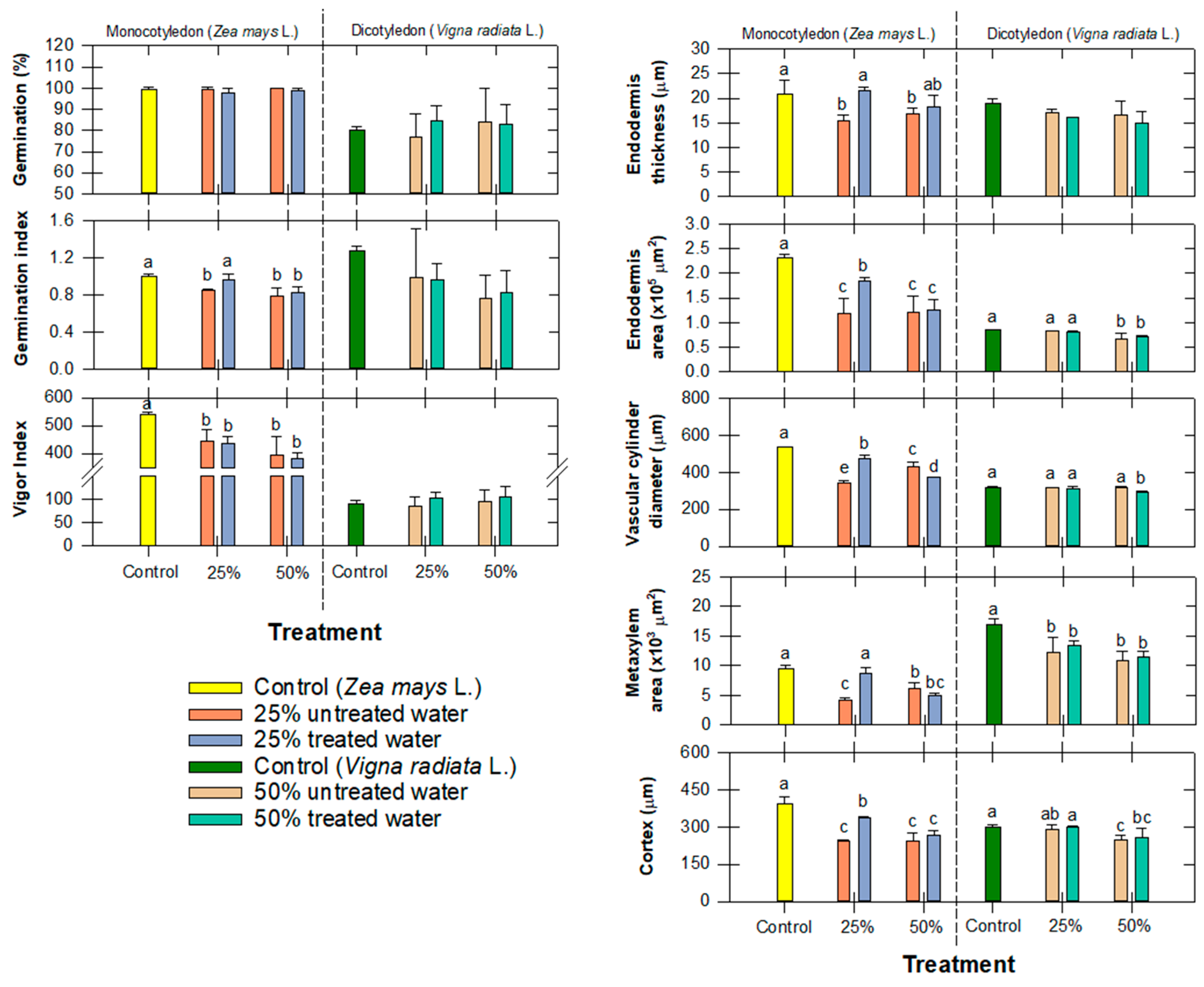
3.5. Effect to Seedling Growth and Root Anatomy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Kumar, A.; Kumar, A.; Balaji, R.; Krishnan, V. Highly Efficient Visible Light Active 2D-2D Nanocomposites of N-ZnO-g-C3N4 for Photocatalytic Degradation of Diverse Industrial Pollutants. ChemistrySelect 2018, 3, 1919–1932. [Google Scholar] [CrossRef]
- Karthikraj, R.; Vasu, A.K.; Balakrishna, K.; Sinha, R.K.; Kannan, K. Occurrence and fate of parabens and their metabolites in five sewage treatment plants in India. Sci. Total Environ. 2017, 593–594, 592–598. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Gaffney, V.; Almeida, C.M.M.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Cardoso, V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015, 72, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Nielsen, O.; Skakkebaek, N.E.; Juul, A.; Andersson, A.-M. UV filters analyzed by isotope diluted TurboFlow-LC–MS/MS in urine from Danish children and adolescents. Int. J. Hyg. Environ. Health 2017, 220, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ying, G.-G.; Zhao, J.-L.; Chen, Z.-F.; Lai, H.-J.; Su, H.-C. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ. Int. 2013, 52, 81–86. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, J.; Zhong, Y.; Ding, T.; Dissanayake, P.D.; Yang, Y.; Tsang, Y.F.; Ok, Y.S. Sorption of pharmaceuticals and personal care products (PPCPs) from water and wastewater by carbonaceous materials: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 727–766. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Qian, H.; Yu, G.; Hou, Q.; Nie, Y.; Bai, C.; Bai, X.; Wang, H.; Ju, M. Ingenious control of adsorbed oxygen species to construct dual reaction centers ZnO@FePc photo-Fenton catalyst with high-speed electron transmission channel for PPCPs degradation. Appl. Catal. B Environ. 2021, 291, 120064. [Google Scholar] [CrossRef]
- Angkaew, A.; Chokejaroenrat, C.; Sakulthaew, C.; Mao, J.; Watcharatharapong, T.; Watcharenwong, A.; Imman, S.; Suriyachai, N.; Kreetachat, T. Two facile synthesis routes for magnetic recoverable MnFe2O4/g-C3N4 nanocomposites to enhance visible light photo-Fenton activity for methylene blue degradation. J. Environ. Chem. Eng. 2021, 9, 105621. [Google Scholar] [CrossRef]
- Huang, G.-X.; Wang, C.-Y.; Yang, C.-W.; Guo, P.-C.; Yu, H.-Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef] [PubMed]
- Angkaew, A.; Sakulthaew, C.; Nimtim, M.; Imman, S.; Satapanajaru, T.; Suriyachai, N.; Kreetachat, T.; Comfort, S.; Chokejaroenrat, C. Enhanced Photo-Fenton Activity Using Magnetic Cu0.5Mn0.5Fe2O4 Nanoparticles as a Recoverable Catalyst for Degrading Organic Contaminants. Water 2022, 14, 3717. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zeng, D.; Zhang, B.; Hassan, M.; Li, P.; Qi, C.; He, Y. Enhanced catalytic activation of photo-Fenton process by Cu0.5Mn0.5Fe2O4 for effective removal of organic contaminants. Chemosphere 2020, 247, 125780. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Zheng, H.; Xie, Q.; Huang, J.; Xiao, L.; Peng, D.-L. 3D graphene encapsulated ZnO-NiO-CuO double-shelled hollow microspheres with enhanced lithium storage properties. J. Alloys Compd. 2018, 765, 1158–1166. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Y.; Tang, H.; Han, X.; Zhu, L.; Wang, N. Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: Efficiency, stability and mechanism. Chem. Eng. J. 2014, 236, 251–262. [Google Scholar] [CrossRef]
- Hashemian, S.; Dehghanpor, A.; Moghahed, M. Cu0.5Mn0.5Fe2O4 nano spinels as potential sorbent for adsorption of brilliant green. J. Ind. Eng. Chem. 2015, 24, 308–314. [Google Scholar] [CrossRef]
- Lasdon, L.S.; Fox, R.L.; Ratner, M.W. Nonlinear optimization using the generalized reduced gradient method. RAIRO Oper. Res 1974, 8, 73–103. [Google Scholar] [CrossRef]
- Chokejaroenrat, C.; Watcharenwong, A.; Sakulthaew, C.; Rittirat, A. Immobilization of Atrazine Using Oxidized Lignite Amendments in Agricultural Soils. Water Air Soil Pollut. 2020, 231, 249. [Google Scholar] [CrossRef]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Textile wastewater treatment by agro-industrial waste: Equilibrium modelling, thermodynamics and mass transfer mechanisms of cationic dyes adsorption onto low-cost lignocellulosic adsorbent. J. Taiwan Inst. Chem. Eng. 2019, 96, 439–452. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, D.; Chen, Y.; Belzile, N.; Bai, Y.; Zhu, J.; Shu, J.; Chen, S. Adsorption behaviors of phenanthrene and bisphenol A in purple paddy soils amended with straw-derived DOM in the West Sichuan Plain of China. Ecotoxicol. Environ. Saf. 2019, 169, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Ansari, S.G.; Foaud, H.; Cho, M.H. Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J. Chem. 2017, 41, 9314–9320. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Ma, J. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard. Mater. 2017, 336, 81–92. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, J.; Niu, S.; Wang, X.; Li, T.; Liu, S.; Lin, Y.; Xie, T.; Dong, S. Comparing dark- and photo-Fenton-like degradation of emerging pollutant over photo-switchable Bi2WO6/CuFe2O4: Investigation on dominant reactive oxidation species. J. Environ. Sci. 2021, 106, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Heterogeneous Fenton-like degradation of tartrazine using CuFe2O4 nanoparticles synthesized by sol-gel combustion. Appl. Surf. Sci. Adv. 2022, 9, 100251. [Google Scholar] [CrossRef]
- Ahmad, H.; Haseen, U.; Umar, K.; Ansari, M.S.; Ibrahim, M.N.M. Bioinspired 2D carbon sheets decorated with MnFe2O4 nanoparticles for preconcentration of inorganic arsenic, and its determination by ICP-OES. Microchim. Acta 2019, 186, 649. [Google Scholar] [CrossRef]
- Ghobadi, M.; Gharabaghi, M.; Abdollahi, H.; Boroumand, Z.; Moradian, M. MnFe2O4-graphene oxide magnetic nanoparticles as a high-performance adsorbent for rare earth elements: Synthesis, isotherms, kinetics, thermodynamics and desorption. J. Hazard. Mater. 2018, 351, 308–316. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, C.; Qin, L.; Fu, Y.; He, J.; Huang, D.; Li, B.; Zhang, M.; Liu, S.; Li, L.; et al. ZIF-8-modified MnFe2O4 with high crystallinity and superior photo-Fenton catalytic activity by Zn-O-Fe structure for TC degradation. Chem. Eng. J. 2020, 392, 124851. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Hussain, I.; White, A.J.P.; Williams, D.J. Synthesis and structure of a copper(II) melamine complex, [Cu(C3H6N6)(µ-OCH3)(ONO2)(HOCH3)]2, with direct Cu–melamine coordination. J. Chem. Soc. Dalton Trans. 1999, 17, 2899–2900. [Google Scholar] [CrossRef]
- Wiles, A.B.; Bozzuto, D.; Cahill, C.L.; Pike, R.D. Copper (I) and (II) complexes of melamine. Polyhedron 2006, 25, 776–782. [Google Scholar] [CrossRef]
- Doddamani, J.S.; Hodlur, R.M.; Rabinal, M.K. Melamine assisted large-scale and rapid synthesis of porous copper oxide nanostructures. Emergent Mater. 2022, 5, 1089–1096. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, J.; Alshehri, S.M.; Ahmad, T. High-Surface-Area Sodium Tantalate Nanoparticles with Enhanced Photocatalytic and Electrical Properties Prepared through Polymeric Citrate Precursor Route. ACS Omega 2019, 4, 19408–19419. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Self-Assembled Interwoven Nanohierarchitectures of NaNbO3 and NaNb1–xTaxO3 (0.05 ≤ x ≤ 0.20): Synthesis, Structural Characterization, Photocatalytic Applications, and Dielectric Properties. ACS Omega 2022, 7, 16952–16967. [Google Scholar] [CrossRef]
- Domínguez, J.R.; Muñoz, M.J.; Palo, P.; González, T.; Peres, J.A.; Cuerda-Correa, E.M. Fenton advanced oxidation of emerging pollutants: Parabens. Int. J. Energy Environ. Eng. 2014, 5, 89. [Google Scholar] [CrossRef]
- Pattanateeradetch, A.; Sakulthaew, C.; Angkaew, A.; Sutjarit, S.; Poompoung, T.; Lin, Y.-T.; Harris, C.E.; Comfort, S.; Chokejaroenrat, C. Fabrication of Ternary Nanoparticles for Catalytic Ozonation to Treat Parabens: Mechanisms, Efficiency, and Effects on Ceratophyllum demersum L. and Eker Leiomyoma Tumor-3 Cells. Nanomaterials 2022, 12, 3573. [Google Scholar] [CrossRef] [PubMed]
- Gmurek, M.; Rossi, A.F.; Martins, R.C.; Quinta-Ferreira, R.M.; Ledakowicz, S. Photodegradation of single and mixture of parabens—Kinetic, by-products identification and cost-efficiency analysis. Chem. Eng. J. 2015, 276, 303–314. [Google Scholar] [CrossRef]
- Angı, A.; Sanlı, D.; Erkey, C.; Birer, Ö. Catalytic activity of copper (II) oxide prepared via ultrasound assisted Fenton-like reaction. Ultrason. Sonochemistry 2014, 21, 854–859. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Brouers, F.; Zarrabi, M. Kinetic modeling of antibiotic adsorption onto different nanomaterials using the Brouers–Sotolongo fractal equation. Environ. Sci. Pollut. Res. 2017, 24, 4048–4057. [Google Scholar] [CrossRef]
- Selmi, T.; Sanchez-Sanchez, A.; Gadonneix, P.; Jagiello, J.; Seffen, M.; Sammouda, H.; Celzard, A.; Fierro, V. Tetracycline removal with activated carbons produced by hydrothermal carbonisation of Agave americana fibres and mimosa tannin. Ind. Crops Prod. 2018, 115, 146–157. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chokejaroenrat, C.; Sakulthaew, C.; Satchasataporn, K.; Snow, D.D.; Ali, T.E.; Assiri, M.A.; Watcharenwong, A.; Imman, S.; Suriyachai, N.; Kreetachat, T. Enrofloxacin and Sulfamethoxazole Sorption on Carbonized Leonardite: Kinetics, Isotherms, Influential Effects, and Antibacterial Activity toward S. aureus ATCC 25923. Antibiotics 2022, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Sakulthaew, C.; Chokejaroenrat, C.; Poapolathep, A.; Satapanajaru, T.; Poapolathep, S. Hexavalent chromium adsorption from aqueous solution using carbon nano-onions (CNOs). Chemosphere 2017, 184, 1168–1174. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Watcharenwong, A.; Chokejaroenrat, C.; Rittirat, A. Leonardite-Derived Biochar Suitability for Effective Sorption of Herbicides. Water Air Soil Pollut. 2021, 232, 36. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Wang, S.; Li, X.; Wang, C.; Liu, B.; Han, F.; Xu, Y.; Yu, P.; Sun, Y. Tetracycline degradation by peroxymonosulfate activated with CoNx active sites: Performance and activation mechanism. Chem. Eng. J. 2022, 431, 133477. [Google Scholar] [CrossRef]
- Bembibre, A.; Benamara, M.; Hjiri, M.; Gómez, E.; Alamri, H.R.; Dhahri, R.; Serrà, A. Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. Chem. Eng. J. 2022, 427, 132006. [Google Scholar] [CrossRef]
- Wang, J.; Zhi, D.; Zhou, H.; He, X.; Zhang, D. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef]
- Chokejaroenrat, C.; Sakulthaew, C.; Chantakulvanich, S.; Angkaew, A.; Teingtham, K.; Phansak, P.; Poompoung, T.; Snow, D.D.; Harris, C.E.; Comfort, S.D. Enhanced degradation of herbicides in groundwater using sulfur-containing reductants and spinel zinc ferrite activated persulfate. Sci. Total Environ. 2023, 892, 164652. [Google Scholar] [CrossRef]
- Chouychai, W.; Paemsom, T.; Pobsuwan, C.; Somtrakoon, K.; Lee, H. Effect of Indole-3-Acetic Acid-Producing Bacteria on Phytoremediation of Soil Contaminated with Phenanthrene and Anthracene by Mungbean. EnvironmentAsia 2016, 9, 128–133. [Google Scholar] [CrossRef]
- Chi, S.L.; Wang, W.Z.; Xu, W.H.; Li, T.; Li, Y.H.; Zhang, C.L. Effects of Tetracycline Antibiotics on Growth and Characteristics of Enrichment and Transformation in Two Vegetables. Huan Jing Ke Xue 2018, 39, 935–943. [Google Scholar] [CrossRef]
- Bao, Y.; Pan, C.; Li, D.; Guo, A.; Dai, F. Stress response to oxytetracycline and microplastic-polyethylene in wheat (Triticum aestivum L.) during seed germination and seedling growth stages. Sci. Total Environ. 2022, 806, 150553. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Richardi, V.S.; Bicalho, E.M.; da Rocha, D.C.; Navarro-Silva, M.A.; Soffiatti, P.; Garcia, Q.S.; Sant’Anna-Santos, B.F. Effects of Ciprofloxacin and Roundup on seed germination and root development of maize. Sci. Total Environ. 2019, 651, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-M.; Huang, X. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Tripthi, D.K.; Varma, R.K.; Singh, S.; Sachan, M.; Guerriero, G.; Kushwaha, B.K.; Bhardwaj, S.; Ramawat, N.; Sharma, S.; Singh, V.P.; et al. Silicon tackles butachlor toxicity in rice seedlings by regulating anatomical characteristics, ascorbate-glutathione cycle, proline metabolism and levels of nutrients. Sci. Rep. 2020, 10, 14078. [Google Scholar] [CrossRef]
- Pandey, A.K.; Zorić, L.; Sun, T.; Karanović, D.; Fang, P.; Borišev, M.; Wu, X.; Luković, J.; Xu, P. The Anatomical Basis of Heavy Metal Responses in Legumes and Their Impact on Plant–Rhizosphere Interactions. Plants 2022, 11, 2554. [Google Scholar] [CrossRef]
- Cole, J.C.; Smith, M.W.; Penn, C.J.; Cheary, B.S.; Conaghan, K.J. Nitrogen, phosphorus, calcium, and magnesium applied individually or as a slow release or controlled release fertilizer increase growth and yield and affect macronutrient and micronutrient concentration and content of field-grown tomato plants. Sci. Hortic. 2016, 211, 420–430. [Google Scholar] [CrossRef]





| Model | Equation | Ref. | Nomenclature |
|---|---|---|---|
| Kinetic Model | : Koble–Corrigan parameter () : Temkin equilibrium binding parameter (L mol–1) : Dubinin-Radushkevich constant (mol2 J–2) : Koble–Corrigan parameter () : Temkin constant (J mol–1) : Langmuir energy constant (L mg–1) : Khan model constant (L mg–1) : constant for intra-particle diffusion kinetic model (mg g–1) : OTC concentration at equilibrium (mg L–1) : Brouers–Sotolongo isotherm constant (L mg–1) : coefficient for intra–particle diffusion kinetic model (mg g–1 h–1/2) : Freundlich constant (mg g–1(L mg–1)1/n) : Hill constant : Jovanovich constant (L mg–1) : Redlich–Peterson isotherm constant (L g–1) : Toth model constant (L mg–1) : rate constant for pseudo first-order kinetic model (h–1) : rate constant for pseudo second-order kinetic model (g mg–1 h–1) : Freundlich adsorption intensity : Koble–Corrigan parameter : Hill cooperativity coefficient : non-integer reaction order : Toth model exponent : amount of OTC adsorbed at equilibrium (mg g–1) : maximum amount of the adsorbate per unit weight of the adsorbent (mg g–1) : amount of OTC adsorbed at time (mg g–1) : universal gas constant (8.314 J K–1 mol–1) : temperature (298 K) : adsorption time (h) : Elovich chemisorption rate (mg g–1 h–1) : Brouers–Sotolongo model exponent : Redlich–Peterson isotherm constant () : Khan model exponent : Elovich desorption rate constant (g mg–1) : Redlich–Peterson model exponent : fractal time exponent : characteristic time (h) | ||
| Pseudo first-order | [18] | ||
| Pseudo second-order | [18] | ||
| Elovich | [18] | ||
| Brouers- Sotolongo | [19] | ||
| Intra-particle diffusion | [18] | ||
| Isotherm model | |||
| Langmuir | [18] | ||
| Freundlich | [18] | ||
| Temkin | [18] | ||
| Dubinin- Radushkevich | [18] | ||
| Jovanovic | [18] | ||
| Koble-Corrigan | [19] | ||
| Khan | [19] | ||
| Hill | [19] | ||
| Brouers- Sotolongo | [19] | ||
| Toth | [19] | ||
| Redlich-Peterson | [19] | ||
| Model | Cu0.5Mn0.5Fe2O4 (g) | Parameters | (mg/g) | (%) | |||
|---|---|---|---|---|---|---|---|
| Pseudo first-order | (mg g−1) | (h−1) | |||||
| 0.006 | 10.14 | 2.41 | 0.48 | 78.98 | |||
| 0.012 | 5.94 | 5.03 | 0.07 | 83.92 | |||
| 0.018 | 4.04 | 7.91 | 0.01 | 79.00 | |||
| Pseudo second-order | (mg g−1) | (g mg−1 h−1) | |||||
| 0.006 | 10.82 | 0.38 | 0.25 | 94.50 | |||
| 0.012 | 6.04 | 3.26 | 0.01 | 99.64 | |||
| 0.018 | 4.06 | 20.72 | <0.01 | 98.35 | |||
| Elovich | (mg g−1 h−1) | (g mg−1) | |||||
| 0.006 | 1.45 × 103 | 0.84 | 0.15 | 97.83 | |||
| 0.012 | 6.79 × 1012 | 5.49 | 0.05 | 90.17 | |||
| 0.018 | 2.78 × 1053 | 31.63 | 0.01 | 92.67 | |||
| = 2) | (mg g−1) | (h) | |||||
| 0.006 | 14.07 | 0.33 | 0.38 | 0.15 | 97.92 | ||
| 0.012 | 6.04 | 0.05 | 0.99 | 0.01 | 99.64 | ||
| 0.018 | 4.07 | 4.50 × 10−3 | 0.78 | <0.01 | 98.73 | ||
| Intra-particle diffusion (two phases) | (mg g−1 h−1/2) | (mg g−1) | |||||
| 0.006 | h | h | 0.07 | 99.52 | |||
| h | h | ||||||
| 0.012 | h | h | 0.03 | 96.84 | |||
| h | h | ||||||
| 0.018 | h | h | <0.01 | 99.12 | |||
| h | h | ||||||
| Model | Cu0.5Mn0.5Fe2O4 (g) | Parameters | (%) | (%) | (mg/g) | ||
|---|---|---|---|---|---|---|---|
| Langmuir | (mg g−1) | (L mg−1) | |||||
| 0.006 | - * | - * | - * | - * | - * | - * | |
| 0.012 | 994.94 | 1.08 × 10−3 | 98.82 | 98.63 | 63.08 | 77.42 | |
| 0.018 | 686.72 | 2.45 × 10−3 | 99.08 | 98.92 | 44.64 | 88.24 | |
| Freundlich | (mg g−1(L mg−1)1/n) | ||||||
| 0.006 | 0.70 | 0.14 | 97.68 | 97.29 | 28.30 | 97.09 | |
| 0.012 | 0.93 | 0.74 | 99.06 | 98.90 | 14.65 | 98.78 | |
| 0.018 | 1.09 | 1.97 | 99.70 | 99.65 | 6.23 | 99.77 | |
| Temkin | (J mol−1) | (L mol−1) | |||||
| 0.006 | 14.68 | 14.47 | 82.87 | 80.01 | 68.60 | 82.87 | |
| 0.012 | 19.48 | 17.17 | 82.15 | 79.17 | 56.09 | 82.15 | |
| 0.018 | 19.98 | 21.34 | 86.60 | 84.37 | 47.66 | 86.60 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokejaroenrat, C.; Sakulthaew, C.; Angkaew, A.; Pattanateeradetch, A.; Raksajit, W.; Teingtham, K.; Phansak, P.; Klongvessa, P.; Snow, D.D.; Harris, C.E.; et al. Adsorptive–Photocatalytic Performance for Antibiotic and Personal Care Product Using Cu0.5Mn0.5Fe2O4. Antibiotics 2023, 12, 1151. https://doi.org/10.3390/antibiotics12071151
Chokejaroenrat C, Sakulthaew C, Angkaew A, Pattanateeradetch A, Raksajit W, Teingtham K, Phansak P, Klongvessa P, Snow DD, Harris CE, et al. Adsorptive–Photocatalytic Performance for Antibiotic and Personal Care Product Using Cu0.5Mn0.5Fe2O4. Antibiotics. 2023; 12(7):1151. https://doi.org/10.3390/antibiotics12071151
Chicago/Turabian StyleChokejaroenrat, Chanat, Chainarong Sakulthaew, Athaphon Angkaew, Apiladda Pattanateeradetch, Wuttinun Raksajit, Kanokwan Teingtham, Piyaporn Phansak, Pawee Klongvessa, Daniel D. Snow, Clifford E. Harris, and et al. 2023. "Adsorptive–Photocatalytic Performance for Antibiotic and Personal Care Product Using Cu0.5Mn0.5Fe2O4" Antibiotics 12, no. 7: 1151. https://doi.org/10.3390/antibiotics12071151
APA StyleChokejaroenrat, C., Sakulthaew, C., Angkaew, A., Pattanateeradetch, A., Raksajit, W., Teingtham, K., Phansak, P., Klongvessa, P., Snow, D. D., Harris, C. E., & Comfort, S. D. (2023). Adsorptive–Photocatalytic Performance for Antibiotic and Personal Care Product Using Cu0.5Mn0.5Fe2O4. Antibiotics, 12(7), 1151. https://doi.org/10.3390/antibiotics12071151










