Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain, Media, and Growth Conditions
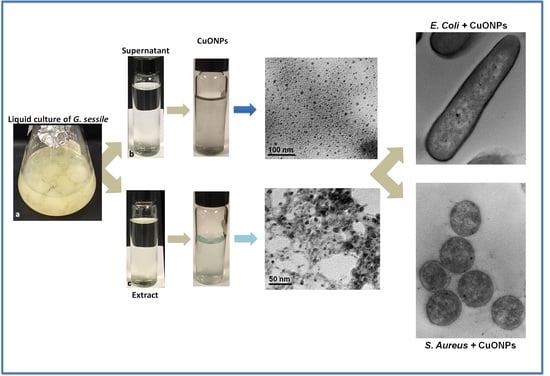
2.2. Obtention of Fungal Supernatant and Extract
2.3. Biosynthesis of Copper Oxide Nanoparticles (CuONPs)
2.4. Characterization of CuONPs
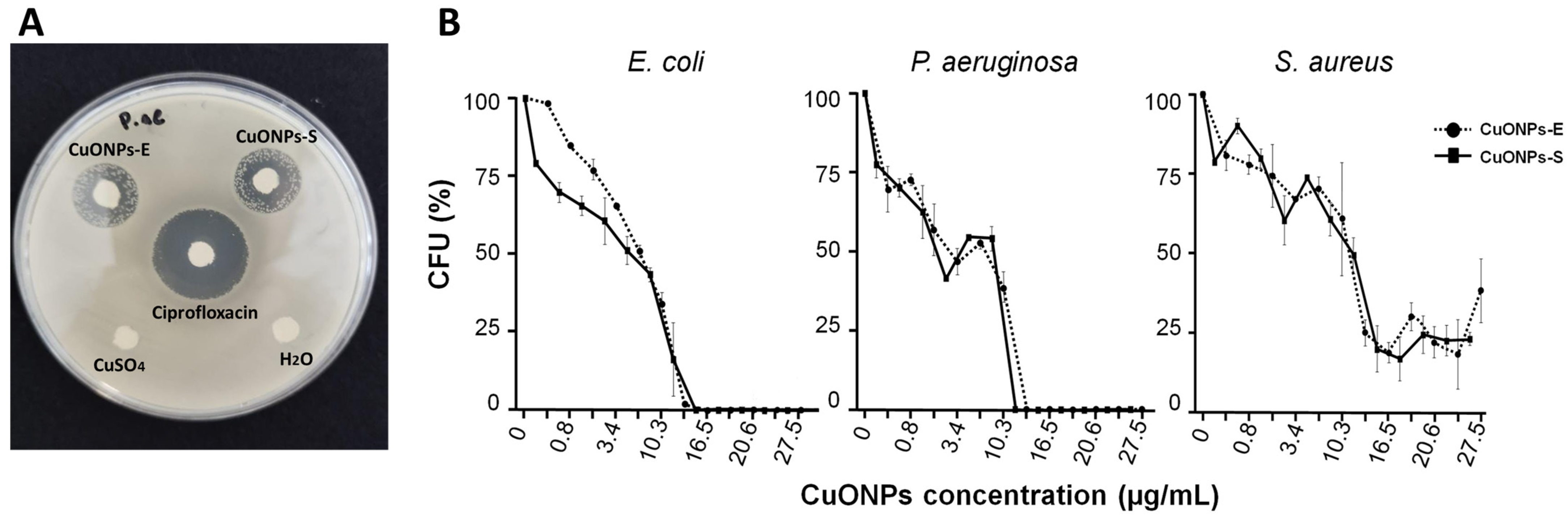
2.5. Evaluation of Antibacterial Activity
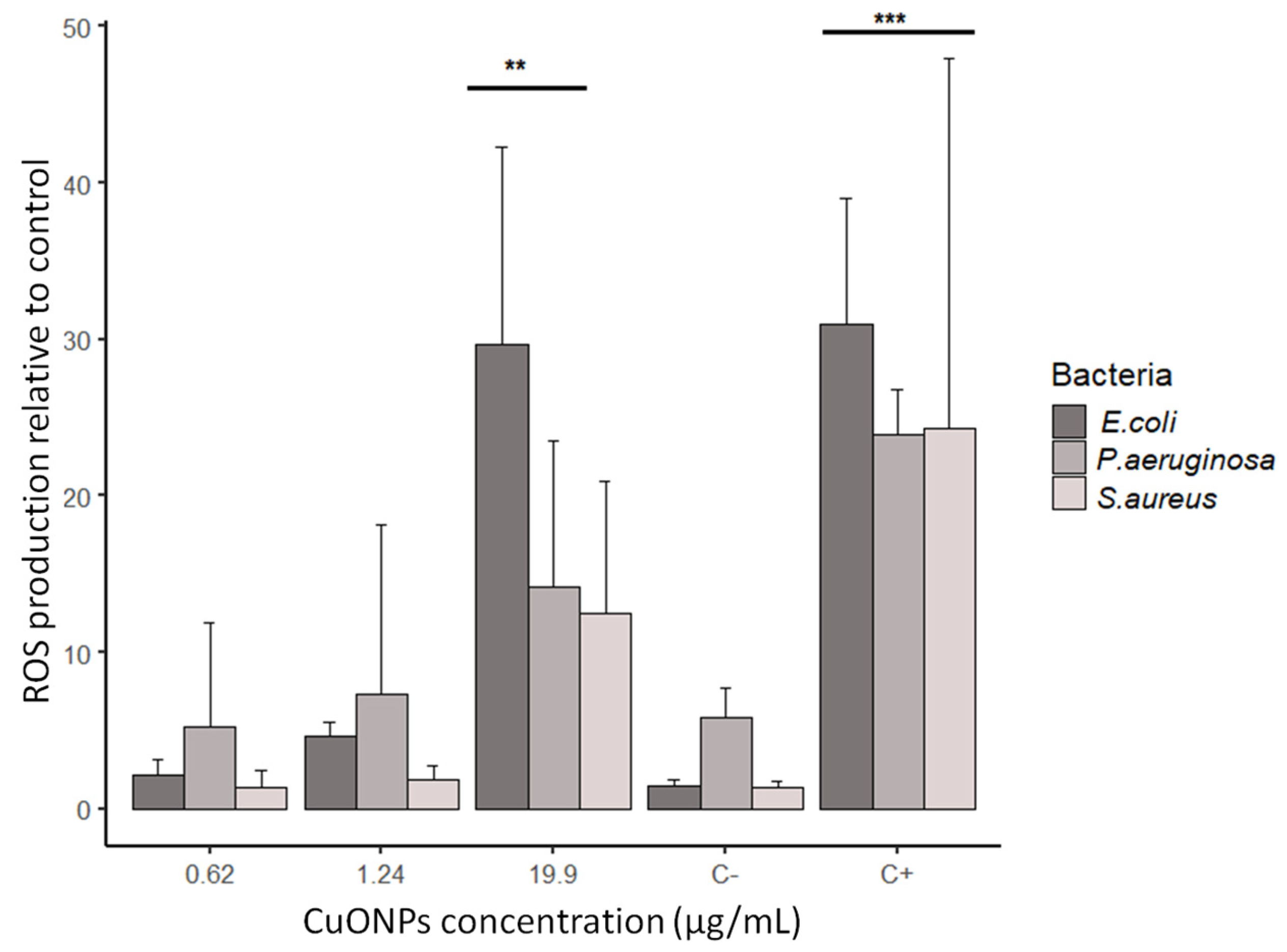
2.6. ROS Production in Bacteria
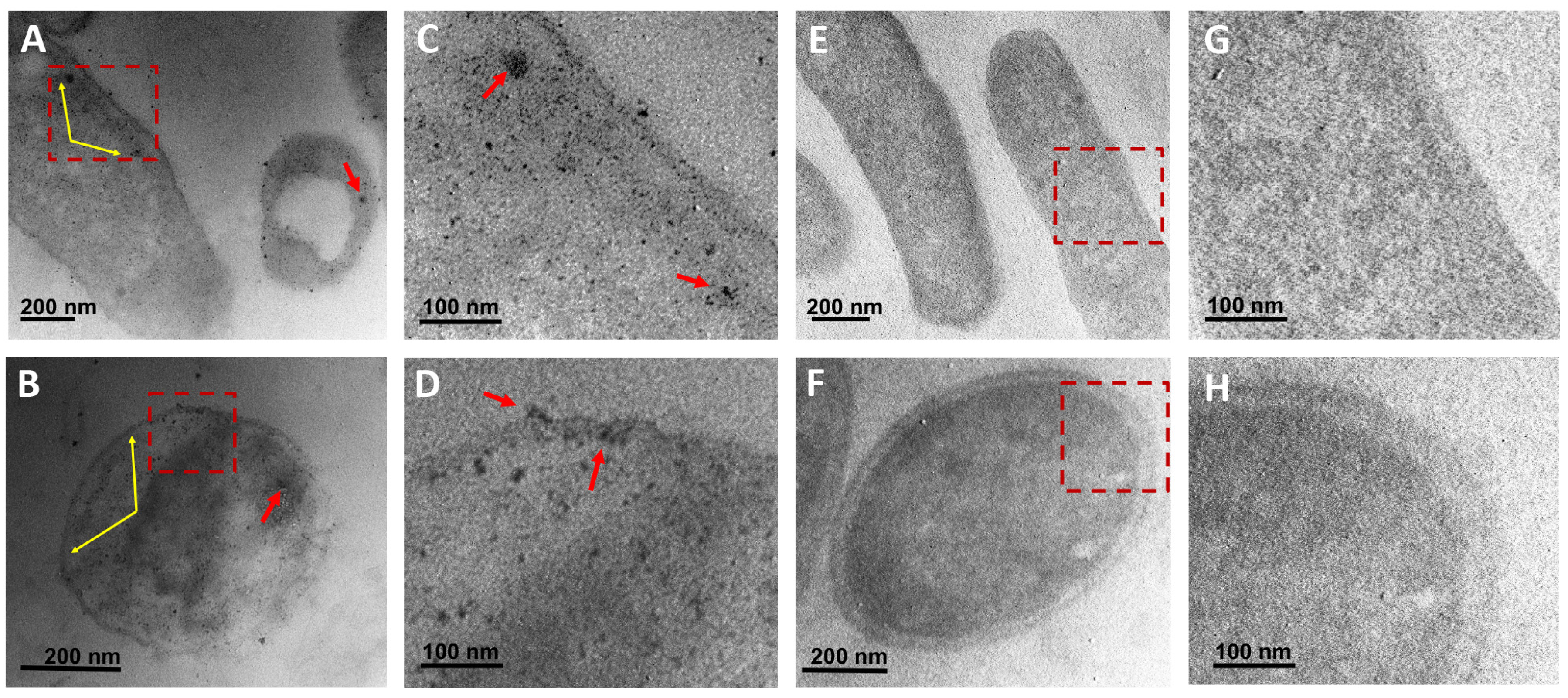
2.7. Ultrastructural Analysis of Bacteria
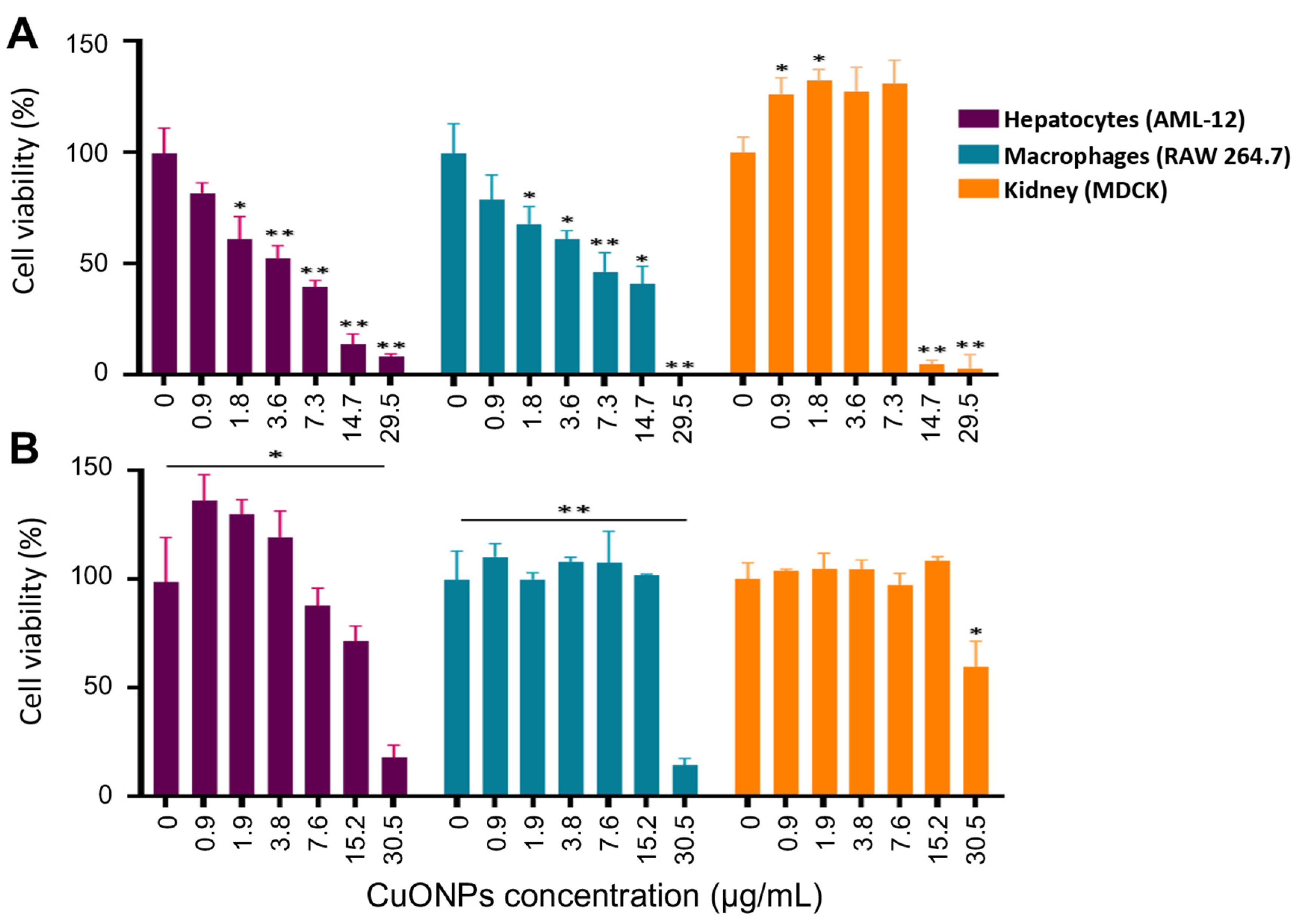
2.8. Biocompatibility of CuONPs in Mammalian Cell Lines
2.9. Statistical Analysis
3. Results
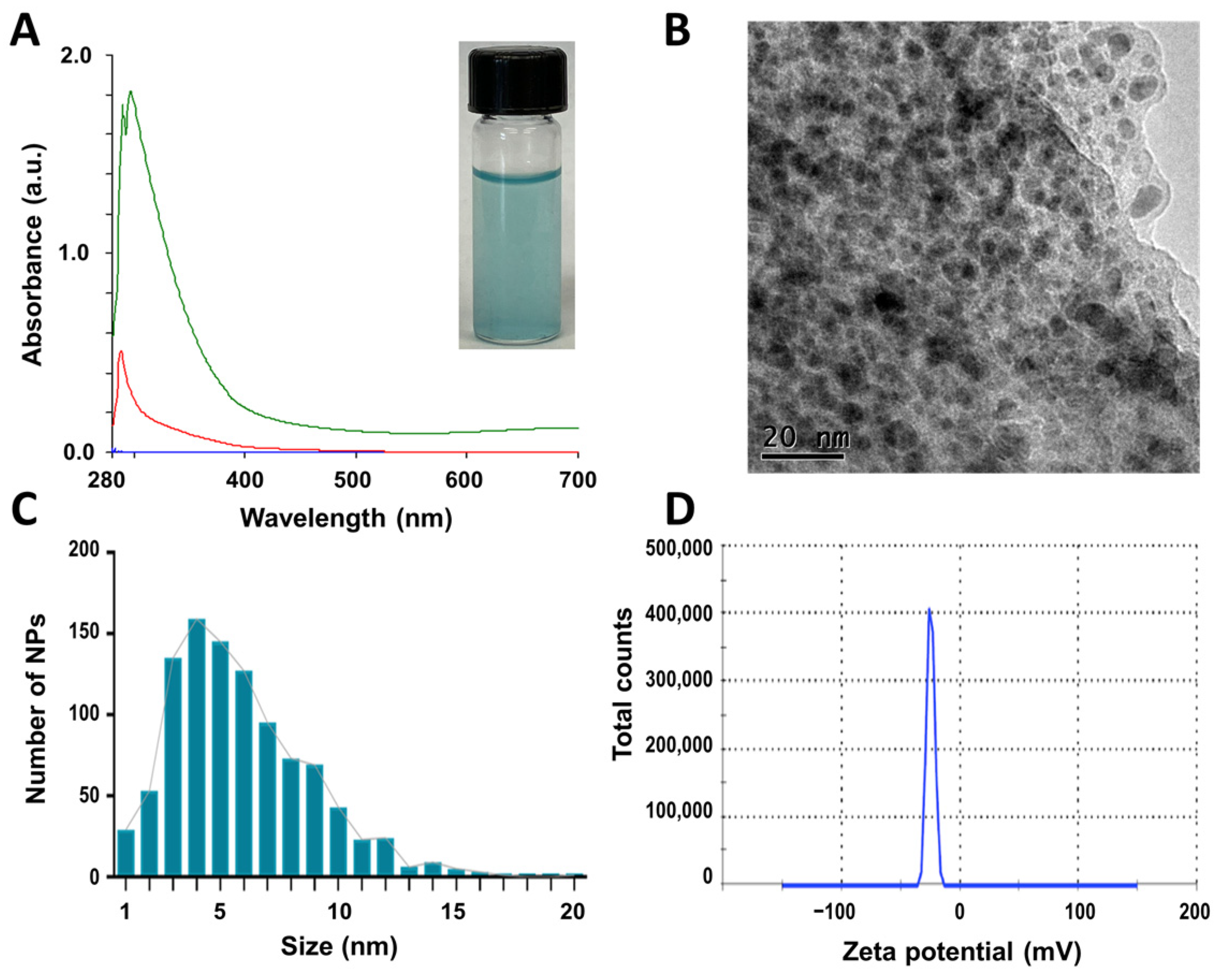
3.1. Nanoparticle Characterization
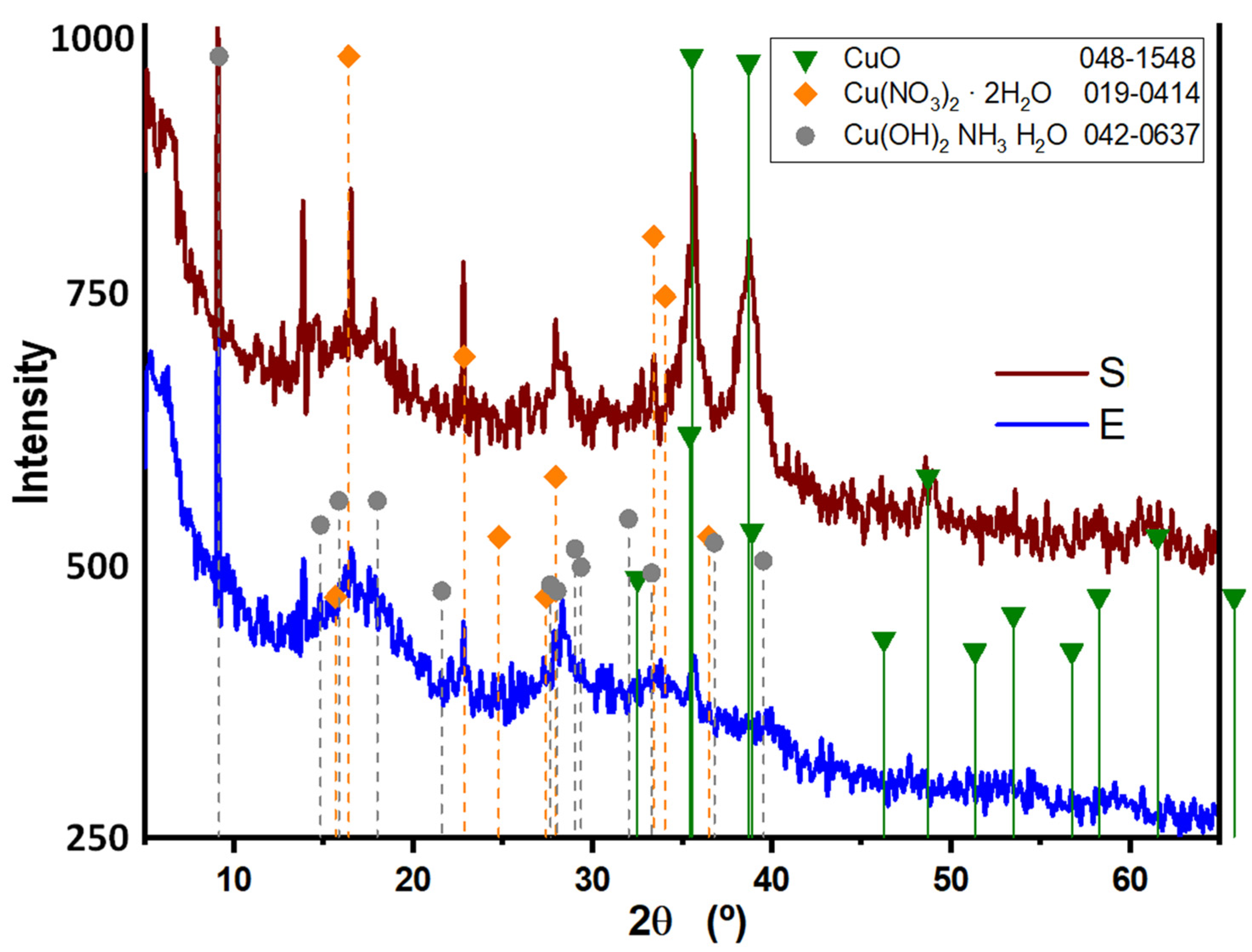
3.2. X-Ray Diffraction (XRD) Patterns of Synthesized CuONPs
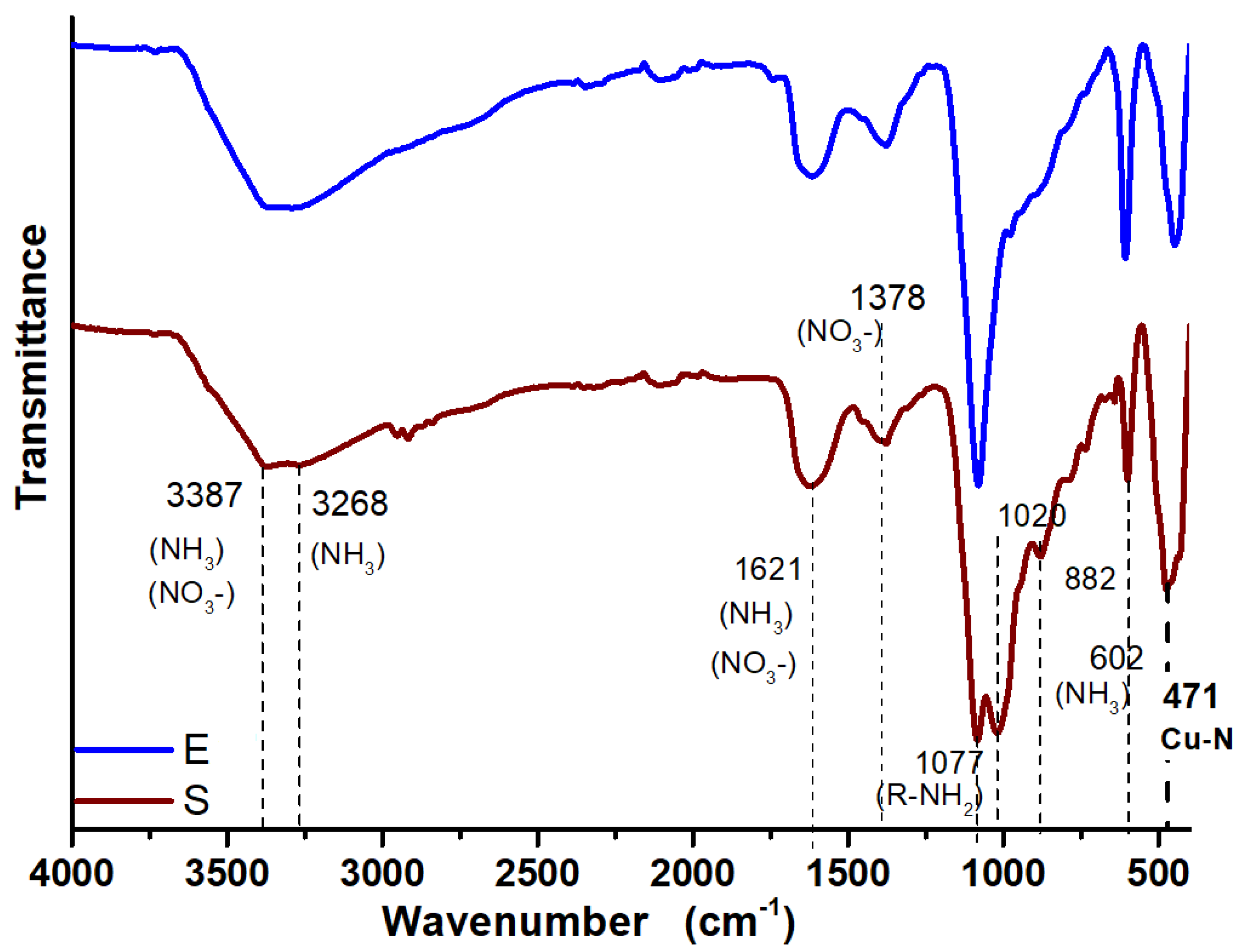
3.3. FTIR Analysis of Synthesized CuONPs
3.4. Antibacterial Capacity
3.5. ROS Production in Bacteria
3.6. Ultrastructural Analysis of Bacteria
3.7. Toxicity of CuONPs in Mammalian Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Kaweeteerawat, C.; Na Ubol, P.; Sangmuang, S.; Aueviriyavit, S.; Maniratanachote, R. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J. Toxicol. Environ. Health-Part A Curr. Issues 2017, 80, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Therapeutic, Nanoparticles in Medicine: Applications and Developments. Educ. Policy Anal. Arch. 2007, 8, 761–769. [Google Scholar]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef]
- Lu, Y.; Lihua, L.; Zhu, Y.; Wang, X.; Li, M.; Zefeng, L.; Xiaoming, H.; Zhang, Y.; Qingshiu, Y.; Chuanbin, M. Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. ACS Appl. Mater. Interfaces 2018, 10, 127–138. [Google Scholar] [CrossRef]
- Norambuena, G.A.; Patel, R.; Karau, M.; Wyles, C.C.; Jannetto, P.J.; Bennet, K.E.; Hanssen, A.D.; Sierra, R.J. Antibacterial and Biocompatible Titanium-Copper Oxide Coating May Be a Potential Strategy to Reduce Periprosthetic Infection: An In Vitro Study. Clin. Orthop. Relat. Res. 2017, 475, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Menon, S.; Venkat Kumar, S.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L.; et al. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. B Biol. 2019, 197. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.H.; Min, J.; Kim, H.J.; Jee, J.P.; Park, B.J. Functionalized ZnO nanoparticles with gallic acid for antioxidant and antibacterial activity against methicillin resistant S. aureus. Nanomaterials 2017, 7, 365. [Google Scholar] [CrossRef]
- Lakshmi, K.R.; Sri Venkata, N.P.; Girija, S.G.; Veerabhadra, S.P.; Venkata, R.M.K. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2019, 7, 42–59. [Google Scholar] [CrossRef]
- Henson, T.E.; Navratilova, J.; Tennant, A.H.; Bradham, K.D.; Rogers, K.R.; Hughes, M.F. In vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models. Nanotoxicology 2019, 13, 795–811. [Google Scholar] [CrossRef]
- Midander, K.; Cronholm, P.; Karlsson, H.L.; Elihn, K.; Möller, L.; Leygraf, C.; Wallinder, I.O. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(ll) oxide particles: A cross-disciplinary study. Small 2009, 5, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G.J.S.A.P.A.M. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Oskam, G. Metal oxide nanoparticles: Synthesis, characterization and application. J. Sol-Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.; Balu, S.K.; Jeevanandham, J.; Muthalagu, M.; Vidyavathy, M.; Chan, Y.S.; Danquah, M.K. Phytosynthesized metal oxide nanoparticles for pharmaceutical applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 755–771. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Shafey, A.M.E. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Castro-Longoria, E. Fungal Biosynthesis of Nanoparticles, a Cleaner Alternative. In Fungal Applications in Sustainable Environmental Biotechnology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 323–351. [Google Scholar]
- Abdel-Wareth, M.T.A. Fungal applications in sustainable environmental biotechnology. En Int. J. Environ. Stud. 2017. [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef]
- Murillo-Rábago, E.I.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Garcia-Marin, L.E.; Quester, K.; Castro-Longoria, E. Optimized synthesis of small and stable silver nanoparticles using intracellular and extracellular components of fungi: An alternative for bacterial inhibition. Antibiotics 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Sudhasree, S.; Shakila Banu, A.; Brindha, P.; Kurian, G.A. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol. Environ. Chem. 2014, 96, 743–754. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Rosado, M.J.; Guerrero, A.; Romero, A. Eco-friendly synthesis of ZnO-nanoparticles using Phoenix dactylifera L.; polyphenols: Physicochemical, microstructural, and functional assessment. New J. Chem. 2023, 47, 4409–4417. [Google Scholar]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci. Rep. 2020, 10, 16680. [Google Scholar] [CrossRef]
- Ashraf, N.; Ahmad, F.; Da-Wei, L.; Zhou, R.B.; Feng-Li, H.; Yin, D.C. Iron/iron oxide nanoparticles: Advances in microbial fabrication, mechanism study, biomedical, and environmental applications. Crit. Rev. Microbiol. 2019, 45, 278–300. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ.-Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Effect of Calcination Temperature and Time on the Synthesis of Iron Oxide Nanoparticles: Green vs. Chem. Method. Mater. 2023, 16, 1798. [Google Scholar]
- Noor, S.; Shah, Z.; Javed, A.; Ali, A.; Hussain, S.B.; Zafar, S.; Ali, H.; Muhammad, S.A. A fungal based synthesis method for copper nanoparticles with the determination of anticancer, antidiabetic and antibacterial activities. J. Microbiol. Methods 2020, 174, 105966. [Google Scholar] [CrossRef]
- Erci, F.; Cakir-Koc, R.; Yontem, M.; Torlak, E. Synthesis of biologically active copper oxide nanoparticles as promising novel antibacterial-antibiofilm agents. Prep. Biochem. Biotechnol. 2020, 50, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tulsawani, R. Ganoderma lucidum aqueous extract prevents hypobaric hypoxia induced memory deficit by modulating neurotransmission, neuroplasticity and maintaining redox homeostasis. Sci. Rep. 2020, 10, 8944. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Shirokov, A.; Bucharskaya, A.; Navolokin, N.; Prilepskii, A.; Burov, A.; Maslyakova, G.; Nikitina, V. Antitumor activity of extracts from medicinal basidiomycetes mushrooms. Int. J. Med. Mushrooms 2016, 18, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.K.S.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard (CLSI docum); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Garcia-Marin, L.E.; Juarez-Moreno, K.; Vilchis-Nestor, A.R.; Castro-Longoria, E. Highly Antifungal Activity of Biosynthesized Copper Oxide Nanoparticles against Candida albicans. Nanomaterials 2022, 12, 3856. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [PubMed]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Hathaway, B.J.; Tomlinson, A.A.G. Copper (II) ammonia complexes. Coord. Chem. Rev. 1970, 5, 1–43. [Google Scholar]
- Bishop, K.S.; Kao, C.H.; Xu, Y.; Glucina, M.P.; Paterson, R.R.M.; Ferguson, L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green synthesis of copper oxide nanoparticles using Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii. Dig. J. Nanomater. Biostructures 2012, 7, 999–1005. [Google Scholar]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Oza, G.; Calzadilla-Avila, A.I.; Reyes-Calderón, A.; Anna, K.K.; Ramírez-Bon, R.; Tapia-Ramirez, J.; Sharma, A. pH-dependent biosynthesis of copper oxide nanoparticles using Galphimia glauca for their cytocompatibility evaluation. Appl. Nanosci. 2020, 10, 541–550. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Zuo, Y.; Chen, J.S.; Song, J.M.; Niu, H.L.; Mao, C.J.; Zhang, S.Y.; Shen, Y.H. Synthesis of metal oxide nanoparticles (CuO and ZnO NPs) via biological template and their optical sensor applications. Appl. Surf. Sci. 2017, 397, 167–174. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar]
- Nagore, P.; Ghotekar, S.; Mane, K.; Ghoti, A.; Bilal, M.; Roy, A. Structural properties and antimicrobial activities of Polyalthia longifolia leaf extract-mediated CuO nanoparticles. BioNanoScience 2021, 11, 579–589. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goyal, D.; Chudasama, B. Antibacterial activity of colloidal copper nanoparticles against Gram-negative (Escherichia coli and Proteus vulgaris) bacteria. Lett. Appl. Microbiol. 2022, 74, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ameh, T.; Gibb, M.; Stevens, D.; Pradhan, S.H.; Braswell, E.; Sayes, C.M. Silver and copper nanoparticles induce oxidative stress in bacteria and mammalian cells. Nanomaterials 2022, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Safronova, A.S.; Mamaeva, P.V.; Kolmogorov, I.M.; Skuredina, A.A.; Kudryashova, E.V. Drug–membrane interaction as revealed by spectroscopic methods: The role of drug structure in the example of rifampicin, levofloxacin and rapamycin. Biophysica 2022, 2, 353–365. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 6003–6009. [Google Scholar]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Humphreys, H. Self-disinfecting and microbiocide-impregnated surfaces and fabrics: What potential in interrupting the spread of healthcare-associated infection? Clin. Infect. Dis. 2014, 58, 848–853. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- World Health Organization. A Global Overview of National Regulations and Standards for Drinking-Water Quality; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Liu, H.; Guo, H.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper Induces Hepatic Inflammatory Responses by Activation of MAPKs and NF-KB Signalling Pathways in the Mouse. Ecotoxicol. Environ. Saf. 2020, 201, 110806. [Google Scholar] [CrossRef]



| NPs | Physicochemical Characteristics | Antimicrobial Effect | Toxicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEM | PDI | ZP (mV) | Bacteria (µg/mL) | Cell Lines | |||||||||
| Size (nm) | Shape | E. coli | P. aeruginosa | S. aureus | AML-12 | RAW-264.7 | MDCK | ||||||
| MIC | IC50 | MIC | IC50 | MIC | IC50 | IC50 | IC50 | IC50 | |||||
| CuONPs-S | 4.5 ± 1.9 | QS | 0.619 | −28.7 | 15.9 | 8.0 | 13.7 | 4.1 | ND | 8.8 | 3.6 | 7.3 | 7.3 |
| CuONPs-E | 5.2 ± 2.1 | QS | 0.674 | −24.8 | 16.5 | 8.5 | 16.5 | 3.4 | ND | 10.2 | 14.7 | 29.5 | 29.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics 2023, 12, 1251. https://doi.org/10.3390/antibiotics12081251
Flores-Rábago KM, Rivera-Mendoza D, Vilchis-Nestor AR, Juarez-Moreno K, Castro-Longoria E. Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics. 2023; 12(8):1251. https://doi.org/10.3390/antibiotics12081251
Chicago/Turabian StyleFlores-Rábago, Karla M., Daniel Rivera-Mendoza, Alfredo R. Vilchis-Nestor, Karla Juarez-Moreno, and Ernestina Castro-Longoria. 2023. "Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile" Antibiotics 12, no. 8: 1251. https://doi.org/10.3390/antibiotics12081251
APA StyleFlores-Rábago, K. M., Rivera-Mendoza, D., Vilchis-Nestor, A. R., Juarez-Moreno, K., & Castro-Longoria, E. (2023). Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics, 12(8), 1251. https://doi.org/10.3390/antibiotics12081251